Abstract
Introduction Otogenic lateral sinus thrombosis is a rare intracranial complication of otitis media in the modern age of antibiotic treatment, but it is potentially a dangerous complication.
Objectives The aim of this study is to focus on the various clinical presentations, management options and sequelae in a series of fifteen patients with otogenic lateral sinus thrombosis.
Methods Retrospective chart review of inpatients treated for otogenic lateral sinus thrombosis at our tertiary care institution between 2010 and 2015.
Results A total of 15 patients (11 males and 4 females) with ages ranging from 9 to 60 years were diagnosed with otogenic lateral sinus thrombosis. The most commonly reported symptoms were headache, ear discharge and hard of hearing, which were experienced by all 15 (100%) patients. In contrast to previous studies found in the literature, 7 (47%) patients in our series presented with neck pain and neck abscess. Imaging studies and microbiological cultures were performed for all patients, who also underwent a mastoidectomy procedure. Internal jugular vein ligation was performed on 5 (33%) patients. Incision and drainage of the neck abscess was performed on 7 (47%) patients. All patients had a satisfactory resolution of their symptoms, and the mortality rate was of 0%.
Conclusions Otogenic lateral sinus thrombosis, though a rare complication, can still occur; therefore, keeping a high level of suspicion is important, especially in developing countries. We also describe the patients with neck abscess associated with this rare condition. Combining parenteral antibiotics with surgical intervention is the treatment of choice.
Keywords: lateral sinus thrombosis, otitis media, cholesteatoma
Introduction
When thromboses occur in the sigmoid and transverse sinuses, they form lateral sinus thrombosis (LST), which is a potentially dangerous complication of otitis media. The rate of this complication has decreased drastically since the introduction of antibiotics for the treatment of otitis media. 1 Before the age of antibiotics, LST was ranked second to meningitis as the most frequent fatal complication of otitis media. Now, it is a rare complication of otitis media, but it poses a danger that requires immediate medical and surgical treatment. The mortality rates for LST in the modern era are lower, but still range from 5 to 10%. 2 Due to the decreased incidence and change in presentation of this complication, a high level of suspicion is required in order to make a diagnosis. The anatomical location of the middle ear and mastoid air cells in the cerebral venous sinuses makes them vulnerable to thrombosis secondary to middle ear infection and inflammation. 3 4 Infection from the mastoid air cell system reaches the perisinus area, resulting in perisinus abscess, which then spreads to the dura and intimal layer of the sinuses, causing mural thrombus. Unless effective treatment is promptly started, the mural thrombus grows and necrotizes, forming an intramural abscess. The mural thrombus within the lumen of the sinus propagates proximally to other cerebral venous sinuses, and extends caudally to the internal jugular and subclavian veins. Embolization of the propagating infected thrombus into the systemic circulation causes septicemia. Lateral sinus thrombosis is frequently associated with other intracranial and extracranial complications. It usually develops as a complication of chronic suppurative otitis media (CSOM) caused by the direct spread of the disease through the adjacent eroded bone, or as a complication of acute suppurative otitis media (ASOM) caused by thrombophlebitic spread through the emissary veins without bony erosion. The mastoidectomy surgeries, intravenous antibiotics, and radiological investigations, such as computed tomography (CT) scans and magnetic resonance imaging (MRI), have all contributed greatly to the current management protocol. Nowadays, the antibiotic therapy masks the typical presentation. The untreated clinical picture is marked by spiking fevers (the so-called picket fence fever curve, due to the periodic release of septic emboli from the infected sinus thrombus), headache, malaise, and progressive anemia.
Objectives
The aim of this study is to focus on the various clinical presentations, management options and sequelae in a series of fifteen patients with otogenic LST.
Materials and Methods
All patients with otogenic LST as the primary diagnosis, who were hospitalized at a tertiary health care center between December 2010 and February 2015, were included in this study. A total of 15 patients was identified.
Results
Patient Demographics and Symptoms
During the 5-year study period, 11eleven (73%) males and 4 (27%) females presented with LST. The patients' age ranged from 9 to 60 years. The most commonly reported symptoms were headache and ear discharge. All patients had hard of hearing, 11 (73%) of them had conductive hearing loss, and 4 (27%) patients had mixed hearing loss. Eleven (73%) patients had fever, and 1 (7%) patient in this group had the characteristic picket fence pattern of fever. One (7%) patient presented with double vision associated with sixth-nerve palsy ( Table 1 ).
Table 1. Symptoms of the patients at admission.
| Symptoms | Number of patients (%) |
|---|---|
| 1. Ear discharge | 15 (100) |
| 2. Headache | 15 (100) |
| 3. Hard of hearing | 15 (100) |
| 4. Nausea and vomiting | 12 (80) |
| 5. Fever | 11 (73) |
| 6. Neck pain and swelling | 7 (47) |
| 7. Giddiness | 6 (40) |
| 8. Visual disturbance (double vision) | 1 (7) |
Signs
All patients had positive otoscopic findings, which included purulent ear discharge, retraction pocket with cholesteatoma debris, congested and retracted pars tensa. All patients had a history of otitis, either recurrent acute or chronic otitis media. One (7%) patient developed LST following the first episode of ASOM. Swelling over the mastoid due to mastoid emissary vein thrombosis (Griesinger sign) was present in 2 patients (13%) ( Table 2 ).
Table 2. Signs of the patients at admission.
| Signs | Number of patients (%) |
|---|---|
| 1. Attic retraction pocket with cholesteatoma | 9 (60) |
| 2. Posterosuperior retraction pocket with cholesteatoma | 5 (33) |
| 3. Congested tympanic membrane (acute suppurative otitis media) | 1 (7) |
| 4. Positive fistula test | 2 (13) |
| 5. Post-aural abscess | 3 (20) |
| 6. Neck abscess | 7 (47) |
| 7. Lateral rectus palsy | 1 (7) |
| 8. Features of internal jugular vein thrombosis | 6 (40) |
| 9. Signs of meningitis | 2 (13) |
| 10. Signs of cerebellar abscess | 2 (13) |
| 11. Griesinger sign | 2 (13) |
| 12. Papilledema | 3 (20) |
Diagnostic Workup
The average duration of the hospital stay was 14 days (range: 12-18 days). All 15 patients underwent contrast-enhanced computed tomography (CECT) of the brain and temporal bones, which showed the “empty delta sign” (central non-enhancing clot surrounded by enhancing dural sinus wall), indicating sinus thrombosis. In eight cases, CECTs of the brain and temporal bone showed the sinus plate erosion ( Fig. 1A and B ). The CECT showed the thrombus confined only to the lateral sinus in 6 (40%) patients, extension to the internal jugular vein in 8 (53%) patients, and extension to mediastinum in 1 (7%) patient ( Fig. 2A, B , and C ). Seven (47%) patients had associated neck abscesses. Three (20%) patients underwent a MRI, and 2 of these patients also underwent a magnetic resonance venography (MRV). Three (20%) of the 15 patients had other intracranial complications, such as frank cerebellar abscess and temporal lobe abscess. Two (13%) patients had meningitis. None of the patients had any evidence of hydrocephalus.
Fig. 1.
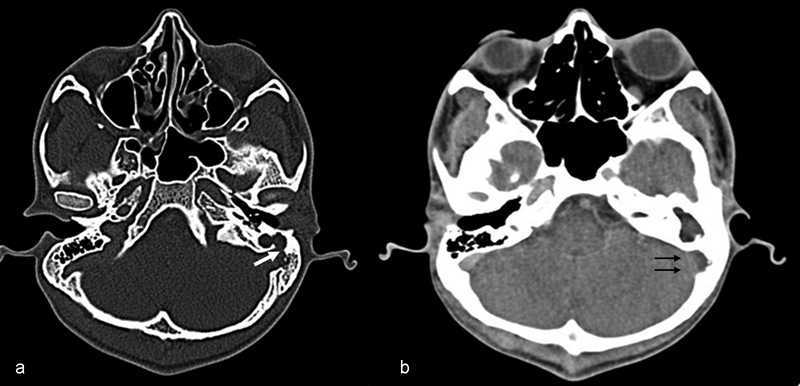
( A ) Computed tomography (CT) brain bone window axial cut showing the density of the soft tissue in the middle ear with a breach of the sigmoid plate (arrow) with cavitation of the mastoid air cell system. ( B ) Post-contrast CT brain soft tissue window axial cut showing central non-enhancement of the clot in the sigmoid sinus observed in the left side, with surrounding enhancing bone and dural sinus wall (double arrow) showing the classic ‘empty delta sign’ in sigmoid sinus thrombosis.
Fig. 2.
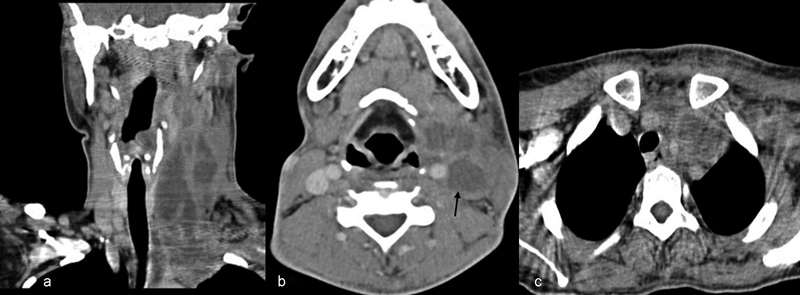
( A ) Contrast-enhanced computed tomography (CECT) neck soft tissue window coronal view showing multiple low attenuated central necrotic components with capsular ring enhancement and surrounding inflammatory changes suggestive of multiple abscess extending to the mediastinum. ( B ) Axial cut of a CECT of the neck showing a non-enhancing thrombosed internal jugular vein (arrow) with multiple abscesses and mass effect with effacement of the adjacent structures. ( C ) CECT of the mediastinum showing an abscess on the left side with mediastinal extension pushing the trachea to the right side.
Complete blood counts showed concentrations of hemoglobin < 10 g/dL in 3 (20%) patients, leukocytosis in 7 (47%) patients (normal range: 4,300–10,800/mL). All patients had normal platelet count (normal range: 1,500,000–4,000,000/mL). Two (13%) patients had altered liver enzymes, and all patients had normal coagulation profiles. Microbiologic cultures were produced from the middle ears of all patients, and 6 (40%) of them had negative cultures. Among the positive cultures, Proteus mirabilis and Pseudomonas aeruginosa were each isolated in 4 (44%) cases, and Escherichia coli and Enterococcus fecalis were each isolated in one (11%) case. One patient had both Proteus mirabilis and Pseudomonas aeruginosa . Blood culture was negative in all cases.
Treatment
All patients with otogenic LST were admitted and treated with broad-spectrum antibiotics effective against both aerobic and anaerobic organisms, followed by oral antibiotics continued for four to eight weeks. We performed an open cavity mastoidectomy for the 14 (93.3%) patients with cholesteatoma. Modified radical mastoidectomy was performed on 11 (73%) adult patients, radical mastoidectomy was performed on 3 (20%) pediatric patients, and cortical mastoidectomy was performed on 1 (7%) patient with ASOM. In all cases, the sigmoid sinus was skeletonized with careful removal of the adjacent granulation tissues. Perisinus abscesses were encountered in 11 (73%) patients and drained. The entire sinus was unroofed for inspection and palpation. The sinus was non-pliable in all cases, and a dry tap was encountered upon aspiration with a small gauge needle and syringe. Incision of the sinus wall and evacuation of the infected clots without traumatizing the medial dural wall was performed in 13 (87%) patients, and in 1 case, clot evacuation was not attempted because the thrombus extension was observed extending to the mediastinum in the CECT. Free bleeding from both ends of the incised sinus was established in 11 (73%) patients. In one case, which developed as a complication of ASOM, the sinus wall was not incised, and the perisinus granulation tissues were removed. The internal jugular vein was ligated in 5 (33%) cases. None of the patients used anticoagulants. Incision and drainage of the neck abscess were performed in 7 (47%) cases ( Fig. 3 ). Associated complications, like labyrinthine fistula, were managed by a single-staged matrix removal, followed by closure of the fistula, and the intracranial abscesses were managed by a multidisciplinary approach ( Table 3 ).
Fig. 3.
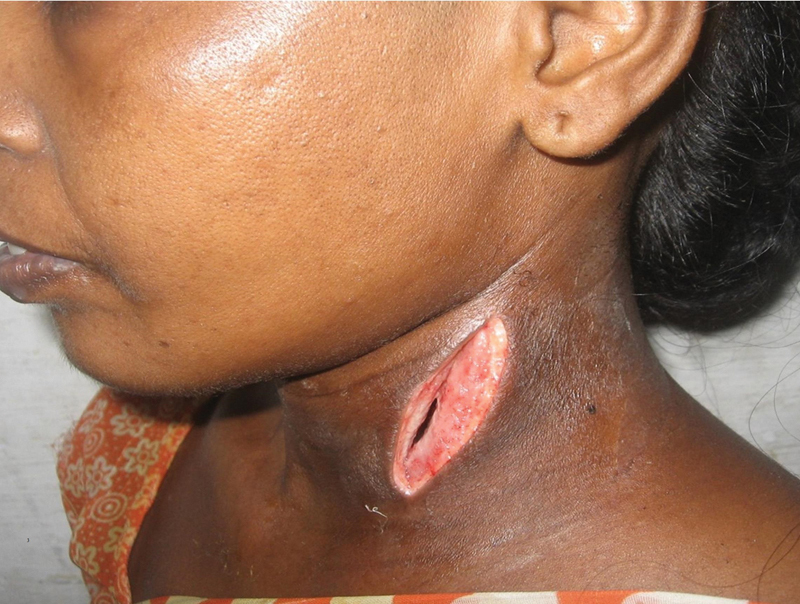
Clinical picture showing post-incision and drainage of the neck abscess.
Table 3. Intraoperative findings in the patients.
| Pathology | Number of patients (%) |
|---|---|
| Perisinus granulation | 15 (100%) |
| Cholesteatoma | 14 (93%) |
| Ossicular erosion | 14 (93%) |
| Perisinus abscess | 11 (73%) |
| Erosion of the sinus plate | 11 (73%) |
| Erosion of the dural plate | 4 (26%) |
| Erosion of the mastoid cortex | 4 (26%) |
| Dehiscent facial nerve | 4 (26%) |
| Lateral semicircular canal fistula | 3 (20%) |
| Promontory fistula | 1 (7%) |
Outcome
All patients recovered satisfactorily, with complete resolution of their symptoms and complications with the control of the middle ear infection. The mortality rate was of 0%. The average length of the follow-up was 16 months (range: 7-36 months). Cholesteatoma recurred in 1 (7%) patient who successfully underwent a revision surgery 2 years later. One (7%) patient had persistent mild lateral gaze diplopia, and 3 (20%) patients had unilateral severe sensorineural hearing loss. One (7%) patient had a long-term papilledema (3 years) that eventually resolved. Eight (53%) patients had long-term follow-up imaging, and 2 of them showed recanalization upon a repeat imaging exam.
Discussion
‘The more things change, the more they stay the same’ is the well-known phrase that aptly applies to LST as it continues to be a problem in the post-antibiotic age. Though the incidence of LST has decreased considerably with good antibiotic availability, the clinical pictures and microbiology of LST have changed. 5 Almost all patients (14; 93%) in our study had a history of otitis media in the weeks preceding admission, and most of them had received antibiotics. In many studies, headache is often cited as the most common symptom at presentation. 6 7 8 This finding was reproduced in our study as well. However, many of our patients also reported otorrhea, hard of hearing, and fever. In looking for a particular temperature pattern, only one patient had picket fence fever curve. A total of 7 (47%) patients had neck abscesses at the time of presentation, and this has not been reported in other studies. It is important to elicit a history of ear infection in patients presenting to the emergency department with neck abscesses.
Beta-hemolytic Streptococcus is no longer the predominant organism causing LST. 9 Because of the use of antibiotics, during prodromal infection, chronic rather than acute infection more commonly precedes LST, and the blood culture is often negative. 10 Culture from the middle ear discharge characteristically yields mixed flora, including Pseudomonas , Proteus , Bacteroides , Staphylococcus , Enterobacteriaceae and other species. 11 In our study, 6 (40%) patients had negative cultures. In patients with positive cultures, Pseudomonas aeruginosa (4 cases; 44%) and Proteus mirabilis (4 cases; 44%) were the predominant organisms, followed by Escherichia coli (1 case; 11%) and Enterococcus fecalis (1 case; 11%). Previous antibiotic treatment might be a major factor in rendering the cultures negative at the time of intervention, as noted in other studies. 8 12
The diagnosis of LST depends on radiographic imaging techniques or direct intraoperative detection. Contrast-enhanced computed tomography of the head and neck is the most commonly performed radiographic investigation when intracranial complications of otitis media are suspected. Lateral sinus thrombosis may be diagnosed by the presence of the pathognomonic “empty delta” sign. Early use of the MRI/MRV for the diagnosis of LST has been advocated in the last decade, because they have more sensitivity in detecting this complication, and are also useful for excluding other intracranial complications like adjacent subdural empyema, cerebritis, or cerebral abscess. The MRI shows increased intraluminal signal intensity on T1- and T2-weighted sequences. 11 13 It also offers the advantage of eliminating the risk of ionizing radiation compared with CECT, which is particularly helpful in the pediatric population. The gold standard for the diagnosis of LST remains MRV, which is a non-invasive method that can obviously outline the patency of the central venous sinuses, and can be performed simultaneously with a brain MRI exam. 14 Magnetic resonance venography enables the distinction between a slow venous flow and an occlusive thrombus, which may be difficult to decide based on spin echo MRI sequences.
In our study, all patients presenting with the complication of otitis media underwent CECT, which enabled the confirmation of venous sinus thrombosis in almost all cases. A total of 3 (20%) patients underwent MRIs, and 2 (13%) underwent MRVs. The imaging exams revealed coalescent mastoiditis in all of the patients studied. The MRI/MRV findings correlated with the CECT findings regarding the detection of associated complications like cerebellar abscess, temporal lobe abscess, dural enhancement and subperiosteal abscess. Neck ultrasounds were routinely performed on an emergency basis, on all complicated cases, to rule out internal jugular vein thrombosis and associated neck abscess.
All patients were treated with antibiotics, even when the microbial cultures were negative. The duration of the antibiotic treatment ranged from four to eight weeks, and was directed by both the culture and the common microbiology of mastoiditis. The surgical management of LST patients in our study included mastoidectomy with incision of the lateral sinus, removal of the clot, and the establishment of free bleeding from both ends of the incised sinus. Removal of all perisinus granulation is obligatory for an effective treatment, thereby reducing the nidus of infection. Neck abscesses were managed with incision and drainage at the time of the mastoidectomy. However, recent reports have questioned this protocol, and have demonstrated that if the surrounding granulation tissues and inflammation are removed through mastoidectomy, the sinus will recanalize. 3 15 Several studies reported similar favorable results when the clot was left untouched, indicating that the natural history of LST is to resolve as soon as the source of infection is removed. 16 17 18 Internal jugular vein ligation was performed on 5 (33%) cases that were associated with deep neck abscess. Internal jugular vein ligation is usually reserved for unresponsive cases with lung thromboembolism, persistent septicemia and deep neck infection. 18
A total of 3 (20%) patients with intracranial abscesses were treated by multidisciplinary approach. In 2 (13%) patients, the abscess was drained by craniotomy, and in 1 (7%) patient, the early stage of the abscess was controlled only with antibiotics in association with mastoidectomy. The current evidence states that the routine use of anticoagulant therapy in LST is not well-supported. 19 Au et al reported a trend in the use of anticoagulation therapy, but lack the statistical difference. 20 The clinician should weigh the risks and benefits of anticoagulation therapy in cases of LST. In our study, the venous sinuses recanalized without anticoagulation following adequate surgical evacuation of the mastoid disease and a six-week course of antibiotics. With our management, all patients in our sample experienced satisfactory outcomes.
The first descriptions of LST reported mortality rates of 100%. This figure dropped dramatically to less than 10% because of prompt surgical intervention. 8 21 22 23 With the age of antibiotics, there has been a further decline in mortality. The mortality rate in our study was of 0%. This dramatic decline in mortality can be attributed to early diagnosis, which is made possible by imaging techniques like the CECT and MRI, and aggressive treatment.
Conclusions
Otogenic LST is a rare intracranial complication of otitis media in the modern age of antibiotic treatment. Combining parenteral antibiotics with surgical intervention is the treatment of choice. In patients with neck abscesses, it is important to elicit history of ear discharge. Though it is rare in the post-antibiotic age, otogenic LST can still occur; therefore, keeping a high level of suspicion is important, especially in developing countries.
References
- 1.Kangsanarak J, Fooanant S, Ruckphaopunt K, Navacharoen N, Teotrakul S. Extracranial and intracranial complications of suppurative otitis media. Report of 102 cases. J Laryngol Otol. 1993;107(11):999–1004. doi: 10.1017/s0022215100125095. [DOI] [PubMed] [Google Scholar]
- 2.Garcia R D, Baker A S, Cunningham M J, Weber A L. Lateral sinus thrombosis associated with otitis media and mastoiditis in children. Pediatr Infect Dis J. 1995;14(07):617–623. doi: 10.1097/00006454-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Smith J A, Danner C J. Complications of chronic otitis media and cholesteatoma. Otolaryngol Clin North Am. 2006;39(06):1237–1255. doi: 10.1016/j.otc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Ooi E H, Hilton M, Hunter G. Management of lateral sinus thrombosis: update and literature review. J Laryngol Otol. 2003;117(12):932–939. doi: 10.1258/002221503322683795. [DOI] [PubMed] [Google Scholar]
- 5.Christensen N, Wayman J, Spencer J. Lateral sinus thrombosis: a review of seven cases and proposal of a management algorithm. Int J Pediatr Otorhinolaryngol. 2009;73(04):581–584. doi: 10.1016/j.ijporl.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh P S, Ghosh D, Goldfarb J, Sabella C. Lateral sinus thrombosis associated with mastoiditis and otitis media in children: a retrospective chart review and review of the literature. J Child Neurol. 2011;26(08):1000–1004. doi: 10.1177/0883073811401745. [DOI] [PubMed] [Google Scholar]
- 7.Bales C B, Sobol S, Wetmore R, Elden L M. Lateral sinus thrombosis as a complication of otitis media: 10-year experience at the children's hospital of Philadelphia. Pediatrics. 2009;123(02):709–713. doi: 10.1542/peds.2008-0280. [DOI] [PubMed] [Google Scholar]
- 8.de Oliveira Penido N, Testa J R, Inoue D P, Cruz O L. Presentation, treatment, and clinical course of otogenic lateral sinus thrombosis. Acta Otolaryngol. 2009;129(07):729–734. doi: 10.1080/00016480802399721. [DOI] [PubMed] [Google Scholar]
- 9.Zanoletti E, Cazzador D, Faccioli C, Sari M, Bovo R, Martini A. Intracranial venous sinus thrombosis as a complication of otitis media in children: Critical review of diagnosis and management. Int J Pediatr Otorhinolaryngol. 2015;79(12):2398–2403. doi: 10.1016/j.ijporl.2015.10.059. [DOI] [PubMed] [Google Scholar]
- 10.Ryan J T, Pena M, Zalzal G H, Preciado D A. Otogenic lateral sinus thrombosis in children: A review of 7 cases. Ear Nose Throat J. 2016;95(03):108–112. [PubMed] [Google Scholar]
- 11.Davison S P, Facer G W, McGough P F, McCaffrey T V, Reder P A. Use of magnetic resonance imaging and magnetic resonance angiography in diagnosis of sigmoid sinus thrombosis. Ear Nose Throat J. 1997;76(07):436–441. [PubMed] [Google Scholar]
- 12.Iseri M, Aydin O, Ustündağ E, Keskin G, Almaç A. Management of lateral sinus thrombosis in chronic otitis media. Otol Neurotol. 2006;27(08):1098–1103. doi: 10.1097/01.mao.0000232002.62997.f3. [DOI] [PubMed] [Google Scholar]
- 13.Kelly K E, Jackler R K, Dillon W P. Diagnosis of septic sigmoid sinus thrombosis with magnetic resonance imaging. Otolaryngol Head Neck Surg. 1991;105(04):617–624. doi: 10.1177/019459989110500414. [DOI] [PubMed] [Google Scholar]
- 14.Magliulo G, Terranova G, Cristofari P, Ronzoni R. Sigmoid sinus thrombosis and imaging techniques. Ann Otol Rhinol Laryngol. 1996;105(12):991–993. doi: 10.1177/000348949610501210. [DOI] [PubMed] [Google Scholar]
- 15.Seven H, Ozbal A E, Turgut S. Management of otogenic lateral sinus thrombosis. Am J Otolaryngol. 2004;25(05):329–333. doi: 10.1016/j.amjoto.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Syms M J, Tsai P D, Holtel M R. Management of lateral sinus thrombosis. Laryngoscope. 1999;109(10):1616–1620. doi: 10.1097/00005537-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Kutluhan A, Kiriş M, Yurttaş V, Kiroğlu A F, Unal O. When can lateral sinus thrombosis be treated conservatively? J Otolaryngol. 2004;33(02):107–110. doi: 10.2310/7070.2004.02094. [DOI] [PubMed] [Google Scholar]
- 18.Tov E E, Leiberman A, Shelef I, Kaplan D M. Conservative nonsurgical treatment of a child with otogenic lateral sinus thrombosis. Am J Otolaryngol. 2008;29(02):138–141. doi: 10.1016/j.amjoto.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Neilan R E, Isaacson B, Kutz J W, Jr, Lee K H, Roland P S. Pediatric otogenic lateral sinus thrombosis recanalization. Int J Pediatr Otorhinolaryngol. 2011;75(06):850–853. doi: 10.1016/j.ijporl.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Au J K, Adam S I, Michaelides E M. Contemporary management of pediatric lateral sinus thrombosis: a twenty year review. Am J Otolaryngol. 2013;34(02):145–150. doi: 10.1016/j.amjoto.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Meltzer P E. Treatment of thrombosis of lateral sinus. Arch Otolaryngol. 1935;22:131–142. [Google Scholar]
- 22.Vijayashree M S, Viswanatha B. Otological manifestations of nonseptic lateral sinus thrombosis:A Review. Indian J Otolaryngol Head Neck Surg. 2016;68(04):400–405. doi: 10.1007/s12070-015-0834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong B Y, Hickman S, Richards M, Jassar P, Wilson T. Management of paediatric otogenic cerebral venous sinus thrombosis: a systematic review. Clin Otolaryngol. 2015;40(06):704–714. doi: 10.1111/coa.12504. [DOI] [PubMed] [Google Scholar]


