Abstract
Type 1 diabetes (T1D) results from the autoimmune destruction of pancreatic beta cells and is partly caused by deficiencies in the Foxp3+ regulatory T-cell (Treg) compartment. Conversely, therapies that increase Treg function can prevent autoimmune diabetes in animal models. The majority of Tregs develop in the thymus (tTregs), but a proportion of Foxp3+ Tregs is generated in the periphery (pTregs) from Foxp3−CD4+ T-cell precursors. Whether pTregs play a distinct role in T1D has not yet been explored. We report here that pTregs are a key modifier of disease in the nonobese diabetic (NOD) mouse model for T1D. We generated NOD mice deficient for the Foxp3 enhancer CNS1 involved in pTreg induction. We show that CNS1 knockout decreased the frequency of pTregs and increased the risk of diabetes. Our results show that pTregs fulfill an important non-redundant function in the prevention of beta cell autoimmunity that causes T1D.
Keywords: Type 1 diabetes, pTreg, Foxp3, CNS1
Graphical abstract
NOD mice, susceptible to autoimmune diabetes, harbor regulatory T cells both of thymic origin (tTregs) and induced in the periphery (pTregs). We deleted the Foxp3 enhancer CNS1 in NOD mice to decrease the frequency of pTregs but not tTregs, and found that this increased the risk of diabetes.

Introduction
Type 1 diabetes (T1D) is caused by autoreactive T cells that destroy pancreatic beta cells, leading to insulin deficiency. The T cells that drive pathogenesis can be held in check by Foxp3+ regulatory T cells (Tregs) [1,2]. Several of the gene variants that increase the risk of T1D do so by diminishing the functionality of the Treg compartment. For example, gene variants in the IL-2 pathway that controls the frequency of circulating Tregs have been shown to associate with disease in mouse and human [3,4]. Therapeutic administration of low-dose IL-2 increases the frequency of Tregs and prevents autoimmune diabetes in mouse models [5]. This approach is now in clinical trial for the prevention of T1D [6]. The success of low-dose IL-2 therapy in mice illustrates how manipulating the Treg compartment is a promising approach for the treatment of T1D. Yet, we do not fully understand the involvement of different Treg populations in the control of autoimmunity. The majority of Tregs emerge as a distinct population during thymic T-cell maturation. Defects in thymic Treg development are a possible cause for the immune dysregulation that leads to autoimmune diabetes. However, a proportion of mature Tregs forms not in the thymus but in the periphery [7,8]. These Tregs, termed peripherally-induced or pTregs, derive from mature Foxp3−CD4+ T cells following the encounter with tolerogenic stimuli, including TGF-β. The highest frequency of pTregs is found in the gut, likely owing to the capacity of gut microbes to promote pTreg induction [9–11]. pTregs are also present in other organs, but to date, a role for pTregs has only been demonstrated in the gut [12–14], in the lungs [12] and in fetal-maternal tolerance [15]. Whether pTregs participate in immune regulation in other organs is unclear. We set out to ask if pTregs have any role in pancreatic autoimmunity in the context of the nonobese diabetic (NOD) mouse model for T1D. Here, we report that the ability to generate pTregs is a critical modifier of autoimmune diabetes.
Results and discussion
pTregs are present in the pancreas of NOD mice
We started by measuring the frequency of thymus-derived Foxp3+CD4+ Tregs (tTregs) and pTregs in different organs in NOD mice. We used helios and neuropilin-1 (Nrp1) to distinguish tTregs from pTregs, as both markers have been shown to be primarily expressed in tTregs [16–18]. As reported previously, gut-associated lymphocytes harbored a high frequency of pTregs (25-30% of all colonic CD4+ T cells, Fig. 1). In contrast, pTregs represented a minority of Tregs in the spleen and mesenteric lymph nodes (mLN). Notably, pTregs were also detectable in the pancreatic lymph node (pLN) and in the pancreas (Fig. 1). The pLN is a major site of activation for autoreactive T cells in autoimmune diabetes. The presence of pTregs in the pLN and in the pancreas, although at low absolute numbers, suggested that pTregs could affect the autoimmune response that underlies T1D.
Figure 1.

pTregs are present in the pancreas of NOD mice. Lymphocytes isolated from the colonic lamina propria (Col. LP in black), mesenteric lymph nodes (mLN in purple), spleen (in orange), pancreatic lymph nodes (pLN in blue) and the pancreas (in green) of NOD WT mice were analyzed by flow cytometry. The full gating strategy is reported in Supporting Information Figure 3. Total Tregs were characterized as Foxp3+ cells (A). pTregs were characterized as Foxp3+Helios− (B) or Foxp3+Nrp1lo cells (C). All samples were gated on live CD4+CD8− lymphocytes. Representative FACS plots are shown on the left, cell frequencies (mean ± SEM) on the right. Data in the histograms are pooled from 3-5 combined individual experiments with with n=11-24 per group (all mice were 6-week old). Exact P-values are shown (unpaired t-test).
CNS1 deletion in NOD mice decreases the frequency of pTregs in the pancreas
The differentiation of pTregs involves tolerogenic stimuli that include TGFβ [19]. TGFβ has a direct effect on expression of Foxp3 that characterizes CD4+ Tregs. De novo expression of Foxp3 in peripheral CD4+ T cells involves the conserved non-coding sequence 1 (CNS1) enhancer in the Foxp3 promoter [19,20], a binding site for Smad3 downstream of TGFβ signaling. CNS1 knockout (KO) decreases the frequency of pTregs without affecting tTregs [12,21]. We used CRISPR-Cas9 genome editing to generate CNS1 KO NOD mice (supporting information Fig. 1). The CNS1 KO allele was inherited with Mendelian frequency and had no deleterious effects on development or fertility. CNS1 deficiency decreased the frequency of pTregs in all organs examined, including blood, colon, spleen, lymph nodes and pancreas (Fig. 2A-E). Of note, CNS1 KO did not affect the frequency of tTregs or the frequency of total Foxp3+ Tregs, except in the colon where the contribution of pTregs to the total Treg population is greatest (Fig. 2D). Frequency and absolute numbers of other lymphocyte populations were comparable in WT and CNS1 KO mice (supporting information Fig. 2). As reported previously [12,21], CNS1 deficiency impaired both the in vitro and in vivo differentiation of naïve CD4+ T cells into Foxp3+ Tregs following tolerogenic stimulation (Fig. 2F,G).
Figure 2.

CNS1 deletion impairs pTreg formation in NOD mice. (A-C) Blood from founder mice (F0) as well as WT and CNS1 KO littermates in the third off-spring generation (F3) was analyzed by flow cytometry. Total Tregs were characterized as Foxp3+ cells (A) and pTregs were characterized as Foxp3+Helios− (B) or Foxp3+Nrp1lo (C) cells, all within live CD4+CD8− lymphocytes. Representative plots (left panels) and cell frequencies (mean ± SEM, right panels) are shown. All mice were 10-12 weeks old. n=12 (WT), n=2 (CNS1 KO) for F0 and n=25 (WT), n=18 (CNS1 KO) for F3 mice. (D, E) Lymphocytes from NOD WT and CNS1 KO colonic lamina propria (Col. LP in black), mesenteric lymph nodes (mLN in purple), spleen (in orange), pancreatic lymph nodes (pLN in blue) and the pancreas (in green) were analyzed by flow cytometry. Total Tregs were characterized as Foxp3+ cells (D). pTregs were characterized as Foxp3+Helios− cells (E). Data are pooled from 3-5 combined individual experiments. All mice were 6 weeks old, n=9-22 mice per group (). (F) Naïve CD4+ T cells from NOD WT or CNS1 KO mice were stimulated in vitro with irradiated splenocytes, anti-CD3, IL-2 and TGFβ. CD4+Foxp3+ Treg frequency was measured by flow cytometry 3 days later. Representative plots (left panels) and cell frequencies (mean ± SEM, right panel) are shown. n=2 mice/group, the value for each mouse was averaged from 3 technical replicates, and results are representative of three similar experiments. (G) Treg-depleted (CD4+CD25−) T cells were isolated from BDC2.5 TCR transgenic mice, labelled and transplanted into NOD mice. T cell recipients were injected i.v. with BDC2.5 mimetope peptide, and Foxp3 expression in labelled BDC2.5 T cells was measured by flow cytometry 7 days later. n=4 mice/group, results are representative of two similar experiments. Exact P-values are shown for all experiments (two-tailed unpaired t-test).
CNS1 deficiency increases the risk of autoimmune diabetes in NOD mice
We generated cohorts of CNS1 KO NOD mice using a breeding scheme that yielded control littermates (WT and heterozygous). Mice were not separated by genotype and were co-housed throughout the study. CNS1 KO causes gastrointestinal pathology in B6 mice [12], but not in a mixed 129/B6 background [21]. Unlike B6 mice, CNS1 KO NOD mice showed no signs of morbidity or weight loss during the 7 months observation period. However, we found that CNS1 deficiency increased the frequency of diabetes in both male and female mice (Fig. 3A). Insulitis was also more severe in pre-diabetic CNS1 KO mice compared with control littermates (Fig. 3B). These data show that pTreg deficiency increases the risk of autoimmune diabetes.
Figure 3.
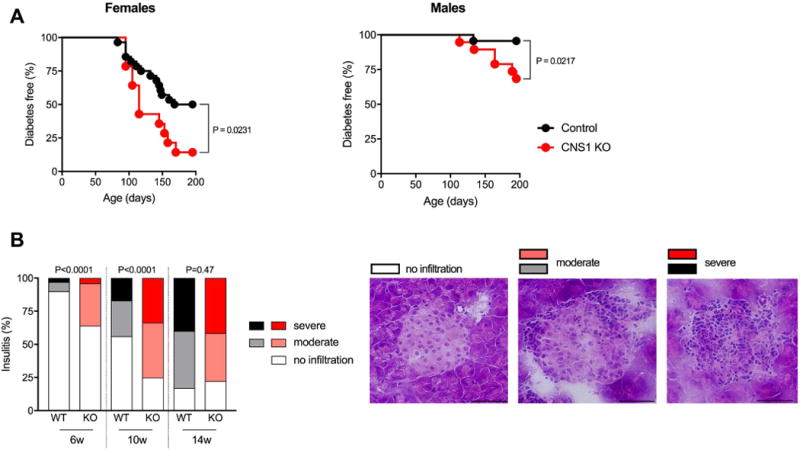
CNS1 KO increases the frequency of diabetes in NOD mice. (A) Spontaneous diabetes frequency in WT or heterozygous (Control, n = 28) and CNS1 KO (KO, n = 14) female mice (left) and WT (n=23) and CNS1 KO (n=19) male mice (right). Disease frequency between groups was compared using the Log-rank test, exact P values are shown. (B) Insulitis in control and CNS1 KO female mice at 6 weeks (214 and 137 islets from 3 mice/group), 10 weeks (493 and 361 islets from 3-4 mice/group) and 14 weeks of age (725 and 391 islets from 4-5 mice/group). Islets were scored as free of insulitis, or as having moderate or severe infiltration, see representative images (40x magnification, scale bars indicate 50μm). Insulitis between genotypes was compared using Fisher’s exact test (comparing the proportion of infiltrated islets in each age group).
Concluding remarks
Treg therapy is a promising avenue for the treatment of autoimmunity. In this context, it is important that we better understand which Tregs can be leveraged for therapy. pTregs constitute a feasible target population, owing to the fact that these cells can be induced within the mature T-cell compartment by various agents, including microbial metabolites [22–24]. However, the extent to which pTregs contribute to the control of pancreatic autoimmunity had not yet been established. We showed here that impairing pTreg formation in NOD mice increased the risk of diabetes. The role of pTregs in islet autoimmunity may relate to the proximity of the pancreas to the gut. The pLN is the primary site of activation for beta cell-reactive T cells in autoimmune diabetes [25]. At the same time, the pLN is readily accessible to antigen and to cells from the gastrointestinal tract [26], providing a direct route by which gut microbe-induced pTregs could impact pancreatic autoimmunity. This putative link could help explain how the gut microbiota impacts the risk of T1D [27–29].
Materials and Methods
Mice
CNS1 KO NOD mice were generated using gRNAs composed of 5’-CACCGAAGACATACACCACCACGG-3’ annealed with 5’- AAACCCGTGGTGGTGTATGTCTTC -3’ and 5’- CACCGCATCAGTCCTCCAGCCAG -3’ annealed with 5’- AAACCTGGCTGGAGGACTGATGC -3’ cloned into the pX330 vector (Addgene), amplified by PCR and transcribed using the Megashortscript T7 transcription kit (Life Technologies). Cas9 mRNA (Trilink Technologies) and gRNAs were purified using the Megaclear clean-up kit (Life Technologies) and injected into the pronucleus of NOD zygotes that were reimplanted into pseudo-pregnant Swiss-Webster mice. Genotyping was performed using the PCR primers 5’-GGCGCTTATGTGGCTTCTTTC-3’, 5’-GAGGTAGCTTCTCATTTTCAAGTGG-3’ and 5’-GGAAGCCAACATGGGGTGAA-3’. See Supporting Information Fig. 1 for a graphical representation of the location of the gRNA sequences and primers relative to the CNS1 region. All experiments were performed with age- and sex-matched mice and approved by the Institutional Animal Care and Use Committee at the Joslin Diabetes Center.
Lymphocyte Isolation
Single cell suspensions were prepared from spleen and lymph nodes by mechanical disruption of tissue followed by red blood cell lysis using ACK buffer. Colonic lamina propria cells were isolated after removal of intraepithelial lymphocytes using EDTA followed by digestion with Collagenase Type VIII (Sigma Aldrich) and DNAse (Roche). Cells were collected using a Percoll (GE Healthcare) density gradient. Intra-pancreatic lymphocytes were isolated from the whole pancreas by digestion using Collagenase P (Roche) followed by a Histopaque-1077 (Sigma) density gradient.
Flow Cytometry
Cell suspensions were pre-incubated with Fc-block antibody (anti-CD16/32, Biolegend), then stained with cell surface antibodies (listed below). Dead cells were excluded using Zombie Fixable Viability dye (Biolegend). Intracellular staining for Foxp3 and Helios was performed with a FoxP3-labelling kit (eBioscience). Data was acquired on a LSRII or LSR Fortessa instrument (BD Biosciences) and analyzed with the FlowJo software. All data are shown using log-scale axes. The list of the antibodies is reported in Tabel 1 and the full gating strategy is reported in Supporting Information Figure 3.
In vitro regulatory T-cell induction
Naïve CD4 T cells were isolated using the naïve CD4 T-cell isolation kit (Miltenyi Biotech). Cells were stimulated in the presence of irradiated T cell-depleted WT splenocytes, 2 μg/ml anti-CD3 (2C11, Biolegend), 200 U/ml IL-2 and 2 ng/ml TGFβ (both from Preprotech) for 3 days, then analyzed by flow cytometry.
In vivo regulatory T-cell induction
CD4+CD25− T cells were isolated from BDC2.5 TCR transgenic WT or CNS1 KO NOD mice by magnetic purification (using the CD4+CD25+ purification kit from Miltenyi Biotech) and labeled with fixable Cell Proliferation Dye eFluor-670 (eBioscience). 107 purified T cells were injected intravenously (i.v.) into WT NOD mice. The following day, mice were injected i.v. with 5 μg BDC2.5 mimetope peptide (RTRPLWVRME, from Anaspec) [30]. Mice were sacrificed 7 days later to measure the frequency of Foxp3+ cells among labelled cells in the spleen by flow cytometry.
Diabetes Frequency Studies
Disease studies were performed with age-matched, contemporary cohorts of mice. Onset of diabetes was monitored by weekly measurements of glycosuria using Diastix (Bayer). Mice with two consecutive readings > 250 mg/dL were considered diabetic.
Insulitis
Pancreatic tissue was frozen in OCT (Fisher Scientific) prior to cryosectioning into 7 μm slices. For insulitis scoring, sections were stained with hematoxylin (Fisher Scientific) and counter-stained with eosin (alcoholic Eosin Y, Fisher Scientific). Pancreatic islets were scored blinded to the genotype as having no infiltration, moderate infiltration or severe infiltration. All sections were acquired on a Olympus BX-60 microscope equipped with an Olympus DP70 camera using the DPManager software.
Statistical Analyses
Data were analysed with the Prism software (Graphpad). Diabetes frequency was compared using the Log-rank test. Insulitis was compared using Fisher’s exact test. All other comparisons were performed using an unpaired t-test, with P < 0.05 considered significant. Exact P values are shown. Sample sizes were approximated in initial experiments, and adjusted to increase power as needed in replicate experiments.
Supplementary Material
Table 1.
Antibodies used for flow cytometry.
| Antibody | Clone | Vendor |
|---|---|---|
| CD4-Brilliant Violet 605 | RM4-5 | Biolegend |
| CD4- Brilliant Violet 785 | RM4-5 | Biolegend |
| CD8-Brilliant Violet 711 | 53-6.7 | Biolegend |
| CD8a-APC/Cy7 | 53-6.7 | Biolegend |
| CD8a-APC | 53-6.7 | Biolegend |
| Helios-Pacific Blue | 22F6 | Biolegend |
| Helios-Alexa Fluor 488 | 22F6 | Biolegend |
| Armenian Hamster IgG Isotype Control-Pacific Blue | HTK888 | Biolegend |
| Foxp3-PE | FJK-16s | eBioscience |
| Rat IgG2a, k Isotype Control-PE | RTK2758 | Biolegend |
| Foxp3-eFluor450 | FJK-16s | eBioscience |
| CD19-APC/Cy7 | 6D5 | Biolegend |
| CD304-PE/Cy7 | 3E12 | Biolegend |
| CD304-PE/Cy7 | 3DS304M | eBioscience |
| Rat IgG2a, k Isotype Control-eFluor450 | eBR2a | eBioscience |
Acknowledgments
The authors wish to thank John Stockton for NOD embryo microinjections and Stephanie Katz for help with mouse colony management. C.S. is the recipient of a postdoctoral fellowship from the Mary K. Iacocca Foundation. This work was supported by NIH funding to the Joslin Diabetes Center (grants P30DK036836 and S10OD021740).
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31(4):654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VES, Gonzalez-munoz A, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2010;39(3):329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JHM, Cutler AJ, Ferreira RC, Reading JL, Cooper NJ, Wallace C, Clarke P, et al. Natural Variation in Interleukin-2 Sensitivity Influences Regulatory T-Cell Frequency and Function in Individuals With Long-standing Type 1 Diabetes. Diabetes. 2015;64(11):3891–3902. doi: 10.2337/db15-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207(9):1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd JA, Evangelou M, Cutler AJ, Pekalski ML, Walker NM, Stevens HE, Porter L, et al. Regulatory T Cell Responses in Participants with Type 1 Diabetes after a Single Dose of Interleukin-2: A Non-Randomised, Open Label, Adaptive Dose-Finding Trial. In: Huizinga TWJ, editor. PLOS Med. 10. Vol. 13. 2016. p. e1002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259(1):88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav M, Stephan S, Bluestone JA. Peripherally induced tregs - role in immune homeostasis and autoimmunity. Front Immunol. 2013;4:232. doi: 10.3389/fimmu.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Östman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36(9):2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 10.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science. 2011;331(6015):337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 12.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482(7385):395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohnmacht C, Park J, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, et al. The microbiota regulates type 2 immunity through RORγt+ T cells. Science. 2015;349:1–9. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 14.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, et al. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015;349(6251):993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic Generation of Regulatory T Cells in Placental Mammals Mitigates Maternal-Fetal Conflict. Cell. 2012;150(1):29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209(10):1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209(10):1723–1742. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N, McGrady G, et al. Conversion of peripheral CD4+CD25- naïve T cells to CD4+C25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9(2):194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furusawa Y, Obata Y, Fukuda S, Endo T, Nakato G, Takahashi D, Nakanishi Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 25.Höglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189(2):331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci U S A. 2005;102(49):17729–17733. doi: 10.1073/pnas.0509006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostic AD, Gevers D, Siljander H, Vatanen T, Hyötyläinen T, Hämäläinen AM, Peet A, et al. The Dynamics of the Human Infant Gut Microbiome in Development and in Progression toward Type 1 Diabetes. Cell Host Microbe. 2015;17(2):260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165(4):842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Judowski V, Pinilla C, Schroder K, Tucker L, Sarventick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a GAD65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 NOD mice. J Immunol. 2001;166(2):908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


