Abstract
The current study examined the role of conceptual knowledge and language in affective instability (AI) associated with Borderline Personality Disorder (BPD). Forty-six females meeting criteria for BPD and 51 nonclinical female control participants without BPD completed a measure of general vocabulary and a Semantic Similarities Task that provided estimates of the degree to which participants weighted information about valence and arousal in their understanding of emotion language. Feelings of valence and arousal were assessed using the Self-Assessment Manikin (SAM) in response to 62 emotionally evocative images, which was used to derive estimates of AI. BPD status was associated with valence and arousal AI at a bivariate level, but not after controlling for language variables (general vocabulary, semantic valence and arousal focus). Participants with stronger as opposed to weaker vocabularies exhibited less AI, and participants who emphasized arousal more in their conceptual representations of emotions exhibited less AI than those who emphasized it to a lesser degree. With the inclusion of language variables in a regression equation with BPD status predicting AI, semantic arousal focus, but not general vocabulary was a significant predictor of AI. Consistent with psychological constructionist models of emotion that specify an active role of language throughout the emotion generation process, these findings suggest that language capacity (general vocabulary and the degree to which arousal influences understanding of emotion words) is an important determinant of the AI associated with BPD.
Keywords: affective instability, psychological constructionist model of emotion, language, Borderline Personality Disorder, emotion regulation
Affective instability (AI) is a feature of a number of psychological disorders, perhaps most notably, Borderline Personality Disorder (BPD; Koenigsberg, 2010). AI refers to frequent and drastic changes in emotions characterized by high affect intensity, rapid emotion rise times, slow rates of return to emotional baseline, and excessive reactivity to psychosocial cues (Koenigsberg, 2010; Trull et al., 2008). In spite of its inclusion as a diagnostic criterion for BPD (Diagnostic and Statistical Manual of Mental Disorders 5th edition, American Psychiatric Association, 2013) the relationship between affective instability and BPD has not been extensively investigated (Ebner-Priemer et al., 2007), and AI has been criticized for its lack of clarity and concision (Koenigsberg, 2010; Renaud & Zacchia, 2012). Affective instability is a form of emotional dysregulation (Koenigsberg, 2010; Trull et al., 2008), and may be a key etiological and sustaining factor of BPD. Linehan’s (1993) explanation of BPD places emotional vulnerability and an inability to regulate emotions as etiological factors (in addition to negative social environments) that lead to maladaptive attempts to regulate intense affective states. Notably, higher affective instability has been implicated in greater suicidality (Koenigsberg, 2010; Yen et al., 2004) and impulsivity in this population (Koenigsberg, 2010).
Several ambulatory monitoring/ecological momentary assessments (e.g., Ebner-Priemer et al., 2007; Trull et al., 2008), as well as imaging studies (e.g. Schulze et al., 2011), have demonstrated that individuals with BPD displayed significantly more variability over time in their positive and negative affect ratings, demonstrated significantly more instability on successive ratings (i.e., large changes) and were more likely to report extreme changes across successive occasions than a comparison group (Trull et al., 2008). Compared to healthy controls, BPD participants demonstrated sudden, large decreases from positive mood states (Ebner-Priemer et al., 2007).
While research has documented affective instability in BPD, the mechanisms contributing to this instability are not well understood. Emotions are complex psychological states involving the combination of several affective processes (e.g. Barrett 2006a, 2006b). According to the Conceptual Act Theory (CAT), a psychological constructionist account of emotion generation, emotions are emergent psychological states that are constructed by a variety of more basic processes including core affect (influenced by several biological processes) and conceptual knowledge of emotion (e.g. Barrett 2006a, 2006b). Though emotions are often experienced as “natural kinds” or tangible entities existing as discreet categories (e.g. Barrett, 2006a) and have been examined this way empirically, CAT posits that emotions occur through a process of combining incoming sensory input (from the body and from the surroundings) with learned, category knowledge (Barrett, 2014). While biological factors and core affect certainly play a role in affective instability (e.g. Koenigsberg, 2010), from the CAT perspective that emotions are influenced by a confluence of factors, it seems pertinent to investigate the role of conceptual knowledge in affective instability. Interestingly, several studies assessing emotional reactions to standardized emotional stimuli have failed to document peripheral physiological correlates of affective instability of BPD (e.g., Herpertz, Kunert, Schwenger, & Sass, 1999; Herpertz et al., 2000, Herpertz et al., 2001; Suvak et al., 2011; for a review see Rosenthal et al., 2008) suggesting that other factors in addition to biological vulnerability play a role in the affective instability component of BPD.
Conceptual knowledge about emotions is intimately intertwined with language and is what a person “knows” conceptually about emotions based on past experience and mental categorization (Barrett, 2006b; Lindquist, 2009). Language provides the labels used to categorize affective states based on their context and on past information (Barrett 2006b). The CAT perspective hypothesizes that the language one possesses to describe emotional experiences will actually influence the emotional experience by constraining how affective states are conceptualized as they are happening (Lindquist, 2009). This is supported by burgeoning research in this area, recently reviewed by Lindquist, Satpute and Gendron (2015). Two studies by Gendron and colleagues demonstrated that perceptual priming of emotional faces was impeded when relevant emotional words were rendered temporarily inaccessible, suggesting that the same face was encoded differently when a word was accessible (Gendron, Lindquist, Barsalou, & Barrett, 2012). Additionally, an empirical test of CAT (Lindquist & Barrett 2008) demonstrated that the activation of emotion knowledge interacted with induced affect in generating an emotional state. When primed with conceptual knowledge of fear, participants experienced an unpleasant core affect induction as evidence that the world was threatening relative to participants who were primed with conceptual knowledge of anger or completed a non-emotional prime. The conceptual prime (i.e., fear, anger, neutral) did not impact the degree to which participants viewed the world as threatening for participants assigned to a neutral affect induction. These studies suggest that conceptual knowledge and emotion words influence emotion experiences.
While Gendron and colleagues’ (2012) study suggests that the activation/accessibility of emotion knowledge impacts emotional processes, relatively little is known about how individual differences in language capabilities and processes impact emotional functioning. Having participants rate the similarity of pairs of emotion terms based on their understanding of the meaning of words, what we refer to hereafter as the semantic similarities test, is one way to examine one’s conceptual organization of emotion knowledge based in language. Similarity judgments can be used to index mental structure anchored in semantic knowledge or language (e.g., Barrett 2004; Barrett & Fossum, 2001; Shepard, 1987). Similarity ratings for pairs of words map the cognitive structure of those words. In multiple studies Barrett (e.g., Barrett, 2004; Barrett & Fossum, 2001) had participants rate the similarity of sixteen emotion terms chosen to equally represent all combinations of valence (pleasant-unpleasant) and arousal (activated-calm) producing 120 similarity ratings (240 if both orders of each pair are presented). Analyses of similarity ratings of emotional terms using factor analytic techniques such as multidimensional scaling consistently support a two factor model identifying valence, or hedonic tone (unpleasant-pleasant) and arousal (activated-calm) as two continuous dimensions that adequately account for the similarity ratings, a model which has been termed the valance-arousal circumplex model (for a review see Russell & Feldman Barrett, 1999).
Research has also demonstrated meaningful individual differences in the valence-arousal circumplex (e.g., Barrett, 2004; Barrett & Fossum, 2001). Although these two dimensions adequately account for the similarity ratings on a nomothetic level, the degree to which people emphasize valence and arousal varies across individuals. Barrett (e.g., 2004) introduced valence focus and arousal focus as two individual differences variables representing variability in the degree to which individuals emphasize valence and arousal in their representations of emotion. For this paper, we use the terms semantic valence focus and semantic arousal focus when referring to the degree to which individuals emphasize valence and arousal in their cognitive structure of emotion language as assessed by similarity ratings. Barrett has demonstrated that semantic valence and semantic arousal focus are only modestly related to the valence and arousal focus estimates derived from experiential sampling techniques (i.e., experiential valence and arousal focus; Barrett, 2004).
From a CAT perspective, one would expect individual differences in language resources and abilities to influence emotional responding (Lindquist et al., 2015). The current study investigated this hypothesis by examining the relationship between affective instability and 1) general vocabulary ability, and 2) semantic valence and semantic arousal focus in a sample of individuals diagnosed with BPD and a healthy control group. Specifically, we hypothesize that better general vocabulary, higher semantic valence focus, and higher semantic arousal focus, represent resources available to help individuals modulate their emotional responses. Therefore, we expect significant negative associations between each of these language-based variables and indices of affective instability such that individuals with more language resources will exhibit less affective instability.
Method
Participants
The current investigation reanalyzed data from a larger laboratory study of emotional processing and BPD (see Suvak et al., 2011). Study procedures were approved by the local Institutional Review Board. Forty-six females meeting DSM-IV criteria for BPD (BPD Group) and 51 nonclinical female control participants without BPD or Axis I pathology (control Group) participated in the study. BPD participants were recruited from the community via advertisements posted on a variety of Internet and community bulletin boards (n = 38) and from an ongoing National Institute of Mental Health (NIMH) funded family study of BPD being conducted at McLean Hospital (n= 8). Control participants were recruited through the community via Internet and flyer advertisements. In exchange for participation, all participants were paid $40.
Inclusion criteria for the BPD group included meeting DSM-IV criteria for BPD as assessed by the Diagnostic Interview for Personality Disorders-IV for Personality Disorders (described below). Inclusion criteria for the control group included endorsing two or fewer criteria of BPD and no current Axis I pathology. Exclusion criteria included a lifetime diagnosis of schizophrenia or schizoaffective disorder, current psychotic symptoms, a manic episode in the past six months, any major medical conditions that could affect physiology or produce psychiatric symptoms, a history of major head injury, current hearing problems, and a history of significant neurological problems (see Suvak et al., 2011 for more details on participant screening and diagnostic verification).
The average age of the final sample was 21.64 years (SD = 3.01, range = 18–33). The majority of participants were Caucasian (58.8%), with fewer numbers of individuals identifying as Asian (10.3%), African-American (6.2%), Hispanic (4.1%), Pacific Islander (2.1%), Biracial (12.4%) and Other (e.g., African, West Indian; 6.2%). Participants completed an average of 15.04 years of education (SD = 1.92; range = 11–20). Median annual family income fell in the range of $60,000–$70,000 per year, with a mode (n = 23) response of “$100,000-above.”
BPD and control participants did not differ in age, t(95) = .77, p = .44, ethnicity, χ2 (6, N = 97) = 1.91, p = .93, or annual family income category, χ2 (12, N = 89) = 10.45, p = .58. While participants with BPD (M = 14.62) reported completing slightly fewer years of education than control participants (M = 15.41), t(95) = .2.06, p = .04, the associated effect size (eta squared = .04) suggests a very small difference.
As expected, participants with BPD demonstrated high rates of comorbidity, with 95.7% of participants with BPD receiving an additional current or lifetime diagnosis. Eight (17.4%) met criteria for a current major depressive episode, 37 (80.4%) for a past major depressive episode, one (2.2%) for a past manic episode, three (6.5%) for panic disorder without agoraphobia, four (8.7%) for panic disorder with agoraphobia, four (8.7%) for agoraphobia without panic disorder, 12 (26.1%) for social phobia, two (4.4%) for OCD, 15 (32.6%) for posttraumatic stress disorder, 18 (39.2%) for generalized anxiety disorder, four (6.5%) for anorexia, four (8.7%) for bulimia, and 7 (13.7%) for an eating disorder not otherwise specified.
Measures
Diagnostic interviews
The BPD module of the Diagnostic Interview for Personality Disorders-IV for Personality Disorders (DIPD-IV; Zanarini, Frankenburg, Sickel, & Yong, 1996) was used to assess the nine DSM-IV criteria for BPD. The use of the DIPD-IV in the Collaborative Longitudinal Personality Disorders Study (CLPS) has provided data in support of its reliability and validity. Median kappa coefficients ranged from .69 to .97 for all Axis II disorders (Zanarini et al., 2000), and factor analytic studies have provided support for four of the DSM-IV Axis-II constructs measured by the DIPD-IV (schizotypal, BPD, avoidant, and obsessive-compulsive; Sanislow et al., 2002; 2009). Select modules (mood disorders, anxiety disorders, psychotic screen, and eating disorders) from the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID; First, Spitzer, Gibbon, & Williams, 2002) were administered to assess the presence of Axis I diagnoses.
Trait measures of emotional behavior
The 20-item Positive and Negative Affect Schedule (PANAS) measured two primary dimensions of mood: Positive affect (PA; 10-items) and negative affect (NA; 10 items). The trait version instructing participants to report “how you feel in general” was used for current study. The PANAS is widely used in experimental studies and has good reliability and validity (Watson et al., 1988). The 40-item Affect Intensity Measure (AIM; Larsen & Diener, 1987) was administered to assess emotional reactions to every day events and mood traits. Although originally conceptualized as a uni-dimensional construct, a series of confirmatory factor analyses identified a three-factor model that included 27 of the original 40 AIM items with subscales labeled as negative intensity, negative reactivity, and positive intensity/reactivity (Bryant, Yarnold, & Grim, 1996). The scoring of the AIM described by Bryant and colleagues (1996) was used in the current study, and we used the negative intensity subscale of the AIM for all analyses reported in the current manuscript1.
General Vocabulary Ability
The Shipley Institute of Living Scale, Vocabulary Subscale (SIL-V; Shipley, 1940) was used to measure overall verbal (vocabulary) performance. For 40 multiple-choice items respondents must choose which one of four words is closest in meaning to a target word (i.e., a synonym). Psychometric data suggest that this scale provides as accurate an estimate of overall vocabulary performance (Dalton, Pederson, & McEntyre, 1987).
Semantic Valence and Arousal Focus
A paper and pencil version of the semantic similarities task was administered at the beginning of the laboratory session. The semantic similarities task involved having participants rate the similarity of all possible pairs of 16 emotion terms that equally represented all octants of the affective circumplex (i.e., all combinations of valence and arousal), resulting in 120 ratings. The terms used in the current study were: afraid, aroused, calm, disappointed, enthusiastic, happy, nervous, peppy, quiet, relaxed, sad, satisfied, sleepy, sluggish, still, and surprised (from Barrett, 2004, Study 2). Participants were instructed to rate the degree to which they thought the words were conceptually similar strictly on the basis of the meanings of words. Ratings were made on a 7-point Likert scale (1: extremely dissimilar, 4: unrelated, 7: extremely similar). The adjective pairs were presented in a single random order. Similarity judgments are believed to represent cognitive organization. Suvak et al. (2011) reported in detail the results of multi-dimensional scaling applied to the semantic similarities data. Consistent with past research, a two-factor valence and arousal solution best fit the data. Most relevant for the current investigation is the application of an individual difference MDS technique (INDSCAL) to derive estimates of Semantic Valence Focus and Semantic Arousal Focus. INDSCAL analyses provide information about what overall structure best accounts for the similarity ratings and computes dimension weights for each individual quantifying the extent a particular attribute or dimension influenced the similarity ratings. With our two-factor valence-arousal solution, INDSCAL weights represent the degree to which individuals weigh valence and arousal when making similarity judgments, in other words, estimates of semantic valence and arousal focus. For more details on the MDS analyses and derivation of estimates of semantic valence and arousal focus, see Suvak et al. (2011).
Self-Assessment Manikin (SAM; Bradley & Lang, 1994; Lang, 1980). Current feelings of valence and arousal were assessed using the Self-Assessment Manikin (SAM). SAM is an animated, interactive computer display that utilizes a cartoon figure that participants use to rate how “happy” or “unhappy” (valence) and how “calm” or “excited” (arousal scale) they felt during the presentation of each picture. Valence and arousal scale scores both ranged from 1 (most unpleasant/least pleasant, most calm/least activated) to 9 (least unpleasant/most pleasant, least calm/most activated). SAM is extensively used as a non-verbal assessment of subjective emotional reactions in investigations of emotion. The SAM ratings were used to derive estimates of AI. AI was operationalized using the mean square successive difference (MSSD) score as described by Jahng, Wood, and Trull (2008). MSSD is calculated by summing the squared differences between the outcome variable at one time point and the outcome at the previous time point and dividing this sum by the number of assessments minus 1. The MSSD has several advantages over other estimates of within-subject variability (e.g., within-person variance) including taking into consideration multiple sources of variability (i.e., variability due to dispersion of states and variability due to temporal order or serial correlation; Jahng et al., 2008). Two estimates of AI were computed using the SAM ratings. AI-Valence was derived by computing the MSSD for the SAM-valence ratings across all pictures, while AI-Arousal was derived by computing the MSSD for the SAM-arousal ratings across all pictures.
Images
Sixty-two images were selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005). Past research that systematically varied valence and arousal using IAPS images was reviewed (Bradley, Codispoti, Cuthbert, & Lang, 2001; Bradley, Codispoti, Sabatinelli, & Lang, 2001; Barrett, 2004). Great care was taken to make sure that within each valence category (i.e., unpleasant and pleasant), valence was matched as closely as possible across arousal conditions (low, medium, and high). In addition, great care was taken to assure that arousal levels matched as closely as possible across valence categories. Normative data of self-reports of valence and arousal in response to the image were used to select the images. The final IAPS set contained 24 pleasant (12 high, 6 medium, and 6 low arousal), 24 unpleasant (same arousal breakdown), and 14 neutral images (12 low and 2 high arousal), and 2 neutral highly arousing images.2
Procedure
Participants were mailed copies of the informed consent form and a packet of questionnaires that assessed trait-like characteristics (e.g., Demographics Questionnaire, PANAS-Trait and AIM). Upon arrival, study procedures were described, and written informed consent was obtained. Consent was followed by the administration of the clinical interviews and completion of a questionnaire packet that included measures assessing state-like processes, which were not used for the current investigation, and the SIL-V.
The tasks of the current study were administered as part of a larger laboratory procedure. We describe in detail only the components of the procedure directly related to the semantic similarities and emotion labeling tasks. After completing the diagnostic interviews and self-report measures, participants were seated in a padded recliner directly in front of a 21-inch computer monitor. Their eyes were positioned at a distance of approximately 40 inches from the monitor, and six inches below the center of the screen such that they were gazing up at a very slight angle. The sensors used for measuring physiological activity were first attached.
The primary component of the laboratory procedure was the picture processing paradigm developed by Peter Lang and colleagues (e.g., Bradley, Codispoti, Cuthbert, & Lang, 2001). A trial consisted of an IAPS image presented for 6 seconds, the presentation of a startle probe occurring 3.5–7 seconds after the onset of the IAPS image (emotion modulation of the eye-blink startle response was the primary physiological indicator of emotion), a recovery period lasting six seconds, a four second delay, and finally SAM and emotion labeling ratings. An inter-trial interval ranging from 12–24 seconds followed the last rating. All participants viewed the 62 pictures described above. To minimize order effects, a complex counterbalancing schema was developed consisting of the creation of six different stimuli presentation orders. The primary goal of counterbalancing was to eliminate order effects (e.g., fatigue, habituation) across each Valence x Arousal combination (7: neutral, pleasant/low, pleasant/medium, pleasant/high, unpleasant/low, unpleasant/medium, and unpleasant/high). Participants were randomly assigned to one of the orders.
Data Analysis
We computed bivariate correlations and conducted a series of hierarchical multiple regression analyses to examine relationships among BPD status, valence and arousal instability, the language variables (general vocabulary ability, semantic valence focus, semantic arousal focus) and dispositional emotional variables (NA, PA, and affect intensity). The primary purposes of the multiple regression analyses were to: 1) examine whether the language variables accounted for differences in valence and arousal AI as a function of BPD status, and 2) identify the most robust predictors of AI with a particular focus on the association between the language variables and AI when controlling for the trait affect measures (NA, PA, and affect intensity). We conducted separate multiple regression analyses for valence AI and arousal AI entering BPD status in Step 1, the language variables in Step 2, and trait emotion variables in Step 3.
Results
Bivariate associations (correlations) and descriptive statistics for all variables are presented in Table 1. Several significant associations emerged between valence and arousal AI and the predictor variables including significant positive relationships between the AI variables and BPD status, NA, and self-reported affect intensity. This indicates that individuals diagnosed with BPD exhibited more instability in affect ratings than those without BPD. Additionally, individuals who reported higher levels of trait NA and affect intensity exhibited higher levels of instability than participants reporting lower levels of these affective traits. Significant negative relationships between both AI variables and general vocabulary suggest that people with better vocabularies tended to exhibit less affective instability than people with poorer vocabularies. Significant and negative associations between both AI variables and semantic arousal focus indicated that participants who tended to emphasize arousal more when making similarity judgments of pairs of emotions terms exhibited less affective instability than those who tended to emphasize arousal less when making similarity judgments. Semantic valence focus and positive affect were not significantly associated with affective instability.
Table 1.
Descriptive Statistics for and Bivariate Relationships Among All Study Variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | M | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Valence AI | --- | 10.02 | 5.34 | ||||||||
| 2. Arousal AI | .75** | --- | 7.30 | 4.52 | |||||||
| 3. Vocabulary (Shipley) | −.34** | −.23* | --- | 31.94 | 3.40 | ||||||
| 4. Semantic valence focus | −.01 | .01 | −.16 | --- | 0.68 | 0.07 | |||||
| 5. Semantic arousal focus | −.32** | −.35** | .42** | −.57** | --- | 0.59 | 0.10 | ||||
| 6. BPD | .26* | .31** | −.33** | −.06 | −.26** | --- | 0.47 | 0.50 | |||
| 7. NA | .22* | .34** | −.33** | −.08 | −.25* | .81** | --- | 20.72 | 10.49 | ||
| 8. PA | .12 | .15 | .00 | .20* | −.11 | −.35** | −.36** | --- | 32.19 | 7.97 | |
| 9. Negative Affect Intensity | .19 | .29** | −.23* | .10 | −.21* | .83** | .86** | −.33** | --- | 3.30 | 1.26 |
Note.
p < .05;
p < .01;
M = Mean; SD = Standard Deviation; AI = Affective Instability; BPD = Borderline Personality Disorder diagnosis; NA = Negative Affect; PA = Positive Affect.
The results of the regression analyses are depicted in Table 2. A few interesting findings emerged in Step 2 of both of the hierarchical multiple regressions. First, the association between BPD status and AI was no longer significant when controlling for the language variables. Second, overall general vocabulary was no longer associated with AI (both valence and arousal) when controlling for semantic valence and arousal focus as well as BPD status. Third, the association between semantic valence focus and both valence AI and arousal AI was statistically significant and negative. This third finding was unexpected, as reported above, the bivariate associations between semantic valence focus and both types of AI was very close to zero and not significant (see Table 1). Therefore, this is an example of a suppressor effect (e.g., MacKinnon, Krull, & Lockwood, 2000), when a non-significant bivariate association becomes statistically significant in multivariate analysis controlling for additional predictor variables.
Table 2.
Summary of Multiple Regression Analyses Predicting Affective Instability
| Variable | Predicting Valence AI | Predicting Arousal AI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| B | SE B | β | p | pr | B | SE B | β | p | pr | |
| Step 1 (R2 = .06, p = .014) | (R2 =.10, p = .002) | |||||||||
| BPD diagnosis | 2.70 | 1.07 | .25 | .01 | .25 | 2.78 | .89 | .31 | .002 | .31 |
| Step 2 (ΔR2 = .15, p = .001) | (ΔR2 = .12, p = .004 ) | |||||||||
| BPD diagnosis | .84 | 1.10 | .08 | .45 | .08 | 1.54 | .93 | .17 | .10 | .17 |
| General Vocabulary | −.31 | .17 | −.20 | .07 | −.19 | −.04 | .14 | −.03 | .80 | −.03 |
| Semantic Valence Focus | −20.83 | 9.41 | −.28 | .03 | −.23 | −17.62 | 7.93 | −.28 | .03 | −.23 |
| Semantic Arousal Focus | −22.52 | 7.82 | −.39 | .01 | −.29 | −22,74 | 6.58 | −.46 | .001 | −.34 |
| Step 3 (ΔR2 = .12, p = .546) | (ΔR2 = .07, p = .051) | |||||||||
| BPD diagnosis | 1.63 | 2.00 | .15 | .42 | .09 | .64 | 1.63 | .07 | .70 | .04 |
| General Vocabulary | −.30 | .17 | −.18 | .09 | −.18 | −.01 | .14 | −.00 | .99 | −.00 |
| Semantic Valence Focus | −21.04 | 9.50 | −.28 | .03 | −.23 | −16.61 | 7.75 | −.26 | .04 | −.22 |
| Semantic Arousal Focus | −20.09 | 8.05 | −.35 | .02 | −.26 | −18.83 | 6.57 | −.38 | .01 | −.29 |
| NA | −.00 | .10 | −.00 | .98 | −.00 | .13 | .09 | .29 | .14 | .16 |
| PA | .10 | .07 | .15 | .15 | .15 | .15 | .06 | .25 | .02 | .26 |
| Negative Affect Intensity | −.04 | .89 | −.01 | .96 | −.01 | −.10 | .72 | −.03 | .89 | −.01 |
Note: B = unstandardized regression coefficient, SE = standard error, β = standardized regression coefficient, pr = partial correlation coefficient, AI = Affective Instability; BPD = Borderline Personality Disorder diagnosis; NA = Negative Affect; PA = Positive Affect.
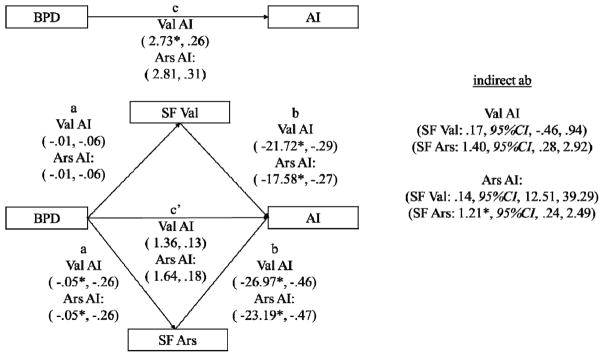
We conducted follow-up analyses to evaluate more precisely how the language variables accounted for the association between BPD and AI. Figure 1 depicts the direct effect from BPD to AI (path c’) and the indirect effects from BPD to AI through semantic valence focus and semantic arousal focus (paths ab). To test the significance of the indirect ab paths the 95% confidence interval for this estimate was computed using the RMediation software program (Tofighi & MacKinnon, 2011). Figure 1 shows that a significant indirect effect through semantic arousal focus, but not semantic valence focus, for both AI variables emerged suggesting that semantic arousal focus may account for at least some of the relationship between BPD and AI. Since semantic arousal focus did partially account for the relationship between BPD status and AI, and semantic valence focus did not, we contend that the significant association between semantic valence focus and AI that emerged in Step 2 of the regression analyses is likely a suppressor effect due to the substantial association between semantic valence and arousal focus (r = −.57) that is not readily interpretable and does not require further interpretation.
Figure 1.
The Indirect Relationship of BPD on AI through Semantic Valence and Arousal Focus. Unstandardized and standardized coefficients presented in parenthesis (unstandardized, standardized). * indicates p < .05. BPD = Borderline Personality Disorder, SF Val = Semantic Valence Focus, SF ARS = Semantic Arousal Focus. AI = Affective Instability, Ars = Arousal, Val = Valence. ab = the indirect effect of SF Val through SF Ars on AI, 95% CI = 95% Confidence Interval.
The most notable results in Step 3 (see Table 2) were that NA and affect intensity were not associated with either of the AI outcomes (both showed significant positive associations to AI at the bivariate level), while the association between both of the semantic focus variables an the AI outcomes remained largely unaffected by adding in the emotional variables into the analyses. A significant association emerged between PA and arousal AI. However, since the bivariate association between PA and arousal AI was not significant, this is likely another suppressor effect. Since the primary reason for Step 3 was to evaluate the language effects when controlling for the emotional variables, this association will not be interpreted. The language effects present in Step 2 maintained when adding the emotional variables into the model.
While we did not have a self-report measure of AI per se, affect intensity as measured by the Negative Affect Intensity subscale of the Affect Intensity Measures was the most similar construct that we did assess via self-report. We conducted one additional regression analysis with the Negative Affect Intensity subscale as the dependent variable (instead of as a predictor in Step 3 as it was in the previously reported regression analyses) to evaluate whether or not the language variables accounted for self-reports of negative affect intensity similarly to the way that they accounted for the AI estimates derived from the SAM ratings. These findings are summarized in Table 3. Step 2 indicated that the language variables did not significantly predict negative affect intensity (individually or combined), and the association between BPD status and negative affect intensity remained statistically significant in all steps. This pattern of results suggests that semantic arousal focus is related to some emotional processes associated with BPD (i.e., assessment-to-assessment changes in valence and arousal), but not others (i.e., the tendency for individuals with BPD to describe themselves as emotionally labile).
Table 3.
Summary of Multiple Regression Analysis Predicting Negative Affect Intensity
| Variable | Predicting Negative Affect Intensity | ||||
|---|---|---|---|---|---|
|
| |||||
| B | SE B | β | p | pr | |
| Step 1 (R2 = .69, p = .000) | |||||
| BPD diagnosis | 2.08 | .15 | .83 | .00 | .83 |
| Step 2 (ΔR2 = .01, p = .636) | |||||
| BPD diagnosis | 2.09 | .16 | .83 | .00 | .81 |
| General Vocabulary | .02 | .03 | .06 | .38 | .09 |
| Semantic Valence Focus | −1.27 | 1.31 | −.07 | .34 | −.10 |
| Semantic Arousal Focus | −.67 | 1.00 | −.05 | .51 | −.07 |
| Step 3 (ΔR2 = .10, p = .000) | |||||
| BPD diagnosis | 1.00 | .21 | .40 | .00 | .45 |
| General Vocabulary | .03 | .02 | .09 | .12 | .17 |
| Semantic Valence Focus | −.37 | 1.09 | −.02 | .74 | −.04 |
| Semantic Arousal Focus | −.07 | .84 | −.01 | .93 | −.01 |
| NA | .07 | .01 | .57 | .00 | .58 |
| PA | .00 | .00 | .02 | .77 | .03 |
Note: B = unstandardized regression coefficient, SE = standard error, β = standardized regression coefficient, pr = partial correlation coefficient, AI = Affective Instability; BPD = Borderline Personality Disorder diagnosis; NA = Negative Affect; PA = Positive Affect.
Discussion
This study examined how BPD, general vocabulary, and conceptual organization of emotion knowledge based in language (i.e., semantic valence and arousal focus) relate to AI. Several interesting findings emerged. First, while BPD status was associated with both valence and arousal AI at the bivariate level, BPD was no longer associated with AI when controlling for the language variables (general vocabulary, semantic valence and arousal focus). General vocabulary and semantic arousal focus, but not semantic valence focus, were negatively associated with both AI outcomes at the bivariate level. Participants with a stronger vocabulary exhibited less AI than those with weaker vocabularies, and participants who emphasized arousal to a greater degree in their conceptual representations of emotions exhibited less AI than those emphasizing arousal to a smaller degree. When all three of these language variables were entered into a regression equation with BPD status predicting AI, general vocabulary was no longer a significant predictor. Semantic arousal, however, remained a significant predictor of AI, and the relationship between semantic valence focus and arousal focus became statistically significant.
Follow-up analyses suggested that the significant association between semantic valence focus and AI in the multiple regression, which was not significant at a bivariate level, was a suppressor effect. Semantic valence focus was likely only associated with AI in the multiple regression because of its association with semantic arousal focus, which showed a substantial bivariate association with both AI variables. The results of the current study suggest that the degree to which participants emphasize arousal in their semantic-based representations of emotion is the most robust predictor of AI. This relationship held when controlling for trait NA, PA, and affective instability. The language variables accounted for the relationship between BPD and AI, as this association was no longer significant when controlling for the language variables. In sum, these results suggest that one’s language capacity (general vocabulary and the degree to which arousal influences understanding of emotion words) is an important determinant of affective instability accounting for the relationship between BPD and AI.
We hypothesized that all three language-based variables would be negatively associated with AI. Semantic arousal focus emerged as the most robust and primary language variable negatively associated with AI. Many theories of emotion describe valence as a basic building block of emotion life (e.g., Barrett, 2006c). Why then would semantic arousal focus be a more robust predictor of AI then valence focus? Many emotion researchers think that valuation, or determining the hedonic tone (i.e., valence) of a stimulus is largely driven by automatic processes to quickly identify potential threats in the environment (Barrett, 2006c; Barrett, Oschner, & Gross, 2007; Ledoux, 2000; Ohman & Mineka, 2001). The robust, nomothetic and automatic role of valence in the construction of emotions likely minimizes the role of individual differences of valence, providing an opportunity for individual differences in arousal to play an important role in emotional responding. This is consistent with previous work that has shown that semantic arousal focus, but not semantic valence focus, was associated with individual differences in emotional granularity, or the ability to make fine-grained distinctions among similarly valenced states (Barrett, 2004).
Traditionally, the primary focus on the association between language and emotion has been the communicative role of emotions (Lindquist et al., 2015). There is a general consensus that the rich information contained in verbal and non-verbal expressions of emotion facilitates communication, and facilitating communication is one of the important functions of emotion. However, recent psychological constructionists models of emotion have proposed an expanded role of language in generating, constructing, and regulating emotional experience (e.g., Barrett, 2014; Lindquist et al., 2015), and research has begun to provide empirical support for this role of language in emotion. For instance, a series of studies used semantic satiation to experimentally manipulate accessibility of emotion word meaning. This procedure reduced the accessibility of words representing basic emotion categories (e.g., “anger,” “fear”) prior to having participants complete emotion perception tasks, such as making judgments of facial behaviors depicting emotion or completing emotional priming tasks. Across five studies, semantic satiation of word meaning interfered with the perception of emotion including reducing the speed and accuracy in perceiving facial behaviors depicting emotion (Lindquist, Barrett, Bliss-Moreau, & Russell, 2006) and the disruption of emotional priming (i.e., the impact of a brief presentation of an emotional stimulus on subsequent cognitive activity; Gendron et al., 2012). These findings suggest that language impacts emotion generation at an early stage, during the initial perception of emotional stimuli.
Other studies have shown that language impacts the generation of emotion in latter stages after an initial response has begun. A number of studies reveal that putting labels to emotions serves an emotion regulation function. For example, Lieberman et al. (2007) showed that affect labeling was associated with decreased amgydala activity and increased activity in the right ventrolateral prefrontal cortex by way of the medial frontal cortex. Exposure to aversive images combined with affect labeling led to greater attenuation in long-term autonomic reactivity compared to exposure alone (Tabibnia, Lieberman & Craske, 2008). Labeling also was associated with reduced skin conductance response and fear responding compared to reappraisal or distraction (Kircanski, Lieberman & Craske, 2012). Participants were unaware of the impact of affect labeling on emotion regulation even after it led to lowered distress (Lieberman, Inagaki, Tabibnia & Crockett, 2011). Affect labeling may also come with a cost, which is dampened affective responses in general, and therefore diminished self-reported pleasure rather than specifically alleviating negative affect (Lieberman et al., 2011).
Recently, Brooks and colleagues (Brooks et al., 2016) conducted a meta-analysis of 386 neuroimaging studies from 1993 to 2014 examining the role of language in the experience and perception of emotion. They found that when emotion words (e.g., ‘anger,’ ‘disgust’) as opposed to affect words (e.g., ‘pleasant,’ ‘unpleasant’) were present, regions related to semantic processing were activated whereas when emotion words were not present, the amygdala and parahippocampal gyrus were more frequently activated. These findings suggest that when emotion concepts are inaccessible, the meaning of affective experiences and perceptions are ambiguous. Amygdala activation signals this uncertainty and emotion concept words may help refine the meaning of otherwise ambiguous affective states allowing a person to know what the affective state means, what to do about it and how to regulate it (Brooks et al., 2016). This process decreases the arousing nature of the stimuli, providing an inherent emotion regulation function (Brooks et al., 2016; Lieberman, 2011). In sum, there is substantial evidence that labeling emotional experience modifies the subsequent course of the emotional experience and expression. An intriguing avenue for future research would be to examine individual difference in labeling emotions and emotion regulation, exploring the possible benefits of being able to label emotions in a precise, nuanced manner (i.e., high in emotion differentiation or emotional granularity, e.g., Barrett, 2004, Smidt & Suvak, 2015). There is some evidence suggesting that the ability to experience emotions in a nuanced manner facilitates emotion regulation (e.g., Barrett, Gross, Christensen, & Benvenuto, 2001); however, the role of language in this association remains unexplored.
The negative association between semantic arousal focus and AI that emerged in the current study not only has implications for our understanding of the role of language in the generation and regulation of emotion broadly, but also has implications for the role of AI in BPD and other types of psychopathology. AI has been a cardinal feature of BPD since it entered the diagnostic nomenclature with the publication of DSM-III (APA, 1980). While a body of research has shown increased AI in individuals diagnosed with BPD compared to healthy controls, recent research suggest that AI may be a transdiagnostic mechanism underlying several different types of psychopathology including posttraumatic stress disorder, bulimia nervosa, and avoidant personality disorder (e.g., Santangelo, 2014, Snir, Bar-Kalifa, Berenson, Downey, & Rafaeli, 2016). The finding that one’s conceptual organization of emotion knowledge based in language is associated with AI supports treatments that teach individuals how to identify and verbally label emotions, such as Dialetical Behavior Therapy (Linehan, 1993). Based on a review of recent neuroimaging studies of the effect of psychotherapy, Messina, Sambin, Beschoner, and Viviani (2016) concluded that areas implicated in coding semantic representations play an important role in enhanced emotion regulation facilitated by therapy. Helping clients develop more nuanced and precise language to describe their emotional experiences may contribute to increased efficacy of interventions for emotional disorders.
What is the extent of the role of language in emotional processes related to BPD? The bivariate associations between BPD status and AI were moderate (r’s = .26 and .31) for valence and arousal AI, respectively, which fall in the medium effect size range according to Cohen (1988). Simultaneously, our follow-up analysis demonstrated that the language variables did not account for the tendency for individuals diagnosed with BPD to describe themselves as experiencing negative emotions intensely via self-report. Although BPD and AI (as operationalized by assessment-to-assessment changes in valence and arousal ratings in response to emotionally evocative images) are only modestly associated, we contend that the findings of the current study are important. As we have described, extant research on emotional processes contributing to BPD is mixed. On the one hand, the tendency for individuals diagnosed with BPD to describe themselves as emotionally volatile/labile is well replicated (e.g., Ebner-Priemer et al., 2007). On the other hand, studies that have relied upon objective measures of emotional responding (such as psychophysiological assessments) or responses to discrete emotional stimuli (in contrast to global, retrospective self-reports) have generally failed to document abnormal emotional functioning in individuals with BPD (e.g., Herpertz, Kunert, Schwenger & Sass, 2010; Kuo & Linehan, 2009). In the context of the larger research literature, documenting a statistically significant relationship between BPD and emotional responding not assessed via global, self-reports, even through modest association, is notable. Our findings suggest that the language variables examined in the current study do not account for all emotional processes contributing to BPD (i.e., language variables may play a role in assessment-to-assessment changes in valence and arousal, but do not account for the tendency for individuals diagnosed with BPD to describe themselves as emotionally labile). However, the findings of the current study suggest that research examining the role of language and emotion is a potentially fruitful avenue for future research attempting to build a comprehensive and coherent account of emotion and BPD.
Examining AI in a sample of individuals meeting criteria for BPD and healthy controls is associated with strengths and weaknesses. AI has been empirically identified as an important feature of BPD, so the findings of the current study can inform efforts to understand emotional processes contributing to BPD. However, we cannot say anything about the generalizability of the findings of the current study. Future research is needed to investigate the role of language and AI in the general population and in a diverse array of clinical populations. The cross-sectional, non-experimental nature of our design limits our ability to draw conclusions about causality. It is possible that affective stability helps individuals develop a more precise understanding of the semantic meaning of emotion words. However, the research cited above examining the impact of language accessibility on emotion perception and the impact of labeling emotions on regulation provides initial support for the causal role of language in the generation and regulation of emotion. Examining very brief reactions to discrete emotionally evocative images in a laboratory setting introduces a degree of experimental control. However, this increased experimental control may come at the expense of costs to external/ecological validity; thus, replication of the current findings in future research using procedures such as ecological momentary assessment (e.g., Ebner-Priemer, & Trull, 2009) is needed. Another limitation of the current study is that only female participants were included. Future research needs to evaluate whether these findings would generalize to men with BPD or are moderated by gender.
A notable strength of the current study is that emotional reactions in response to the images used to derive AI estimates were assessed via SAM ratings, an extensively used non-verbal assessment of subjective emotional reactions, reducing contamination that may have been introduced by using a more language-based assessment of emotional reactions. The findings of the current study are consistent with current psychological constructionists models of emotion that specify an active role in language throughout the emotion generation process. Future research is needed to explore the generalizability of these findings, which can inform whether AI should be conceptualized as a transdiagnostic or a disorder-specific mechanism, and whether individual differences in language contribute to AI across a range of conditions. The current findings also support interventions designed to increase the precision of the use of emotion labels, a strategy that is at the heart of many psychotherapeutic interventions. An exciting direction for future research would be to evaluate whether semantic representations of emotion can change as the result of psychological interventions and whether these changes lead to reductions in AI.
Footnotes
We conducted all analyses using three different scales/subscales of the AIM: the total AIM score, and negative intensity and negative reactivity subscales. The pattern of results was identical, with only minor variations in the strength of associations. We decided to use the negative intensity score in the revised manuscript because this was the AIM score that exhibited the strongest bi-variate association with BPD status (.83 for negative intensity, .63 for total AIM, and .31 for negative reactivity).
The list of the IAPS slides used can be obtained from the corresponding author.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Barrett LF. Feelings or words? Understanding the content in self-report ratings of emotional experience. Journal of Personality and Social Psychology. 2004;87:266–281. doi: 10.1037/0022-3514.87.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Are emotion natural kinds? Perspectives on Psychological Science. 2006a;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Solving the emotion paradox: Categorization and the experience of emotion. Personality and Social Psychology Review. 2006b;10:20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Valence as a basic building block of emotional life. Journal of Research in Personality. 2006c;40:35–55. doi: 10.1016/j.jrp.2005.08.006. [DOI] [Google Scholar]
- Barrett LF. The conceptual act theory: A précis. Emotion Review. 2014;6:292–297. doi: 10.1177/1754073914534479. [DOI] [Google Scholar]
- Barrett LF, Fossum T. Mental representations of affect knowledge. Cognition & Emotion. 2001;15(3):333–363. doi: 10.1080/02699930125711. [DOI] [Google Scholar]
- Barrett LF, Gross J, Christensen TC, Benvenuto M. Knowing what you’re feeling and knowing what to do about it: Mapping the relation between emotion differentiation and emotion regulation. Cognition & Emotion. 2001;15(6):713–72. doi: 10.1080/02699930143000239. [DOI] [Google Scholar]
- Barrett LF, Ochsner KN, Gross JJ. On the automaticity of emotion. In: Bargh J, editor. Social psychology and the unconscious: The automaticity of higher mental processes. New York: Psychology Press; 2007. pp. 173–217. [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276. doi: 10.1037//1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1(3):300. doi: 10.1037//1528-3542.1.3.300. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavioral Therapy & Experiential Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brooks JA, Shablack H, Gendron M, Satpute AB, Parrish MH, Lindquist KA. The role of language in the experience and perception of emotion: a neuroimaging meta-analysis. Social Cognitive and Affective Neuroscience. 2016:nsw121. doi: 10.1093/scan/nsw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant FB, Yarnold PR, Grimm LG. Toward a measurement model of the Affect Intensity Measure: A three-factor structure. Journal of Research in Personality. 1996;30:223–247. doi: 10.1006/jrpe.1996.0015. [DOI] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, N.J: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Dalton JE, Pederson SL, McEntyre WA. A comparison of the Shipley vs. WAIS-R subtests in predicting WAIS-R Full Scale IQ. Journal of Clinical Psychology. 1987;43(2):278–280. doi: 10.1002/1097-4679(198703)43:2<278::aid-jclp2270430220>3.0.co;2-d. [DOI] [Google Scholar]
- Ebner-Priemer UW, Trull TJ. Ecological momentary assessment of mood disorders and mood dysregulation. Psychological Assessment. 2009;21(4):463. doi: 10.1037/a0017075. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Welch SS, Grossman P, Reisch T, Linehan MM, Bohus M. Psychophysiological ambulatory assessment of affective dysregulation in borderline personality disorder. Psychiatry Research. 2007;150(3):265–275. doi: 10.1016/j.psychres.2006.04.014. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Gendron M, Lindquist KA, Barsalou L, Barrett LF. Emotion words shape emotion percepts. Emotion. 2012;12(2):314. doi: 10.1037/a0026007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: A functional MRI study. Biological Psychiatry. 2001;50:292–298. doi: 10.1016/S0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Kunert HJ, Schwenger UB, Sass H. Affective responsiveness in borderline personality disorder. A psychophysiological approach. American Journal of Psychiatry. 1999;156:1550–1556. doi: 10.1176/ajp.156.10.1550. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Schwenger UB, Kunert HJ, Lukas G, Gretzer U, Nutzmann J, Sass H. Emotional responses in patients with borderline as compared with avoidant personality disorder. Journal of Personality Disorders. 2000;14(4):339–351. doi: 10.1521/pedi.2000.14.4.339. [DOI] [PubMed] [Google Scholar]
- Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: Indices using successive difference and group comparison via multilevel modeling. Psychological Methods. 2008;13(4):354. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Lieberman MD, Craske MG. Feelings into words: contributions of language to exposure therapy. Psychological Science. 2012;23(10):1086–1091. doi: 10.1177/0956797612443830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW. Affective instability: toward an integration of neuroscience and psychological perspectives. Journal of Personality Disorders. 2010;24(1):60–82. doi: 10.1521/pedi.2010.24.1.60. [DOI] [PubMed] [Google Scholar]
- Kuo JR, Linehan MM. Disentangling emotion processes in borderline personality disorder: Physiological and self-reported assessment of biological vulnerability, baseline intensity, and reactivity to emotionally evocative stimuli. Journal of Abnormal Psychology. 2009;118:531–544. doi: 10.1037/a0016392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in mental healthcare delivery systems. Norwood, NJ: Ablex; 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual (Technical Report A6) Gainesville: University of Florida; 2005. [Google Scholar]
- Larsen RJ, Diener E. Affect intensity as an individual difference characteristic: A review. Journal of Research in Personality. 1987;21(1):1–39. doi: 10.1016/0092-6566(87)90023-7. [DOI] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words. Psychological Science. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki TK, Tabibnia G, Crockett MJ. Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion. 2011;11(3):468. doi: 10.1037/a0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA. Language is powerful. Emotion Review. 2009;1(1):16–18. doi: 10.1177/1754073908097177. [DOI] [Google Scholar]
- Lindquist KA, Barrett LF. Constructing emotion the experience of fear as a conceptual act. Psychological Science. 2008;19(9):898–903. doi: 10.1111/j.1467-9280.2008.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Barrett LF, Bliss-Moreau E, Russell JA. Language and the perception of emotion. Emotion. 2006;6(1):125. doi: 10.1037/1528-3542.6.1.125. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Gendron M. Does language do more than communicate emotion? Current directions in psychological science. 2015;24(2):99–108. doi: 10.1177/0963721414553440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press; 1993. [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prevention Science. 2000;1(4):173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina I, Sambin M, Beschoner P, Viviani R. Changing views of emotion regulation and neurobiological models of the mechanism of action of psychotherapy. Cognitive, Affective, & Behavioral Neuroscience. 2016;16(4):571–587. doi: 10.3758/s13415-016-0440-5. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108(3):483–522. doi: 10.1037/0033-295X.108.3.483. [DOI] [PubMed] [Google Scholar]
- Renaud SM, Zacchia C. Toward a Definition of Affective Instability. Harvard Review of Psychiatry. 2012;20(6):298–308. doi: 10.3109/10673229.2012.747798. [DOI] [PubMed] [Google Scholar]
- Rosenthal MZ, Gratz K, Kosson DS, Cheavens JS, Lejuez CW, Lynch TR. Borderline personality disorder and emotional responding. A review of the research literature. Clinical Psychology Review. 2008;28:75–91. doi: 10.1016/j.cpr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. Journal of Personality and Social Psychology. 1999;76(5):805. doi: 10.1037/0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Grilo CM, Morey LC, Bender DS, Skodol AE, Gunderson JG, … McGlashan TH. Confirmatory factor analysis of DSM-IV criteria for borderline personality disorder: findings from the collaborative longitudinal personality disorders study. American Journal of Psychiatry. 2002;159(2):284–290. doi: 10.1176/appi.ajp.159.2.284. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Little TD, Ansell EB, Grilo CM, Daversa M, Markowitz JC, … McGlashan TH. Ten-year stability and latent structure of the DSM–IV schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders. Journal of Abnormal Psychology. 2009;118(3):507. doi: 10.1037/a0016478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo P, Reinhard I, Mussgay L, Steil R, Sawitzki G, Klein C, … Ebner-Priemer UW. Specificity of affective instability in patients with borderline personality disorder compared to posttraumatic stress disorder, bulimia nervosa, and healthy controls. Journal of Abnormal Psychology. 2014;123(1):258–272. doi: 10.1037/a0035619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Domes G, Krüger A, Berger C, Fleischer M, Prehn K, … Herpertz SC. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biological Psychiatry. 2011;69(6):564–573. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Shepard R. Toward a universal law of generalization for psychological science. Science. 1987;237:1317– 1323. doi: 10.1126/science.3629243. [DOI] [PubMed] [Google Scholar]
- Shipley WC. A self-administered scale for measuring intellectual impairment and deterioration. Journal of Psychology. 1940;9:371–377. doi: 10.1080/00223980.1940.9917704. [DOI] [Google Scholar]
- Smidt KE, Suvak MK. A brief, but nuanced, review of emotional granularity and emotion differentiation research. Current Opinion in Psychology. 2015;3:48–51. doi: 10.1016/j.copsyc.2015.02.007. [DOI] [Google Scholar]
- Snir A, Bar-Kalifa E, Berenson KR, Downey G, Rafaeli E. Affective instability as a clinical feature of avoidant personality disorder. Personality Disorders: Theory, Research, And Treatment. 2016 doi: 10.1037/per0000202. [DOI] [PubMed] [Google Scholar]
- Suvak MK, Litz BT, Sloan DM, Zanarini MC, Barrett LF, Hofmann SG. Emotional granularity and borderline personality disorder. Journal of Abnormal Psychology. 2011;120:414–426. doi: 10.1037/a0021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Lieberman MD, Craske MG. The lasting effect of words on feelings: words may facilitate exposure effects to threatening images. Emotion. 2008;8(3):307. doi: 10.1037/1528-3542.8.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Solhan MB, Tragesser SL, Jahng S, Wood PK, Piasecki TM, Watson D. Affective instability: Measuring a core feature of borderline personality disorder with ecological momentary assessment. Journal of Abnormal Psychology. 2008;117(3):647. doi: 10.1037/a0012532. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yen S, Shea MT, Sanislow CA, Grilo CM, Skodol AE, Gunderson JG, … Morey LC. Borderline personality disorder criteria associated with prospectively observed suicidal behavior. American Journal of Psychiatry. 2004;161(7):1296–1298. doi: 10.1176/appi.ajp.161.7.1296. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Sickel AE, Yong L. The diagnostic interview for DSM-IV personality disorders (DIPD-IV) Belmont, MA: McLean Hospital; 1996. p. 340. [Google Scholar]
- Zanarini MC, Skodol AE, Bender D, Dolan R, Sanislow C, Schaefer E, … Gunderson JG. The collaborative longitudinal personality disorders study: Reliability of axis I and II diagnoses. Journal of Personality Disorders. 2000;14(4):291–299. doi: 10.1521/pedi.2000.14.4.291. [DOI] [PubMed] [Google Scholar]