Abstract
Objective
Hypersensitivity with repeated exposure to platinum agents is common and can preclude continued treatment, even in patients with disease that remains platinum sensitive. We sought to compare the effects of prophylactic, extended carboplatin infusion versus standard infusion on the rate of carboplatin hypersensitivity reactions (HSRs) in women with recurrent ovarian cancer.
Methods
This was a single-institution, randomized, non-blinded trial comparing a graded, 3-hour extended infusion of carboplatin with a standard 30-minute infusion in patients with recurrent ovarian cancer who were enrolled from 1/2011–4/2015. The study was designed to detect a decrease in the HSR rate from 20% (standard infusion) to 5% (extended infusion) assuming a type 1 error of 10% and power of 80% using a one-sided test.
Results
Of 146 enrolled patients, 114 were evaluable. Fifteen (13%) had an HSR—11% (6/56) in the extended-infusion and 16% (9/58) in the standard-infusion groups (P=0·582). Planned treatment completion was achieved in 50 (89%) of 56 patients and 49 (84%) of 58 patients, respectively. Of 25 patients who received single-agent carboplatin, 8 (32%) had an HSR (53% of all patients who had an HSR [8/15]). Of 23 patients who received carboplatin with gemcitabine, 4 (17%) had an HSR (27% of all patients who had an HSR [4/15]). Of 8 patients who received carboplatin with paclitaxel, 3 (38%) had an HSR (20% of all patients who had an HSR [3/15]). There were no HSRs with pegylated liposomal doxorubicin, the most commonly given concurrent chemotherapy (46% of all patients).
Conclusion
A prophylactic, extended carboplatin infusion was not associated with a decreased HSR rate. The overall low HSR rate suggests that premedication may help reduce HSRs.
Keywords: ovarian cancer, carboplatin, hypersensitivity, hypersensitivity reactions, extended infusion
Introduction
Recurrent ovarian cancer is distinct from most solid tumors in that it often retains sensitivity to platinum chemotherapeutic agents over multiple relapses [1]. An important prognostic factor in recurrent ovarian cancer is platinum sensitivity, which is defined as relapse 6 or more months from the date of last platinum treatment [2]. Patients with recurrent platinum-sensitive ovarian cancer are usually retreated with carboplatin in combination with a taxane, gemcitabine or liposomal doxorubicin, or as a single agent [3–5]. The risk of carboplatin hypersensitivity reaction (HSR) increases with repeated exposure to carboplatin [6, 7]. The reported rates of carboplatin HSR range from 12% to 44% [6, 8–12]. Clinical manifestations are variable and usually require treatment with antihistamines, corticosteroids, and in severe cases, epinephrine [13, 14]. There have been reported deaths associated with carboplatin HSRs, despite aggressive resuscitative efforts [15]. The sudden development of a carboplatin HSR is particularly distressing for patients whose cancer remains platinum sensitive. Select patients who experience carboplatin HSRs may undergo retreatment with carboplatin using desensitization schedules, but these are complex and burdensome regimens, and breakthrough HSRs are common [16–20]. Moreover, these prolonged desensitization schedules must be maintained with each dose and are often not available outside larger treatment centers, precluding platinum retreatment for many patients.
We hypothesized that an abbreviated desensitization schedule, administered preventatively in the form of a graded-challenge, 3-hour infusion of carboplatin might reduce the incidence of initial HSRs, facilitating the successful administration of this critical ovarian cancer chemotherapy. Findings from a large retrospective review of 707 patients with ovarian cancer who had undergone platinum retreatment showed that the use of an extended carboplatin infusion, compared with standard infusion, was associated with a statistically significant reduction in the rate of HSRs (3% vs. 21%, respectively [21]. Similar results were seen in another retrospective study of 326 patients, with an HSR rate of 40% in the standard-infusion arm and 24% in the 3-hour extended-infusion arm [9]. On regression analysis, receiving a triple premedication regimen of a corticosteroid, an antihistamine, and an H2 antagonist prior to carboplatin treatment was the only variable significantly associated with fewer HSRs.
To determine the role of a prophylactic, extended infusion of carboplatin to reduce the incidence of HSRs, we conducted a prospective randomized clinical trial that compared an extended infusion with a standard infusion of carboplatin. The primary objective of this study was to determine whether the prophylactic use of an extended infusion was associated with a clinically meaningful lower rate of HSRs compared with the standard 30-minute schedule.
Methods
Study Design and Participants
Eligible patients included women with histologically confirmed ovarian, fallopian tube, or primary peritoneal carcinoma who had received at least one prior platinum-containing regimen. Participants were required to have a Karnofsky Performance Status of ≥ 70% and adequate hematologic, hepatic, and renal function. Exclusion criteria included a history of platinum HSR, uncontrolled concurrent illness, a life expectancy of less than 12 weeks, pregnancy or lactation, or receipt of another investigational agent. All study patients had previously received at least 6 cycles of a platinum-containing regimen. The full list of inclusion and exclusion criteria are provided in the appendix. Demographic information is shown in Table 1.
Table 1.
Patient demographics by carboplatin infusion type
| Patient Demographics | Extended Infusion (n=56) | Standard Infusion (n=58) |
|---|---|---|
| Median age, years (range) | 61 (35–79) | 61 years (41–78) |
| FIGO stage at diagnosis, n (%) | ||
| I or II | 3 (5%) | 5 (9%) |
| III | 42 (75%) | 32 (55%) |
| IV | 11 (19%) | 21 (36%) |
| Karnofsky Performance Score, median (range) | 90 (70–100) | 90 (70–100) |
| Race, n (%) | ||
| White | 49 (88%) | 52 (90%) |
| Asian | 3 (5%) | 1 (1.5%) |
| Black/African American | 4 (7%) | 1 (1.5%) |
| Other | 0 (0%) | 4 (7%) |
| Histologic subtype, n (%) | ||
| High-grade serous | 54 (96%) | 53 (91%) |
| Carcinosarcoma | 0 (0%) | 1 (2%) |
| Endometrioid | 0 (0% | 2 (3%) |
| Clear cell | 2 (4%) | 0 (0%) |
| Low-grade serous | 0 (0%) | 2 (4%) |
| Concomitant regimen | ||
| Single-agent carboplatin | 13 (23%) | 12 (20%) |
| Gemcitabine | 12 (21%) | 11 (19%) |
| Gemcitabine plus bevacizumab | 2 (4%) | 2 (3%) |
| Pegylated liposomal doxorubicin | 25 (45%) | 27 (47%) |
| Paclitaxel | 3 (5%) | 5 (9%) |
| Bevacizumab | 1 (2%) | 1 (2%) |
| Number of prior platinum regimens | ||
| 1 | 39 (70%) | 42 (72%) |
| 2 | 16 (29%) | 12 (22%) |
| 3 | 0 (0%) | 2 (3%) |
| 4 | 1 (1%) | 2 (3%) |
FIGO, International Federation of Gynecology and Obstetrics
Procedures
The trial was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by our institutional review board, and the trial was registered with ClinicalTrials.gov (NCT01248962). The study was closed and the database locked on October 20, 2017. This was a single-institution, randomized, non-blinded trial comparing a graded, extended (3-hour) infusion of carboplatin (extended arm) with a standard (30-minute) infusion (standard arm) in women with recurrent ovarian, fallopian tube, or primary peritoneal cancer slated for treatment with a carboplatin-containing chemotherapy regimen.
All patients in both arms received identical chemotherapy premedications: montelukast 10 mg once daily for 3 days prior, dexamethasone 20 mg the night before and day of the carboplatin infusion, and either ranitidine 50 mg IV or famotidine 20 mg IV before the carboplatin infusion (Table 2). Patients also received diphenhydramine 50 mg IV prior to the infusion. If a patient was unable to tolerate diphenhydramine 50 mg IV, she was given a reduced dose of 25 mg IV or hydroxyzine 25 mg orally. Previously published data have shown that the incidence of HSRs can be reduced with the administration of premedication [22, 23], and therefore all patients on the study received premedication as per our standard. At the treating physician’s discretion, patients received carboplatin alone or in combination with pegylated liposomal doxorubicin, paclitaxel, gemcitabine +/− bevacizumab, or bevacizumab. The dosing of these agents was based on institutional guidelines, with a plan for 5 to 8 cycles.
Table 2.
Carboplatin premedication and extended-infusion schedules
| Carboplatin Premedication Schedule | |
|---|---|
| Day | Premedication drug |
| Three days prior to carboplatin | Montelukast 10 mg orally |
| Night before and day of carboplatin | Dexamethasone 20 mg orally |
| Day of carboplatin, prior to infusion | Diphenhydramine 50 mg IV Ranitidine 50 mg IV or Famotidine 20 mg IV |
| Extended-Infusion Carboplatin Schedule | |
| Infusion time | Percentage of carboplatin infused |
| 1st hour | 1% |
| 2nd hour | 9% |
| 3rd hour | 90% |
| Total: 3-hour infusion | 100% |
Patients on the extended arm were treated per a graded protocol (Table 2). The initial infusion contained 10% of the total carboplatin dose in 100 mL of D5W. Ten mL (1% of the total dose) was infused during the first hour. If there was no evidence of an HSR, the remaining 90 mL (9% of the total dose) was infused over the second hour. If tolerated, the second infusion containing 90% of the total dose in 250 mL of D5W was infused over the third hour (Table 2). Patients on the standard arm were treated per institutional guidelines: 100% of the carboplatin dose was administered in 250 mL of D5W over 30 minutes. Per protocol, patients who experienced an HSR of grade 1 or 2 on the standard arm were permitted to crossover to the extended arm at the discretion of the treating physician.
An HSR was defined as any allergic or anaphylactic reaction, regardless of grade, deemed unlikely, possibly, potentially, or definitely attributed to the carboplatin. HSRs were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE; version 4.0). All patients with an HSR were evaluated by a treating physician and managed according to standard institutional practice. Patients underwent standard clinical, laboratory, and toxicity evaluations prior to day 1 of every cycle. Criteria for removal from the study included substantial non-compliance with the requirements of the study, treatment delay of more than 30 days, the development of an intercurrent illness or situation that would affect assessments of clinical status and study endpoints to a significant degree, progression of disease, or the development of a grade 3 or 4 HSR. Patients were instructed on the premedication regimen at study entry and throughout the study. Premedication compliance was evaluated through patient diaries, which were reviewed at the start of each cycle.
Outcomes
The primary objective of the study was to determine the rate of HSRs in each group. The secondary endpoint was to determine the rate of planned treatment completion of carboplatin in each group. Exploratory endpoints were to determine the correlation, if any, between HSR and patient history of atopy, food allergies, drug allergies, number of prior platinum regimens, and the duration of time since the last platinum and concomitant chemotherapy agent. Patients were considered evaluable for the study endpoint if they had an HSR during any cycle or if they completed at least 5 cycles of carboplatin-based chemotherapy.
Randomization and Masking
Patients were randomized to receive an extended or standard infusion of carboplatin in a 1:1 manner. Randomization was accomplished by the method of random permuted block. Patients were stratified based on concurrent taxane treatment, as prior data had shown an increased rate of carboplatin HSR with taxane-containing doublets [8]. We anticipated approximately 5–10% of patients would receive a concomitant taxane. The second stratification factor was based on previous carboplatin exposure.
Statistical Analysis
We anticipated that 80% of the patients enrolled on this study would receive their second platinum-based regimen and 20% their third or greater platinum line of treatment. We determined that 114 patients would be required in order to show a decrease in the HSR rate from 20% to 5%, assuming a type 1 error of 10% and a power of 80% using a one-sided test. We employed continuous significant testing with a significance level of 12% to monitor the HSR rate within each arm separately, and we defined stopping boundaries for an unacceptable rate of HSR. The stopping boundary was set at ≥ 23%. Fischer’s exact test was used to analyze categorical values. Univariate logistic regression was performed in the exploratory analysis of the relationship of baseline variables to the HSR rate.
Results
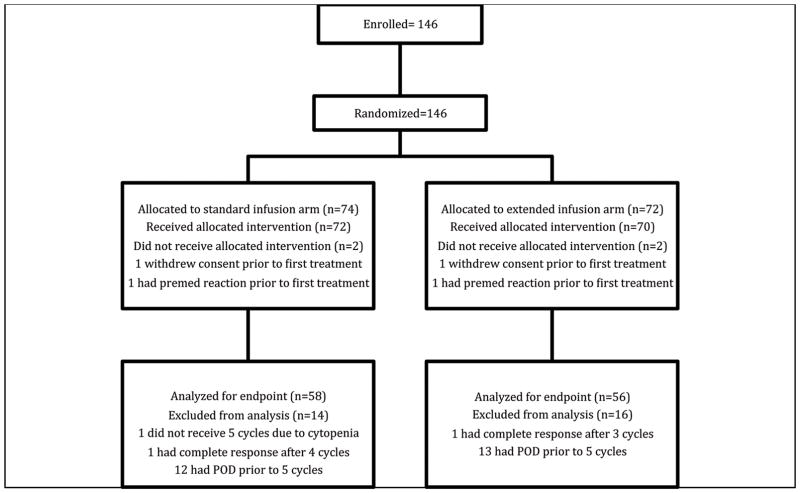
Of the 146 patients who were enrolled on the study between 1/2011 and 4/2015, 114 (78%) were evaluable for the primary study endpoint. Of the patients who were not evaluable, 25 had progression of disease prior to receiving 5 cycles of carboplatin-based treatment, 2 withdrew consent, 2 had a complete response prior to 5 cycles, leading to cessation of treatment, and 3 were taken off of the study at their treating physician’s discretion (Figure 1). The median age of the participants was 60 years (range, 35–79), and the majority were white (88%) and had a diagnosis of a high-grade serous malignancy (97%). Demographic data are summarized in Table 1. The majority of patients (71%) had been treated with one prior platinum-based regimen. Among the remaining patients, 25% had 2, 2% had 3, and 3% had 4 prior lines of platinum-based treatment. These percentages were similar between the two arms.
Figure 1.
Consort Diagram
POD, progression of disease
The carboplatin doublets used were liposomal doxorubicin (46%, n=52), gemcitabine (20%, n=23), gemcitabine plus bevacizumab (4%, n=4), paclitaxel (7%, n=8), and bevacizumab (2%, n=2). Twenty-five patients (22%) received single-agent carboplatin.
Among the 114 evaluable patients, 15 (13%) experienced an HSR—6 (11%) of 56 patients in the extended-infusion group and 9 (16%) of 58 in the standard-infusion group (P=0.582). HSR onset most frequently occurred during cycle 2 (n=5, 33%) but was observed as late as cycle 6 (Table 3). The completion of planned carboplatin-based treatment was achieved in 89% (50/56) of the patients in the extended-infusion arm and 84% (49/58) in the standard-infusion arm. Two of the 9 patients in the standard arm who experienced an HSR crossed over to the extended-infusion arm and completed therapy without an HSR recurrence.
Table 3.
Characteristics associated with carboplatin hypersensitivity reactions by infusion type
| Extended Infusion (n=6) | Standard Infusion (n=9) | |
|---|---|---|
| HSR Grade | n (%) | n (%) |
| 1 | 2 (33) | 2 (22) |
| 2 | 3 (50) | 7 (78) |
| 3 | 1 (17) | 0 (0) |
| 4 | 0 (0) | 0 (0) |
| Symptoms/Signs | ||
| Flushing/rash | 6 (100) | 9 (100) |
| Chest tightness | 0 (0) | 2(22) |
| Pruritus | 2 (33) | 5 (56) |
| Orofacial edema | 0 (0) | 1 (11) |
| Burning sensation | 1 (17) | 1 (11) |
| Nausea | 3 (50) | 0 (0) |
| Abdominal pain | 1 (17) | 0 (0) |
| Back pain | 1(17) | 0 (0) |
| Hospitalized | 0 (0) | 0 (0) |
| HSR by Cycle | ||
| 1 | 1 (17) | 0 (0) |
| 2 | 3 (50) | 2 (22) |
| 3 | 1 (17) | 2 (22) |
| 4 | 0 (0) | 3 (33) |
| 5 | 0 (0) | 1 (11) |
| 6 | 1 (17) | 1 (11) |
HSR, hypersensitivity reaction
The overall premedication compliance rate was 72%. The premedication compliance rate was 77% (43/56) in the extended-infusion arm and 67% (39/58) in the standard-infusion arm. The rate was 80% (12/15) in the HSR group and 71% (70/99) in the remaining patients.
HSR events most frequently occurred in patients treated with single-agent carboplatin. Twenty-five patients received single-agent carboplatin, of whom 8 experienced an HSR (32%), representing 53% of all of the patients who experienced an HSR (8/15). Twenty-three patients received carboplatin with gemcitabine, of whom 4 experienced an HSR (15%), representing 27% of all of the patients who experienced an HSR (4/15). Eight patients received carboplatin with paclitaxel, of whom 3 experienced an HSR (38%), representing 20% of all of the patients who experienced an HSR (3/15). There were no HSRs among patients treated with pegylated liposomal doxorubicin, despite this being the most commonly given concurrent chemotherapy (46% of all patients). There were no HSRs among patients treated with bevacizumab either when given with carboplatin alone or in triplet therapy with gemcitabine (Table 4). Univariate logistic regression was performed as part of the exploratory analyses looking at the association of baseline variables with HSR. There was no association with number of prior platinum regimens, platinum-free interval, history of drug allergies, history of food allergies, or history of atopy (Table 5).
Table 4.
Concurrent chemotherapy by carboplatin infusion type and occurrence of a hypersensitivity reaction
| Concurrent Chemotherapy/Biologic Agent with Carboplatin | Extended Infusion (n=56) | Standard Infusion (n=58) | Overall (n=114) | HSR (n=15) |
|---|---|---|---|---|
| Carboplatin single agent | 13 (23%) | 12 (21) | 25 (22%) | 8 (53%) |
| Bevacizumab | 1 (2%) | 1 (2%) | 2 (2%) | 0 (0%) |
| Gemcitabine | 12 (21%) | 11 (19%) | 23 (20%) | 4 (27%) |
| Gemcitabine/bevacizumab | 2 (4%) | 2 (3%) | 4 (4%) | 0 (0%) |
| Liposomal doxorubicin | 25 (45%) | 27 (47%) | 52 (46%) | 0 (0%) |
| Paclitaxel | 3 (5%) | 5 (9%) | 8 (7%) | 3 (20%) |
HSR, hypersensitivity reaction
Table 5.
Relationship of baseline variables to the rate of carboplatin hypersensitivity reactions
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Number of prior platinum-based regimens (>2 vs 1) | 2.5 | 0.8–7.5 | 0.1 |
| Prior cisplatin regimen | 1.5 | 0.5–4.4 | 0.5 |
| Platinum-free interval | 1 | 0.96–1 | 0.6 |
| History of drug allergies | 0.8 | 0.3–2.4 | 0.7 |
| History of food allergies | 2.2 | 0.5–9.2 | 0.3 |
| History of atopy | 2 | 0.6–5.8 | 0.2 |
Discussion
In this randomized controlled trial, a prophylactic, extended infusion of carboplatin was not associated with a decreased HSR rate in patients undergoing treatment for recurrent ovarian cancer. This is the first randomized, prospective trial evaluating the prophylactic use of an extended-infusion carboplatin regimen to reduce the incidence of HSRs. An important finding of this study is the overall low HSR rate (13%), which is lower than many reported rates in the literature [6, 8, 9]. One possible reason for this low rate could be the high use of liposomal doxorubicin and carboplatin, which is associated with a lower rate of HSR. Another possible reason may be that all of the patients in our study received a standard premedication regimen that included a leukotriene antagonist. Previously published data have shown that the incidence of HSRs can be reduced with the administration of premedication [22, 23], and therefore all patients on the study received premedication as per our standard. While the extended carboplatin infusion was not associated with a lower HSR rate, it is possible that the observed low HSR rate was due to the standardized premedication regimen and the relatively good patient compliance with at-home premedications before chemotherapy administration. Since all patients received premedications, the contribution of the premedication cannot be fully assessed in this study. While the extended carboplatin infusion was not associated with a lower HSR rate, it is possible that the observed low HSR rate was due to the standardized premedication regimen and the relatively good patient compliance with at-home premedications before chemotherapy administration. In a study by Lax et al. [8], in which a 40% HSR rate was observed among 15 patients treated on an extended-infusion protocol, patients received similar premedications immediately prior to chemotherapy, but they did not receive any home premedications. In a study by Pasternak et al. [9], the use of premedications, which were similar to those used in our study (triple therapy with H2 antagonist, corticosteroid, and antihistamine), was associated with a lower rate of HSRs, with an odds ratio of 0.59 (95% CI, 0.36–0.97). As all of our patients received premedication, this limits our ability to ascertain the contribution of home premedications on the low HSR rate observed. However, the findings reported by Pasternak et al., along with the results of our study, suggest that the use of a home premedication regimen could help reduce the rate of carboplatin HSRs. Ensuring compliance with home medications was a challenge in our study; however, the use and careful review of a patient pill diary helped with adherence.
The rate of carboplatin HSRs in our standard arm was lower than anticipated. Based on our retrospective study, we had powered our prospective trial for a 20% HSR rate in the standard arm, but the actual rate was 15% [21]. Therefore, it is possible that our study was underpowered to show a difference between the arms.
Prior studies have shown an association between baseline variables such as number of prior platinum regimens, platinum-free interval, history of drug or food allergies, and history of atopy [7, 24–27] with an increased risk of HSR. Our exploratory analysis did not show a correlation between HSR rate and these baseline variables. The analysis may have been limited by the overall very low number of HSRs observed, with only 15 patients for HSR analysis. Previous data have shown higher rates of HSR with taxanes compared to liposomal doxorubicin-containing carboplatin doublets, as was seen in our study [11, 28, 29]. We saw a trend towards a higher rate of HSRs with single-agent carboplatin, gemcitabine, and paclitaxel. There were no carboplatin HSRs with pegylated liposomal doxorubicin or in bevacizumab-containing regimens. The mechanism of carboplatin hypersensitivity is not fully understood, but it appears to be a type 1 immunoglobulin E-mediated reaction. Previous data have suggested that the pegylation may lower the immunologic response by the masking of antigenic sites, and the encapsulated doxorubicin may selectively damage T-cells responsible for the accelerated blood clearance phenomenon that is seen in the immediate immune response [11]. The mechanism for decreased HSR with bevacizumab is not known. Approximately half of the patients evaluated received pegylated doxorubicin, and given its protective effect, this may limit the applicability of the results to other regimens and may also contribute to the overall low HSR rate seen.
Data from our study show that a prophylactic, graded, extended infusion of carboplatin, compared with a shorter, standard infusion, is not associated with a reduced rate of HSRs among patients with no history of carboplatin allergy. The study was not designed to address the potential utility of graded, extended carboplatin for patients with a history of prior carboplatin HSRs. For patients with confirmed prior carboplatin HSRs, many institutions have introduced a 12-step chemotherapy desensitization protocol for use in appropriately selected patients [19].
The overall low rate of carboplatin HSRs observed in this study is encouraging and suggests that a robust premedication regimen that incorporates at-home leukotriene plus dexamethasone followed by immediate premedication with H1 and H2 blockers may help to reduce the rates of this potentially life-threatening and treatment-limiting side effect of carboplatin therapy.
Acknowledgments
We acknowledge Dr Garry Duffy from the National University of Ireland, Galway, who reviewed the manuscript.
Funding
This work was supported in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 and the Kaleidoscope of Hope Ovarian Cancer Foundation.
Footnotes
Disclosures
The authors have declared no conflicts of interest.
References
- 1.Markman M, Kennedy A, Webster K, et al. Continued chemosensitivity to cisplatin/carboplatin in ovarian carcinoma despite treatment with multiple prior platinum-based regimens. Gynecol Oncol. 1997;65:434–436. doi: 10.1006/gyno.1997.4708. [DOI] [PubMed] [Google Scholar]
- 2.Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–393. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 3.Pignata S, Scambia G, Raspagliesi F, et al. The MITO8 phase III international multicenter randomized study testing the effect on survival of prolonging platinum-free interval (PFI) in patients with ovarian cancer (OC) recurring between 6 and 12 months after previous platinum-based chemotherapy: A collaboration of MITO, MANGO, AGO, BGOG, ENGOT, and GCIG. J Clin Oncol. 2016;34(15_suppl):5505. [Google Scholar]
- 4.Parmar MK, Ledermann JA, Colombo N, et al. ICON and AGO Collaborators. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2. 2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 5.Dizon DS, Hensley ML, Poynor EA, et al. Retrospective analysis of carboplatin and paclitaxel as initial second-line therapy for recurrent epithelial ovarian carcinoma: application toward a dynamic disease state model of ovarian cancer. J Clin Oncol. 2002;20:1238–1247. doi: 10.1200/JCO.2002.20.5.1238. [DOI] [PubMed] [Google Scholar]
- 6.Markman M, Kennedy A, Webster K, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 7.Navo M, Kunthur A, Badell ML, et al. Evaluation of the incidence of carboplatin hypersensitivity reactions in cancer patients. Gynecol Oncol. 2006;103:608–613. doi: 10.1016/j.ygyno.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Lax T, Dizon DS, Birrer M, et al. Extended carboplatin infusion does not reduce frequency of hypersensitivity reaction at initiation of retreatment in patients with recurrent platinum-sensitive ovarian cancer. J Allergy Clin Immunol Pract. 2017;5:177–178. doi: 10.1016/j.jaip.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Pasternak AL, Link NA, Richardson CM, Rose PG. Effect of prophylactic extended-infusion carboplatin on incidence of hypersensitivity reactions in patients with ovarian, fallopian tube, or peritoneal carcinomas. Pharmacotherapy. 2016;36:723–730. doi: 10.1002/phar.1769. [DOI] [PubMed] [Google Scholar]
- 10.Bergamini A, Pisano C, Di Napoli M, et al. Cisplatin can be safely administered to ovarian cancer patients with hypersensitivity to carboplatin. Gynecol Oncol. 2017;144:72–76. doi: 10.1016/j.ygyno.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Joly F, Ray-Coquard I, Fabbro M, et al. Decreased hypersensitivity reactions with carboplatin-pegylated liposomal doxorubicin compared to carboplatin-paclitaxel combination: analysis from the GCIG CALYPSO relapsing ovarian cancer trial. Gynecol Oncol. 2011;122:226–232. doi: 10.1016/j.ygyno.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Morgan JS, Adams M, Mason MD. Hypersensitivity reactions to carboplatin given to patients with relapsed ovarian carcinoma. Eur J Cancer. 1994;30:1205–1206. doi: 10.1016/0959-8049(94)90489-8. [DOI] [PubMed] [Google Scholar]
- 13.Caiado J, Castells M. Presentation and diagnosis of hypersensitivity to platinum drugs. Curr Allergy Asthma Rep. 2015;15:15. doi: 10.1007/s11882-015-0515-3. [DOI] [PubMed] [Google Scholar]
- 14.Banerji A, Lax T, Guyer A, et al. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2:428–33. doi: 10.1016/j.jaip.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Gadducci R, Tana G, Teti G, et al. Analysis of the pattern of hypersensitivity reactions in patients receiving carboplatin retreatment for recurrent ovarian cancer. Int J Gynecol Cancer. 2008;18:615–620. doi: 10.1111/j.1525-1438.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 16.Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol Pract. 2008;122:574–580. doi: 10.1016/j.jaci.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Cohn D, Waller A, et al. Outpatient rapid 4-step desensitization for gynecologic oncology patients with mild to low-risk, moderate hypersensitivity reactions to carboplatin/cisplatin. Gynecol Oncol. 2014;135:90–94. doi: 10.1016/j.ygyno.2014.07.104. [DOI] [PubMed] [Google Scholar]
- 18.Robinson JB, Singh D, Bodurka-Bevers DC, et al. Hypersensitivity reactions and the utility of oral and intravenous desensitization in patients with gynecologic malignancies. Gynecol Oncol. 2001;82:550–558. doi: 10.1006/gyno.2001.6331. [DOI] [PubMed] [Google Scholar]
- 19.O’Malley DM, Vetter MH, Cohn DE, et al. Outpatient desensitization in selected patients with platinum hypersensitivity reactions. Gynecol Oncol. 2017;145:603–610. doi: 10.1016/j.ygyno.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Lee CW, Matulonis UA, Castells MC. Carboplatin hypersensitivity: a 6-h 12-step protocol effective in 35 desensitizations in patients with gynecological malignancies and mast cell/IgE-mediated reactions. Gynecol Oncol. 2004;95:370–376. doi: 10.1016/j.ygyno.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 21.O’Cearbhaill R, Zhou Q, Iasonos A, et al. The prophylactic conversion to an extended infusion schedule and use of premedication to prevent hypersensitivity reactions in ovarian cancer patients during carboplatin retreatment. Gynecol Oncol. 2010;116:326–331. doi: 10.1016/j.ygyno.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jerzak KJ, Deghan Manshadi S, et al. Prevention of carboplatin-induced hypersensitivity reactions in women with ovarian cancer. J Oncol Pharm Pract. 2016 Nov 17; doi: 10.1177/1078155216679028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Mach CM, Lapp EA, Weddle KJ, et al. Adjunct histamine blockers as premedications to prevent carboplatin hypersensitivity reactions. Pharmacotherapy. 2016;36:482–487. doi: 10.1002/phar.1739. [DOI] [PubMed] [Google Scholar]
- 24.Moon DH, Lee JM, Noonan AM, et al. Deleterious BRCA1/2 mutation is an independent risk factor for carboplatin hypersensitivity reactions. Br J Cancer. 2013;109:1072–1078. doi: 10.1038/bjc.2013.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markman M, Zanotti K, Kulp B, et al. Relationship between a history of systemic allergic reactions and risk of subsequent carboplatin hypersensitivity. Gynecol Oncol. 2003;89:514–516. doi: 10.1016/s0090-8258(03)00155-0. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz JR, Bandera C, Bradley A, et al. Does the platinum-free interval predict the incidence or severity of hypersensitivity reactions to carboplatin? The experience from Women and Infants’ Hospital. Gynecol Oncol. 2007;105:81–83. doi: 10.1016/j.ygyno.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 27.Koshiba H, Hosokawa K, Kubo A, et al. Incidence of carboplatin-related hypersensitivity reactions in Japanese patients with gynecologic malignancies. Int J Gynecol Cancer. 2009;19:460–465. doi: 10.1111/IGC.0b013e3181a1bf2e. [DOI] [PubMed] [Google Scholar]
- 28.Bafaloukos D, Linardou H, Aravantinos G, et al. A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group study. BMC Med. 2010;8:3. doi: 10.1186/1741-7015-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrie TA, Bryant A, Cameron A, et al. Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database Syst Rev. 2013;(7):CD006910. doi: 10.1002/14651858.CD006910.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]