Abstract
Background: We often experienced early recurrence in patients with completely resected N2-positive non-small-cell lung cancer (NSCLC). Loss of muscle mass is a poor prognostic factor in patients with several stages of NSCLC. This study aimed to investigate the relationship between preoperative loss of muscle mass and postoperative early recurrence in patients with N2-positive NSCLC.
Methods: We retrospectively analyzed 47 male patients with completely resected pathological N2-positive NSCLC. Early recurrence was defined as that diagnosed within 1 year after the operation. We used the L3 muscle index (cross-sectional area of muscle at the L3 level, normalized for height) as a clinical measurement of loss of muscle mass (cutoff value, 52.4 cm2/m2).
Results: In all, 18 patients with early recurrence had significantly poorer outcomes compared with those without (P <0.01). In univariate analysis, loss of muscle mass (P = 0.023), carcinoembryonic antigen (CEA) level >5.0 ng/mL (P = 0.002), and absence of postoperative chemotherapy (P = 0.042) were predictors of postoperative early recurrence. In multivariate analysis, loss of muscle mass (P = 0.004) and CEA level >5.0 ng/mL (P = 0.001) were independent predictors.
Conclusions: Loss of muscle mass is an independent predictor of postoperative early recurrence in pathological N2-positive NSCLC patients.
Keywords: non-small-cell lung cancer, early recurrence, loss of muscle mass, mediastinal lymph node metastasis
Introduction
Survival after surgery of non-small-cell lung cancer (NSCLC) with mediastinal lymph node (N2) metastasis remains poor. We often experienced postoperative early recurrence in patients with completely resected N2positive NSCLC. Prediction of early recurrence may contribute to select a good prognostic group in these patients. Thus, we consider prediction of early recurrence as a significantly meaningful action in patients with N2-positive NSCLC.
Loss of muscle mass is a poor prognostic factor in patients with clinical stage III to IV NSCLC or small-cell lung cancer who are treated with chemotherapy.1,2) We previously reported that loss of muscle mass was a significant postoperative poor prognostic factor in patients with stage I NSCLC.3) There is no report investigating the correlation between loss of muscle mass and postoperative early recurrence.
In the present study, we analyzed the relationship between preoperative loss of muscle mass and postoperative early recurrence in patients with completely resected N2-positive NSCLC.
Materials and Methods
Of 725 patients who underwent definitive pulmonary resection with mediastinal lymph node dissection for primary lung cancer at our hospital from January 2003 to December 2012, we retrospectively analyzed 47 male patients with pathological N2-positive NSCLC. Patients who had preoperative treatments or whose tumors were not completely removed were excluded. Patients who underwent pneumonectomy, pulmonary segmentectomy, or partial resection were also excluded. Informed consent for application of the patients’ examination outcomes and data of clinical courses to clinical studies was obtained before surgery in all of the patients. This study was approved by the local institutional ethical committee.
The mediastinal lymph nodes were clinically diagnosed as positive for metastasis when more than 10 mm in short axis was calculated using enhanced computed tomography (CT). Patients with distant metastasis, mediastinal lymph node metastasis at more than two stations, bulky mediastinal lymph node metastasis and contralateral mediastinal lymph node metastasis were not candidate for surgery. Patients with a predicted postoperative forced expiratory volume in 1 second of less than 40% were also ineligible for surgery. We used the 7th edition of the tumor node metastasis (TNM) Classification of Malignant Tumors to determine the pathological stage.
After discharge, all of the patients got follow-up examination every 2 to 4 months by chest X-ray and for tumor markers, and every 6 months by CT. Recurrence diseases were commonly detected by imaging methods. Early recurrence diseases were defined as those detected within 1 year after operation. The last follow-up review was conducted on August 31, 2017.
We used the cross-sectional area (cm2) of skeletal muscle at the third lumbar vertebral (L3) level on CT as a barometer of systemic muscle mass.4) The L3 muscle index (L3MI) is a clinical indicator of loss of muscle mass, and calculated by the cross-sectional area of muscle at the L3 level with normalized for height (cm2/m2; as with body mass index).5) Yoshizumi et al.6) reported that the measured skeletal muscle area at L3 was correlated with body surface area (BSA) significantly. They created a formula to calculate skeletal muscle area at L3 using BSA as follows: BSA (m2) = 71.84 × height0.725 × bodyweight0.425 × 10−4. Skeletal muscle area at L3 (cm2) for males = 126.9 × BSA − 66.2. We used this formula to calculate the L3MI. The cutoff value for the L3MI was 52.4 cm2/m2 for males according to a previous report,5) and patients with an L3MI <52.4 cm2/m2 were defined as those with loss of muscle mass.
The prognostic nutritional index (PNI) is a clinical indicator of nutritional status, and calculated by the serum albumin level and the total peripheral blood lymphocyte count as follows: PNI = 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (/uL).7)
Platinum-doublet chemotherapy was performed as postoperative adjuvant therapy. However, because an indication for adjuvant chemotherapy was not established, the decision was depended on each physician’s empirical concept.
We collected blood samples within 1 month before surgery, and measured body height and weight on admission. Median age in this study population (68 years) was used as a cutoff value for age. We used 18.5 kg/m2 as a cutoff value for body mass index according to the guidelines of the World Health Organization.8) We defined cutoff values for tumor markers according to previous reports.9–11)
We used χ2 test and Mann–Whitney U-test to compare the clinicopathologic factors of patients with and without early recurrence. The Kaplan–Meier method was used to investigate overall survival and the log-rank test was used to assess differences. Logistic regression analysis was used for univariate analysis and multiple logistic regression was used for multivariate analysis. Odds ratios were used to estimate the relative risk for early recurrence. P <0.05 was considered significant. Statistical analyses were performed using JMP 10 software (SAS Institute, Cary, NC, USA).
Results
The median follow-up period was 37 months, during which 37 patients had recurrent disease and 18 had early recurrence. The first recurrent sites were the lungs in 10, bone in 9, lymph nodes in 9, liver in 3, brain in 3, adrenal gland in 2, and kidney and carcinomatous pleuritis in one patient each. The first recurrent sites in early recurrence cases were bone in seven, lymph nodes, lung, and liver in three each, and the adrenal gland, brain, and carcinomatous pleuritis in one patient each. The patients with early recurrence had a significantly poorer prognosis compared with those without early recurrence, with 5-year survival rates of 6% and 64%, respectively (P <0.01; Fig. 1). Characteristics of patients according to the presence of early recurrence are shown in Table 1. Early recurrence was significantly associated with L3MI values, tumor size, and the status of postoperative chemotherapy. Postoperative complications were observed in 15 patients, including prolonged air leakage in eight, arrhythmia in three, and pneumonia and chylothorax in two patients each. There was no significant difference in the presence of complications according to the presence of early recurrence (P = 0.968).
Fig. 1. Overall survival curves according to the presence or absence of early recurrence.
Table 1. Characteristics of patients according to the presence of early recurrence.
| Characteristics | Early recurrence | |||
|---|---|---|---|---|
| All (n = 47) | Yes (n = 18) | No (n = 29) | P value | |
| Age | 68 (35, 84) | 73 (35, 80) | 63 (47, 84) | 0.463 |
| Performance status | ||||
| 0 | 38 | 14 | 24 | 0.376 |
| 1 | 8 | 3 | 5 | |
| 2 | 1 | 1 | 0 | |
| Smorking index | 890 (0, 2580) | 810 (0, 2800) | 0.983 | |
| BMI (Kg/m2) | 20.9 (16.7, 29.8) | 22.6 (15.9, 30.9) | 0.108 | |
| L3MI (m2/cm2) | 51.2 (44.7, 62.4) | 53.9 (45.6, 64.5) | 0.043 | |
| PNI | 53.2 (44.5, 65.5) | 54.9 (40.0, 63.9) | 0.441 | |
| Respiratory function | ||||
| %VC (%) | 106 (81, 127) | 104 (70, 142) | 0.717 | |
| %FVC (%) | 106 (71, 127) | 104 (67, 142) | 0.824 | |
| FEV1.0% (%) | 76 (56, 93) | 75 (55, 96) | 0.851 | |
| Clinical stage | ||||
| I | 31 | 11 | 20 | 0.266 |
| II | 8 | 5 | 3 | |
| III | 8 | 2 | 6 | |
| CEA (ng/mL) | 10.9 (2.8, 131) | 4.6 (1.2, 1194) | 0.072 | |
| CYFRA (ng/mL) | 2.2 (0.7, 13.7) | 1.9 (0.5, 31.4) | 0.718 | |
| Tumor size (mm) | 37 (15, 83) | 43 (24, 83) | 32 (15, 70) | 0.035 |
| Histological subtypes | ||||
| Adenocarcinoma | 37 | 14 | 23 | 0.901 |
| Non-adenocarcinoma | 10 | 4 | 6 | |
| Pathological stage | ||||
| IIIA | 37 | 13 | 24 | 0.400 |
| IIIB | 10 | 5 | 5 | |
| Status of N2 nodal extension | ||||
| Skip/sequential | 5/13 | 20/9 | 0.812 | |
| Single/multiple | 12/6 | 18/11 | 0.749 | |
| Postoperative chemotherapy | ||||
| Yes / No | 7/11 | 20/9 | 0.042 | |
Values are median (range). BMI: body mass index; L3MI: L3 muscle index; PNI: prognostic nutritional index; %VC: percent of vital capacity; %FEV1.0: percent of forced expiratory volume in 1 second; FEV1.0%: forced expiratory volume in 1 second as a percent of forced vital capacity; CEA: carcinoembryonic antigen; CYFRA: cytokeratin 19 fragment
In univariate analysis, an L3MI <52.4 cm2/m2, carcinoembryonic antigen (CEA) levels >5.0 ng/mL, and absence of postoperative chemotherapy were significant predictors of postoperative early recurrence. When these factors and tumor size >37 mm were subjected to multivariate analysis, an L3MI <52.4 cm2/m2 and CEA levels >5.0 ng/mL were independent predictors (Table 2).
Table 2. Univariate and multivariate analyses of risk factors for early recurrence.
| n | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Age | >68 | 23 | 2.23 | 0.68–7.69 | 0.187 | |||
| ≤68 | 24 | |||||||
| Performance status | 0 | 38 | 0.73 | 0.17–3.37 | 0.675 | |||
| 1-2 | 9 | |||||||
| BMI (kg/m2) | ≥18.5 | 40 | 0.40 | 0.07–2.08 | 0.273 | |||
| <18.5 | 7 | |||||||
| L3MI (m2/cm2) | ≥52.4 | 28 | 0.24 | 0.07–0.82 | 0.023 | 0.10 | 0.01–0.50 | 0.004 |
| <52.4 | 19 | |||||||
| PNI | ≥50 | 39 | 0.59 | 0.12–2.88 | 0.506 | |||
| <50 | 8 | |||||||
| CEA (ng/mL) | >5.0 | 29 | 9.23 | 2.10–65.68 | 0.002 | 19.3 | 2.95–236.54 | 0.001 |
| ≤5.0 | 18 | |||||||
| CYFRA (ng/mL) | >3.5 | 32 | 1.25 | 0.34–4.50 | 0.733 | |||
| ≤3.5 | 15 | |||||||
| Tumor size (mm) | >37 | 23 | 3.27 | 0.99–11.89 | 0.054 | 3.09 | 0.69–16.84 | 0.143 |
| ≤37 | 24 | |||||||
| Postoperative chemotherapy | Yes | 27 | 0.28 | 0.08– 0.96 | 0.042 | 0.57 | 0.12–2.74 | 0.477 |
| No | 20 | |||||||
HR: hazard ratio; CI: confidence interval; BMI: body mass index; L3MI: L3 muscle index; PNI: prognostic nutritional index; CEA: carcinoembryonic antigen; CYFRA: cytokeratin 19 fragment
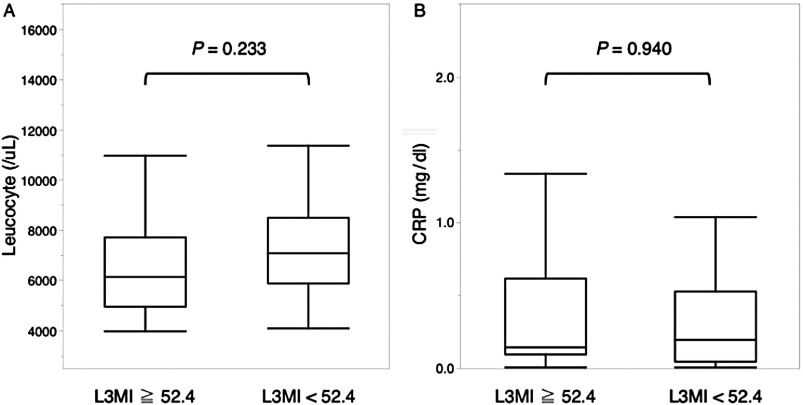
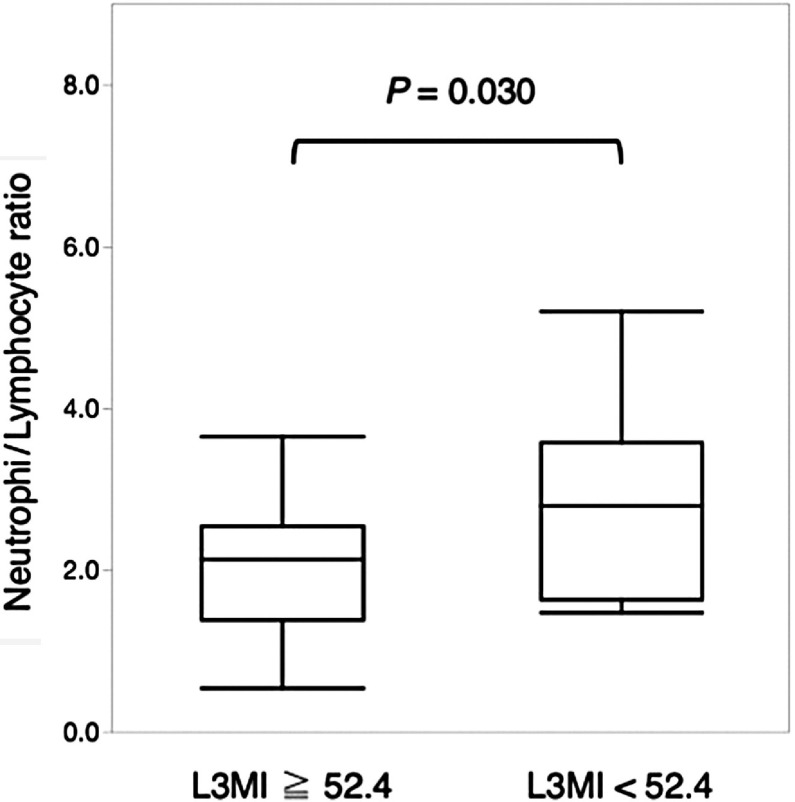
There were no significant differences in blood leucocyte count and C-reactive protein levels (Fig. 2). The neutrophil/lymphocyte ratio (NLR) was significantly higher in patients with loss of muscle mass than in those without (P = 0.030, Fig. 3).
Fig. 2. The preoperative leucocyte level (A) and the C-reactive protein level (B) in the blood according to the presence or absence of loss of muscle mass. CRP: C-reactive protein; L3MI: L3 muscle index.
Fig. 3. The preoperative neutrophil/lymphocyte ratio in the blood according to the presence or absence of loss of muscle mass. L3MI: L3 muscle index.
Discussion
Several clinicopathological factors12) and proliferative molecular factors13,14) were reported as significant predictors of postoperative recurrence in N2-positive NSCLC. This is the first report to investigate the relationship between early recurrence and loss of muscle mass in patients with completely resected N2-positive NSCLC. We showed a poor prognostic effect of decreased muscle mass on cancer recurrence. In patients with muscle deficiency, operations should be selected after careful deliberation, and more frequent postoperative follow-up examinations may be required.
We showed that the NLR was significantly higher in patient with loss of muscle mass compared with those without. Elevation of the NLR indicates infiltration of neutrophils into the tumor microenvironment and suppression of the lymphocyte-mediated anticancer immune response.15) Elevation of the NLR is reported to be correlated with postoperative outcomes and cancer recurrence.16,17) Systemic inflammatory signals, including interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), are involved in the pathogenesis of tumorassociated inflammation and provide a high NLR.18) Tumor-associated depletion of skeletal muscle is considered to be induced by tumor-associated inflammation. IL-6 and TNF-α activate muscular nuclear factor-kappa signaling and subsequent ubiquitin proteasome systemmediated proteolysis.19) Tumor-associated inflammation may be a trigger for loss of muscle mass and elevation in the NLR, and these lead to early recurrence and poor prognosis. Additionally, because muscle cells generate antiproliferative mediators, depression of these mediators may also be associated with cancer recurrence in patients with loss of muscle mass.20)
In our study, significantly fewer patients with loss of muscle mass were treated with postoperative adjuvant chemotherapy compared with those without. Because a criterion for avoidance of adjuvant chemotherapy was not established, judgment depended on each physician’s empirical concept. A relatively high median age of patients with muscle deficiency may be one of the reasons of avoidance of adjuvant chemotherapy. A beneficial effect of aggressive introduction of conventional adjuvant chemotherapy on postoperative early recurrence remains unclear in patients with muscle deficiency. A criterion of indication for adjuvant chemotherapy should be established and accumulation of patients’ data is necessary. If tumor-associated inflammation is the cause of muscle deficiency and a poor prognosis, anti-inflammatory treatments should be introduced to improve the prognosis. IL-6 is an important pro-inflammatory cytokine, and plays a crucial role in cancer progression. An anti-IL-6 antibody has been reported to have beneficial effect on cancer treatments either as a single agent or in combination with other anticancer drugs.21)
This study has some limitations. First, this was a retrospective study that included only a small number of patients. Second, we used 52.4 cm2/m2 as the cutoff value for L3MI because this value was widely used in several articles.5) However, Kimura et al.1) reported that the L3MI cutoff values in Japanese patients with inoperable stages III to IV NSCLC was 41.0 cm2/m2 for males. It may be important to use appropriate cutoff values based on disease stage, cancer type, and ethnicity. Third, the formula which calculated skeletal muscle area using BSA was produced by physical findings in healthy adults. Because almost all patients in this study were categorized as PS 0 and 1, we used this formula. The measurement error of the L3MI could be reduced by use of this formula and an unnecessary CT at the lumbar vertebral level could be avoided. Forth, although we considered tumor-associated inflammation might be a crucial reason for the loss of muscle mass, other unrevealed reasons, such as tumor-unrelated inflammation, should be explored in further examinations.
In conclusion, loss of muscle mass is an independent predictor of early recurrence in patients with pathological N2-positive NSCLC. Tumor-related inflammation might be an important reason for muscle deficiency and poor prognosis. Loss of muscle mass may become a novel biomarker for determining the treatment strategy for patients with N2-positive NSCLC.
Disclosure Statement
The authors have no relevant financial or other potential conflicts of interest in this manuscript.
References
- 1).Kimura M, Naito T, Kenmotsu H, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer 2015; 23: 1699-708. [DOI] [PubMed] [Google Scholar]
- 2).Kim EY, Kim YS, Park I, et al. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol 2015; 10: 1795-9. [DOI] [PubMed] [Google Scholar]
- 3).Tsukioka T, Nishiyama N, Izumi N, et al. Sarcopenia is a novel poor prognostic factor in male patients with pathological stage I non-small cell lung cancer. Jpn J Clin Oncol 2017; 47: 363-8. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 4).Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004; 97: 2333-8. [DOI] [PubMed] [Google Scholar]
- 5).Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008; 9: 629-35. [DOI] [PubMed] [Google Scholar]
- 6).Yoshizumi T, Shirabe K, Nakagawara H, et al. Skeletal muscle area correlates with body surface area in healthy adults. Hepatol Res 2014; 44: 313-8. [DOI] [PubMed] [Google Scholar]
- 7).Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi 1984; 85: 1001-5. (in Japanese) [PubMed] [Google Scholar]
- 8).WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157-63. [DOI] [PubMed] [Google Scholar]
- 9).Inoue M, Takakuwa T, Minami M, et al. Clinicopathologic factors influencing postoperative prognosis in patients with small-sized adenocarcinoma of the lung. J Thorac Cardiovasc Surg 2008; 135: 830-6. [DOI] [PubMed] [Google Scholar]
- 10).Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012; 76: 138-43. [DOI] [PubMed] [Google Scholar]
- 11).Kawai T, Ohkubo A, Hasegawa S, et al. Study of standard level, cut off level, diagnostic specificity and sensitivity for a new tumor marker CYFRA in lung cancer measured by EIA. Kiki Shiyaku Jpn 1993; 16: 1232-8. (in Japanese) [Google Scholar]
- 12).Sonobe M, Date H, Wada H, et al. Prognostic factors after complete resection of pN2 non-small cell lung cancer. J Thorac Cardiovasc Surg 2013; 146: 788-95. [DOI] [PubMed] [Google Scholar]
- 13).Matsuwaki R, Ishii G, Zenke Y, et al. Immunophenotypic features of metastatic lymph node tumors to predict recurrence in N2 lung squamous cell carcinoma. Cancer Sci 2014; 105: 905-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Chen G, Liu XY, Wang Z, et al. Vascular endothelial growth factor C: the predicator of early recurrence in patients with N2 non-small-cell lung cancer. Eur J Cardiothorac Surg 2010; 37: 546-51. [DOI] [PubMed] [Google Scholar]
- 15).Nagaraj S, Schrum AG, Cho HI, et al. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol 2010; 184: 3106-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Wang J, Kalhor N, Hu J, et al. Pretreatment neutrophil to lymphocyte ratio is associated with poor survival in patients with stage I-III non-small cell lung cancer. PLoS ONE 2016; 11: e0163397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Takahashi Y, Kawamura M, Hato T, et al. Neutrophil-lymphocyte ratio as a prognostic marker for lung adenocarcinoma after complete resection. World J Surg 2016; 40: 365-72. [DOI] [PubMed] [Google Scholar]
- 18).Tomita M, Shimizu T, Ayabe T, et al. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res 2012; 32: 3535-8. [PubMed] [Google Scholar]
- 19).Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12: 249-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Fishman P, Bar-Yehuda S, Vagman L. Adenosine and other low molecular weight factors released by muscle cells inhibit tumor cell growth. Cancer Res 1998; 58: 3181-7. [PubMed] [Google Scholar]
- 21).Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 2014; 141: 125-39. [DOI] [PubMed] [Google Scholar]