Abstract
Background
The extent to which short interval intracortical inhibition (SICI) responds to low-frequency repetitive transcranial magnetic stimulation (rTMS) remains inconclusive with reports of increased, decreased and unchanged response following modulation. The aim of this study was to systematically investigate if the variability of SICI following rTMS is explained by the interstimulus interval (ISI) and/or the conditioning stimulus intensity (CSI).
Methods
Two experiments with pre/post-testing and an rTMS session (1 Hz, 90% RMT, 900 pulses) were done. Experiment I (N=15): SICI with multiple ISIs (1.0-4.0 ms, 0.2ms increment). Experiment II (N=15): SICI with CSIs (50-95% of RMT, 5% increment). In both experiments, the cortical silent period (cSP) was also collected.
Results
After low-frequency rTMS, no significant change (p>0.10) in SICI at any specific ISI or CSI was observed, nor did the optimal ISI or CSI change. However, a significant decrease was observed in SICI responses when assessed under the range of ISIs (p=0.0001), but not CSIs. cSP inhibition increased significantly (p<0.0015) for both experiments.
Conclusions
The optimal ISI or CSI did not shift or reveal SICI changes after inhibitory rTMS. However, when the whole curve of SICI responses were evaluated from a wide range of ISIs, a decrease in inhibition was found. The contrast between the results of individual ISI tests and the wide range of ISI assessment may be due to higher inter-subject variability of SICI and/or sample size, rendering traditional SICI testing methods ineffective for measuring changes in inhibition. Further, it is possible that rTMS modulates GABAA and GABAB mediated inhibitory processes differently, which would explain the conflicting results for SICI and cSP.
Keywords: SICI, TMS, rTMS, ISI, CSI, Neuromodulation, short interval intracortical inhibition, transcranial magnetic stimulation, interstimulus interval, conditioning intensity
Introduction
Short interval intracortical inhibition (SICI) is a common motor cortical excitability assessment method that reflects the intracortical inhibitory circuits mediated by gamma-aminobutyric acid-A (GABAA) receptors1. It employs paired transcranial magnetic stimulation (TMS) pulses which consists of a subthreshold conditioning stimulus applied to the motor cortex followed by a suprathreshold test stimulus at a short interstimulus interval (ISI) between 1 and 5 ms2. Due to the effect of the conditioning stimulus, the amplitude of the induced motor evoked potential (MEP) is suppressed compared to single pulse stimulation. The level of this suppression is represented by the ratio of the MEP amplitude between the conditioned (paired pulse) and unconditioned (single pulse) response. Besides GABAA, other factors that influence SICI response include indirect (I)-wave phase3 and high inter-subject variability4. Another factor important to consider is that as the ISIs lengthen, there is a shift from inhibited response to the facilitated response known as intracortical facilitation (ICF)5. As an integrated effect of these factors, it has been proposed that the average parameters to induce the most suppressed response in healthy individuals are 2.5 ms ISI and 80% of resting motor threshold (RMT) conditioning stimulus intensity (CSI)6,7.
Cortical excitability can be modulated by repetitive transcranial magnetic stimulation (rTMS)8–10. Low-frequency (≥1 Hz) rTMS applied over the primary motor cortex acts on the cortical spinal tract neurons11 and induces a ‘long term depression (LTD)-like’ inhibition9. As a mainstream neuromodulation technique, low-frequency rTMS has been widely tested for its potential to serve as treatment for a variety of neurological disorders10. Using SICI to measure this neuromodulatory effect yields controversial results because variable findings have been reported12. Following low-frequency rTMS, many reports have noted no change in SICI13–17; an increase in SICI18, meaning the brain is more inhibited; or a decrease in SICI19–21, meaning the brain is less inhibited. These mixed results diminish the utility of SICI as a measure to determine the effects of neuromodulatory interventions and may cause confusion in the interpretation of findings12. A potential reason for these mixed results is that the previous studies utilized a uniform ISI (2.5 ms or 3.0 ms) and CSI (80% of RMT) for all participants12. However, individuals can have different optimal ISI and CSI4 which may diminish the validity of uniform SICI testing parameters. Also, the neuromodulatory effect induced by rTMS may change the I-wave phase as well as the dynamic of the transition between SICI and ICF. Thus, we hypothesized that the lack of consistent SICI change may be because rTMS modulates SICI by altering the optimal ISI and CSI which would make uniform values unsuitable for pre- and post-tests comparisons.
The purpose of this study was to compare SICI responses under individualized optimal ISI and CSI, before and after 1-Hz rTMS. We hypothesized that low-frequency rTMS would: 1) change individual’s optimal ISI and CSI; and 2) modulate SICI if tested using the optimized parameters.
Methods
Participants
Twenty-one healthy participants (age, 26.1±5.2 yrs; 13 females) were recruited and gave written, informed consent prior to participation according to the Declaration of Helsinki22. Participants were excluded if they presented the following: history of epilepsy in the past two years; convulsion and/or seizure disorder; cochlear implants; pregnancy; metal in the brain; implanted neurostimulators; cardiac pacemaker and/or intracardiac lines; medication infusion devices23. The study was approved by the Clinical and Translational Science Institute and the Institutional Review Board of the University of Minnesota.
Devices
During the experiments, participants were comfortably seated in a semi-reclining chair. Surface Ag-AgCl bipolar electrodes (EL254, BIOPAC System Inc., Aero Camino Goleta, CA) were placed over the dominant first dorsal interosseous (FDI) muscle. Electromyogram (EMG) signals were amplified (Y03-2, Motion Lab System, Inc., Baton Rouge, LA) with the gain of ×300, filtered (band-passed with 15 to 2000 Hz) and digitalized (NI 9234, National Instruments, Austin, TX) at 6.4k Hz for offline analysis. All data were collected using a custom LabVIEW program (v2012, National Instruments, Austin, TX).
For the pre- and post-test excitability assessments in both experiments, TMS was applied using a 70-mm figure-of-eight coil (Double 70mm Remote Control Coil, Magstim Co., Whitland, UK) connected to a Magstim Bistim2 magnetic stimulator Magstim Co., Whitland, UK). To improve coil localization accuracy, a frameless stereotactic neuronavigation system (Brainsight, Rogue Research Co., Canada) was employed with phantom common template T1 structural magnet resonance image data.
1-Hz rTMS was delivered with a figure-of-eight coil (70mm Double Air Film Coil, Magstim Co., Whitland, UK) connected to a Magstim Rapid2 magnetic stimulator (Magstim Co., Whitland, UK) under the guidance of the aforementioned neuronavigation system.
Experimental design and excitability measures
To test our hypotheses, we designed two experiments to examine ISI and CSI respectively. In experiment I (N=15), SICI responses under multiple ISIs were collected. In experiment II (N=15), SICI responses under multiple CSIs were collected. Both experiments included a pre-test, 1-Hz rTMS session, and a post-test performed in the same day. Experiments I and II were performed in two separate days no less than 3 months apart.
For thresholds and hotspot determination, previously established procedures were used19,21. Briefly, the RMT was determined as the lowest intensity that generated MEPs with the peak-to-peak amplitude ≥ 50 μV in 5 out of 10 consecutive trials. The 1-mV threshold was determined by using a similar protocol with the MEP amplitude response ≥ 1 mV. The hotspot was defined as the location in which the RMT was determined, corresponding to the optimal site to evoke an MEP in the FDI muscle.
In Experiment I, the SICI protocol consisted of paired pulses delivered at a series of ISIs between 1.0 ms and 4.0 ms in 0.2 ms increments, with the CSI of 80% RMT and the testing intensity of 1-mV threshold held constant. The intensity of CSI and testing pulses were selected based on previous work24. In Experiment II, paired pulses were delivered at a series of CSIs between 50% and 95% of RMT in 5% increments with the ISI of 2.5 ms (CSI2.5) and the testing intensity of 1-mV threshold held constant. For both SICI protocols, fifteen trials of SICI at each ISI/CSI and fifteen trials of single pulse responses at 1-mV threshold were conducted in random order. For the 9 subjects who participated in both experiments, SICI under multiple CSIs were also collected with the individual’s optimal ISI (CSIOptISI) as determined in Experiment I.
To provide additional evidence that the rTMS induced neuromodulatory effects in the motor cortex, single pulse (different from the single pulses included in the SICI protocols) and cortical silent period (cSP) were also collected. In both pre- and post-tests, twenty trials of single pulse TMS responses at the 1-mV threshold were collected. The 1-mV threshold was determined at pre-test and held constant at post-test. Twenty trials of cSP were collected by following a previously established protocol19,25–27. During each trial, participants performed an isometric contraction of the FDI and maintained the EMG intensity at 20% of maximum voluntary contraction. Single pulse stimulation with the intensity of 120% RMT (determined at pre-test and readjusted at post-test) was applied approximately 3 s after contraction initiation and participants were instructed to relax ~2 s after stimulation. There was a minimum 10-second rest interval between each trial. For the SICI and cSP post-test, the thresholds were re-established after rTMS to ensure comparable relative stimulation intensities. This procedure was done because the inhibitory neuromodulation effects induced by the low-frequency rTMS may result in a new threshold for each individual19. All measures were tested by following the order: pre-test (thresholds determination -> single pulse -> cSP -> SICI) -> rTMS -> post-test (threshold determination -> single pulse -> cSP -> SICI).
Repetitive Transcranial Magnetic Stimulation
In both experiments, immediately following pre-test, all participants received low-frequency rTMS. The coil was positioned over the TMS hotspot with the handle positioned at 45º posteriolateral to the mid-sagittal line. rTMS included 15-min (900 pulses) of 1-Hz rTMS at 90% of the RMT28. The post-test was conducted immediately after rTMS.
Data processing
The MEP peak-to-peak amplitude was used to quantify SICI by following previously established procedures19,21. For both pre- and post-tests, the ratio between the individual SICI trials and the average value of the single pulse responses collected within the SICI protocol were calculated according to the following formula: paired-pulse MEP/average 1-mV single pulse MEP. A ratio value less than 1 indicates an inhibited response and a value greater than 1 indicates a facilitated response. Thus, a higher SICI value corresponds to a less inhibited response, or decreased SICI, and vice versa. Optimal ISI and CSI were defined as the ISI and CSI values that resulted in the lowest average SICI value (the most inhibited SICI) of each participant. For both experiments, optimal ISI/CSI and the corresponding SICI value were calculated at pre- and post-tests for comparison.
For cSP processing, previously published methods were used19,21. Briefly, the EMG data were first rectified, and a 10-ms moving SD window was applied to slide through the rectified EMG curve and generate an SD curve of the signal. The average value of this SD curve during the pre-stimulus period (−100ms to −5ms) was calculated. This value was defined as the pre-stimulus level and was used to determine the offset of the cSP which is the point that the EMG signal returns to pre-stimulus level. The stimulus delivery defined the onset of the cSP. The duration of cSP was calculated by subtracting the onset from the offset of the cSP.
Data analysis
All tests were conducted with JMP Pro (v13.0. SAS Institute Inc. USA) with a significance level of p<0.05. Parametric statistical analyses were used for comparisons since all outcome measures were normally distributed as determined by the Shapiro-Wilk W-test. For SICI responses under multiple ISI/CSI values, RMANOVA was used with ISI/CSI as interaction factor to examine Time (pre- and post-) effect and Tukey’s HSD as the post-hoc to test individual ISI/CSI effect as appropriate. Paired t-tests were used to test pre- and post-test differences in single pulse and cSP in both experiments.
For hypothesis 1, optimal ISI and optimal CSI values were compared between pre- and post-tests by paired t-test to examine if the optimal ISI and CSI changed after rTMS. The distribution pattern of the optimal ISI and CSI were evaluated by Shapiro-Wilk W-test and were visually compared between pre- and post-tests, to check any changes in optimal ISI and CSI distribution pattern after rTMS.
For hypothesis 2, the SICI values at optimal ISI as tested in Experiment I and at optimal CSI as tested in Experiment II were compared between pre- and post-tests by paired t-test to examine if SICI was modulated by rTMS as measured under individual optimal parameters (either optimal ISI or optimal CSI). For those nine participants who participated in both experiments, SICI responses were compared under both individual optimal ISI and CSI conditions.
Results
All participants tolerated the procedures without adverse effects. All participants self-identified as right handed. Average TMS parameters and outcomes for both experiments are listed in Table 1.
Table 1.
Average TMS outcomes (mean±SD)
| Experiment I | Experiment II | |||
|---|---|---|---|---|
|
| ||||
| Pre | Post | Pre | Post | |
| RMT (%MSO) | 44.5±8.0 | 45.9±7.6 | 44.3±7.0 | 46.6±7.6 |
| 1mVT (%MSO) | 60.7±11.5 | 62.6±11.7 | 57.5±9.1 | 58.8±8.9 |
| SP (μV) | 1116.4±588.7 | 1114.3±836.8 | 1072.2±679.6 | 869.1±464.2 |
| cSP (ms) | 135.1±28.9 | 153.5±22.4* | 141.7±26.9 | 158.9±25.0* |
| Optimal ISI (ms) | 2.55±0.35 | 2.59±0.35 | N/A | N/A |
| Optimal CSI2.5 (%RMT) | N/A | N/A | 86.3±7.4 | 82.7±7.9 |
| Optimal CSIOptISI (%RMT) | N/A | N/A | 81.7±5.8 | 82.8±9.5 |
| SICI under Optimal ISI (ratio) | 0.13±0.09 | 0.16±0.14 | N/A | N/A |
| SICI under Optimal CSI2.5 (ratio) | N/A | N/A | 0.13±0.12 | 0.15±0.15 |
| SICI under Optimal CSIOptISI (ratio) | N/A | N/A | 0.19±0.11 | 0.19±0.16 |
RMT: resting motor threshold. 1mVT: 1 mV threshold. SP: single pulse. CSP: cortical silent period. ISI: interstimulus interval. CSI: conditioning stimulus intensity. TMS: transcranial magnetic stimulation. MSO: maximum stimulator output. CSI2.5: SICI tested under multiple conditioning intensities with the ISI of 2.5 ms. CSIOptISI: SICI tested under multiple conditioning intensities with the individual optimal ISI tested in Experiment I. N/A: not applicable.
significantly increased as compared with pre-test (p<0.05), indicating increased inhibition after low-frequency rTMS.
Experiment I
No differences in SICI values under optimal ISI between pre- and post-tests were revealed (t14=1.0345, p=0.3185), meaning SICI under optimal ISI did not change after low-frequency rTMS (Figure 1B). Data for optimal ISI were normally distributed as tested by using the Shapiro-Wilk W-test (pre-test, W=0.9413, p=0.3997; post-test, W=0.9251, p=0.2308), which means the distribution pattern of the optimal ISI did not change after rTMS (Figure 1A). The SICI ISI results did not support our hypotheses of a potential change in optimal ISI after low-frequency rTMS.
Figure 1.
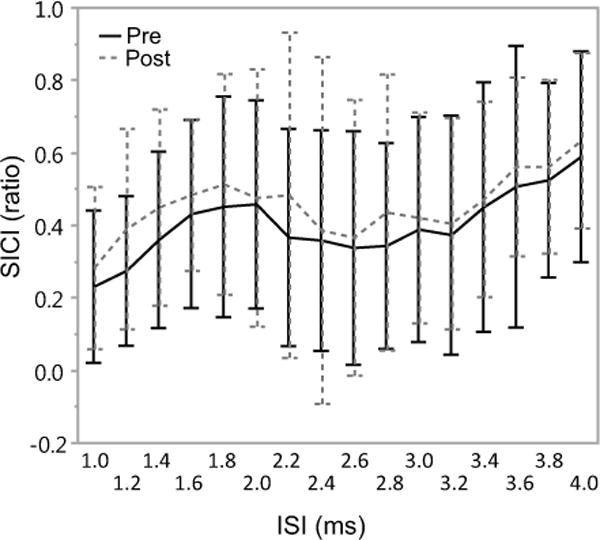
Mean (SD) of short interval intracortical inhibition (SICI) under multiple interstimulus intervals (ISI) before (Pre, solid) and after (Post, dashed) 1-Hz rTMS (N=15). Significant Time effect was revealed (p=0.0001), suggesting a reduction of inhibition after low-frequency rTMS. Post-hoc analysis revealed no significant differences at any individual ISI, indicating that SICI decreased, however, remained the same if measured at a specific ISI after low-frequency rTMS.
The RMANOVA test for SICI under multiple ISIs revealed significant effect of Time (F(224, 1)=15.1207, p=0.0001). No significant effects were observed for the interaction between Time and ISI factors (F(224, 15)=0.3306, p=0.9917). These indicate that after rTMS there was an overall decrease in the SICI, however, there were no differences between pre- and post-tests when SICI was measured at any specific ISI (Figure 2).
Figure 2.
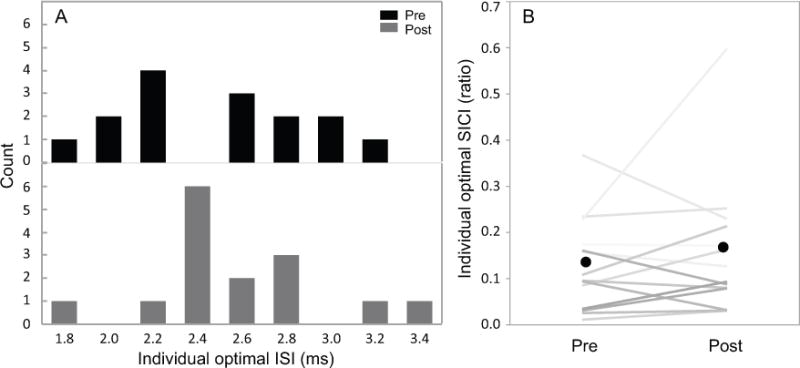
Individual optimal interstimulus interval (ISI) comparison before (Pre) and after (Post) 1-Hz rTMS (N=15). A: Optimal ISI values distribution. B: Short interval intracortical inhibition (SICI) values under optimal ISI (grade represents individual participant). Black dots represent the mean SICI values. No difference in the individual optimal ISI distribution pattern between pre- and post-tests was observed and no SICI difference was revealed as measured by using individual optimal ISI. These findings indicate that SICI responses remained unchanged after 1-Hz rTMS.
Paired t-test revealed significantly longer cSP duration at post-test as compared to pre-test (t14=4.0092, p=0.0015), indicating increased inhibition induced by rTMS. Paired t-test for single pulse responses showed no differences between pre- and post-tests (t14=−0.0105, p=0.9917), suggesting the corticospinal tract excitability did not change.
Experiment II
Data for the optimal CSI2.5 and CSIOptISI were normally distributed as tested by using Shapiro-Wilk W-test (CSI2.5, pre-test, W=0.9106, p=0.1381; CSI2.5, post-test, W=0.9234, p=0.2171; CSIOptISI, pre-test, W=0.8541, p=0.0826; CSIOptISI, post-test, W=0.8467, p=0.0686), meaning the distribution pattern of the optimal CSI did not change after low-frequency rTMS (Figure 3A). No differences in SICI values under optimal CSI2.5 or CSIOptISI between pre- and post-tests were revealed (CSI2.5, t14=0.4524, p=0.6579; CSIOptISI, t8=0.0612, p=0.9527), indicating that SICI under optimal CSI value did not change after low-frequency rTMS (Figure 3B). The SICI CSI results did not support our hypotheses.
Figure 3.
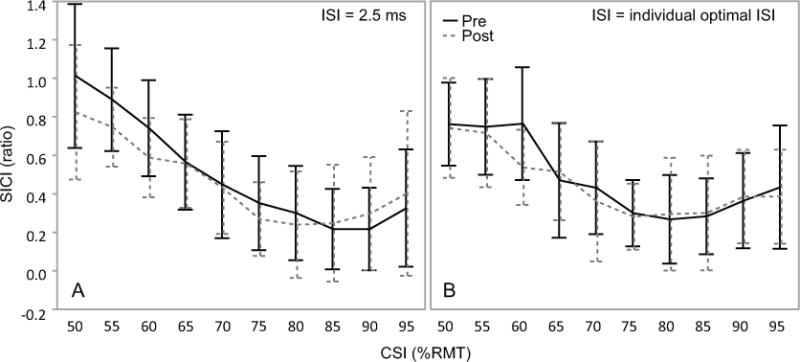
Mean (SD) of short interval intracortical inhibition (SICI) under multiple conditioning stimulus intensities (CSI) responses before (Pre, solid) and after (Post, dashed) 1-Hz rTMS. A: SICI responses measured with an ISI of 2.5ms (N=15). B: SICI responses measured with the optimal ISI as tested in Experiment I (N=9). ISI: interstimulus interval. No significant effect was revealed by RMANOVA test with either CSI2.5 or CSIOptISI. These findings indicate that SICI responses remained the same as measured by either the whole CSI range or at a specific CSI after 1-Hz rTMS.
RMANOVA test for SICI under multiple CSI2.5 revealed no significant effect of Time (F(140, 1)=3.6684, p=0.5750) or the interaction between Time and CSI factors (F(140, 15)=1.5331, p=0.1419). RMANOVA test for SICI under multiple CSIOptISI revealed no significant effect of Time (F(80, 1)=1.3649, p=0.2462) or the interaction between Time and CSI factors (F(80, 9)=0.8749, p=0.5511) (Figure 4). These results indicate that SICI remained the same as measured by CSI curve both with the ISI of 2.5 ms and the individual optimal ISI after low-frequency rTMS.
Figure 4.
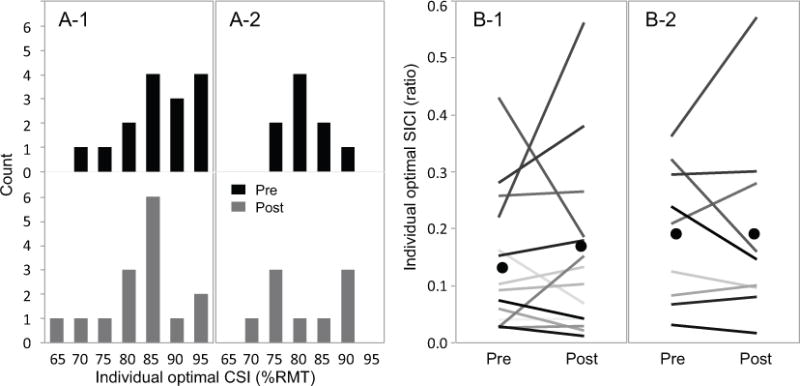
Individual optimal conditioning intensity (CSI) comparison before (Pre) and after (Post) 1-Hz rTMS. A: optimal CSI values distribution. A-1: optimal CSI2.5 (N=15) at pre-test (black) and post-test (grey). A-2: optimal CSIOptISI (N=9) distribution. B: short interval intracortical inhibition (SICI) values under optimal CSI. B-1: individual SICI values (grade represents individual participant) at optimal CSI2.5. B-2: individual SICI values (grade represents individual participant) at optimal CSIOptISI. Black dots represent mean SICI values. CSI2.5: SICI tested under multiple conditioning intensities with the ISI of 2.5 ms. CSIOptISI: SICI tested under multiple conditioning intensities with the individual optimal ISI tested in Experiment I. No difference in the individual optimal CSI distribution pattern between pre- and post-tests were observed and no SICI response difference was revealed as measured by using individual optimal CSI. These findings indicate that SICI responses remained unchanged after 1-Hz rTMS.
Paired t-test revealed significant longer cSP duration at post-test as compared to pre-test (t14=4.4930, p=0.0001), indicating increased inhibition induced by rTMS. Paired t-test for single pulse MEP amplitude showed no differences between pre- and post-tests (t14=−1.6819, p=0.1033), suggesting the corticospinal excitability did not change.
Discussion
The aim of this study was to investigate if the previously reported variability of SICI responses to low-frequency rTMS is explained by ISI and/or the CSI parameters. To our knowledge, this is the first study that systematically evaluated SICI responses to low-frequency rTMS using multiple ISIs and CSIs and individual optimal parameter analysis. The results did not reveal any shift of the optimal ISI or CSI, nor any changes of SICI measured under the optimal ISI/CSI. However, the SICI response under multiple ISIs showed an overall significant effect of Time, indicating a decrease in overall inhibition of cortical excitability after low-frequency rTMS, although, post-hoc analysis did not reveal significant differences in SICI at any individual ISI (Experiment I). Additionally, no significant differences were observed in multiple CSI testing (Experiment II). Although the primary findings did not support our hypotheses, the contrasting findings between the ISI (significant Time effect) and CSI (non-significant Time effect) experiments deserve further discussion.
Short interval intracortical inhibition
Interstimulus interval
The significant Time effect indicated a general decrease in inhibition after low-frequency rTMS, but there was no significant difference at any specific ISI. Previous studies testing SICI at uniform ISI settings for each participant have reported similar results that suggest SICI is unchanged after neuromodulation13–17. The contrast between the results tested under multiple ISIs and at a specific ISI may be due to the high inter-subject variability of SICI and/or sample size4. When SICI testing was completed at a specific ISI with a smaller sample size (i.e. N=15), SICI was not sensitive enough to reveal neuromodulation after effects. However, when measured with all ISIs collapsed together, the sample size greatly increased (i.e. N=240, with ISIs treated as subjects). This large increase in sample size compensated for the variability in SICI responses and produced a significant effect. However, it is important to consider that the time required for such a large sample may render SICI impractical to examine neuromodulation effects in many experiments.
The finding of decreased SICI is unexpected and, to some degree, counter intuitive because low-frequency rTMS is an inhibitory intervention as shown by other measures. A potential explanation is a floor effect if SICI is already at a maximally inhibited state, it may not be physiologically possible to be further inhibited. If that is true, then the only possibilities are to be unchanged or decreased. Another potential explanation is that rTMS may suppress the GABAA mediated inhibitory process. Other methodological reasons may also contribute, such as re-thresholding13,17 at post-test vs not14–16. This unexpected decreased in SICI after rTMS is, however, consistent with many other reports19–21. The mechanism of this phenomenon is outside the scope of this work, but is worthy of further investigation.
In our findings, SICI assessed under multiple ISIs showed a two-phasic inhibitory pattern with the average optimal ISI in the second inhibitory phase of 2.5 - 2.6 ms (Figure 1), consistent with previous reports29. The optimal ISI for participants remained stable during the study, suggesting that SICI may not be affected by low-frequency rTMS in terms of the timing of inhibitory signaling. It has been reported that the timing of the ISI is related to I-wave facilitation5. Based on this, we speculated that rTMS may modulate the SICI optimal ISI by altering the I-wave phasic timing, however, this was not supported by the findings.
Conditioning stimulus intensity
After low-frequency rTMS, SICI responses under multiple CSIs did not change and optimal CSI did not shift. Lack of change in SICI was also reported in previous work13–17. It has been hypothesized that lower CSI values may excite some pyramidal tract neurons and render them partially refractory to the test pulse, leading to an inhibited MEP response30,31; higher CSI values (>85% of RMT) are proposed to be within the transition range between SICI and ICF6. Considering these data from previous studies, we initially speculated that 1-Hz rTMS would modulate optimal CSI via the dynamic between the low and high end CSIs. However, this hypothesis was not supported by the findings. It is noteworthy that the effects of changing the intensity of the testing pulse were not tested in the current study.
It is interesting that ISI and CSI group effects were different. It appears that the ISI effect is due to a consistent effect of decreased inhibition (although not statistically significant) across all ISIs (note, Figure 2). In contrast, the CSIs did not have a consistent response across CSIs as illustrated in Figure 4-A. At the lower intensities, there was an inhibited response at post-test which switched to a less inhibited response at 90% CSI. This contrast may be due to the different effects ISI and CSI have on SICI. In the ISI Experiment (I), the CSI was a fixed value for multiple ISIs; in the CSI Experiment (II), the ISI was a fixed value for multiple CSIs. Modulating ISI with a fixed CSI induces a more consistent SICI response across all ISI’s tested, allowing a group effect to be observed. In contrast, modulating CSI with a fixed ISI does not produce a consistent SICI response across the tested CSIs, thus eliminating potential group effects. The physiologic mechanisms behind this response are beyond the scope of this study, but worthy of future investigation.
Cortical silent period and single pulse
cSP and single pulse measures were included to provide additional evidence that the rTMS induced neuromodulatory effects in the motor cortex. The duration of cSP was significantly increased after rTMS indicating an increased inhibition within the cortical neuronal circuits32 and demonstrating the effectiveness of the rTMS in inducing inhibition. Similar findings have been reported in numerous studies17,19,21,33. Single pulse response modulation was not observed after low-frequency rTMS which was unexpected, however consistent with many previous reports13,34,35.
The contrast between cSP and single pulse responses may be due to the differences in the mechanisms of the two measures. The single pulse MEP amplitude is an excitatory transynaptic response to TMS which can be affected by several variables such as pre-stimulus muscle activity12 and degree of attention36, thus the lack of changes are likely due to the variability both within and between subjects. This explanation is supported by the lack of significant change in either 1-mV threshold or RMT after rTMS. In contrast, cSP is a reliable and consistent measure that can reflect the inhibitory neuromodulation effects induced by low-frequency rTMS19,21, which reflects the inhibitory postsynaptic potentials mainly modulated by GABAB receptor mediated mechanisms1,37. The increased or prolonged cSP duration provides evidence that rTMS modulated inhibitory pathways in the motor cortex. Given that there was no sham rTMS included in the study design, it is also possible that the significant Time effects of the cSP and SICI under multiple ISIs were due to repeat testing or a general effect associated with the long-lasting protocol (~3 hours).
Limitations
The study design did not include sham rTMS as a control condition. This is due to the time needed to conduct the study. However, this limitation was partially reduced by the inclusion of two other measures (single pulse and cSP); whereby the increased cSP duration at post-test indicated the effectiveness of low-frequency rTMS to modulate cortical inhibition.
There was only one post-test included in the study, all measures were tested immediately after rTMS. This means no information regarding longitudinal effects were obtained. Delayed neuromodulatory effects have been reported by other studies with multiple post-tests. However, considering the data amount and time required in the SICI protocol adopted in this work, we did not include multiple post-tests. This can be done in future studies when a simplified SICI protocol is established.
Conclusions
The group effect in the ISI curve found here indicates that SICI can reflect low-frequency rTMS neuromodulatory effects when measured under multiple ISIs, but this modulatory effect is not reflected by the changes in optimal ISI or CSI, and is opposite of the anticipated response to inhibitory neuromodulation. Overall, the findings reported indicate that SICI, as typically measured using one set of parameters with 10-20 repetitions, is not effective at measuring changes in inhibition. What may be required to demonstrate changes after neuromodulation is a wide range of ISI values and hundreds of trials. Further, the contrasting results between SICI ISI curve and cSP indicate that rTMS may modulate GABAA and GABAB mediated inhibitory activity in different ways. These findings provide a comprehensive assessment of SICI response to 1Hz rTMS that may be used to guide future studies that evaluate the complex influence of neuromodulation on corticospinal, intracortical and interhemispheric excitability.
Acknowledgments
We acknowledge Erin Horwath, Jackie Jaspers, Jon Heimdal, Kory Lutz, Laura Gengler, Taylor Hilbrant and Katrina Olson for their help with the data collection.
Funding support: This work was partly supported by National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114, the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative, and São Paulo Foundation (Process nº 2015/16744-4).
Footnotes
Authorship Statement: Mo Chen and Teresa Kimberley designed the study. Mo Chen conducted the study, including patient recruitment, data collection, data processing and data analysis. Maira C. Lixandrao helped with patient recruitment, data collection and data processing. Mo Chen prepared the manuscript draft with important intellectual input from Maira C. Lixandrao, Cecilia N. Prudente, Rebekah LS Summers and Teresa J. Kimberley. All authors approved the final manuscript. Teresa J. Kimberley provided funding for the study. Mo Chen analyzed the data with input from Maira Lixandrao, Cecilia N. Prudente, Rebekah LS Summers and Teresa J. Kimberley. Mo Chen, Maira Lixandrao, Cecilia N. Prudente, Rebekah LS Summers and Teresa J. Kimberley had complete access to the study data.
Conflict of Interest: The authors report no conflicts of interest.
References
- 1.Ziemann U, Lönnecker S, Steinhoff B, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109(1):127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 2.Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545(1):153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothwell JC, Day BL, Thompson PD, Kujirai T. Short latency intracortical inhibition: one of the most popular tools in human motor neurophysiology. J Physiol. 2009;587(1):11–12. doi: 10.1113/jphysiol.2008.162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du X, Summerfelt A, Chiappelli J, Holcomb HH, Hong LE. Individualized Brain Inhibition and Excitation Profile in Response to Paired-Pulse TMS. J Mot Behav. 2014;46(1):39–48. doi: 10.1080/00222895.2013.850401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peurala SH, JF M-D, Arai N, et al. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119(10):2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 6.MacKinnon CD, Gilley EA, Weis-McNulty A, Simuni T. Pathways mediating abnormal intracortical inhibition in Parkinson’s disease. Ann Neurol. 2005;58(4):516–524. doi: 10.1002/ana.20599. [DOI] [PubMed] [Google Scholar]
- 7.Ni Z, Chen R. Short-interval intracortical inhibition: A complex measure. Clin Neurophysiol. 2008;119(10):2175–2176. doi: 10.1016/j.clinph.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549–1560. doi: 10.2147/NDT.S67477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoogendam JM, Ramakers GMJ, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3(2):95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Lepping P, Schönfeldt-Lecuona C, Sambhi RS, et al. A systematic review of the clinical relevance of repetitive transcranial magnetic stimulation. Acta Psychiatr Scand. 2014;130(5):326–341. doi: 10.1111/acps.12276. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol. 1994;481(Pt 1):243–250. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 13.Daskalakis Z, Möller B, Christensen BKB, et al. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174(3):403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald PB, Brown TL, Daskalakis ZJ. The application of transcranial magnetic stimulation in psychiatry and neurosciences research. Acta Psychiatr Scand. 2002;105(5):324–340. doi: 10.1034/j.1600-0447.2002.1r179.x. [DOI] [PubMed] [Google Scholar]
- 15.Brighina F, Giglia G, Scalia S, Francolini M, Palermo A, Fierro B. Facilitatory effects of 1 Hz rTMS in motor cortex of patients affected by migraine with aura. 2005;161(1):34–38. doi: 10.1007/s00221-004-2042-7. [DOI] [PubMed] [Google Scholar]
- 16.Romero J, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clinical. 2002;113(1):101–107. doi: 10.1016/S1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- 17.Stinear C, Byblow W. Impaired modulation of corticospinal excitability following subthreshold rTMS in focal hand dystonia. 2004;23(3-4):527–538. doi: 10.1016/j.humov.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Khedr EM, Gilio F, Rothwell J. Effects of low frequency and low intensity repetitive paired pulse stimulation of the primary motor cortex. Clin Neurophysiol. 2004;115(6):1259–1263. doi: 10.1016/j.clinph.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Deng H, Schmidt RL, Kimberley TJ. Low-Frequency Repetitive Transcranial Magnetic Stimulation Targeted to Premotor Cortex Followed by Primary Motor Cortex Modulates Excitability Differently Than Premotor Cortex or Primary Motor Cortex Stimulation Alone. Neuromodulation Technol Neural Interface. 2015;18(8):678–685. doi: 10.1111/ner.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modugno N, Currà A, Conte A, et al. Depressed intracortical inhibition after long trains of subthreshold repetitive magnetic stimuli at low frequency. Clin Neurophysiol. 2003;114(12):2416–2422. doi: 10.1016/S1388-2457(03)00262-1. [DOI] [PubMed] [Google Scholar]
- 21.Summers RLS, Chen M, Kimberley TJ. Corticospinal excitability measurements using transcranial magnetic stimulation are valid with intramuscular electromyography. In: Yuan T, editor. PLoS One. 2. Vol. 12. 2017. p. e0172152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association. World Medical Association Declaration of Helsinki. JAMA. 2013;310(20):2191. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 23.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borich M, Arora S, Kimberley TJ. Lasting effects of repeated rTMS application in focal hand dystonia. Restor Neurol Neurosci. 2009;27(1):55–65. doi: 10.3233/RNN-2009-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimberley TJ, Schmidt RLS, Chen M, Dykstra DD, Buetefisch CM. Mixed effectiveness of rTMS and retraining in the treatment of focal hand dystonia. Front Hum Neurosci. 2015;9:385. doi: 10.3389/fnhum.2015.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samargia S, Schmidt R, Kimberley TJ. Shortened Cortical Silent Period in Adductor Spasmodic Dysphonia: Evidence for Widespread Cortical Excitability. 2014;560 doi: 10.1016/j.neulet.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefaucheur J, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125(11):2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151(3):330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- 30.Di Lazzaro V, Restuccia D, Oliviero A, et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119(2):265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- 31.Di Lazzaro V, Restuccia D, Oliviero A, et al. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998;508(2):625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romeo S, Gilio F, Pedace F, et al. Changes in the cortical silent period after repetitive magnetic stimulation of cortical motor areas. Exp brain Res. 2000;135(4):504–510. doi: 10.1007/s002210000. [DOI] [PubMed] [Google Scholar]
- 34.Gerschlager W, Siebner H, Rothwell J. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57(3):449–455. doi: 10.1212/WNL.57.3.449. [DOI] [PubMed] [Google Scholar]
- 35.Inghilleri M, Conte A, Frasca V, Scaldaferri N. Altered response to rTMS in patients with Alzheimer’s disease. Clinical. 2006;117(1):103–109. doi: 10.1016/j.clinph.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92(1):66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- 37.Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21(9):1209–1212. doi: 10.1002/(SICI)1097-4598(199809)21:9<1209::AID-MUS15>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]


