Abstract
Acne vulgaris is a common chronic skin disease in young adults caused by infection of the pilosebaceous unit, resulting in pimples and possibly permanent scarring on the skin. Minocycline, a common antibiotic, has been widely utilized as a systemic antimicrobial treatment for acne via oral administration. Recently, a topical minocycline gel (BPX-01) was developed to directly deliver minocycline through the epidermis and into the pilosebaceous unit to achieve localized treatment with lower doses of drug. As the effectiveness of the drug is directly related to its successful delivery, there is a need to evaluate the pharmacokinetics at the cellular level within tissue. Advantageously, minocycline is naturally fluorescent and can be directly visualized using microscopy-based approaches. Due to high endogenous autofluorescence, however, imaging of weakly emitting fluorescent molecules such as minocycline in skin tissue can be challenging. Here, we demonstrate a method for the selective visualization of minocycline within human skin tissue by utilizing two-photon excitation fluorescence (TPEF) microscopy and fluorescence lifetime imaging microscopy (FLIM). To demonstrate the feasibility of this approach, ex vivo human facial skin samples treated with various concentrations of BPX-01 were investigated. From the TPEF analysis, we were able to visualize relatively high levels of drug uptake within facial skin. However, minocycline fluorescence could be overwhelmed by endogenous fluorescence that complicates TPEF quantitative analysis, making FLIM more advantageous for visualizing drug uptake. Importantly, we found a unique signature of minocycline uptake via FLIM analysis that enabled the successful differentiation of the drug and enabled the extraction of drug local distribution from the endogenous fluorescence using a non-Euclidean phasor analysis method. Based on these results, we believe that the drug local distribution visualization method using TPEF and FLIM with phasor analysis can play an important role in studying the pharmacokinetics and pharmacodynamics of a topically applicable drug.
OCIS codes: (180.0180) Microscopy, (190.4180) Multiphoton processes, (100.0100) Image processing, (170.0170) Medical optics and biotechnology, (170.1870) Dermatology
1. Introduction
Minocycline is a tetracycline class antibiotic that is frequently used for the treatment of acne vulgaris [1, 2]. This drug is believed to have both antibacterial and anti-inflammatory properties. Currently, commercially available minocycline is orally administered to target Propionibacterium acnes (P. acnes), a type of bacterium that normally resides in the pores and hair shafts of the skin that contributes to the etiology of acne, and the associated cutaneous inflammation. Systemic oral administration of an antibiotic to address a cutaneous disease may not be particularly efficient, with high doses of minocycline required to drive sufficient concentration of the drug to its intended target. This is problematic for patients as minocycline is associated with systemic side effects that include dizziness, nausea, joint and muscle pain, and skin discoloration [3–5]. To avoid these side-effects and improve the efficiency of delivery into the skin, a topical minocycline gel (BPX-01; at press time BPX-01 is a new drug product limited by United States law to investigational use only) has been developed that aims to drive minocycline to the microenvironments where P. acnes resides. BPX-01’s mechanism of action is intended to deliver minocycline through the stratum corneum of the skin and into pores and hair shafts. The key question driving this study was to determine if and how the BPX-01 formulation could deliver minocycline to the sebaceous glands. Interestingly, minocycline has a characteristic yellow-green fluorescence that becomes brighter when chelated to magnesium ions, and this fluorescence has been used in the past to detect minocycline within minocycline-induced skin hyperpigmentation using conventional fluorescence microscopy [4, 5].
Two-photon excitation fluorescence microscopy (TPEF) has been widely utilized for non-invasive imaging both in vitro and in vivo because of its ability to image deeply in tissue while substantially decreasing the photobleaching and photodamage associated with one-photon imaging tools [6, 7]. As a nonlinear imaging method, TPEF provides intrinsic three-dimensional sectioning that can be used to build “pharmacokinetic (PK) tomograms” of drug delivery [7–9]. However, skin contains various endogenous autofluoroescent species, such as collagens, elastins, melanins, nicotinamide adenine dinucleotides (NAD(P)H), porphyrins, and flavin adenine dinucleotides (FAD) that generate strong endogenous fluorescence in the 400-600 nm spectral range [10, 11]. This substantial endogenous fluorescence within skin can overwhelm the signals arising from drugs of interest, such as minocycline, thereby limiting the ability to clearly visualize drug distribution over time, particularly at low local concentrations.
Two-photon excited fluorescence lifetime imaging microscopy (FLIM) is a powerful imaging tool that enables differentiation and visualization of specific fluorophores within a sample based on their fluorescence lifetime and can be readily applied to heterogenous samples with multiple sources of fluorescence, such as skin. The emission of each fluorophore has an associated fluorescence lifetime, and by measuring both the number of emitted photons in addition to their arrival times, FLIM offers an additional source of molecular contrast that can enable differentiation of fluorophores in a heterogenous sample that may otherwise be indistinguishable based solely on spectrum and fluorescence intensity. Importantly, the fluorescence lifetime of a fluorophore can vary depending on its environment and molecular interactions, such as when a coenzyme binds to a protein. This property can be utilized to investigate processes such as the metabolic activity of cells [12, 13] and the evaluation of drug activity by measuring intermolecular Förster resonance energy transfer (FRET) between protein-bound fluorophores [14, 15].
Here, we report the visualization of drug distribution of the active pharmaceutical ingredient (API), minocycline, in ex vivo human facial skin at the equivalent of daily application doses using TPEF and FLIM. We found that TPEF allows for the identification of high dosage minocycline uptake in facial skin samples, but a similar approach for the analysis of a single daily-dose (low-dose) treated skin was unsuccessful due to the interfering effects of tissue autofluorescence. At these lower dosages of treatment, FLIM was found to allow for identification and visualization of minocycline. We identified a unique fluorescence lifetime signature of minocycline via FLIM phasor analysis that is distinct from that of the surrounding endogenous fluorescence, which enabled the extraction of the drug contribution within the skin, even in the presence of a strong autofluorescence background. This study demonstrates that a combination of TPEF and FLIM can be used as a promising tool to further study the pharmacokinetics and pharmacodynamics of topically applied drugs with subcellular resolution and molecular specificity.
2. Materials and methods
2.1 Preparation of facial skin with BPX-01 treatment
Excised human periauricular facial skin from facelift patients was obtained and stored at −80°C. Prior to experiment, the specimens were thawed and then portioned with a scalpel. BPX-01 formulations containing 1%, 2% or 4% minocycline, as well as a BPX-01 vehicle, were dispensed onto the skin’s surface at 2.5 mg/cm2 or 60 mg/cm2. Formulations were rubbed on the surface of the skin. Tissues receiving 60 mg/cm2 doses included a ring barrier to contain the formulation. Samples were incubated on a damp gauze pad at 32°C for 24 hours. The skin surface was then gently cleansed with isopropyl alcohol (70%) to remove residual formulation, and the sample was then trimmed. Next, the tissues were embedded in optimal cutting temperature compound and frozen. The frozen tissues were cross-sectioned along the plane perpendicular to the skin surface with a thickness of 30 µm using a cryostat (AVANTIK QS11, Springfield Township, NJ) to explore the delivery of minocycline throughout the entire depth of skin while the cellular morphological structures of interest were retained. The sections were then mounted on microscope slides for TPEF microcopy and FLIM measurements.
2.2 Two-photon excitation fluorescence (TPEF) microscopy and spectral analysis
A confocal laser scanning microscope (Olympus FV1000, Center Valley, PA) with a 60X water-immersion 1.20 NA objective lens (Olympus UPLSAPO 60XW, Center Valley, PA) was used in this study. For nonlinear optical imaging, the emission from a widely tunable ultrafast femtosecond laser source (Spectra-Physics InSight DeepSee, Santa Clara, CA) was introduced into the microscope. The laser source was tuned to an excitation wavelength of 780 nm; the power was maintained below 30 mW at the sample plane for all imaging experiments, with a pixel dwell time of 4 µs to avoid any photodamage. Photobleaching of minocycline was not observed under these conditions. At the side output port of the microscope, a 680 nm shortpass filter (Chroma E680SP-2P, Bellows Falls, VT) was used to reject any remaining 780 nm light from the excitation source. The emitted light was then split by a 480 nm longpass dichroic mirror. In the shortpass channel, a bandpass filter centered at 475 nm with a 60 nm bandwidth (Chroma HQ475/60M, Bellows Falls, VT) was used to filter emission light prior to detection with a photomultiplier tube (PMT; Hamamatsu H7422P-40, Hamamatsu City, Japan). In the longpass channel, another bandpass filter centered at 620 nm with a bandwidth of 60 nm (Chroma HQ620/60M, Bellows Falls, VT) was used to filter the longer wavelength fluorescence prior to detection with a second PMT (Hamamatsu H7422P-50, Hamamatsu City, Japan). The PMT signals were amplified with high-speed trans-impedance amplifiers (Femto HCA-4M-500K, Berlin, Germany) and fed into the Olympus FV1000 analog-to-digital converter (ADC) input ports for acquiring two-photon excitation fluorescence (TPEF) images. For quantitative analysis of TPEF images, a consistent set of acquisition conditions including PMT gain and acquisition time was used for all TPEF measurements.
TPEF spectra corresponding to TPEF images were acquired with 10-s acquisition time by a spectrometer (Ocean Optics QE65000, Largo, FL) coupled at the side output port of the microscope via an optical fiber (Thorlabs M25L02, 200 μm silica core, NA 0.22, Newton, NJ) in conjunction with a collimation lens (Thorlabs F810SMA-635, NA 0.25, Newton, NJ), while the excitation laser was continuously scanning the sample yielding a ~200 × 200 μm field of view (FOV). Prior to detection via the spectrometer, the 480 nm longpass dichroic mirror was replaced with 750 nm longpass dichroic mirror to collect TPEF signals from the sample below 750 nm.
2.3 Two-photon excitation fluorescence lifetime imaging microscopy (FLIM)
To collect FLIM data, the same microscopy setup described above for TPEF was used. For FLIM acquisition, however, the output from the “longpassed” PMT was instead routed into a commercial time-correlated single-photon counting (TCSPC) system (Becker & Hickl SPC-150, Berlin, Germany). The FLIM images consisted of 256 × 256 pixels within the 200 × 200 μm FOV, and each pixel was associated with a fluorescence decay trace histogram with 256 time bins across the 12.5 ns pulse repetition period of the laser source. The instrument response function of the FLIM system was calibrated using a solution of fluorescein with a known lifetime of 4.05 ns at pH 9.0. FLIM images were acquired with a 90 s acquisition time by detecting the emitted fluorescence in the 590 to 650 nm spectral window as previously described. This integration time was set such that the average number of photons collected per pixel was approximately 300, such that a 3x3 binning analysis provided more than 2500 photons which is considered adequate for a two-component lifetime fit [16].
2.4 Non-Euclidean phasor analysis for fluorescence contribution separation
The non-Euclidean phasor analysis method has been described in a previously published article [17]. Briefly, the phasor analysis algorithm begins by transforming each temporal fluorescence decay trace into a pair of phasor coordinates (G, S), where G and S respectively correspond to the normalized real and imaginary components of the Fourier transform of the fluorescence decay traces evaluated at the laser repetition frequency. This phasor transform is applied to each pixel of 3 input images: (i) the test sample itself, i.e. a sample treated with the topical gel with minocycline; (ii) an endogenous fluorescence reference, i.e. a sample that is treated with vehicle only without minocycline; and (iii) an exogenous fluorescence reference consisting of minocycline alone. The endogenous and exogenous reference samples yield two distinct reference clusters in phasor space, while the test sample results in a broadened phasor cluster spanning the space between the two references. The Mahalanobis distances between each test sample phasor to the phasor clusters associated with the endogenous and exogenous references are then calculated. This distance is a non-Euclidean metric that allows one to measure the distance between a single observation and a population. This is ensured by taking into account the covariance of the reference cluster in addition to its mean coordinates, whereas a Euclidean distance only considers a pair of observations (or one observation versus the mean of a reference population) [18]. The calculated Mahalanobis distance between each test phasor is then normalized by the Mahalanobis distance between the two reference clusters, resulting in an array of coefficients ranging from 0 to 1 for each of the test sample image's pixels. These coefficients quantify the relative signal contribution from either endogenous or exogenous fluorescence, depending on how the user defines 0 or 1 to correspond to endogenous or exogenous fluorescence signal. Performing this calculation on a pixel-by-pixel basis thus results in the construction of an intuitive visual map that conveniently illustrates the topical agent's fluorescence intensity contribution throughout the sample. As there are numerous factors that modulate fluorescence intensity (e.g. local environment, pH, ionic strength, chelation, etc) this intensity contribution calculation is used to build a map of the molecule’s spatial distribution, rather than provide a quantitative metric of concentration.
3. Results and discussion
3.1 Characterization of minocycline and facial skin using TPEF spectroscopy and microscopy
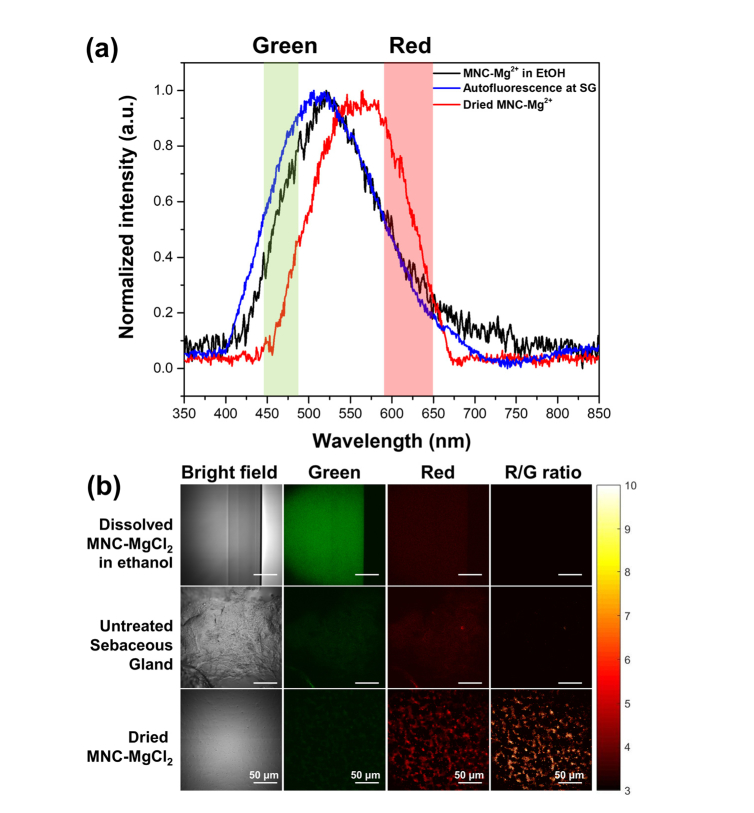
Minocycline chelated with magnesium ions (MNC-Mg2+) shows a yellow-green fluorescence [4, 5] that can be utilized to visualize minocycline uptake within skin. To characterize the fluorescent properties of minocycline, the fluorescence excitation-emission matrix (EEM) of MNC-Mg2+ was obtained experimentally and revealed to have maximum excitation and emission at 390 and 500 nm, respectively. Based on this result, we experimentally determined the optimal two-photon excitation wavelength for MNC-Mg2+ by observing a maximum two-photon excitation signal at 780 nm. In order to characterize the fluorescent properties of the drug and sebaceous glands, the TPEF spectra of MNC-Mg2+ (both dissolved in ethanol as well as in its dried form) and a thin section of human facial sebaceous gland were measured while the ultrafast laser was continuously scanned over the field of view. As shown in Fig. 1(a), the MNC-Mg2+ dissolved in ethanol shows a characteristic emission peak centered around 520 nm that is consistent with previous reports [4, 5] and is strikingly similar to the autofluorescence emission spectrum of the sebaceous gland (peak ca. 510 nm). However, we found that the MNC-Mg2+ in its dried form shows a red-shifted fluorescence emission relative to its dissolved form in ethanol, with a new maximum at 560 nm. In order to exploit this unique property for visualization of MNC-Mg2+ uptake within the skin, the BPX-01 treated skin samples were cryosectioned, mounted on a glass slide and then allowed to dry in ambient atmospheric conditions for more than 10 minutes. This drying period was found to be sufficient to dry the dissolved MNC-Mg2+ within the anatomical features of the skin sample. Thus, it would be expected that the spectral features of the dried form of MNC-Mg2+ could be observed in the skin samples. To test this hypothesis and visualize MNC-Mg2+ within skin, TPEF images were acquired from two fluorescence channels: 445 to 480 nm (designated green channel, G), and 590 to 650 nm (designated red channel, R), as described in Section 2.2. The signals captured in the 445 to 480 nm fluorescence channel arise predominantly from the autofluorescence of skin. Signals in the 590 to 650 nm channel, on the other hand, are expected to have a stronger contribution from MNC-Mg2+ in its dried form, rather than tissue autofluorescence (see Fig. 1). Thus, by dividing the red channel image by the green channel image (R/G ratio image), the contribution of the dried MNC-Mg2+ in the red fluorescence channel might be visualized (R/G ratio images in Fig. 1(b)). In order to set a baseline for the autofluorescence, the endogenous R/G ratio was determined by averaging R/G ratio values of the untreated sebaceous gland image. By setting a fixed value for the threshold of the R/G ratio value, the dried MNC-Mg2+ contribution could potentially be discriminated from the autofluorescence contribution in the red channel.
Fig. 1.
Characterization of fluorescence from MNC-Mg2+, either dissolved in ethanol or dried, and from a sebaceous gland within facial skin tissue at sebaceous gland (SG) by two-photon excitation fluorescence (TPEF) (a) spectra and (b) images. The spectra were obtained with 10-s acquisition time while the laser continuously scanned the sample. The TPEF images were acquired at the same field of view as the TPEF spectra from two fluorescence channels: 445-480 nm (green) and 590-650 nm (red). The R/G ratio images were generated by dividing the red channel images by the green channel images on a pixel-by-pixel basis.
3.2 Visualization of minocycline within skin using TPEF microscopy
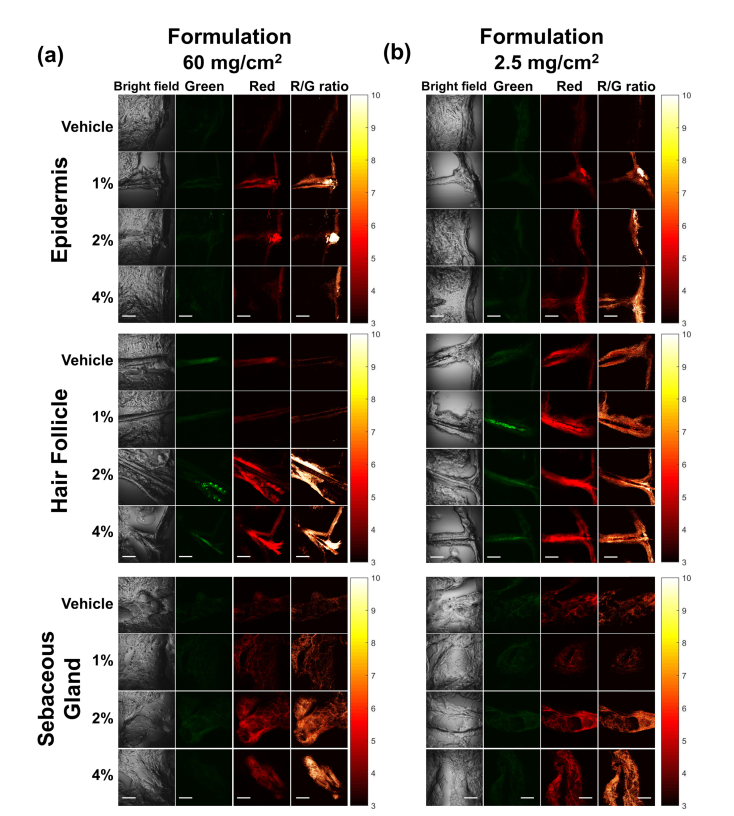
To test the possibility of visualizing the uptake of MNC-Mg2+ within skin using TPEF, a pilot experiment was conducted using two skin samples from a single donor treated with either a low dose (2.5 mg/cm2, i.e. the recommended daily dose) or a high dose (60 mg/cm2) of BPX-01 followed by a 24-hour incubation period. Skin samples were subsequently cross-sectioned and imaged by TPEF (see Fig. 2). To study the delivery and distribution of topically applied minocycline within human skin, three types of anatomical features (epidermis, hair follicle, and sebaceous gland) were imaged. In order to measure the tissue’s baseline autofluorescence, TPEF images of a skin sample treated with vehicle only (i.e. topical BPX-01 gel without MNC-Mg2+) were acquired from each anatomical feature. As shown in Fig. 2(a) and 2(b), the epidermis of the vehicle-treated sample showed low autofluorescence signals in both channels, whereas the BPX-01-treated skin samples exhibited stronger fluorescence signals in the red channel compared to the green channel. This tendency can be more clearly observed in the R/G ratio images. As expected, the strong signals in the red (drug) channel were primarily localized to the superficial layers of the epidermis, i.e. the layer directly exposed to BPX-01, as well as the hair follicle. However, the hair follicles and the sebaceous glands of vehicle-treated skin samples showed intense fluorescence signals in both the red (drug) and green (autofluorescence) channels, indicating the presence of strong tissue autofluorescence at these tissue sites.
Fig. 2.
TPEF images of facial skin samples treated with (a) 60 mg/cm2 and (b) 2.5 mg/cm2 of BPX-01 formulation with 0% (vehicle), 1%, 2%, and 4% of MNC-Mg2+ for 24 hours at three anatomical sites: epidermis, hair follicle, and sebaceous gland. The TPEF images were acquired from two fluorescence channels: 445-480 nm (green) and 590-650 nm (red). The R/G ratio images were generated by dividing the red channel images by the green channel images on a pixel-by-pixel basis. The scale bar is 50 μm.
In an attempt to quantitatively measure the uptake of MNC-Mg2+, the ratio of fluorescence signals between the green and red channels were computed and averaged. Pixels having a R/G ratio value below the threshold determined from the untreated sebaceous gland skin sample were excluded from the calculation. However, the uptake of MNC-Mg2+ was not successfully quantified for epidermis, hair follicles or sebaceous glands for any treatment group when compared to vehicle (pairwise t-test with Holm-Bonferroni correction method and non-pooled standard deviation, p > 0.05). In these cases, the relatively small fluorescence signal from MNC-Mg2+ was overwhelmed by the tissue autofluorescence. Though this experiment had a small sample size, the massive contribution from the observed tissue autofluorescence made it clear that TPEF alone would be inadequate for minocycline tissue quantification.
3.3 Visualization of minocycline within skin using time-domain analysis of FLIM data
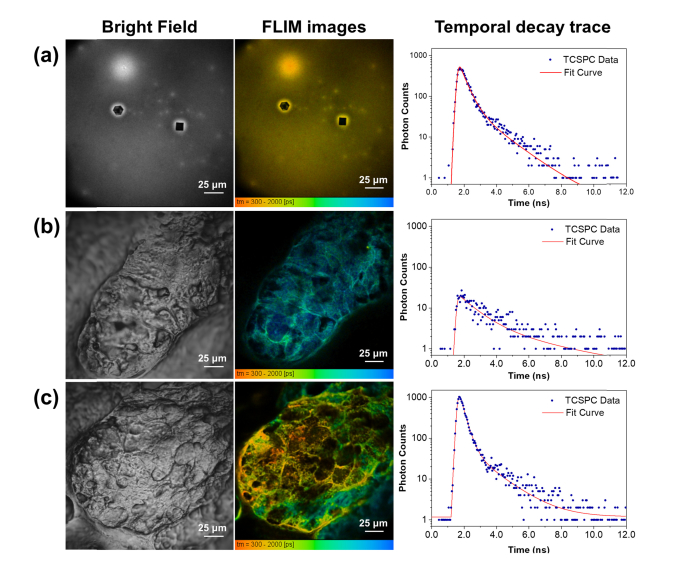
To overcome these challenges, we utilized two-photon excitation FLIM to search for a unique signature of MNC-Mg2+ that could be used to track its uptake within skin. As every fluorophore has an associated fluorescence lifetime, FLIM can identify exogenous fluorescent compound if its lifetime is distinct from those of the surrounding endogenous fluorophores. To investigate the applicability of FLIM for visualizing MNC-Mg2+ uptake within skin, a 780 nm ultrafast pulsed laser excitation was used to acquire FLIM data in the red channel from dried MNC-Mg2+, an untreated sebaceous gland, and a sebaceous gland treated with MNC-Mg2+ post-sectioning. Next, all fluorescence lifetimes were calculated by fitting a double exponential decay to each pixel’s associated fluorescence decay traces using SPCImage software (Fig. 3) in an attempt to model the various sources of fluorescence from the samples. The resulting figures illustrate TPEF signal intensity via pixel brightness, with pixel color corresponding to the mean weighted lifetime of the fitted decay curve for each pixel (rightmost column in Fig. 3). The dried MNC-Mg2+ showed a mean lifetime on the order of 400-500 ps (yellowish-orange in Fig. 3(a)) that was readily distinguishable from that of the sebaceous gland, which tends to be on the order of 1.5 ns (greenish-blue in Fig. 3(b)).
Fig. 3.
Bright field and FLIM images, as well as temporal decay traces of (a) dried MNC-Mg2+, (b) an untreated sebaceous gland, and (c) a sebaceous gland treated with MNC-Mg2+. The FLIM images were constructed from the red fluorescence channel (590-650 nm). All FLIM images were generated by fitting a double-exponential decay function to each pixel’s decay trace using SPCImage software.
The source of the tissue autofluorescence here is primarily attributed to flavins, FAD in particular, which can be efficiently two-photon excited at 780 nm, where FAD fluorescence emission peaks at 525 nm, but the emission spectrum extends until roughly 650 nm [17, 19, 20]. Additionally, epidermis and hair follicles contain keratins, elastins, and collagens, which may also interfere with the exogenous MNC-Mg2+ signal in the red TPEF channel. As a proof-of-concept, a 10 μL ethanol solution of 5 mg/mL MNC-Mg2+ was directly applied onto a cross-sectioned skin sample and left to dry. Consequently, as shown in Fig. 3(c), the highly concentrated MNC-Mg2+ within regions of the exposed sebaceous gland can be distinguished by its yellowish-orange color indicating short lifetime, consistent with the fluorescence lifetime measured from dried MNC-Mg2+ alone. It follows that pixels with an intermediate color, i.e. green rather than yellow or blue, contain signal contributions from both MNC-Mg2+ as well as autofluorescence. As such, FLIM offers a means to distinguish between these two competing sources of fluorescence. The fluorescence signals from MNC-Mg2+ can thus be extracted for accurate and specific visualization within skin despite intense background autofluorescence.
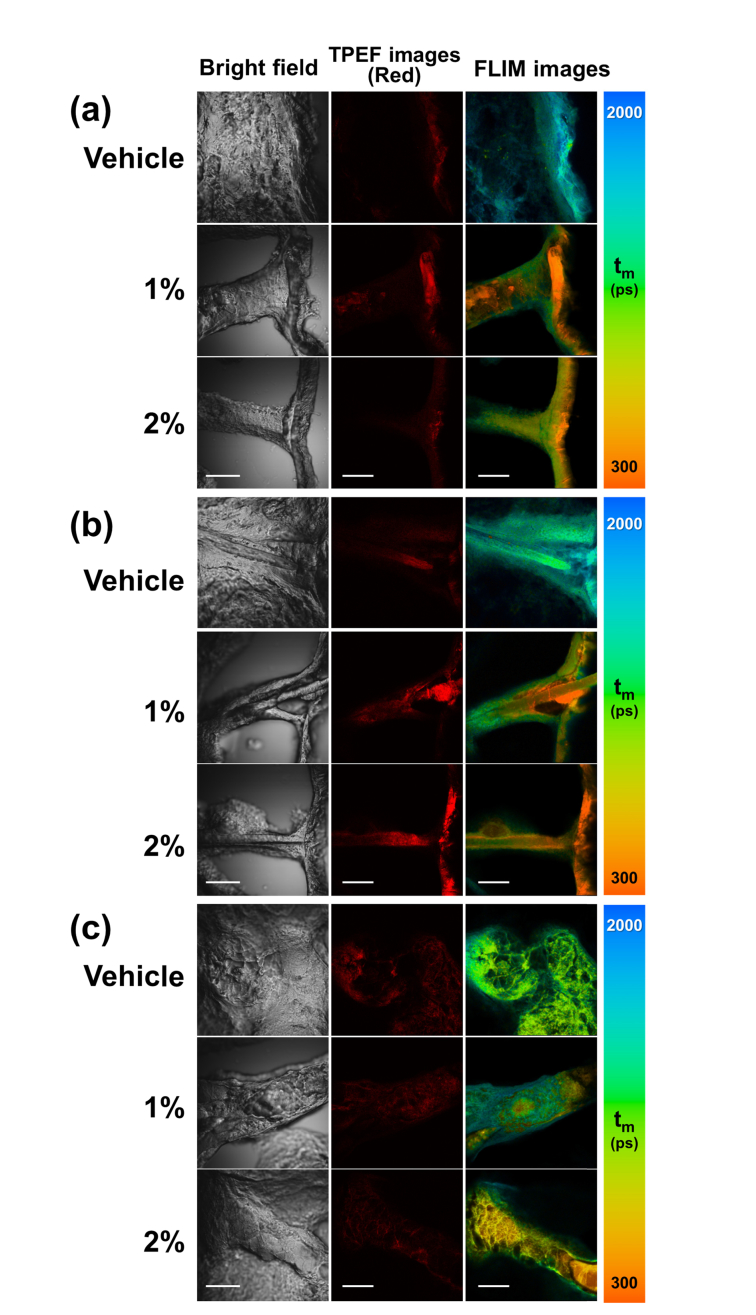
To verify the applicability of FLIM for visualizing MNC-Mg2+ uptake in a clinically relevant context, ex vivo skin specimens were treated with 2.5 mg/cm2 BPX-01 (a recommended daily dose) and imaged using FLIM. As shown in Fig. 4, the MNC-Mg2+ uptake corresponds to the characteristic yellowish-orange color within the epidermis, hair follicles, and sebaceous glands of the treated skin samples. The vehicle-treated skin sample did not show evidence of a predominantly short-lived fluorescent compound. Importantly, the MNC-Mg2+ fluorescence signal was found to be highly concentrated at the topmost layers of the epidermis (rightmost column in Fig. 4(a)), and the deep layers of skin strictly along the hair shaft and into the follicle. Similarly, at the sebaceous gland, the MNC-Mg2+ uptake flows from the top of the gland progressively down into its deeper layers. Interestingly, the sample treated with the 2% BPX-01 dose showed a strikingly greater uptake than the 1% treatment, as shown in Fig. 4.
Fig. 4.
Uptake of the active pharmaceutical ingredient (MNC-Mg2+) in facial skin, as illustrated by the TPEF and FLIM images of BPX-01 treated facial skin at three anatomical sites: (a) epidermis, (b) hair follicle, and (c) sebaceous gland. The facial skin was treated with 2.5 mg/cm2 BPX-01 containing 0% (vehicle), 1%, and 2% MNC-Mg2+ for 24 hours. The TPEF and FLIM images were acquired from the red fluorescence channel (590-650 nm). All FLIM images were obtained with 90-s acquisition time and generated by fitting a double-exponential decay function to the decay trace of each pixel using SPCImage software. The scale bar is 50 μm.
3.4 Visualization of minocycline with skin using non-Euclidean phasor analysis of FLIM data
Although the uptake of MNC-Mg2+ within skin was successfully visualized using FLIM, there remained several challenges for accurate discrimination and separation of the MNC-Mg2+ signal from autofluorescence. Analysis of FLIM data in the time domain identifies a compound’s fluorescence lifetime by fitting its fluorescence decay trace with a single, double, or triple exponential decay curve. Thus, in order to compute the fluorescence lifetime at each pixel of a FLIM image with high accuracy, a sufficient number of photons per pixel must be collected, and the number of molecular species contributing to the fluorescence signal must be known a priori [21]. However, human skin contains a wide variety of endogenous fluorophores, and its various molecular environments further complicate the characterization of skin autofluorescence in terms of fluorescence lifetime. Additionally, the fluorescence lifetime of MNC-Mg2+ also depends on magnesium concentration, thereby increasing lifetime variance and complicating accurate visualization of MNC-Mg2+ within skin using traditional curve-fitting in the time domain.
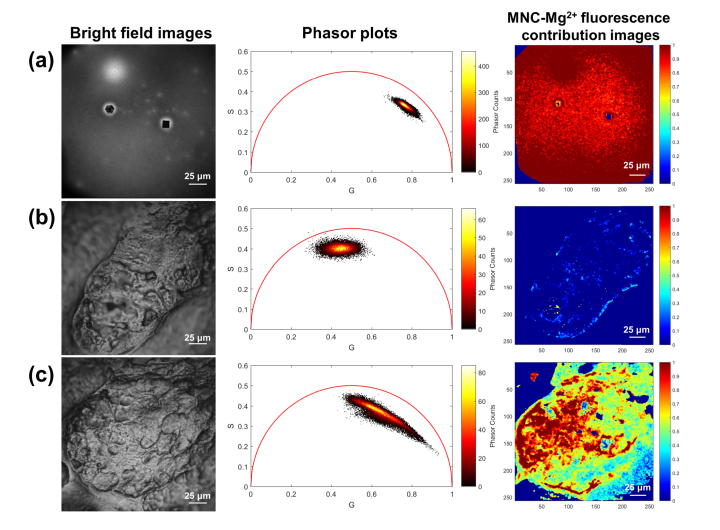
Therefore, we applied a non-Euclidean phasor analysis approach to process the acquired FLIM data in a renewed attempt to separate exogenous from endogenous fluorescent species without a priori knowledge of the sample. The phasor analysis method transforms a fluorescence decay trace into a pair of phasor coordinates via a Fourier transform rather than computing the fluorescence lifetime by exponential fitting in the time domain. Using this transform, each pixel of a FLIM image can be plotted on a graph based on its phasor coordinates (G,S). For pixels containing a single fluorophore with a well-defined lifetime, these coordinates lie along the circumference of the so-called universal semicircle, centered at (0.5,0) with a radius of 0.5 [13, 22, 23]. In the case of two fluorescent species colocalized within the same pixel, the relative contributions of each fluorophore can be computed by measuring the relative distance from the pixel’s phasor to the phasors of the two individual fluorescent species. If the two fluorescent species have a well-defined lifetime, then their respective phasors will lie at precise locations along the universal semi-circle; calculating relative contributions is then straightforward, as pixels containing both species lie along a line connecting the two original phasors. If the two fluorophores do not have well-defined lifetimes, as is the case for MNC-Mg2+ and most sources of tissue autofluorescence, then the reference phasor coordinates are likely to lie within the universal semicircle with broad two-dimensional distributions. Recently, we reported a non-Euclidean phasor analysis method for FLIM data that can be used to distinguish skin autofluorescence from the fluorescence of an exogenous compound [17]. The collected FLIM images were processed using this separation algorithm in order to improve MNC-Mg2+ visualization and extract drug local distribution information from the treated samples. First, reference phasor clusters of exogenous and endogenous sources of fluorescence were identified from FLIM image data of dried MNC-Mg2+ and a sebaceous gland, respectively. As shown in Fig. 5(a) and 5(b), the dried MNC-Mg2+ sample data generated an elongated elliptical phasor cluster in the top-right region of the phasor plot, while the sebaceous gland data generated a phasor cluster in the central topmost region. In the case of Fig. 5(c), the data from the MNC-Mg2+ treated sebaceous gland yielded a broad phasor cluster that spans the space between the two reference phasor clusters.
Fig. 5.
Bright field images, phasor plots, and MNC-Mg2+ fluorescence contribution images of (a) dried MNC-Mg2+, (b) untreated sebaceous gland, and (c) sebaceous gland treated with MNC-Mg2+. The phasor plots and MNC-Mg2+ fluorescence contribution images were generated from the red fluorescence channel (590-650 nm) using a non-Euclidean phasor analysis algorithm developed in Matlab [17]. All FLIM images were obtained with 90 s acquisition time.
By computing the Mahalanobis distance between a given phasor and each of the reference phasor clusters, the contribution of MNC-Mg2+ in each pixel of the treated sample FLIM image can be calculated into a value between 0 and 1, corresponding to the fraction of that pixel’s fluorescence that was contributed by the drug, as discussed above in Section 2.4. As shown in Fig. 5, the fluorescence contribution from the drug in the dried MNC-Mg2+ sample approached 1 across the field of view, given that the signal arose entirely from MNC-Mg2+; conversely, the untreated sebaceous gland showed virtually no contribution from MNC-Mg2+, as evidenced by the fraction of drug fluorescence contribution approaching 0 across the field of view. The MNC-Mg2+ treated sebaceous gland, however, showed a range of pixel values ranging from 0 to 1, where redder regions of the color-coded image indicate areas where fluorescence is dominated by MNC-Mg2+ rather than autofluorescence.
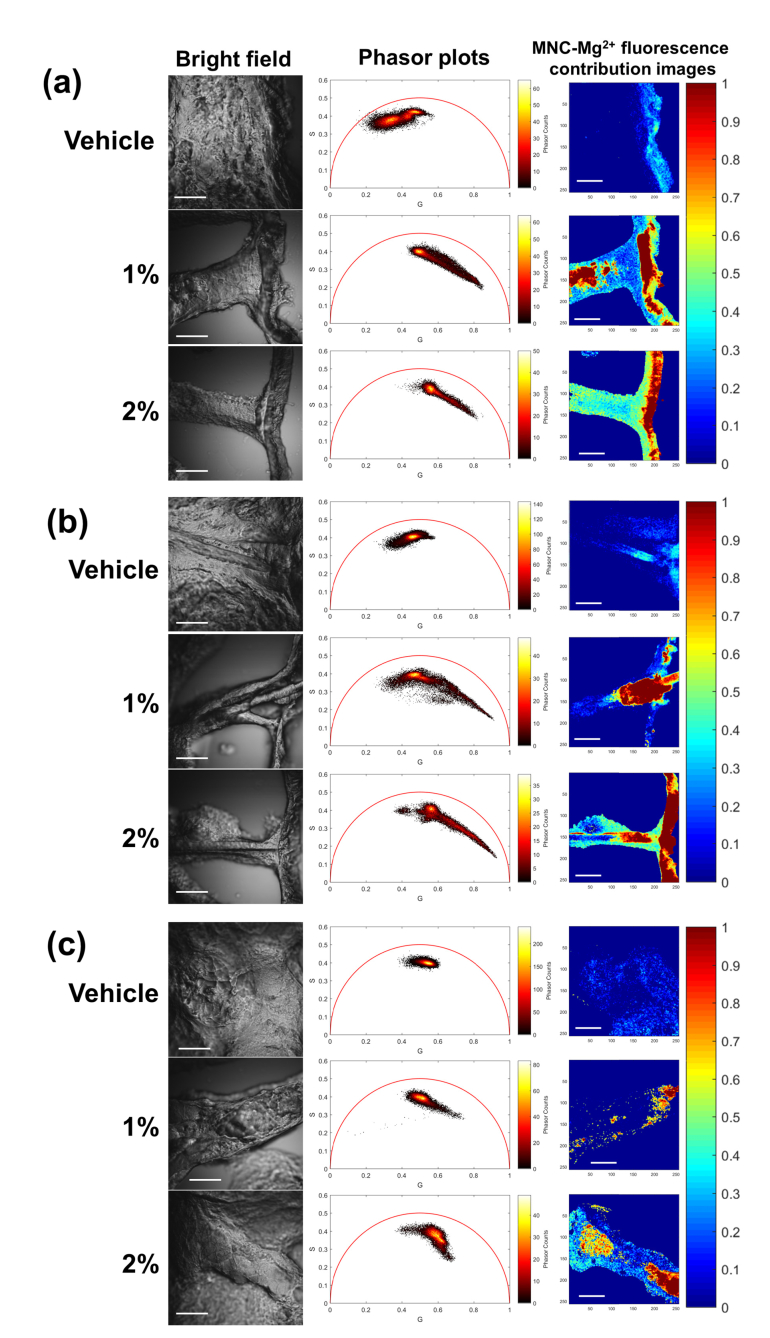
Last, clinically representative ex vivo skin samples treated with 2.5 mg/cm2 BPX-01 samples were processed using this non-Euclidean phasor analysis method (Fig. 6). The epidermis, hair follicle, and sebaceous gland samples treated with vehicle only showed phasor clusters consistent with tissue autofluorescence [24]. In contrast, the minocycline-treated skin sample revealed a phasor cluster corresponding to autofluorescence accompanied by an elongated tail extending towards the coordinates of the previously described dried MNC-Mg2+ phasor cluster. To generate images of minocycline contribution using this non-Euclidean phasor analysis method, the phasor clusters from each anatomical feature of the vehicle-treated sample were used as the reference of endogenous fluorescence for each respective feature; the dried MNC-Mg2+ reference cluster was used as the exogenous fluorescence reference in the analysis. In the MNC-Mg2+ fluorescence contribution images (rightmost column in Fig. 6), the drug distribution in the ex vivo skin samples treated with various concentrations of MNC-Mg2+ could be clearly visualized and distinguished from tissue autofluorescence; this clear separation could not otherwise be achieved using time-domain analysis of the FLIM data. Furthermore, in the MNC-Mg2+ fluorescence contribution images of the treated epidermis and hair follicle samples (Fig. 6), the MNC-Mg2+ was clearly visualized with high contrast and found to be localized at the superficial layers of the epidermis, as well as within the hair follicle. Of note, the diffusion and spatial distribution of MNC-Mg2+ within sebaceous glands were more distinctly observed in the drug fluorescence contribution images; this supports the applicability of FLIM analysis with non-Euclidean phasor analysis to improve visualization of MNC-Mg2+ distribution within human skin as a PK tomographic toolkit. Collectively, these results support the conclusion that minocycline applied on the skin in BPX-01 travels through the hair shaft to eventually reach sebaceous glands.
Fig. 6.
Bright field images, phasor plots, and MNC-Mg2+ fluorescence contribution images of BPX-01 treated facial skin at three anatomical sites: (a) epidermis, (b) hair follicle, and (c) sebaceous gland. The facial skin was treated with 2.5 mg/cm2 BPX-01 containing 0% (vehicle), 1%, and 2% MNC-Mg2+ for 24 hours. The phasor plots and minocycline uptake images were generated from the red fluorescence channel (590-650 nm) using a non-Euclidean phasor analysis algorithm developed in Matlab. All FLIM images were obtained with 90 s acquisition time. The scale bar is 50 μm.
4. Conclusion
In this study, we developed and demonstrated a method for the selective visualization of minocycline uptake within human skin using TPEF microscopy and FLIM combined with a non-Euclidean phasor analysis approach. Minocycline is inherently fluorescent, enabling non-invasive and label-free visualization of minocycline uptake within skin; however, the drug’s fluorescence emission profile is similar to that of the endogenous skin fluorescence. Using TPEF microscopy alone and comparing TPEF signals between the two fluorescence channels from 445 to 480 nm and 590 to 650 nm, the uptake of minocycline could be visualized at the epidermis with high and low dose treatments of minocycline in BPX-01, but could not be conclusively detected within the hair follicle and sebaceous glands due to competing autofluorescence. FLIM offers an additional source of contrast by measuring fluorescence lifetime that enables the distinction of minocycline fluorescence from tissue autofluorescence.
Additionally, using a non-Euclidean phasor analysis approach to FLIM data resulted in superior discrimination and separation of minocycline, allowing for clear visualization and relative quantification of drug uptake and distribution. In this manner, minocycline uptake can be visualized based on the drug’s fluorescence contribution without interference from heterogeneous tissue autofluorescence. This allows for the investigation of minocycline delivery and spatial distribution within ex vivo and potentially in vivo human skin in a non-invasive and label-free manner with subcellular resolution.
Based on these developed methods, we were able to selectively visualize the distribution and uptake of minocycline in human facial skin treated with a recommended daily dose of BPX-01 gel. It is important to note that the drug distribution is generated based on fluorescence contribution from the drug and is not necessarily in a 1:1 ratio with the concentration of minocycline. This effect arises naturally from the myriad of spatially heterogenous effects that can modulate molecular fluorescence, ranging from the local environment of the molecule to factors including pH and magnesium ion chelation. Here, this was not considered a limitation, as the key study goal was to determine if and how the drug was delivered to sebaceous glands. The drug distribution maps in Fig. 6 demonstrate that the topically applied minocycline from the BPX-01 gel was indeed delivered to sebaceous glands via hair follicles from the superficial layers of epidermis. The study results additionally suggest higher uptake of minocycline within sebaceous glands from the 2% dose than the 1% dose, as was expected.
Excitingly, these results support a BPX-01 Phase 2b multi-center randomized, double-blind, vehicle-controlled dose-ranging study outcomes in which the 2% BPX-01 treatment arm (n = 72) exhibited 58.5% reduction in inflammatory lesions from baseline versus that of 43.8% reduction in the vehicle arm (n = 74) at week 12 based on LOCF (Last Observation Carried Forward), with statistical significance (p = 0.0256) analyzed using ANCOVA (pairwise comparisons of the treatment groups were generated with Bonferroni adjustment for multiplicity). Interestingly, the 2% BPX-01 arm also demonstrated an early onset of 43.3% reduction in inflammatory lesions with statistical significance (p = 0.0047) over the vehicle arm’s 25.3% at week 4 based on LOCF. The 1% BPX-01 (n = 73) treatment exhibited 54.4% reduction in inflammatory lesions from baseline at week 12 based on LOCF, but interestingly was not statistically significantly different from that of the vehicle arm (p = 0.0765). Based on these results, we believe that this selective visualization and distribution method using a combination of TPEF and FLIM with non-Euclidean phasor analysis can be used as a promising tool for pharmacokinetics and pharmacodynamics analyses of a topically applied drug onto skin and potentially serves as a preclinical guiding tool for future translational research or clinical development.
It is also worth mentioning that this established method may be able to play a role for investigating and evaluating bioequivalence of generic drugs and biologics, which could be helpful in achieving FDA approval. In particular, portable multiphoton FLIM systems are already commercially available and are suitable for visualizing and quantitatively evaluating the uptake of the generic version of drug within its intended target in the large-scale clinical investigation [25, 26]. In this manner, the drug’s effectiveness could thus be directly evaluated. Furthermore, in future studies, this method could readily be extended into a multifunctional PK/PD tomographic toolkit in conjunction with coherent Raman imaging modalities to improve signal specificity and image acquisition speed.
Acknowledgments
This study was funded via a sponsored research agreement between Massachusetts General Hospital and BioPharmX, Inc.
Funding
Massachusetts General Hospital; BioPharmX.
Disclosures
The authors declare no competing financial interests.
References and links
- 1.Goulden V., Glass D., Cunliffe W. J., “Safety of long-term high-dose minocycline in the treatment of acne,” Br. J. Dermatol. 134(4), 693–695 (1996). 10.1111/j.1365-2133.1996.tb06972.x [DOI] [PubMed] [Google Scholar]
- 2.Eady E. A., Cove J. H., Holland K. T., Cunliffe W. J., “Superior antibacterial action and reduced incidence of bacterial resistance in minocycline compared to tetracycline-treated acne patients,” Br. J. Dermatol. 122(2), 233–244 (1990). 10.1111/j.1365-2133.1990.tb08270.x [DOI] [PubMed] [Google Scholar]
- 3.Smith K., Leyden J. J., “Safety of doxycycline and minocycline: A systematic review,” Clin. Ther. 27(9), 1329–1342 (2005). 10.1016/j.clinthera.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 4.Okada N., Sato S., Sasou T., Aoyama M., Nishida K., Yoshikawa K., “Characterization of pigmented granules in minocycline-induced cutaneous pigmentation: observations using fluorescence microscopy and high-performance liquid chromatography,” Br. J. Dermatol. 129(4), 403–407 (1993). 10.1111/j.1365-2133.1993.tb03166.x [DOI] [PubMed] [Google Scholar]
- 5.Dodiuk-Gad R. P., de Morentin H. M., Schafer J., Harel A., Neudorfer M., Misonzhnik F., Gitstein G., Rozenman D., Tur E., Brenner S., “Minocycline-induced cutaneous hyperpigmentation: confocal laser scanning microscope analysis,” J. Eur. Acad. Dermatol. Venereol. 20(4), 435–439 (2006). 10.1111/j.1468-3083.2006.01436.x [DOI] [PubMed] [Google Scholar]
- 6.Helmchen F., Denk W., “Deep tissue two-photon microscopy,” Nat. Methods 2(12), 932–940 (2005). 10.1038/nmeth818 [DOI] [PubMed] [Google Scholar]
- 7.Yu B., Kim K. H., So P. T., Blankschtein D., Langer R., “Visualization of oleic acid-induced transdermal diffusion pathways using two-photon fluorescence microscopy,” J. Invest. Dermatol. 120(3), 448–455 (2003). 10.1046/j.1523-1747.2003.12061.x [DOI] [PubMed] [Google Scholar]
- 8.Svoboda K., Yasuda R., “Principles of two-photon excitation microscopy and its applications to neuroscience,” Neuron 50(6), 823–839 (2006). 10.1016/j.neuron.2006.05.019 [DOI] [PubMed] [Google Scholar]
- 9.Bender J., Simonsson C., Smedh M., Engström S., Ericson M. B., “Lipid cubic phases in topical drug delivery: visualization of skin distribution using two-photon microscopy,” J. Control. Release 129(3), 163–169 (2008). 10.1016/j.jconrel.2008.04.020 [DOI] [PubMed] [Google Scholar]
- 10.Na R., Stender I. M., Henriksen M., Wulf H. C., “Autofluorescence of human skin is age-related after correction for skin pigmentation and redness,” J. Invest. Dermatol. 116(4), 536–540 (2001). 10.1046/j.1523-1747.2001.01285.x [DOI] [PubMed] [Google Scholar]
- 11.Na R., Stender I.-M., Ma L., Wulf H. C., “Autofluorescence spectrum of skin: component bands and body site variations,” Skin Res. Technol. 6(3), 112–117 (2000). 10.1034/j.1600-0846.2000.006003112.x [DOI] [PubMed] [Google Scholar]
- 12.Lakner P. H., Monaghan M. G., Möller Y., Olayioye M. A., Schenke-Layland K., “Applying phasor approach analysis of multiphoton FLIM measurements to probe the metabolic activity of three-dimensional in vitro cell culture models,” Sci. Rep. 7, 42730 (2017). 10.1038/srep42730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stringari C., Cinquin A., Cinquin O., Digman M. A., Donovan P. J., Gratton E., “Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue,” Proc. Natl. Acad. Sci. U.S.A. 108(33), 13582–13587 (2011). 10.1073/pnas.1108161108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peter M., Ameer-Beg S. M., Hughes M. K., Keppler M. D., Prag S., Marsh M., Vojnovic B., Ng T., “Multiphoton-FLIM quantification of the EGFP-mRFP1 FRET pair for localization of membrane receptor-kinase interactions,” Biophys. J. 88(2), 1224–1237 (2005). 10.1529/biophysj.104.050153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grecco H. E., Roda-Navarro P., Girod A., Hou J., Frahm T., Truxius D. C., Pepperkok R., Squire A., Bastiaens P. I., “In situ analysis of tyrosine phosphorylation networks by FLIM on cell arrays,” Nat. Methods 7(6), 467–472 (2010). 10.1038/nmeth.1458 [DOI] [PubMed] [Google Scholar]
- 16.Walsh A. J., Sharick J. T., Skala M. C., Beier H. T., “Temporal binning of time-correlated single photon counting data improves exponential decay fits and imaging speed,” Biomed. Opt. Express 7(4), 1385–1399 (2016). 10.1364/BOE.7.001385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osseiran S., Roider E. M., Wang H., Suita Y., Murphy M., Fisher D. E., Evans C. L., “Non-Euclidean phasor analysis for quantification of oxidative stress in ex vivo human skin exposed to sun filters using fluorescence lifetime imaging microscopy,” J. Biomed. Opt. 22(12), 1–10 (2017). 10.1117/1.JBO.22.12.125004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahalanobis P. C., “On the Generalized Distance in Statistics,” Proc. Natl. Inst. Sci. 2, 49–55 (1936). [Google Scholar]
- 19.Skala M. C., Riching K. M., Gendron-Fitzpatrick A., Eickhoff J., Eliceiri K. W., White J. G., Ramanujam N., “In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia,” Proc. Natl. Acad. Sci. U.S.A. 104(49), 19494–19499 (2007). 10.1073/pnas.0708425104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S., Heikal A. A., Webb W. W., “Two-Photon Fluorescence Spectroscopy and Microscopy of NAD(P)H and Flavoprotein,” Biophys. J. 82(5), 2811–2825 (2002). 10.1016/S0006-3495(02)75621-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker W., “Fluorescence lifetime imaging--techniques and applications,” J. Microsc. 247(2), 119–136 (2012). 10.1111/j.1365-2818.2012.03618.x [DOI] [PubMed] [Google Scholar]
- 22.Digman M. A., Caiolfa V. R., Zamai M., Gratton E., “The phasor approach to fluorescence lifetime imaging analysis,” Biophys. J. 94(2), L14–L16 (2008). 10.1529/biophysj.107.120154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stringari C., Nourse J. L., Flanagan L. A., Gratton E., “Phasor fluorescence lifetime microscopy of free and protein-bound NADH reveals neural stem cell differentiation potential,” PLoS One 7(11), e48014 (2012). 10.1371/journal.pone.0048014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fereidouni F., Bader A. N., Colonna A., Gerritsen H. C., “Phasor analysis of multiphoton spectral images distinguishes autofluorescence components of in vivo human skin,” J. Biophotonics 7(8), 589–596 (2014). 10.1002/jbio.201200244 [DOI] [PubMed] [Google Scholar]
- 25.Koenig K., “Hybrid multiphoton multimodal tomography of in vivo human skin,” Intravital 1(1), 11–26 (2012). 10.4161/intv.21938 [DOI] [Google Scholar]
- 26.König K., Raphael A. P., Lin L., Grice J. E., Soyer H. P., Breunig H. G., Roberts M. S., Prow T. W., “Applications of multiphoton tomographs and femtosecond laser nanoprocessing microscopes in drug delivery research,” Adv. Drug Deliv. Rev. 63(4-5), 388–404 (2011). 10.1016/j.addr.2011.03.002 [DOI] [PubMed] [Google Scholar]