Abstract
Background
Screening for colorectal cancer (CRC) reduces mortality, yet over one-third of age-eligible Americans are unscreened.
Objective
To examine the effect of a digital health intervention (mPATH-CRC) on rates of CRC screening.
Design
Randomized clinical trial.
Setting
Six community-based primary care practices.
Participants
450 patients (223 mPATH-CRC, 227 usual care) scheduled for a primary care visit and due for routine CRC screening.
Intervention
An iPad app that displays a CRC screening decision aid, lets patients order their own screening tests, and sends automated follow-up electronic messages to support patients.
Measurements
Chart-verified completion of CRC screening within 24 weeks (primary outcome); ability to state a screening preference, intention to receive screening, screening discussions, and orders for screening tests (secondary outcomes). All outcome assessors were blinded to randomization arm.
Results
Baseline characteristics were similar between groups and included 37% with limited health literacy and 53% with annual incomes < $20,000. CRC screening was completed by 30% of mPATH-CRC participants and 15% of those receiving usual care (logistic regression OR 2.5, 95% CI 1.6 – 4.0). Compared to usual care, more mPATH-CRC participants could state a screening preference, planned to be screened within 6 months, discussed screening with their provider, and had a screening test ordered. Half of mPATH-CRC participants (53%; 118 of 223) “self-ordered” a test via the program.
Limitations
Study was conducted among English-speaking individuals within a single healthcare system.
Conclusions
A digital health intervention that allows patients to “self-order” tests can increase receipt of CRC screening. Future research should identify methods for implementing similar interventions into clinical care.
Trial registration
Primary Funding Source
National Cancer Institute
INTRODUCTION
Screening for colorectal cancer (CRC) reduces incidence and mortality.(1) Several CRC tests have been shown to be cost-effective,(2–5) and widely-accepted guidelines recommend routine screening for adults starting at age 50.(6–8) Despite broad support for CRC screening, over one-third of age-eligible Americans are unscreened, and CRC remains the second-leading cause of cancer death in the United States.(9,10)
Multiple barriers contribute to the low screening rates observed. Some patients have negative attitudes about CRC screening tests or are unaware of their need for screening or the tests available.(11–15) Busy clinicians lack the time to fully discuss screening options and explore patients’ concerns.(16,17) Additionally, once patients leave the office, there is little support for those who encounter difficulties preparing for or completing their screening tests.
Barriers to CRC screening are even greater in vulnerable populations, such as those with limited health literacy or low incomes.(10,18,19) Over one-third of Americans have limited health literacy, or a decreased capacity to obtain, understand, and process health information.(20,21) Patients with limited literacy are less likely to understand medical information, less likely to ask questions, and less likely to receive preventive services.(22,23) Accordingly, CRC screening rates are even lower among those with low health literacy, low education, or low income.(24,25)
Decision aids are a partial solution. Decision aids address patient knowledge and attitude barriers well, but fail to address many of the provider and system barriers.(26) In our prior-randomized controlled trial of a web-based decision aid in vulnerable patients, the decision aid increased patients’ desire to receive CRC screening, yet orders for screening tests and completion of screening increased by only 9% and 5% respectively, differences that were not statistically significant.(27) To meaningfully increase receipt of CRC screening, additional efforts are needed that address these multilevel barriers and reach broadly across populations.
Recent increases in mobile device ownership create new opportunities for developing novel effective and efficient interventions. Over half of American adults own a tablet device, over three-quarters own a smartphone, and 95% own a cellphone.(28) Cellphone ownership is consistent across major sociodemographic groups,(28) giving interventions that incorporate text messaging broad reach.
To leverage patients’ growing familiarity with mobile devices, we developed a digital health CRC screening intervention that addresses patient, provider, and system barriers. The intervention, called mobile Patient Technology for Health – CRC (mPATH-CRC) is an iPad app that informs patients of their need for screening, helps them make a screening decision, lets them “self-order” a screening test, and sends automated electronic messages to help them complete their chosen test. Patients use mPATH-CRC at their primary care provider’s office on a device owned by the practice, with the opportunity to receive follow-up support on their own devices. We examined the efficacy of mPATH-CRC in a socioeconomically diverse patient population.
METHODS
Design Overview
We conducted a parallel design randomized-controlled trial to determine the impact of mPATH-CRC on receipt of screening within 24 weeks. Participants, enrolled between June 2014 and May 2016, were assigned with equal probability to either usual care or mPATH-CRC. Follow-up was complete in October 2016. Participants were told the study was being done “to determine the best way to teach people about important health topics.” Study interviewers and outcome assessors were blinded to participant allocation. The Wake Forest Baptist Health Institutional Review Board approved the study, and all participants provided informed consent.
Setting and Participants
We conducted the study in 6 community-based primary care practices affiliated with a large academic health system in North Carolina. Three practices serve a primarily urban/suburban population, and three practices serve a rural population. The health system has a strong commitment to CRC screening. All practices share a common electronic health record (EHR) that notifies providers if CRC screening is due.
We queried the EHR to identify English-speaking individuals aged 50 to 74 years who were scheduled to see a primary care provider and were due for CRC screening. We excluded patients with a prior history of CRC or colonic polyps, a family history of CRC, recent rectal bleeding, a functional impairment that would prevent them from using an iPad, or a medical condition that predicted shorter life-expectancy (dementia, recent treatment for cancer, advanced stage cancer, or end-stage renal disease). A research assistant called these patients to confirm eligibility and invite them to participate. Patients agreeing to participate were asked to arrive 45–60 minutes prior to their scheduled medical visit to enroll in the study and use the program.
Randomization and Interventions
The study program randomly assigned participants, stratified by site, to either the mPATH-CRC or Control Program with equal probability using variably sized permuted block randomization with random block sizes of 2 or 4. The random allocation sequences were generated by the study statistician using nQuery Advisor 7.0 and stored on the iPads used at each site in files accessible only by the study programmer. Two iPads were used at the largest clinic, each with its own allocation sequence.
On the day of enrollment, participants met with a research assistant in a private room at their primary care clinic. The research assistant launched the study app on the iPad, which displayed the program determined by the randomization, then handed the iPad to the participant and waited outside the door. After completing the assigned program, patients immediately proceeded to their scheduled medical visits.
Details of the two programs (mPATH-CRC and the Control Program) and their usability have been previously published.(29) Each program includes an identical baseline self-survey and post-program self-survey to collect demographic information and assess short-term outcomes described below. The survey estimates health literacy level using the single item health literacy screening question, “How confident are you filling out medical forms by yourself?”(30)
mPATH-CRC Program
Participants who interacted with mPATH-CRC viewed a previously validated 8.6 minute decision aid about CRC screening that reviewed the two most commonly used CRC screening tests, fecal testing for blood and colonoscopy.(25,31) The mPATH-CRC program then let patients “order” their own screening test (either fecal testing or colonoscopy). If patients self-ordered a screening test, mPATH-CRC requested a cellphone number or email address for follow-up electronic messages designed to help them complete the screening procedure.
When the participant finished using mPATH-CRC, the program informed the research staff of the patient’s decision. If the patient “self-ordered” a test, the research assistant immediately entered a co-signature required order under the primary care provider’s name in the EHR. In the EPIC-based EHR used by the study practices, any co-signature required order is automatically sent to the provider’s digital “in basket” for approval or denial with a single click. The research assistant also attached a half-page flier to the patient’s clinic paperwork to alert the primary care provider of the decision made.
A separate series of electronic messages was developed for the three screening tests used in the study clinics: guaiac-based fecal occult blood testing (gFOBT), fecal immunohistochemical testing, and colonoscopy. A computerized protocol automatically sent messages to give patients key information at specific times. For example, a message in the colonoscopy pathway delivered the day before the procedure encouraged patients to complete the bowel prep and reminded them they would need a driver. Patients could receive 3 to 10 messages depending on the test ordered and their responses to the messages.
Control Program
The control program included a 4.3 minute video about diet and exercise produced by the Centers for Disease Control.(32) Control participants were not given the option to self-order screening tests or receive follow-up electronic messages.
Outcomes and Follow-up
The next business day after the primary care visit, a research assistant called all participants to administer a telephone survey assessing whether they discussed CRC screening with their provider and their satisfaction with any decisions made. Twenty-four weeks after the visit, a final telephone survey was administered by a research assistant to all participants, and study staff reviewed charts to determine if CRC screening had been ordered and completed. If screening was ordered but not completed, participants were asked why. All interviewers and outcome assessors were blinded to study group assignment.
The primary outcome was chart-verified completion of a CRC screening test within 24 weeks of study enrollment. Participants who reported receiving screening outside the health system on the final survey were asked for permission to obtain their records for verification. A second chart review was conducted for any patient not reached on the final survey or for any disagreements between the final survey and initial chart review. Any discrepancies were resolved by the consensus of a blinded adjudication committee. Discrepancies related to test completion occurred for less than 2% of participants (7/450).
Secondary outcomes were assessed for all participants and included patients’ ability to state a screening preference and their intention to receive screening as measured on the post-program iPad survey. We determined ability to state a preference with the single item, “If you were going to be tested for colon cancer, which test would you want to have?” with possible answers including “stool test for blood”, “colonoscopy”, “I never want to be tested”, and “I don’t know enough to decide.” Intention to receive screening was measured with the item “Are you seriously thinking about getting tested for colon cancer?” with possible answers ranging from “Yes, within the next 30 days” to “No, I am not thinking of getting tested.” Additional secondary outcomes included participant discussions of CRC screening with their provider (from the next day telephone survey), and CRC screening tests ordered (from chart review). Program usability, decisional satisfaction, and costs were also collected but are not reported here.
Statistical analysis
Our target sample size of 450 participants was chosen to detect a 12% difference in screening completion (20% vs 32%) with 80% power at the 5% two-sided level of significance. There was no adjustment for loss to follow-up as the primary outcome was being determined by chart review.
We used logistic regression to assess the effect of mPATH-CRC on completion of screening. Because our study was randomized and stratified by clinic, the primary model only included the intervention arm and clinic site as covariates. Simple chi-square tests were used to assess group differences in the secondary outcomes. The intervention effect was estimated as the difference in proportions with corresponding confidence intervals. These analyses were also used to assess differences in other independent groups, such as test completion rates for ordered fecal tests vs ordered colonoscopies.
Only five of the self-survey demographic questions had some responses of “don’t know” or missing data. The number of missing responses ranged from 1 (0.2%) for cellphone ownership to 15 (3.3%) for date of last routine health checkup. These data were not used in the primary analysis. We purposefully designed mPATH-CRC for use in populations prone to health disparities.(29) Therefore, we conducted subgroup analyses of the effect of mPATH-CRC in those with low income (<$20,000 per year), limited literacy, and black/African-American race. Logistic regression was used to assess the interactions of intervention arm with income, literacy and race. All analyses were done using SAS version 9.4 (Cary, North Carolina) with 2-sided tests and an alpha of 0.05. Procedures used included Proc Freq with the riskdiff option for assessing unadjusted differences, and Proc Logistic for assessing the difference in the primary outcome adjusted for clinic.
Role of the funding source
The study received funding and support from the National Cancer Institute (R01CA178941), the Wake Forest Clinical and Translational Science Institute study coordinator pool (UL1TR001420), and the shared resources provided by the Wake Forest Comprehensive Cancer Center (CCSG P30CA012197). No funding organization played a role study conduct, manuscript preparation, or decision to submit for publication.
RESULTS
Research assistants screened 1183 patients to identify 640 eligible individuals, of whom 450 enrolled.(Figure 1) Approximately half (n=223) were randomized to mPATH-CRC and half (n=227) to the control program. All participants completed the assigned iPad programs, including the embedded self-surveys. Almost all participants completed the 24 week follow-up survey (86% [192/223] for mPATH and 90% [204/227] for control). Research staff blinded to randomization completed chart-reviews for all 450 participants.
Figure 1.
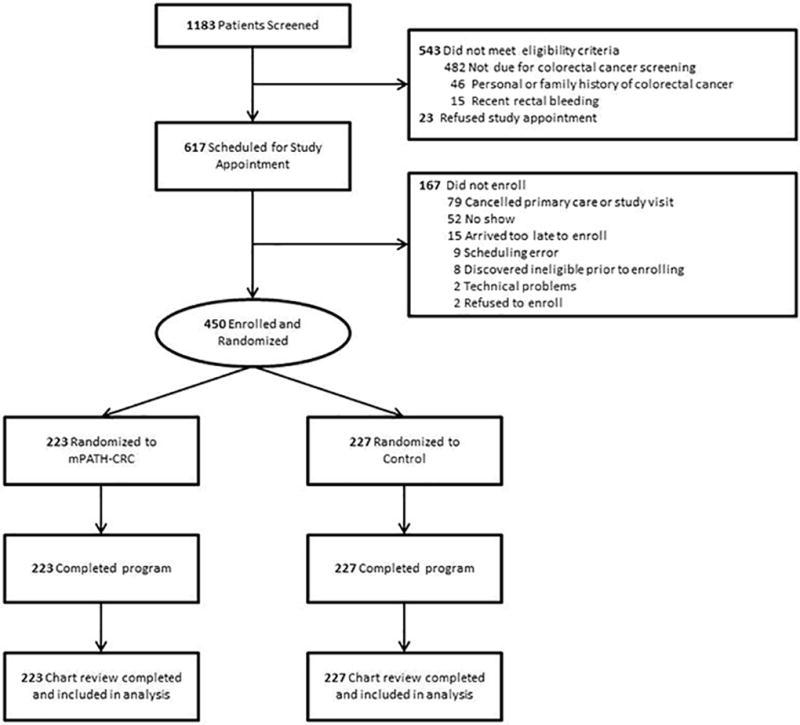
CONSORT flowchart
Enrolled participants (median age 57 years, range 50–74) exhibited racial and socioeconomic diversity. Overall, 38% of participants self-identified as African-American, 37% had limited health literacy, and 53% had annual household incomes less than 20,000 US dollars. Patient characteristics were similar between the two groups.(Table 1)
Table 1.
Baseline Characteristics of Trial Participants.
| Characteristic* | mPATH-CRC (n=223) |
Control (n=227) |
Total (N=450) |
|---|---|---|---|
|
| |||
| # (%) | # (%) | # (%) | |
| Age, Median (Range) | 58 (50–74) | 57 (50–74) | 57 (50–74) |
| Female | 124 (56) | 118 (52) | 242 (54) |
| Race/Ethnicity | |||
| White, non-Hispanic | 125 (56) | 133 (59) | 258 (57) |
| African-American | 87 (39) | 82 (36) | 169 (38) |
| Hispanic/Latino | 5 (2) | 5 (2) | 10 (2) |
| Other | 6 (3) | 7 (3) | 13 (3) |
| Clinic Site | |||
| 1 | 89 (40) | 92 (41) | 181 (40) |
| 2 | 56 (25) | 57 (25) | 113 (25) |
| 3 | 18 (8) | 18 (8) | 36 (8) |
| 4 | 13 (6) | 14 (6) | 27 (6) |
| 5 | 33 (15) | 32 (14) | 65 (14) |
| 6 | 14 (6) | 14 (6) | 28 (6) |
| Education | |||
| < High school | 46 (21) | 49 (22) | 95 (21) |
| High school | 70 (31) | 74 (33) | 144 (32) |
| Some college or more | 107 (48) | 104 (46) | 211 (47) |
| Married/Living as Married | 104 (47) | 105 (46) | 209 (46) |
| Health insurance | |||
| Uninsured | 28 (13) | 33 (15) | 61 (14) |
| Medicaid | 46 (21) | 34 (15) | 80 (18) |
| Medicare | 58 (26) | 50 (22) | 108 (24) |
| Commercial | 91 (41) | 110 (48) | 201 (45) |
| Employed | 85 (38) | 83 (37) | 168 (37) |
| Annual Household Income < $20,000† | 118 (55) | 113 (51) | 231 (53) |
| Limited health literacy | 90 (40) | 76 (33) | 166 (37) |
| Cell phone ownership‡ | 191 (86) | 207 (92) | 398 (89) |
| Internet use last 30 days§ | 137 (62) | 148 (66) | 285 (64) |
| Last visit for routine checkup‖ | |||
| Within last year | 139 (64) | 135 (62) | 274 (63) |
| One to two years ago | 33 (15) | 44 (20) | 77 (18) |
| More than two years ago | 45 (21) | 39 (18) | 84 (19) |
| General Health¶ | |||
| Very good to excellent | 51 (23) | 55 (25) | 106 (24) |
| Good | 87 (40) | 84 (38) | 171 (39) |
| Poor to fair | 81 (37) | 83 (37) | 164 (37) |
| Lives in rural area** | 51 (23) | 51 (22) | 102 (23) |
A few participants responded “don’t know” or skipped some items on the self-surveys;
n=214 in the mPATH-CRC arm and 223 in the Control arm;
n=223 and 226;
n=222 and 223;
n=217 and 218;
n=219 and 222
rural dwelling defined by Rural-Urban Continuum Areas codes ≥ 4(43)
Screening test completion
mPATH-CRC participants were twice as likely as control participants to complete a screening test (30% [67/223] vs. 15% [34/227], difference=15%, 95% CI 7% to 23%). The odds ratio for completing screening was 2.5 (95% CI 1.6 – 4.0) in favor of mPATH participants after accounting for the stratification factor (clinic). Results were fairly consistent across clinics (interaction p-value = 0.82). Only one clinic had an intervention effect of less than 13%.(Table 2)
Table 2.
Differences in colorectal cancer screening rates between intervention groups
| mPATH-CRC | Control | |||||
|---|---|---|---|---|---|---|
| Characteristic | n | % Screened (#) | n | % Screened (#) | Difference | (95% CI) |
| Overall | 223 | 30% (67) | 227 | 15% (34) | 15% | (7%, 23%) |
| Clinic | ||||||
| 1 | 89 | 29% (26) | 92 | 15% (14) | 14% | (2%, 26%) |
| 2 | 56 | 21% (12) | 57 | 9% (5) | 13% | (0%, 26%) |
| 3 | 18 | 50% (9) | 18 | 22% (4) | 28% | (−2%, 58%) |
| 4 | 13 | 46% (6) | 14 | 21% (3) | 25% | (−10%, 59%) |
| 5 | 33 | 27% (9) | 32 | 25% (8) | 2% | (−19%, 24%) |
| 6 | 14 | 36% (5) | 14 | 0% (0) | 36% | (11%, 61%) |
| Health Literacy | ||||||
| Limited | 90 | 24% (22) | 76 | 16% (12) | 9% | (−3%, 21%) |
| Adequate | 133 | 34% (45) | 151 | 15% (22) | 19% | (9%, 29%) |
| Income | ||||||
| < 20K | 118 | 25% (29) | 113 | 15% (17) | 10% | (−1%, 20%) |
| ≥20K | 96 | 38% (36) | 110 | 15% (17) | 22% | (10%, 34%) |
| Race/Ethnicity | ||||||
| Not NHW* | 98 | 34% (33) | 94 | 19% (18) | 15% | (2%, 27%) |
| NHW | 125 | 27% (34) | 133 | 12% (16) | 15% | (6%, 25%) |
NHW = non-Hispanic White
Screening rates stratified by health literacy level, income, and race/ethnicity are also shown in Table 2. While not always statistically significant due to reduced sample sizes, the screening rates consistently favored the mPATH-CRC arm in all subgroups. There was no statistically significant interaction between the intervention and health literacy, income, or race/ethnicity.
Colorectal neoplasia detection rates were 2.6% in the control group (6 adenomas) and 7.2% in the mPATH-CRC group (15 adenomas, 1 carcinoma) (difference=4.5%, 95% CI 0.6% to 8.5%). This difference in neoplasia detection reflects the increased screening associated with mPATH-CRC.
Screening test preferences, intention, and discussions
On the post program survey, 97% (216/223) of mPATH-CRC participants could state a screening preference compared to 71% (162/227) of control participants (difference=26%, 95% CI 19% – 32%). Participants who used mPATH-CRC were more likely to prefer fecal testing than control participants (53% [119/223] vs. 28% [63/227], difference=26%, 95% CI 17% – 34%). There was no difference in the percentage of participants who preferred colonoscopies (43% [95/223] vs. 42% [96/227], difference=0.3%, 95% CI −9% to 9%).
Compared to controls, more mPATH-CRC participants planned to be screened for CRC within the next 6 months (62% [138/223] vs. 49% [112/227], difference=13%, 95% CI 3% to 22%), and more mPATH-CRC participants discussed CRC screening with their providers immediately after using the program (76% [150/197] vs. 48% [103/213], difference = 28%, 95% CI 19% to 37%).
Screening test orders
Test ordering was also higher in the mPATH-CRC arm (69% [153/223] vs. 32% [72/227], difference=37%, 95% CI 28% to 45%), and both fecal blood tests and colonoscopies were ordered more frequently for mPATH-CRC patients.(Table 3) Overall, participants in both arms were more likely to complete an ordered colonoscopy than an ordered fecal test (61% [44/72] vs. 26% [21/81], difference=35%, 95% CI 20% to 50% for mPATH-CRC; and 53% [25/47] vs. 32% [8/25], difference=21%, 95% CI −2% to 44% for Control). For participants who failed to complete a screening fecal test, the most commonly cited reasons were losing or not having the kit (20%), not wanting to complete the kit or finding it embarrassing/distasteful (13%), forgetting to do the kit (11%), and encountering difficulty adhering to required dietary or medication restrictions (11%).
Table 3.
Screening tests ordered by intervention group (n=450)
| mPATH-CRC | Control | ||
|---|---|---|---|
| % (#) | % (#) | Difference (95% CI) | |
| Total in group | 100% (223) | 100% (227) | |
| Screening Test Ordered | |||
| Fecal blood test | 36% (81) | 11% (25) | 25% (18% – 33%) |
| Colonoscopy | 32% (72) | 21% (47) | 12% (3% – 20%) |
| Any test (fecal test or colonoscopy) | 69% (153) | 32% (72) | 37% (28% – 45%) |
As stated previously, mPATH-CRC participants were given the option to “self-order” screening tests and to sign up for follow-up email or text messages to help them complete the screening process. In the mPATH-CRC arm, participants self-ordered 118 screening tests. Primary care providers changed the “self-orders” for 11% (8/74) of those requesting fecal tests (instead ordering colonoscopy) and for 9% (4/44) of those requesting colonoscopies (instead ordering fecal tests). Among participants who self-ordered tests, 81% (96/118) registered for the follow-up electronic messages. In a post-hoc analysis of the participants who self-ordered tests, more of those who signed up for electronic messages completed screening than those who did not, but the difference was not statistically significant (43% [41/96] vs. 27% [6/22], difference=15%, 95% CI −6% to 37%).
CONCLUSIONS
In this randomized-controlled trial, the mPATH-CRC digital health program doubled the proportion of patients who completed CRC screening, primarily due to an increase in test orders. Two components of mPATH-CRC directly encourage screening orders: the decision aid, which increases patients’ intention to receive screening, and the ability of patients to “self-order” tests, which decreases barriers to order entry. We had previously studied the decision aid alone in a similar population, and found that while patients’ intention to receive screening increased, test ordering and completion only increased by a non-statistically significant 9% and 5% respectively.(27) In contrast, in this current trial that combined the decision aid with system interventions, test orders increased by 37% and test completion by 15%. This magnitude of effect is consistent with prior findings that system-level and structural changes often result in the greatest increases in CRC screening.(33)
Time constraints are cited as a major barrier to delivering preventive services.(17,34) We designed mPATH-CRC with the intent of saving providers and staff time by empowering patients to order their own screening independent of a medical encounter. Wide-spread adoption of mPATH-CRC could allow clinicians to spend their time more efficiently and effectively. Over two-thirds of screening tests ordered for mPATH-CRC participants were self-ordered before the primary care visit, obviating or reducing the amount of clinical time required to discuss screening options and order tests. Additional novel interventions like mPATH-CRC are needed to serve as visit extenders, offloading routine tasks from the clinical encounter for the majority of patients who are comfortable and capable of managing a preventive care need.
The majority of CRC screening in the United States is done with colonoscopy,(25) but patients who used mPATH-CRC were as likely to choose a fecal test as colonoscopy, a result consistent with other studies of patient preference. In survey studies, one third to one half of patients choose fecal testing as their preferred screening option.(35,36) In contrast, in an analysis of over 400 audio-recorded CRC screening provider-patient discussions, 99% of providers favored colonoscopy, and only 4% asked patients about their preferences.(37) Therefore, it is not surprising that patients are more likely to receive the CRC screening test their doctor wants rather than the one they want.(38) However, patients are more likely to complete screening when they are given a choice.(39) Population-based interventions such as mass mailing fecal test kits fail to offer a choice and require annual completion to remain effective. Decision aids, such as the one included in mPATH-CRC, are a valuable tool for ensuring patients are aware of their options.
The gap between the number of tests ordered and tests completed indicates that sending text messages or emails to help patients complete screening had a smaller effect. Both mPATH-CRC and control participants completed approximately half of all ordered screening tests. Overall, participants were more likely to complete a colonoscopy than a fecal test, a result we found surprising. This difference could indicate an increased commitment to screening among patients choosing colonoscopy, or it could reflect the routine appointment phone call reminders that patients receive prior to their colonoscopy appointments. A recent study found that a CRC screening decision aid coupled with patient navigation significantly increased CRC screening, the majority of which was done with fecal testing.(40) Adding basic navigation support for patients who fail to return their test kits may yield higher screening rates than we observed.
The mPATH-CRC app currently runs external to the EHR. This approach allows mPATH-CRC to be used in any practice, but it also requires staff to manually enter scheduled colonoscopy dates into the text messaging platform. Updated data exchange standards such as Fast Healthcare Interoperability Resources (FHIR) and application programming interfaces (API’s) will make integrating mPATH-CRC with EHRs easier in the future.(41)
Two features of our study may have limited the difference in screening rates observed. First, for most of the study time period, the clinics were using gFOBT and not the newer fecal immunohistochemical tests which have simpler collection procedures and higher completion rates.(42) Second, our use of patient level randomization may have caused some contamination leading to higher screening rates in the control group. Therefore, our estimate of the effect of mPATH-CRC on screening is likely conservative.
Selection bias could affect our results since recruited patients had to agree to arrive to the clinic early to enroll. In addition, the post-program survey assessing preferences may have triggered some patients to discuss CRC screening with their providers. However, both these biases would be similar in both arms. While 90% of “self-ordered” tests were enacted by providers, we are unable to determine the reasons why providers changed some requests for self-ordered tests. Lastly, our study was conducted in a single large health system and was limited to English-speaking individuals.
Conclusions
In this study that included many individuals with low income and limited health literacy, mPATH-CRC doubled the proportion of patients who completed CRC screening. While screening increased substantially, approximately half of patients failed to complete their ordered tests. Incorporating more strategies to help patients complete ordered tests could further increase the effectiveness of mPATH-CRC. Future research should identify methods for implementing digital health interventions such as mPATH-CRC into clinical care.
Acknowledgments
Financial Support:
The study received funding from the National Cancer Institute (R01CA178941). The study was supported by the Wake Forest Clinical and Translational Science Institute study coordinator pool (UL1TR001420) and by the Wake Forest Comprehensive Cancer Center shared resources (CCSG P30CA012197).
The authors are grateful to Don Babcock for his work programming the mPATH-CRC program.
Footnotes
Reproducible Research Statements
Protocol: Available from Dr. Miller (dmiller@wakehealth.edu)
Statistical code and data set: Available to approved persons through agreement with the authors (dmiller@wakehealth.edu)
References
- 1.Inadomi JM. Screening for Colorectal Neoplasia. N Engl J Med. 2017 Jan 12;376(2):149–56. doi: 10.1056/NEJMcp1512286. [DOI] [PubMed] [Google Scholar]
- 2.Ladabaum U, Mannalithara A. Comparative Effectiveness and Cost Effectiveness of a Multitarget Stool DNA Test to Screen for Colorectal Neoplasia. Gastroenterology. 2016 Sep;151(3):427–439.e6. doi: 10.1053/j.gastro.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Lin JS, Piper MA, Perdue LA, Rutter C, Webber EM, O’Connor E, et al. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. Report No: 14-05203-EF-1. [PubMed] [Google Scholar]
- 4.Patel SS, Kilgore ML. Cost Effectiveness of Colorectal Cancer Screening Strategies. Cancer Control J Moffitt Cancer Cent. 2015 Apr;22(2):248–58. doi: 10.1177/107327481502200219. [DOI] [PubMed] [Google Scholar]
- 5.Sharaf RN, Ladabaum U. Comparative Effectiveness and Cost-Effectiveness of Screening Colonoscopy vs. Sigmoidoscopy and Alternative Strategies. Am J Gastroenterol. 2013 Jan;108(1):120–32. doi: 10.1038/ajg.2012.380. [DOI] [PubMed] [Google Scholar]
- 6. [cited 2017 May 30];American Cancer Society Recommendations for Colorectal Cancer Early Detection. [Internet] Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html.
- 7.US Preventive Services Task Force. Screening for colorectal cancer: Us preventive services task force recommendation statement. JAMA. 2016 Jun 21;315(23):2564–75. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017 Jul;112:1016–30. doi: 10.1038/ajg.2017.174. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 10.White A. Cancer Screening Test Use—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017 Mar 3;66(8):201–6. doi: 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLachlan S-A, Clements A, Austoker J. Patients’ experiences and reported barriers to colonoscopy in the screening context—A systematic review of the literature. Patient Educ Couns. 2012 Feb;86(2):137–46. doi: 10.1016/j.pec.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Guessous I, Dash C, Lapin P, Doroshenk M, Smith RA, Klabunde CN. Colorectal cancer screening barriers and facilitators in older persons. Prev Med. 2010 Jan;50(1–2):3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Berkowitz Z, Hawkins NA, Peipins LA, White MC, Nadel MR. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307–14. doi: 10.1111/j.1532-5415.2007.01547.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-Reported Barriers to Colorectal Cancer Screening: A Mixed-Methods Analysis. Am J Prev Med. 2010 May;38(5):508–16. doi: 10.1016/j.amepre.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bromley EG, May FP, Federer L, Spiegel BMR, van Oijen MGH. Explaining persistent under-use of colonoscopic cancer screening in African Americans: A systematic review. Prev Med. 2015 Feb;71:40–8. doi: 10.1016/j.ypmed.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Østbye T, Yarnall KSH, Krause KM, Pollak KI, Gradison M, Michener JL. Is There Time for Management of Patients With Chronic Diseases in Primary Care? Ann Fam Med. 2005 May 1;3(3):209–14. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarnall KSH, Pollak KI, Østbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003 Apr;93(4):635–41. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, et al. National Institutes of Health state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. Ann Intern Med. 2010 May 18;152(10):663–7. doi: 10.7326/0003-4819-152-10-201005180-00237. [DOI] [PubMed] [Google Scholar]
- 19.von Wagner C, Semmler C, Good A, Wardle J. Health literacy and self-efficacy for participating in colorectal cancer screening: The role of information processing. Patient Educ Couns. 2009 Jun;75(3):352–7. doi: 10.1016/j.pec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen-Bohlman L, Panzer AM, Kindig DA, editors. Health Literacy: a Prescription to End Confusion. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 21.Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The Prevalence of Limited Health Literacy. J Gen Intern Med. 2005 Feb;20(2):175–84. doi: 10.1111/j.1525-1497.2005.40245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107. doi: 10.7326/0003-4819-155-2-201107190-00005. [DOI] [PubMed] [Google Scholar]
- 23.Katz MG, Jacobson TA, Veledar E, Kripalani S. Patient Literacy and Question-asking Behavior During the Medical Encounter: A Mixed-methods Analysis. J Gen Intern Med. 2007 Jun 1;22(6):782–6. doi: 10.1007/s11606-007-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold CL, Rademaker A, Bailey SC, Esparza JM, Reynolds C, Liu D, et al. Literacy barriers to colorectal cancer screening in community clinics. J Health Commun. 2012;17(Suppl 3):252–64. doi: 10.1080/10810730.2012.713441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of Colorectal Cancer Test Use, Including CT Colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Prev Biomark. 2012 Jun 1;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volk RJ, Linder SK, Lopez-Olivo MA, Kamath GR, Reuland DS, Saraykar SS, et al. Patient Decision Aids for Colorectal Cancer Screening: A Systematic Review and Meta-analysis. Am J Prev Med. 2016 Nov;51(5):779–91. doi: 10.1016/j.amepre.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller DP, Spangler JG, Case LD, Goff DC, Singh S, Pignone MP. Effectiveness of a Web-Based Colorectal Cancer Screening Patient Decision Aid. Am J Prev Med. 2011 Jun 1;40(6):608–15. doi: 10.1016/j.amepre.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pew Research Center. Mobile Fact Sheet. [cited 2017 Feb 2];Pew Research Center: Internet, Science & Tech. 2017 Available from: http://www.pewinternet.org/fact-sheet/mobile/
- 29.Miller DP, Jr, Weaver KE, Case LD, Babcock D, Lawler D, Denizard-Thompson N, et al. Usability of a Novel Mobile Health iPad App by Vulnerable Populations. JMIR MHealth UHealth. 2017 Apr 11;5(4):e43. doi: 10.2196/mhealth.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, et al. Validation of Screening Questions for Limited Health Literacy in a Large VA Outpatient Population. J Gen Intern Med. 2008 Mar 12;23(5):561–6. doi: 10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med. 2009 Jul;37(1):8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC. CDC-TV Video: Finding Balance. [cited 2016 Jul 21];Centers for Disease Control and Prevention. 2013 [Internet] Available from: http://www.cdc.gov/cdctv/healthyliving/healthyeating/finding-balance-obesity.html.
- 33.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic Review: Enhancing the Use and Quality of Colorectal Cancer Screening. Ann Intern Med. 2010 May 18;152(10):668–76. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 34.Ayres CG, Griffith HM. Perceived barriers to and facilitators of the implementation of priority clinical preventive services guidelines. Am J Manag Care. 2007 Mar;13(3):150–5. [PubMed] [Google Scholar]
- 35.DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based Preferences for Stool Cards versus Colonoscopy in Colorectal Cancer Screening. J Gen Intern Med. 2007 Dec 21;23(2):169–74. doi: 10.1007/s11606-007-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawley ST, McQueen A, Bartholomew LK, Greisinger AJ, Coan SP, Myers R, et al. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer. 2012 May 15;118(10):2726–34. doi: 10.1002/cncr.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lafata JE, Cooper GS, Divine G, Flocke SA, Oja-Tebbe N, Stange KC, et al. Patient–Physician Colorectal Cancer Screening Discussions: Delivery of the 5A’s in Practice. Am J Prev Med. 2011 Nov;41(5):480–6. doi: 10.1016/j.amepre.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawley S, Lillie S, Cooper G, Elston Lafata J. Managed care patients’ preferences, physician recommendations, and colon cancer screening. Am J Manag Care. 2014 Jul;20(7):555–61. [PMC free article] [PubMed] [Google Scholar]
- 39.Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012 Apr 9;172(7):575–82. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reuland DS, Brenner AT, Hoffman R, McWilliams A, Rhyne RL, Getrich C, et al. Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population: A Randomized Clinical Trial. JAMA Intern Med. 2017 Jul 1;177(7):967–74. doi: 10.1001/jamainternmed.2017.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connecting Health and Care for the Nation. A Shared Nationwide Interoperability Roadmap. [cited 2017 Jun 15];The Office of the National Coordinator for Health Information. 2015 [Internet] Available from: https://www.healthit.gov/sites/default/files/hie-interoperability/nationwide-interoperability-roadmap-final-version-1.0.pdf.
- 42.Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015 Aug 1;64(8):1327–37. doi: 10.1136/gutjnl-2014-308074. [DOI] [PubMed] [Google Scholar]
- 43.United States Department of Agriculture, Economic Research Service. [cited 2017 Nov 15];Rural-Urban Continuum Codes. [Internet] Available from: http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx#.U7K2RPldXRN.


