Abstract
A 66-year-old woman underwent right nephrectomy for treatment of renal cell carcinoma (RCC). Two years later, she underwent wedge resection of the right lung for treatment of metastatic RCC and primary adenocarcinoma of the lung. She began oral sorafenib for the remaining nodules of the left lung, which were suspected to be metastatic RCC. Two years later, the sorafenib was changed to everolimus because of slight enlargement of the left pulmonary nodules. The carcinoembryonic antigen (CEA) concentration then increased to 25.7 ng/mL, and chest computed tomography (CT) revealed ground-glass opacities (GGO) in the bilateral lungs. Everolimus-induced lung injury was suspected, and she discontinued the everolimus. Two months later, the serum CEA concentration decreased to almost within the reference range at 5.9 ng/mL, and the GGO disappeared on chest CT. In conclusion, we encountered a patient who developed an elevated serum CEA concentration caused by everolimus-induced lung injury.
Keywords: everolimus, carcinoembryonic antigen, tumor marker, drug-induced lung injury
Introduction
Everolimus is an orally administered inhibitor of the mammalian target of rapamycin (mTOR) and has been approved for the treatment of metastatic renal cell carcinoma (RCC).1) No reports have described everolimusassociated elevations in tumor markers such as carcinoembryonic antigen (CEA). We herein report a patient who developed an elevated serum CEA concentration caused by everolimus-induced lung injury during the postoperative follow-up period after treatment of metastatic RCC and primary adenocarcinoma of the lung.
Case Report
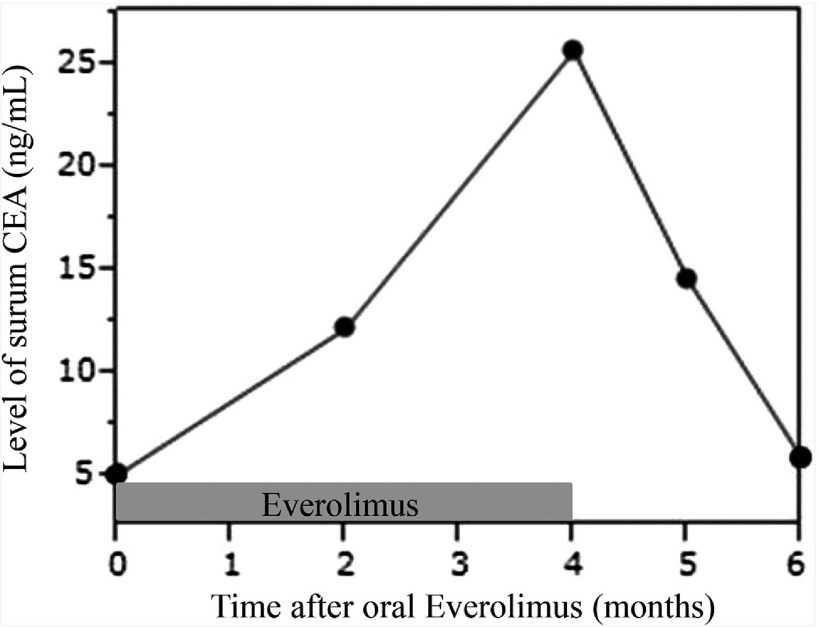
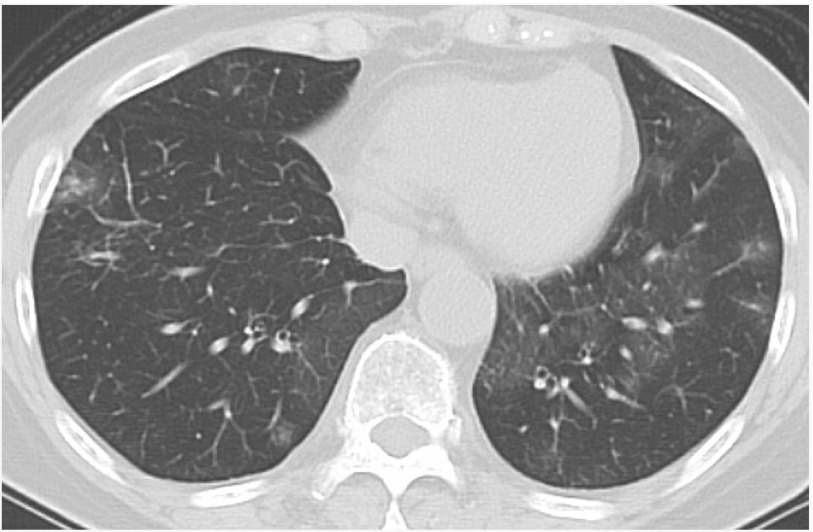
A 66-year-old woman was referred to our affiliated hospital because of macrohematuria. Abdominal computed tomography (CT) revealed a right renal tumor, and she underwent right nephrectomy. Histologic examination of the resected specimen showed RCC. Two years after the right nephrectomy, chest CT revealed multiple pulmonary nodules: one 10-mm lobulated nodule in the right middle lobe, one 5-mm round nodule in the right lower lobe, and two round nodules in the left upper lobe (diameters of 4 and 5 mm, respectively). For diagnosis and therapy, she underwent wedge resection of the two nodules in the right lung. Histologic examination of each resected specimen revealed primary adenocarcinoma of the right middle lobe and metastatic RCC of the right lower lobe. The remaining two nodules in the left lung were both round shaped, which were similar to the nodule diagnosed as metastatic RCC in the right lower lobe. Therefore, they were suspected to be metastatic RCC, and she began treatment with oral sorafenib after the right pulmonary surgery. Two years after beginning oral sorafenib, chest CT revealed slight enlargement of the left pulmonary nodules. Therefore, she discontinued the oral sorafenib and began oral everolimus. Laboratory examination showed a serum CEA concentration of 5.1 ng/mL. Two and four months after beginning the oral everolimus, the serum CEA concentration gradually increased to 12.2 and 25.7 ng/mL, respectively (Fig. 1). The concentrations of all other serum tumor markers, including carbohydrate antigen 19-9, cytokeratin 19 fragment, sialyl LewisX, s-pancreas-1 antigen, and duke pancreatic monoclonal antigen type 2, were within their reference ranges. The white blood cell count was 5300/mm3, and the serum C-reactive protein concentration was slightly elevated at 1.71 mg/dL. Chest CT revealed ground-glass opacities (GGO) in the lower lobes of the bilateral lungs (Fig. 2). Chest and abdominal CT, brain magnetic resonance imaging, positronemission tomography, gastrointestinal endoscopy, and colonoscopy revealed neither recurrent lesions nor other malignant tumors. Although neither bronchoscopic nor histologic examination was performed, everolimusinduced lung injury was suspected. She had a dry cough classified as a grade 2 adverse event according to the Common Terminology Criteria for Adverse Events, version 4.0. Therefore, she discontinued the oral everolimus 4 months after beginning the everolimus. One and two months after discontinuing the oral everolimus, her serum CEA concentration gradually decreased to 14.6 and 5.9 ng/mL, respectively (Fig. 1). Chest CT showed disappearance of the GGO.
Fig. 1. Graph showing chronological change in serum carcinoembryonic antigen concentration after administration of everolimus. CEA: carcinoembryonic antigen.
Fig. 2. Chest CT showing GGO in the lower lobes of the bilateral lungs. CT: computed tomography; GGO: ground-glass opacities.
Discussion
Activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway has been detected in a wide range of cancers, including RCC.2) Everolimus is an orally administered inhibitor of mTOR, which is a component of an intracellular signaling pathway that regulates cellular metabolism, growth, proliferation, and angiogenesis.1) Everolimus has antiangiogenic properties and induces decreased levels of proangiogenic proteins (plateletderived growth factor AB and insulin growth factorbinding protein 2) and increased levels of antiangiogenic proteins (angiostatin, pentraxine 3, and platelet factor 4).3) The most common side effects of everolimus are stomatitis, rash, fatigue or asthenia, and diarrhea. With respect to laboratory abnormalities, hypercholesterolemia, hyperglycemia, lymphopenia, and hypophosphatemia were significantly more frequent in the everolimus than placebo group in a randomized phase III study.1) No reports have described elevated tumor markers such as CEA associated with everolimus. CEA can reportedly affect proangiogenic endothelial cell behavior and induce tumor angiogenesis,4) and an elevated serum CEA concentration is incompatible with the antiangiogenic properties of everolimus. Thus, it cannot be assumed that everolimus directly elevates the serum CEA concentration.
CEA is the most widely used tumor marker in patients with non-small-cell lung cancer.5) One study of patients who had undergone complete resection of non-small-cell lung cancer showed that measuring the serum CEA concentration during the follow-up period is useful for predicting postoperative recurrence and prognosis.6) In our patient, the serum CEA concentration was measured following pulmonary resection of primary lung cancer, and an elevated serum CEA concentration was revealed 2 months after beginning oral administration of everolimus. Whole-body examinations were performed to rule out lung cancer recurrence, and neither recurrent lesions nor other malignant tumors were found. Because everolimus-induced lung injury was suspected, the patient discontinued the oral everolimus. Two months later, the serum CEA concentration had decreased to almost within the reference range, and the GGO had disappeared on chest CT. With the exception of pulmonary inflammation, no other causes of the elevated serum CEA concentration, such as smoking, other benign diseases, or other drugs, were found. An elevated serum CEA level has been reported to occur in association with various benign lung diseases, such as interstitial pneumonia.7) Noninfectious pneumonitis has been reported as an adverse event associated with everolimus administration in 8%–12% of patients.1,8) In a retrospective radiographic study involving chest CT, 132 of 245 patients (53.9%) receiving everolimus reportedly showed new or worsening radiographic changes of pneumonitis.9) Therefore, in the present case, we believe that everolimus caused noninfectious pneumonitis, which led to the elevated serum CEA concentration. Although few reports have described an elevated serum CEA concentration caused by drug-induced lung injury,10,11) our case suggests that drug-induced pneumonitis elevates the serum CEA concentration by the same mechanism as in previously reported benign lung diseases, such as interstitial pneumonia. The serum CEA concentration is rarely measured during the follow-up period of RCC. We believe that the elevated serum CEA concentration caused by everolimus-induced lung injury may not be rare, if it is examined in all the patients with everolimus.
Conclusion
We encountered a patient who developed an elevated serum CEA concentration caused by everolimus-induced lung injury. Importantly, the serum CEA concentration may become elevated due to drug-induced lung injury as shown by measurement of this parameter during the follow-up period of many cancers.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1).Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008; 372: 449-56. [DOI] [PubMed] [Google Scholar]
- 2).Garcia JA, Danielpour D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol Cancer Ther 2008; 7: 1347-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Seront E, Rottey S, Sautois B, et al. Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. Ann Oncol 2012; 23: 2663-70. [DOI] [PubMed] [Google Scholar]
- 4).Bramswig KH, Poettler M, Unseld M, et al. Soluble carcinoembryonic antigen activates endothelial cells and tumor angiogenesis. Cancer Res 2013; 73: 6584-96. [DOI] [PubMed] [Google Scholar]
- 5).Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012; 76: 138-43. [DOI] [PubMed] [Google Scholar]
- 6).Ozeki N, Fukui T, Taniguchi T, et al. Significance of the serum carcinoembryonic antigen level during the follow-up of patients with completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg 2014; 45: 687-92. [DOI] [PubMed] [Google Scholar]
- 7).Fahim A, Crooks MG, Wilmot R, et al. Serum carcinoembryonic antigen correlates with severity of idiopathic pulmonary fibrosis. Respirology 2012; 17: 1247-52. [DOI] [PubMed] [Google Scholar]
- 8).Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012; 366: 520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).White DA, Camus P, Endo M, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med 2010; 182: 396-403. [DOI] [PubMed] [Google Scholar]
- 10).Noonan SA, Sachs PB, Camidge DR. Transient asymptomatic pulmonary opacities occurring during osimertinib treatment. J Thorac Oncol 2016; 11: 2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Hasegawa Y, Ota T, Tsukuda H, et al. Drug-induced pneumonitis following the administration of TAS-102. Intern Med 2016; 55: 2855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]