Abstract
Prior research suggests that episodic memory can guide value-based decisions when single episodes are encoded in relation to the specific reward-context in which they were experienced. The current experiments examine the role that a flexible recombination-related retrieval mechanism that allows one to link together distinct events plays in the misattribution of specific reward-contexts across distinct episodes. To determine whether the same recombination-related retrieval mechanism supports both successful inference and transfer of reward-context across episodes, we developed a modified version of an associative inference paradigm in which participants encoded overlapping associations (AB, BC) that could later be linked to support inferential retrieval (AC), where one element (‘A’) was tied to reward. Our key experimental manipulation concerned whether value memory (Experiments 1 and 2) or decision bias tests (Experiment 3) were probed before or after the associative inference test, thereby allowing us to assess whether false value transfer and decision bias scores increased after as compared to before successful versus unsuccessful inference. Results revealed that participants more frequently misattributed the specific reward-context (‘A’) to unrewarded items (‘C;’ Experiments 1 and 2) and showed higher decision bias scores when asked to choose between two previously unrewarded items (‘C;’ Experiment 3) for successful compared with unsuccessful inference, but only when the value memory and decision bias tests were given after the associative inference test. These results suggest that a recombination-related retrieval mechanism that supports successful inference also contributes to the misattribution of reward-context in memory and further biases participants’ novel value-based decisions.
Keywords: inference, false memory, value, decision-making, episodic memory
Decisions are often guided by memory for past experiences. If a past choice led to a positive outcome, people are more likely to make that same choice again. Recent research in the field of value-based decision-making has examined this link between choice and memory (Doll, Shohamy & Daw, 2015; Duncan & Shohamy, 2016; Palombo, Keane & Verfaellie, 2015; Wimmer & Shohamy, 2012). In these studies, participants learned stimulus-reward relationships gradually within the context of hundreds of repeated experiences. Because participants were exposed to many repeated trials over which they were able to incrementally learn the stimulus-reward association, it is difficult to determine exactly how specific valued events affect the interaction of episodic memory and future value-based choices. In everyday life, individuals often are not presented with multiple repetitions of past experiences prior to making decisions. Rather, individuals commonly make decisions based on limited prior information, where they may have only experienced the decision-relevant episode once. In contexts where the decision-relevant episode has only been experienced once, value-based choice has been proposed to depend on a mechanism that flexibly samples specific reward-related representations stored in memory (Bornstein, Khaw, Shohamy & Daw, 2017; Bornstein & Norman, 2017; Gluth, Sommer, Reiskamp & Büchel, 2015; Murty, FeldmanHall, Hunter, Phelps & Davachi, 2016; Wimmer & Büchel, 2016).
Such proposals linking episodic memory and value-based decision-making require that single episodes be encoded in relation to the specific reward-context in which they occurred (Gluth, Sommer, Reiskamp & Büchel, 2015; Murty et al., 2016; Wimmer & Büchel, 2016). Recent behavioral experiments have found that (1) participants are indeed able to remember specific associations between an item and its reward-context and (2) memory for these detailed item-reward-context associations guide value-based decisions, particularly in situations where the participant decides between two options that they have never previously considered in relation to one another (e.g., novel choice between a previously experienced option and a new, never before experienced option; Murty et al., 2016; Wimmer & Büchel, 2016). For example, when participants were asked to select between a lottery that was previously associated with either a high or low reward outcome (i.e., ‘old’ lottery) and a new lottery during a novel decision-making task, adaptive decisions were identified as decisions where participants selected ‘old’ lotteries that were associated with high-reward outcomes or avoided ‘old’ lotteries that were associated with low-reward outcomes, such that these decisions increased the likelihood that participants would receive a high-value reward (Murty et al., 2016). Importantly, the likelihood that participants made these adaptive decisions (i.e., chose high-value lotteries and avoided low-value lotteries) depended on their memory for the association between an ‘old’ lottery and its specific reward-context (i.e., whether the lottery was associated with a high- or low-value outcome), rather than item memory, where participants remember individual features of a lottery without retrieving any details of the context in which the lottery was previously experienced (Murty et al., 2016). In sum, these results suggest that at the time of the decision, participants retrieve rich associative memories consisting of relationships between specific features of a previous experience, such that they are able to remember the specific lottery (item) and its associated reward-context (source), thus providing participants with a detailed representation of a single past experience that they can flexibly apply to guide subsequent value-based choice (Murty et al., 2016).
Previous research suggests that such novel decisions (i.e., decisions between two options that have never been considered in relation to one another), based on single past experiences, may be supported by two key episodic memory mechanisms. The first is reward-related learning, where the relationship between the anticipated reward (i.e., reward-context) and the currently presented cue (i.e., the lottery) is encoded, thus linking a specific item to its potential future value (Murty et al., 2016; Wimmer & Büchel, 2016; Wimmer & Shohamy, 2012; Wolosin, Zeithamova & Preston, 2012, 2013). The second is a flexible recombination mechanism that has been previously linked with episodic simulation of hypothetical experiences (Schacter & Addis 2007a, 2007b), where participants either simulate possible future scenarios that might occur as the result of a choice, or simulate alternative scenarios of what might have occurred as a result of having chosen differently in the past (for review, see Schacter, Benoit, De Brigard & Szpunar, 2015). Let us consider each mechanism in turn.
With respect to reward-related learning, specific reward-contexts presented during encoding affect explicit measures of memory for the source of the information, such that participants are able to remember the specific value associated with an item or episode (i.e., whether items were no-, low-, or high-value; Murty et al., 2016; Wimmer & Büchel, 2016). Consistent with this behavioral finding, research suggests that distributed patterns of activation within the hippocampus may reflect the specific value of the reward-context associated with each individual event, such that representations within the hippocampus differentiate between low- and high-value reward-contexts (Wolosin, Zeithamova & Preston, 2013). Reward-context can also affect implicit measures of value-based decision bias, where participants’ financial risk-taking or choice of a familiar lottery increased (as compared to a new lottery) when they were primed with the previously learned high-value associations (Wimmer & Büchel, 2016), as well as when they could explicitly remember such associations (Murty et al., 2016). Thus, representations of previously learned associations include specific information identifying the reward-context in which the information was encoded (Wolosin, et al., 2013) and further guide future value-based decision-making (Murty et al., 2016; Wimmer & Büchel, 2016).
Flexible recombination processes support our ability to link together related information acquired in distinct episodes in order to construct simulations of novel future events (Schacter & Addis, 2007a, 2007b). We frequently encounter and simulate possible future scenarios in which we are required to make a choice between two options that we have never previously considered. In such instances of novel decision-making, flexible recombination during retrieval may play a particularly adaptive role, allowing one to recombine elements of past experiences in order to simulate the prospective scenario (Schacter, 2012; Schacter & Addis, 2007a, 2007b). This simulated mental representation can then guide future-oriented or novel decisions by allowing one to predict the expected value associated with the decision (Benoit, Gilbert &Burgess, 2011) and further allows one to link the prospective scenario to past and expected future rewards, allowing for an immediate experience of the future decision’s affective value (Boyer, 2008). This simulated mental representation can then guide future-oriented or novel decisions (Benoit, Gilbert &Burgess, 2011; Schacter et al., 2015; Schacter, Benoit, & Szpunar, 2017).
This kind of flexible recombination that allows one to simulate future events is quite similar to the flexible recombination processes examined in studies of associative inference that allow one to link together related information acquired in distinct episodes in order to make novel connections that have not been directly experienced. In previous studies utilizing the associative inference paradigm, which requires participants to reactivate and flexibly recombine elements of overlapping episodes, participants learned direct associations between two items (e.g., individual ‘A’ and child ‘B’) and then learned overlapping associations between a member of the previously studied pair and a new item (e.g., child ‘B’ and individual ‘C;’ for evidence and review see Carpenter & Schacter, 2017; Schlichting & Preston, 2015; Zeithamova & Preston, 2010; Zeithamova, Schlichting, & Preston, 2012). Participants were also instructed to learn the indirect relationships between the ‘A’ and ‘C’ items that are mediated by the item ‘B’ (i.e., associative inference). Later, participants completed a memory test for both the directly learned associations (AB and BC) and the associative inferences (AC). Flexibly recombining and linking related information acquired in distinct episodes allows for novel connections that have not been directly experienced. For example, if one sees two different individuals (‘A’ and ‘C’) walking with the same child (‘B’) on two different days, retrieving and recombining details of the two episodes allows one to infer that the two individuals are related in some way by their relationship with the child. Further, if you have had a positive past experience with the first individual who was with the child but no prior experience with the second individual, retrieving and recombining details of these two episodes not only allows you to infer the relationship between the two individuals, but may transfer affective value associated with the first individual to your mental representation of the second individual, thereby updating the associated reward-context and perhaps leading to a choice to interact with the second individual. Reward-related learning in concert with such flexible recombination processes during retrieval could provide a mechanism by which previously rewarded associations can systematically update the value of unrewarded items from distinct yet related episodes. That is, unrewarded items may gain a positive value simply by way of flexibly recombining elements of previously separate experiences, which may further bias novel value-based decisions (for an example of simulation-based recombination see Benoit, Szpunar & Schacter, 2014).
Recent work from Martinez, Mack, Gelman and Preston (2016) provides evidence for the role of memory-guided decision bias in such social interactions. Specifically, results revealed that the reactivation of prior memories (e.g., remembering that individual ‘A’ cooperates) during new learning (e.g., learning that individual ‘A’ is friends with individual ‘B’) allowed for the transfer of social reputation from individual ‘A’ to the associated individual ‘B,’ such that if individual ‘A’ cooperates then individual ‘B’ was treated as if they also cooperate (Martinez et al., 2016). That is, memories of past interactions and learned social connections biased participants’ decisions to interact with a member of a shared social group, even when they had not interacted with that particular group member in the past.
Indeed, a related line of research suggests that false ‘spreading’ or transfer of value can occur when a rewarded item (‘A’) was previously paired with an unrewarded item (‘B’), resulting in a preference for item ‘B’ even though it was never directly linked to receipt of the reward (Wimmer & Shohamy, 2012). Importantly, items ‘A’ and ‘B’ were originally learned in the same context (i.e., similar to how participants learned the direct relationship between ‘A’ and ‘B’ during the associative inference paradigm) and thus did not require participants to flexibly recombine elements of distinct episodes in order to learn the relationship between the two items, or to simulate the outcomes of possible future choices. Therefore, results suggest that value can be transferred within associative pairings, as participants showed a greater decision bias for unrewarded items that were directly paired with a subsequently rewarded item as a result of reactivating the previously learned A-B association during the reward-learning phase (Wimmer & Shohamy, 2012). That is, the reward-context associated with a specific item can be spread or transferred to a related item that was presented within a single reactivated episode.
However, it is currently unknown whether the same flexible recombination processes that allow for novel associative inference, where the relationship between two items (‘A’ and ‘C’) is mediated by a third item (‘B’), also support the biased updating of specific value representations across distinct contexts when episodes are only experienced once. Consistent with this possibility, in recent experiments using a modified associative inference paradigm, we directly linked such flexible recombination during retrieval to source memory errors: details of an overlapping scene were mistakenly attributed to memory for the original scene as a consequence of flexible recombination processes that support successful associative inference (Carpenter & Schacter, 2017). That is, participants were more prone to false memories that resulted from mistakenly combining scene details from related episodes when they made successful (versus unsuccessful) inferences about the relations between these episodes, but only when details were probed after (versus before) the associative inference test.
Similar to the way in which contextual scene details are mistakenly transferred across distinct past experiences following flexible retrieval supporting associative inference, we suggest that the specific reward-context of an original event may be misattributed to memory of an unrewarded overlapping event. If so, we would also predict that following successful inference, when participants are confronted with a novel decision between two previously unrewarded items, they will be biased to choose the unrewarded item that was linked indirectly to a rewarded item learned in a distinct context.
While our previous research implicates the role of recombination-related retrieval processes on subsequent source memory errors, there are two ways that participants can perform successfully on an associative inference test (Carpenter & Schacter, 2017). First, participants may integrate the AB and BC representations during encoding such that an integrated representation (ABC) is later retrieved during the test (i.e., integrative encoding; e.g., Shohamy & Wagner, 2008). Alternatively, participants may flexibly retrieve and recombine the previously studied AB and BC pairs during the associative inference test (i.e., recombination processes during retrieval; Carpenter & Schacter, 2017; Zeithamova & Preston, 2010). Prior neuroimaging studies suggest that both integrative encoding and recombination processes during retrieval play a role in successful associative inference (Zeithamova, Dominick & Preston, 2012; Zeithamova & Preston, 2010).
To directly test the role of flexible recombination processes in reward-related learning and subsequent novel decision-making, we used a modified version of an associative inference task that targets flexible recombination (Carpenter & Schacter, 2017; Preston, Shrager, Dudukovic, & Gabrieli, 2004; Zeithamova et al., 2012; Zeithamova & Preston, 2010) and, critically, incorporates aspects of the monetary incentive encoding task that targets reward-related learning mechanisms (Adcock, Thangavel, Whitfield-Gabrieli, Knutson & Gabrieli, 2006; Wimmer & Shohamy, 2012).
In our version of the associative inference paradigm, during an initial session participants study person-object associations (AB) where the ‘A’ item is either linked to a value or no-value reward-context (Experiment 1) or to a high-value or low-value reward-context (Experiment 2). Participants then study overlapping person-object associations (BC) where the ‘C’ item is either linked to a no-value or value reward-context, respectively. Participants are instructed to learn both the direct associations between each person and object (AB and BC) and the indirect association between the two people based on the shared object (AC). Additionally, participants are instructed to learn the reward-context associated with each item (i.e., value or no-value). After a delay, participants return for a second session in which they are tested for direct associations (AB, BC) and perform an associative inference test for novel combinations that are linked via the ‘B’ item (AC; see Figure 1).
Figure 1.
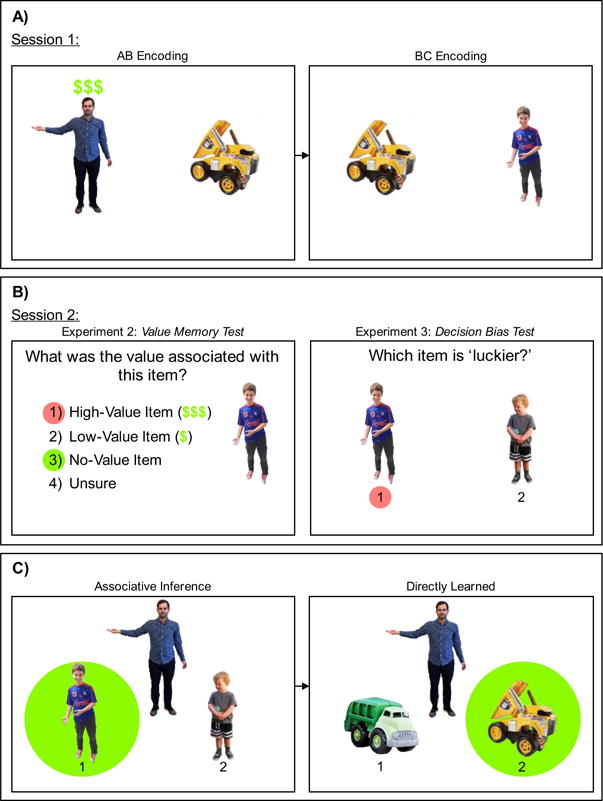
Illustration of materials, stimuli, and test displays from Experiments 2 and 3. (A) The Session 1 section shows one example of an AB image in which the man is item ‘A’ and the toy truck is item ‘B’ and the corresponding BC images in which the boy is item ‘C.’ Only the ‘A’ item (man) is identified as a high-value item. (B) The Session 2 section shows the Value Memory Test used in Experiment 2 (Experiment 1: 1) Valued Item ($$$), 2) No-Value Item and 3) Unsure) and the Decision Bias Test used in Experiment 3. For each value memory question participants saw a cutout of an item (A, B or C) presented to the right of the question in order to indicate which event the question referred to. In Experiments 1 and 2, false value transfer occurred when participants chose the specific value (i.e., Experiment 1: valued; Experiment 2: high- or low-value) that was associated with the ‘A’ (or ‘C’) individual and attributed this value to the unrewarded ‘C’ (or ‘A’) individual – as indicated by the red (dark grey) circles. True memories occurred when participants correctly indicated that the unrewarded ‘C’ individual was indeed a “no-value” item – as indicated by the green (light grey) circles. In Experiment 3, participants were instructed to choose between two unrewarded ‘C’ items. One of the unrewarded ‘C’ items was indirectly linked to a high-value item (i.e., unrewarded high-value) whereas the other item was indirectly linked to a low-value item (i.e., unrewarded low-value). False decision bias occurred when participants chose the unrewarded high-value item as the “luckier” item meaning this item was more likely to lead to a high-value reward at the end of the second session – as indicated by the red (dark grey) circle. No decision bias occurred when participants equally chose the unrewarded high- and low-value items as the “luckier” item. (C) The green (light grey) circles indicate the correct answer for the associative inference and directly learned questions. Participants saw these images without the red (dark grey) and green (light grey) circles. Panel C of Figure 1 is reprinted from Carpenter, A.C. & Schacter, D.L. (2017). Flexible retrieval: When true inferences produce false memories. Journal of Experimental Psychology: Learning, Memory & Cognition, 43(3), 335–349. Reprinted with permission of the American Psychological Association.
In order to test whether retrieval-related recombination processes underlying successful inference contribute to the transfer of positive value across event boundaries to previously unrewarded items, in Experiments 1 and 2 participants’ memory for the specific value of each item (i.e., ‘A,’ ‘B’ and ‘C’) is explicitly probed. For one half of the AB and BC pairs, explicit value memory tests, where participants are asked to remember the specific reward-context associated with a previously learned item (i.e., Experiment 1: value or no-value; Experiment 2: high-value, low-value, no-value), are given before the test of direct (AB, BC) and indirect (AC) associations, and for the other half, the value memory tests are given after the tests of direct and indirect associations.
The critical comparisons concern the proportions of what we call false value transfer (e.g., when participants attributed the specific reward-context of previously rewarded item ‘A’ to their memory of unrewarded item ‘C,’ thus remembering item ‘C’ as having been rewarded) on the value memory test given before versus after the associative inference test, for correct as compared to incorrect associative inference trials (i.e., AC). We distinguish among three competing hypotheses:
If recombination during retrieval both enhances associative inference performance and increases false value transfer, then false value transfer scores should be higher for correct than incorrect inference trials, but only when the value memory test is given after the associative inference test (during which recombination occurs); there should be no difference in the proportion of false value transfer scores for correct vs. incorrect inference trials when the value memory test is given before the associative inference test.
If false value transfer scores are higher for correct as compared to incorrect inference trials when the value memory tests are given both before and after the associative inference test, then these effects are consistent with integrative encoding processes.
If there is no link at all between recombination during retrieval and false value transfer then there should be no difference between the proportion of false value transfer scores for correct and incorrect inference trials regardless of when the value memory tests are given.
To test these hypotheses, we conducted three experiments that used the same basic paradigm and differed only in whether false value transfer was assessed with respect to explicit value memory using a value memory test (Experiments 1 and 2) or implicit decision bias using a decision bias test (Experiment 3; see figure 2 for a visualization of the predicted patterns of results for each hypothesis).
Figure 2.
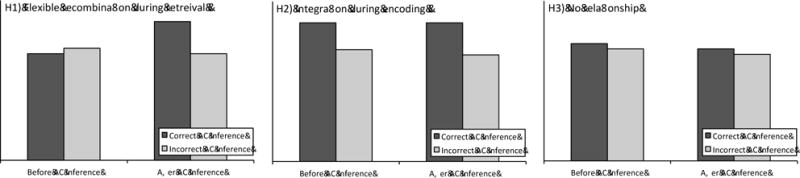
Schematic depiction of predicted false value transfer (Experiments 1 and 2) and decision bias (Experiment 3) results for each hypothesis. (H1) If flexible recombination during retrieval both enhances associative inference performance and increases false value transfer (or decision bias), then false value transfer (or decision bias) scores should be higher for correct than incorrect inference trials, but only when the value memory (or decision bias) test is given after the associative inference test (during which recombination occurs); there should be no difference in the proportion of false value transfer (or decision bias) scores for correct vs. incorrect inference trials when the value memory test is given before the associative inference test. (H2) If integration during encoding supports both successful associative inference and increases false value transfer (or decision bias), then false value transfer (or decision bias) scores should higher for correct as compared to incorrect inference trials when the value memory (or decision bias) tests are given both before and after the associative inference test. (H3) If there is no link at all between recombination during retrieval or integration during encoding and false value transfer (or decision bias) then there should be no difference between the proportion of false value transfer (or decision bias) scores for correct and incorrect inference trials regardless of when the value memory (or decision bias) tests are given.
All three experiments provided evidence in favor of the first hypothesis outlined above: both the proportion of false value transfer and decision bias scores were higher for correct than incorrect inference trials, but only when the tests were given after the associative inference test, during which recombination occurs. These findings implicate recombination mechanisms during retrieval in both successful associative inference and false transfer of value across distinct episodes to previously unrewarded items, which further bias novel value-based decisions.
Experiment 1
Methods
Participants
A power analysis (Faul, Erdfelder, Lang & Buchner, 2007) based on effect sizes from our previous related work (Carpenter & Schacter, 2017) for the key predicted effect of interest revealed that a sample size of 20 would provide the ability to detect an effect after as compared to before successful inference conditions with power of >.80. Thus, we aimed for a sample of 24 usable participants. 25 young adults (mean age = 21.20, SD = 2.09; 19 female) were recruited via advertisements at Boston University and Harvard University. All had normal vision and no history of neurological impairment. They gave informed consent, were treated in accordance with guidelines approved by the ethics committee at Harvard University, and received either course credit or pay for completing the study.
Reward-Related AB and BC Encoding
Both experimental sessions were executed on an Apple desktop computer using PsychoPy2 (v1.80.03). Stimuli consisted of 160 pairs of color images depicting people and common objects (e.g., toy truck). 80 total unique ABC triads (80 AB pairs, 80 BC pairs) were constructed. Overlapping AB and BC pairs were constructed such that two individuals (‘A’ and ‘C’) shared an association with an overlapping object (‘B;’ i.e., one ABC triad). Presentation locations were counterbalanced across participants such that each item was presented on the right/left equally often for both AB and BC pairs. Participants received one of two versions of the AB encoding task, which consisted of 80 pairs (i.e., AB) followed by the corresponding BC encoding task, which consisted of 80 pairs (i.e., BC; see Figure 1). Each pair was randomly presented for 5 seconds within each encoding block (i.e., AB encoding and BC encoding; see Figure 1). 40 pairs of the AB encoding task identified the ‘A’ item as a valued item (i.e., $$$ - $2.00 reward), whereas the alternate 40 pairs identified the ‘A’ item as a no-value item (i.e., $0.00 reward). For the 40 no-value AB pairs, the corresponding ‘C’ items during the BC encoding task were identified as valued items. If a triad’s ‘A’ item was associated with a value (i.e., valued-item), the corresponding ‘C’ item was not associated with any value (i.e., no-value item). All value associations were counterbalanced across participants, such that each ‘A’ or ‘C’ item was associated with a value or no-value equally often.
Participants were instructed to learn both the direct associations (i.e., AB, BC) and the indirect associations (i.e., AC) along with whether an item was valued or not. Participants were told that if they were able to remember all of the information that was tied to a valued item (i.e., value information, direct association and indirect association) they would receive this reward at the end of the second session. Importantly, participants were instructed to use the same encoding strategy for valued and no-value pairs (Wolosin et al., 2013) in order to control for the possibility of a strategic tradeoff between learning valued as compared to no-value pairs (Hennessee, Castel & Knowlton, 2017). Following each pair, participants were asked to provide a judgment of learning on a scale from 1 to 4 (1 = definitely forget, 4 = definitely remember). These judgments were collected in order to ensure participants’ attention during the encoding phase.
Value Memory Test
Following a 48-hour delay, participants came to the lab to complete the second session. Three value memory questions were constructed for each of the 80 ABC triads (one question related to each distinct item – ‘A’, ‘B’ or ‘C’). A cutout of the individual (‘A’ or ‘C’) or object (‘B’) was presented to the right of the value memory question in order to indicate to which item the question referred. Participants were asked to explicitly remember whether an item was valued or not valued and were given three possible answer choices: 1) Valued-item ($$$), 2) No-value item, or 3) Unsure. Immediately following participants’ value memory response they were asked to rate their confidence in their response on a scale from 1 to 4 (1 = very unsure, 4 = very sure). The presentation order of the value memory questions was randomized for each participant and the questions were self-paced.
Participants answered the value memory questions for one half of the 80 ABC triads before being tested on the directly learned and associative inference trials. After participants were tested on the directly learned and associative inference trials, they completed the value memory questions for the alternate half of the 80 ABC triads.
Directly Learned and Associative Inference Trials
Following the first half of the value memory questions, participants were tested on directly learned (AB and BC) and associative inference trials (AC). During each directly learned trial, a single cue individual (e.g., an ‘A’ or ‘C’ individual) was presented at the top of the screen and two choice objects were presented at the bottom of the screen (e.g., the correct ‘B’ object pairing and a lure ‘B’ object from a different ABC triad; see Figure 1). On the associative inference trials, a cue individual (‘A’) was presented along with two individuals at the bottom of the screen (i.e., the correct ‘C’ individual from the ABC triad and a lure ‘C’ individual from another triad). Participants were instructed on associative inference trials that the association between the cue (‘A’) and the correct choice (‘C’) was indirect, mediated through an object (‘B’) that shared an association with both the cue and the correct choice during encoding. Participants were instructed to select one of the two choice objects/individuals presented or to respond “neither” when they believed that the items had not been previously paired. The lure choice options were pseudo-randomly sampled from different triads with the constraint being that each lure option was of the same trial type as the correct option (e.g., if the correct option was a ‘B’ item then the lure option was another familiar ‘B’ item from a different triad). All directly learned and associative inference trials were self-paced. Importantly, for both directly learned and associative inference trials, the incorrect choice was a familiar item that had been studied in the context of another individual independent from the cue. Thus, correct responses required retrieval of learned associations and could not be made based on the familiarity of the choice. The presentation order of the trials was randomized with the only constraint being that AC associative inference trials were shown before their corresponding AB and BC directly learned trials in order to ensure that participants were not able to form an association between ‘A’ and ‘C’ individuals during test. Following each of the directly learned and associative inference trials, participants rated their confidence in their response on a scale from 1 to 4 (1 = very unsure, 4 = very sure).
Coding of True Memory and False Value Transfer
True value memory was defined as value memory questions for which the participant chose the correct value that was associated with the currently cued item. False value transfer was defined as value memory questions for which the participant chose the correct value associated with the overlapping ‘A’ (or ‘C’) item and attributed the value to the currently cued unrewarded ‘C’ (or ‘A’) item. False value transfer was analyzed for ABC triads for which participants correctly inferred the relationship between ‘A’ and ‘C’ compared to triads for which the inference was not correctly made. Additionally, false value transfer was evaluated both before explicit retrieval of the inference (i.e., before AC associative inference trials) and after inferential retrieval in order to selectively compare the distinct effects of integration during encoding and flexible recombination at retrieval on subsequent false value transfer.
Results and Discussion
Directly Learned and Associative Inference Trials
First we evaluated overall accuracy on directly learned and associative inference trials. On average, participants were accurate on 73% of directly learned trials (Mdirect = 0.73, SE = 0.02; range: 0.55 to 0.87) and responded ‘neither’ on 2% of directly learned trials (Mneither = 0.02, SE = 0.007; range: 0 to 0.13). On average, participants were accurate on 63% of associative inference trials (Massociative inference = 0.63, SE = 0.02; range: 0.33 to 0.75) and responded ‘neither’ on 7% of associative inference trials (Mneither = 0.07, SE = 0.02; range: 0 to 0.38). Importantly, memory performance for directly learned valued pairs was positively correlated with performance for directly learned non-valued pairs (r = 0.58, p = .002), suggesting that there was not a tradeoff between learning valued and non-valued pairs. Consistent with previous research (Carpenter & Schacter, 2017; Zeithamova & Preston, 2010), we found that reaction times on associative inference trials (Massociative inference = 4700 msec, SE = 408) were significantly longer than directly learned trials (Mdirect = 3364 msec, SE = 251), suggesting that an additional recombination-related retrieval mechanism was used for inferential versus direct retrieval (t(24) = 6.52, p < .001, mean difference = 1336, 95% CI = [913, 1759], d = 1.30). Further, participants assigned significantly higher confidence ratings to their responses on directly learned (Mdirect = 2.84, SE = 0.09) as compared to associative inference trials (Massociative inference = 2.34, SE = 0.10), indicating that participants were more confident in their memory for events that they had directly experienced as compared to those resulting from recombination (t(24) = 10.93, p < .001, mean difference = 0.50, 95% CI = [0.41, 0.60], d = 2.18).
False Value Transfer to unrewarded ‘A’ and ‘C’ items
To examine the effects of flexible recombination during associative inference on subsequent false value transfer across event boundaries, we examined the proportion of value memory questions for which the participant chose the correct value associated with the overlapping ‘A’ (or ‘C’) item and attributed the value to the currently cued unrewarded ‘C’ (or ‘A’) item with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures analysis of variance (ANOVA). Importantly, only trials for which participants correctly remembered the directly learned association were included in subsequent analyses. Results revealed no main effect of time, F(1,24) < 1, p > .25, ηp2 = 0.03, no main effect of inference, F(1,24) = 2.91, p = .10, ηp2 = 0.11, and a significant time by inference interaction, F(1,24) = 5.69, p = .025, ηp2 = 0.19 (see Fig. 2). Participants more frequently falsely attributed value to the overlapping event after successful inference retrieval (Mafter = 0.49, SE = 0.04) than before successful inference retrieval (Mbefore = 0.40, SE = 0.04; t(24) = 2.21, p = .037, mean difference = 0.09, 95% CI = [0.006, 0.17], d = 0.44). Further, participants did not falsely attribute value more frequently to the overlapping event after unsuccessful inference retrieval (Mafter = 0.37, SE = 0.05) than before unsuccessful inference retrieval (Mbefore = 0.39, SE = 0.05; t(24) < 1, p > .25, mean difference = 0.03, 95% CI = [−0.07, 0.12], d = 0.11). Similarly, participants did not falsely attribute value more frequently to the overlapping event before successful inference retrieval (Mcorrect = 0.40, SE = 0.04) than before unsuccessful inference retrieval (Mincorrect = 0.39, SE = 0.05; t(24) < 1, p > .25, mean difference = −0.01, 95% CI = [−0.09, 0.07], d = 0.05). Critically, participants falsely attributed value more often to the overlapping event after successful inference retrieval (Mcorrect = 0.49, SE = 0.04) than after unsuccessful inference retrieval (Mincorrect = 0.37, SE = 0.05; t(24) = 2.44, p = .022, mean difference = 0.12, 95% CI = [0.02, 0.22], d = 0.49), suggesting that recombination processes underlying successful inference at retrieval can also lead to false transfer of value to unrewarded items (see Figure 3 and Supplemental Table 1 for means and raw trial numbers).
Figure 3.
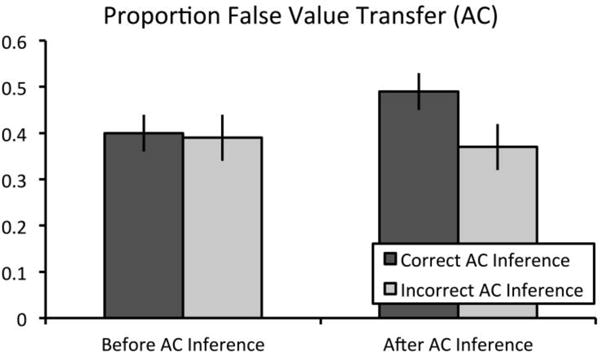
Proportion of false value transfer scores in Experiment 1. Performance on the value memory test was examined both before and after either successful or unsuccessful inference. Importantly, only trials for which participants responded correctly to directly learned trials were included in this analysis. Results revealed a significant time by inference interaction in Experiment 1. Subsequent t-tests confirm that false value transfer scores selectively increased only following successful associative inference. Error bars represent ± 1 SEM.
False Value Transfer to unrewarded ‘B’ items
To examine the effects of flexible recombination during associative inference on subsequent false value transfer within associative pairs, we examined the proportion of value memory questions for which the participant chose the correct value associated with the overlapping ‘A’ (or ‘C’) item and attributed the value to the currently cued unrewarded ‘B’ item with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA. Results revealed no main effect of time, F(1,24) < 1, p > .25, ηp2 = 0.02, no main effect of inference, F(1,24) = 1.82, p = .19, ηp2 = 0.07, and no time by inference interaction, F(1,24) = 1.67, p = .21, ηp2 = 0.07. Thus, false value transfer scores for unrewarded ‘B’ items were similar both before (Mbefore = 0.47, SE = 0.06) and after successful inference retrieval (Mafter = 0.46, SE = 0.06). Additionally, false value transfer scores for unrewarded ‘B’ items were similar both before (Mbefore = 0.44, SE = 0.06) and after unsuccessful inference retrieval (Mafter = 0.41, SE = 0.05).
True Value Memory
To examine the effects of flexible recombination during retrieval on subsequent true value memory, we examined correct responses on the value memory questions with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA. Results revealed no main effect of time, F(1,24) < 1, p > .25, ηp2 = 0.001, no time by inference interaction, F(1,24) = 1.01, p > .25, ηp2 = 0.04, but a main effect of inference, F(1,24) = 4.49, p = .045, ηp2 = 0.16, where true memory scores were moderately higher for unsuccessful (M = 0.52, SE = 0.03) than successful inference (M = 0.49, SE = 0.02). True memory scores were similar both before (Mbefore = 0.50, SE = 0.02) and after successful inference retrieval (Mafter = 0.58, SE = 0.03). Additionally, true memory scores were similar both before (Mbefore = 0.51, SE = 0.04) and after unsuccessful inference retrieval (Mafter = 0.53, SE = 0.02; see Figure 4).
Figure 4.
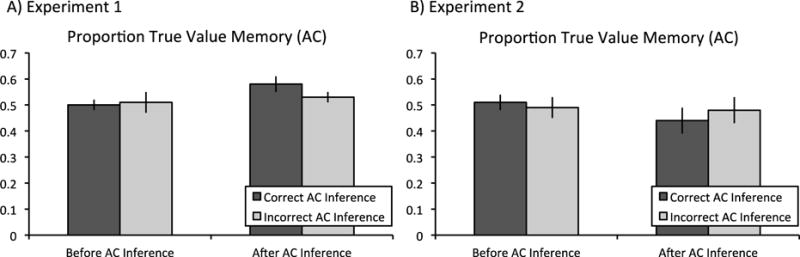
Proportion of true value memory scores in Experiments 1 and 2. Performance on the value memory test was examined both before and after either successful or unsuccessful inference. Importantly, only trials for which participants responded correctly to directly learned trials were included in this analysis. Error bars represent ± 1 SEM.
In summary, the results of Experiment 1 showed that false value transfer scores were higher for correct than incorrect inference trials, but only when the value memory test was given after the associative inference test, during which recombination occurs. These findings help to distinguish among the three competing hypotheses enumerated in the Introduction. Our results support hypothesis 1 that recombination occurring during retrieval both enhances associative inference performance and increases false transfer of value to memories for unrewarded items. By contrast, the results failed to support hypothesis 2 that integrative encoding is responsible for false value transfer, because this hypothesis predicts that false value transfer scores should be higher for correct as compared to incorrect inference trials regardless of whether the value memory tests are given before or after the associative inference test. The results also allow us to reject hypothesis 3, which claims no relation between recombination during retrieval and false value transfer, because we found clear evidence for such a relation. Thus, Experiment 1 extends to the novel domain of false value transfer a parallel pattern of results previously reported for contextual details by Carpenter and Schacter (2017), who found that participants were more prone to false memories that resulted from mistakenly combining contextual scene details from related episodes following successful inferences about the relations between these episodes, but only when these details were probed after the associative inference test.
Experiment 2
In Experiment 2, we attempt to replicate and extend the critical results of Experiment 1 by investigating the specificity of the effects produced by retrieval-related recombination processes underlying successful inference on false transfer of value to previously unrewarded items. To address this issue, participants learned overlapping AB and BC pairs where one item in the ABC triad was either a high- or low-value item (compared to valued or no-value in Experiment 1). During both value-transfer tests, given before and after the directly learned and associative inference trials, participants were asked to remember the specific value (i.e., high- or low-value) associated with each individual item. That is, Experiment 2 investigated whether the falsely transferred reward-context tied to a previously unrewarded item includes information as to the context’s specific degree of value (i.e., high- or low-value), thus going beyond the valued/no-value distinction used in Experiment 1.
Method
Participants
25 young adults (mean age = 20.70, SD = 2.39; 14 female) were recruited via advertisements at Boston University and Harvard University. The procedures regarding recruitment, eligibility criteria, informed consent and ethical guidelines were identical to Experiment 1. One participant was excluded from all subsequent analyses because they were accurate on less than 4 percent of the associative inference trials; thus, our final sample consisted of 24 participants.
Summary of the Procedure
Participants came to the lab for two sessions, separated by a 48-hour delay. The design parameters and stimuli presented during the first session were the same as in Experiment 1 with one modification: 20 pairs of the AB encoding task identified the ‘A’ item as a high-value item (i.e., $$$ - $2.00 reward), whereas 20 pairs identified the ‘A’ item as a low-value item (i.e., $ - $0.10 reward). The remaining 40 pairs did not associate any value with the ‘A’ item (i.e., no-value item). For the 40 no-value pairs, 20 of the corresponding ‘C’ items during the BC encoding task were identified as high-value items, whereas the other 20 ‘C’ items were identified as low-value items. If a triad’s ‘A’ item was associated with any value (i.e., either high- or low-value items), the corresponding ‘C’ item was not associated with any value (i.e., no-value item). Participants received one of four versions of the AB encoding task such that all value associations were counterbalanced across participants, where each ‘A’ or ‘C’ item was associated with high-, low-, or no-value equally often.
Similar to Experiment 1, participants were instructed to learn both the direct associations (i.e., AB, BC) and the indirect associations (i.e., AC) along with the specific value associated with each item. Participants were told that if they were able to remember all of the information that was tied to valued item (i.e., specific value information, direct association and indirect association) they would receive this reward at the end of the second session. Identical to Experiment 1, participants were instructed to use the same encoding strategy for high-value and low-value pairs (Hennessee, Castel & Knowlton, 2017; Wolosin et al., 2013) and were asked to provide a judgment of learning following each pair.
The design parameters and stimuli presented during the second session were the same in Experiment 2 as in Experiment 1 with one modification: Participants were asked to explicitly remember the specific value of the currently cued item and were given four possible answer choices: 1) High-value item ($$$), 2) Low-value item ($), 3) No-value item, or 4) Unsure. The test of directly learned (AB and BC) and associative inference trials (AC) was the same in Experiment 2 as in Experiment 1.
Coding of True Memory and False Value Transfer
True value memory was defined as value memory questions for which the participant chose the correct specific value (i.e., high- vs. low-value) that was associated with the currently cued item. False value transfer was defined as value memory questions for which the participant chose the correct specific value (i.e., high- vs. low-value) associated with the overlapping ‘A’ (or ‘C’) item and attributed the value to the currently cued unrewarded ‘C’ (or ‘A’) item. False value transfer was analyzed for ABC triads for which participants correctly inferred the relationship between ‘A’ and ‘C’ compared to triads for which the inference was not correctly made. Additionally, false value transfer was evaluated both before explicit retrieval of the inference (i.e., before AC associative inference trials) and after inferential retrieval in order to selectively compare the distinct effects of integration during encoding and flexible recombination at retrieval on subsequent false value transfer.
Results and Discussion
Directly Learned and Associative Inference Trials
First we evaluated overall accuracy on directly learned and associative inference trials. On average, participants were accurate on 67% of directly learned trials (Mdirect = 0.67, SE = 0.03; range: 0.43 to 0.84) and responded ‘neither’ on 9% of directly learned trials (Mneither = 0.09, SE = 0.03; range: 0 to 0.53). On average, participants were accurate on 54% of associative inference trials (Massociative inference = 0.54, SE = 0.03; range: 0.14 to 0.77) and responded ‘neither’ on 20% of associative inference trials (Mneither = 0.20, SE = 0.05; range: 0 to 0.78). Importantly, memory performance for directly learned high-value pairs was positively correlated with performance for directly learned low-value pairs (r = 0.80, p < .001), suggesting that there was not a tradeoff between learning high-value and low-value pairs. Consistent with previous research (Carpenter & Schacter, 2017; Zeithamova & Preston, 2010), we found significantly longer reaction times on associative inference trials (Massociative inference = 4491 msec, SE = 251) as compared to directly learned trials (Mdirect = 3037 msec, SE = 118), suggesting that an additional recombination-related retrieval mechanism was used for inferential versus direct retrieval (t(23) = 8.17, p < .001, mean difference = 1.45, 95% CI = [1.09, 1.82], d = 1.67). Further, participants assigned significantly higher confidence ratings to their responses on directly learned (Mdirect = 2.83, SE = 0.10) as compared to associative inference trials (Massociative inference = 2.39, SE = 0.09), indicating that participants were more confident in their memory for events that they had directly experienced as compared to those resulting from recombination (t(23) = 7.20, p < .001, mean difference = 0.44, 95% CI = [0.31, 0.56], d = 1.47).
False Value Transfer to unrewarded ‘A’ and ‘C’ items
To examine the effects of flexible recombination during associative inference on subsequent false value transfer across event boundaries, we examined the proportion of value memory questions for which the participant chose the correct value associated with the overlapping ‘A’ (or ‘C’) item and attributed the value to the currently cued unrewarded ‘C’ (or ‘A’) item with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures analysis of variance (ANOVA). Importantly, only trials for which participants correctly remembered the directly learned association were included in subsequent analyses. Results revealed no main effect of time, F(1,23) = 1.23, p > 0.25, ηp2 = 0.05, no main effect of inference, F(1,23) < 1, p >.25, ηp2 = 0.005, but a significant time by inference interaction, F(1,23) = 11.53, p = .002, ηp2 = 0.33 (see Fig. 2). Participants more frequently falsely attributed value to the overlapping event after successful inference retrieval (Mafter = 0.28, SE = 0.03) than before successful inference retrieval (Mbefore = 0.20, SE = 0.02; t(23) = 2.48, p = .018, mean difference = 0.08, 95% CI = [0.01, 0.14], d = 0.51). Further, participants did not falsely attribute value more frequently to the overlapping event after unsuccessful inference retrieval (Mafter = 0.23, SE = 0.03) than before unsuccessful inference retrieval (Mbefore = 0.24, SE = 0.03; t(23) < 1, p > .25, mean difference = −0.02, 95% CI = [−0.08, 0.05], d = 0.09). Similarly, participants did not falsely attribute value more frequently to the overlapping event before successful inference retrieval (Mcorrect = 0.20, SE = 0.02) than before unsuccessful inference retrieval (Mincorrect = 0.24, SE = 0.03; t(23) = 1.73, p = .10, mean difference = −0.04, 95% CI = [−0.09, 0.008], d = 0.34). Critically, participants falsely attributed value more often to the overlapping event after successful inference retrieval (Mcorrect = 0.28, SE = 0.03) than after unsuccessful inference retrieval (Mincorrect = 0.23, SE = 0.03; t(23) = 2.34, p = .028, mean difference = 0.05, 95% CI = [0.006, 0.10], d = 0.47), suggesting that recombination processes underlying successful inference at retrieval can also lead to false transfer of value to unrewarded items (see Figure 5 and Supplemental Table 1 for means and raw trial numbers).
Figure 5.
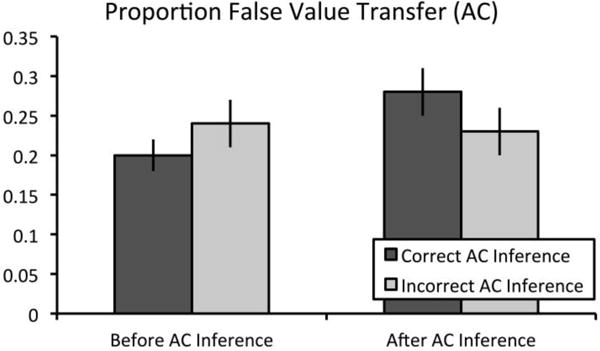
Proportion of false value transfer scores in Experiment 2. Performance on the value memory test was examined both before and after either successful or unsuccessful inference. Importantly, only trials for which participants responded correctly to directly learned trials were included in this analysis. Results revealed a significant time by inference interaction in Experiment 2. Subsequent t-tests confirm that false value transfer scores selectively increased only following successful associative inference. Error bars represent ± 1 SEM.
False Value Transfer to unrewarded ‘B’ items
To examine the effects of flexible recombination mechanisms during associative inference on subsequent false value transfer within associative pairs, we examined the proportion of value memory questions for which the participant chose the correct value associated with the overlapping ‘A’ (or ‘C’) item and attributed the value to the currently cued unrewarded ‘B’ item with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA. Results revealed no main effect of time, F(1,23) < 1, p > .25, ηp2 = 0.001, no main effect of inference, F(1,23) = 1.32, p = 0.26, ηp2 = 0.05, and no time by inference interaction, F(1,23) < 1, p > .25, ηp2 = 0.002. Thus, false value transfer scores for unrewarded ‘B’ items were similar both before (Mbefore = 0.30, SE = 0.03) and after successful inference retrieval (Mafter = 0.30, SE = 0.04). Additionally, false value transfer scores for unrewarded ‘B’ items were similar both before (Mbefore = 0.26, SE = 0.03) and after unsuccessful inference retrieval (Mafter = 0.27, SE = 0.03).
True Value Memory
To examine the effects of flexible recombination mechanisms during retrieval on subsequent true value memory, we examined correct responses on the value memory questions with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA. Results revealed no main effect of time, F(1,23) = 1.31, p > .25, ηp2 = 0.05, no main effect of inference, F(1,23) < 1, p > .25, ηp2 = 0.01, and no time by inference interaction, F(1,23) = 1.56, p = .22, ηp2 = 0.06. Thus, true memory scores were similar both before (Mbefore = 0.51, SE = 0.03) and after successful inference retrieval (Mafter = 0.44, SE = 0.05; t(23) = 1.74, p = .096, mean difference = 0.07, 95% CI = [−0.01, 0.16], d = 0.36). Additionally, true memory scores were similar both before (Mbefore = 0.49, SE = 0.04) and after unsuccessful inference retrieval (Mafter = 0.48, SE = 0.05; t(23) < 1, p > .25, mean difference = 0.01, 95% CI = [−0.09, 0.11], d = 0.06; see Figure 4).
In summary, the results of Experiment 2 provide further evidence that flexible recombination processes required for successful associative inference also produce increases in the frequency with which participants falsely transfer value to an overlapping event under conditions in which the falsely transferred reward-context includes information about the context’s specific degree of value (i.e., high- or low-value): false value transfer scores increased significantly after but not before successful compared to unsuccessful inferential retrieval. These findings build on those from Experiment 1 showing false value transfer for a valued/no-value contrast, and again extend our earlier false memory effects for contextual scene details (Carpenter & Schacter, 2017), and provide additional evidence for a link between processes that support successful inferential retrieval and those that allow for the updating of the reward-contexts associated with previously unrewarded events. If associative inference related processes did not affect participants’ memory for the value associated with previously learned events, then false value transfer scores would not have differed for correct and incorrect inferences, but they did. As in Experiment 1, our finding that the observed increase in false value transfer scores occurred only after the associative inference test was given implicates recombination during retrieval as the source of the effect, rather than integration during encoding.
Further, true memory scores and false value transfer to unrewarded ‘B’ items showed no relationship to correct versus incorrect inferential retrieval either before or after the associative inference test was presented. The lack of a difference in true memory scores indicates that the observed updating of reward-context as a result of successful inference is selective to the updating of events that were previously unrewarded. The lack of a difference in false value transfer to unrewarded ‘B’ items indicates that a retrieval-related recombination mechanism that supports successful inference is selectively involved in the transfer or false binding of reward-context across event boundaries. Thus, our results specifically speak to the transfer of a positive reward-context across distinct events to an unrewarded item that was indirectly linked to the rewarded item following the reactivation and recombination of both AB and BC events that allows for successful inference.
Experiment 3
Experiments 1 and 2 have provided evidence for false value transfer using tests that probe explicit recall of value context. In Experiment 3, we asked whether retrieval-related recombination processes underlying successful inference also contribute to implicit biasing of future decisions between two previously unrewarded items. To address this issue, participants were asked to choose between two unrewarded items, selecting the “luckier” item for potential winnings awarded at the end of the experiment (i.e., decision bias test; Wimmer & Shohamy, 2012). Previous research using a similar decision bias test indeed found evidence for false value transfer to an unrewarded item that was directly paired with a rewarded item following multiple repetitions during encoding (Wimmer & Shohamy, 2012). In contrast, our manipulation of interest concerns single-trial learning and targets recombination mechanisms during retrieval rather than integration during encoding. In order to directly test the role of recombination during retrieval as compared to integration during encoding on subsequent decision bias, implicit decision bias tests were given before the test of direct (AB, BC) and indirect (AC) associations for one half of the AB and BC pairs, and for the other half, the preference tests were given after the tests of direct and indirect associations. Because the results of Experiment 2 showed that false value transfer following successful inference was specific to the level of value associated with the reward context (i.e., high- vs. low-value reward context), in Experiment 3 we utilized this manipulation of degree of value and compared decision bias scores for unrewarded items from high- and low-value triads (rather than unrewarded items from valued vs. no-value triads from Experiment 1).
Our logic for Experiment 3 closely follows that of Experiment 1 and Experiment 2:
If recombination during retrieval both enhances associative inference performance and biases implicit decisions, then decision bias scores should be higher for correct than incorrect inference trials, but only when the decision bias test is given after the associative inference test (during which recombination occurs).
If decision bias scores are higher for correct as compared to incorrect inference trials independent on whether the decision bias tests are given before or after the associative inference test, then these effects are consistent with integrative encoding processes.
If there is no link at all between recombination during retrieval and implicit decision bias then there should be no difference between the decision bias scores for correct and incorrect inference trials regardless of when the decision bias tests are given.
Method
Participants
24 young adults (mean age = 20.20, SD = 1.79; 16 female) were recruited via advertisements at Boston University and Harvard University. The procedures regarding recruitment, eligibility criteria, informed consent and ethical guidelines were identical to Experiments 1 and 2.
Summary of the Procedure
Participants came to the lab for two sessions, separated by a 48-hour delay. The design parameters and stimuli presented during the first session were the same in Experiment 3 as in Experiment 2. During the second session, Experiment 3 utilized a decision bias test before and after the test of directly learned (AB and BC) and associative inference trials (AC). The test of directly learned and associative inference trials was the same in Experiment 3 as in Experiment 2. Following both sets of decision bias trials and all of the directly learned/associative inference trials, participants completed all of the value memory test trials for the 80 ABC triads in the same manner as described for Experiment 2.
Decision Bias Test
In accordance with previous research utilizing a similar decision bias test, participants were presented with 160 pairs of items that they had previously seen during the first session and were asked to select the “luckier” item for potential winnings at the end of the second session. Participants were given a short response time (2.5s) to make their choice, in order to ensure that they were not recalling the direct and indirect associations for both options before making their choice (Wimmer & Shohamy, 2012). Following each decision, participants were asked to rate the level of their preference for the item that they previously chose on a scale from 1 to 4 (1 = no preference, 4 = high preference). Participants’ responses on the preference rating were self-paced. For items that were rewarded, high-value items were always paired with a low-value item of the same item type. For example, a high-value ‘A’ item was always paired with a low-value ‘A’ item, whereas a high-value ‘C’ item was always paired with a low-value ‘C’ item. For items that were previously unrewarded, items that were indirectly associated with a high-value item were always paired with items that were indirectly associated with a low-value item of the same item type. For example, if the ‘A’ item in the triad was a high-value item, the corresponding ‘C’ item in that triad was paired with another unrewarded ‘C’ item that was indirectly linked to a low-value ‘A’ item. For these critical trials of interest, participants were asked to choose between two unrewarded ‘C’ items and thus should have no significant level of decision bias.
In accordance with previous research using a similar decision bias task, all trials were presented at random and participants completed four repetitions of each trial randomly interspersed (Wimmer & Shohamy, 2012). Participants answered the decision bias questions for one half of the 80 ABC triads before being tested on the directly learned and associative inference trials. After participants were tested on the directly learned and associative inference trials, they completed the decision bias questions for the alternate half of the 80 ABC triads.
Results
Directly Learned and Associative Inference Trials
Again we evaluated overall accuracy on directly learned and associative inference trials. On average, participants were accurate on 67% of directly learned trials (Mdirect = 0.67, SE = 0.03; range: 0.44 to 0.86) and responded ‘neither’ on 3% of directly learned trials (Mneither = 0.03, SE = 0.02; range: 0 to 0.33). On average, participants were accurate on 61% of associative inference trials (Massociative inference = 0.61, SE = 0.03; range: 0.30 to 0.79) and responded ‘neither’ on 5% of associative inference trials (Mneither = 0.05, SE = 0.03; range: 0 to 0.59). Importantly, memory performance for directly learned high-value pairs was positively correlated with performance for directly learned low-value pairs (r = 0.83, p < .001), again suggesting that there was not a tradeoff between learning high-value and low-value pairs. We found significantly longer reaction times on associative inference trials (Massociative inference = 3638 msec, SE = 324) as compared to directly learned trials (Mdirect = 2737 msec, SE = 190), suggesting an additional recombination-related retrieval mechanism is elicited for inferential versus direct retrieval (t(23) = 5.17, p < .001, mean difference = 0.90, 95% CI = [0.54, 1.26], d = 1.06). Further, participants assigned significantly higher confidence ratings to their responses on directly learned (Mdirect = 2.78, SE = 0.06) as compared to associative inference trials (Massociative inference = 2.32, SE = 0.07), indicating that participants were more confident in their memory for events that they had directly experienced as compared to those resulting from recombination (t(23) = 8.63, p < .001, mean difference = 0.47, 95% CI = [0.35, 0.58], d = 1.76).
Decision Bias Scores
To examine the effects of flexible recombination during associative inference on subsequent implicit decision bias, we examined the proportion of decision bias trials for which the participant chose the unrewarded item that was indirectly linked to a high-value item as compared to a low-value item with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA. Importantly, only trials for which participants correctly remembered the directly learned association were included in subsequent analyses. Results revealed no main effect of time, F(1,23) < 1, p > .25, ηp2 = 0.004, a main effect of inference, F(1,23) = 5.13, p = .03, ηp2 = 0.18, and a significant time by inference interaction, F(1,23) = 4.39, p = .05, ηp2 = 0.16 (see Fig. 3). Participants more frequently chose the unrewarded item that was indirectly linked to the high-value item after successful inference retrieval (Mafter = 0.61, SE = 0.04) than before successful inference retrieval (Mbefore = 0.52, SE = 0.03; t(23) = 2.07, p = .05, mean difference = 0.09, 95% CI = [0.00, 0.18], d = 0.42). Further, participants did not choose the unrewarded high-value item more frequently after unsuccessful inference retrieval (Mafter = 0.43, SE = 0.05) than before unsuccessful inference retrieval (Mbefore = 0.50, SE = 0.04; t(23) = 1.09, p > .25, mean difference = −0.07, 95% CI = [−0.20, 0.06], d = 0.22). Similarly, participants did not choose the unrewarded high-value item more often before successful inference retrieval (Mcorrect = 0.52, SE = 0.03) than before unsuccessful inference retrieval (Mincorrect = 0.50, SE = 0.04; t(23) < 1, p > .25, mean difference = 0.02, 95% CI = [−0.09, 0.13], d = 0.07). Critically, participants chose the unrewarded high-value item more frequently after successful inference retrieval (Mcorrect = 0.61, SE = 0.04) than after unsuccessful inference retrieval (Mincorrect = 0.43, SE = 0.05; t(23) = 2.87, p = .009, mean difference = 0.18, 95% CI = [0.05, 0.31], d = 0.59), suggesting that recombination processes underlying successful inference at retrieval can also bias participants choice toward unrewarded items that were indirectly tied to a high-value item (see Figure 6 and Supplemental Table 1 for means and raw trial numbers).
Figure 6.
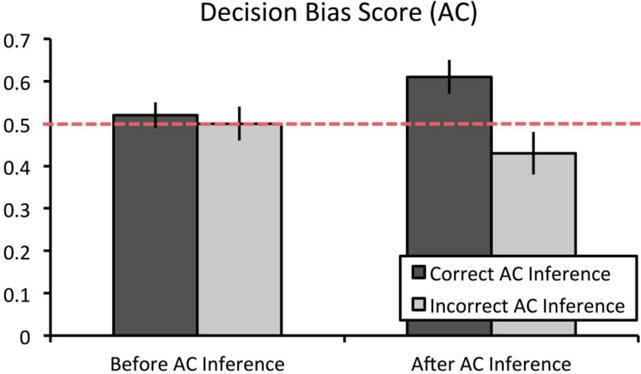
Proportion of decision bias scores in Experiment 3. Performance on the decision bias test was examined both before and after either successful or unsuccessful inference. Importantly, only trials for which participants responded correctly to directly learned trials were included in this analysis. Results revealed a significant time by inference interaction in Experiment 3. Subsequent t-tests confirm that decision bias scores selectively increased only following successful associative inference. Dashed red (light grey) line represents decision bias scores at chance levels (0.50). Error bars represent ± 1 SEM.
Importantly, in order to determine whether our decision bias scores were significantly different from chance we conducted a one-sample t-test comparing all conditions to chance (i.e., 0.50). Critically, the only condition in which decision bias scores significantly differed from chance was after successful inference (Mcorrect = 0.61, SE = 0.04; t(23) = 2.88, p = .008, mean difference = 0.11, 95% CI = [0.03, 0.19], d = 0.59). No significant differences from chance were found after unsuccessful inference (Mincorrect = 0.43, SE = 0.05; t(23) = 1.43, p = .17, mean difference = −0.07, 95% CI = [−0.17, 0.03], d = 0.29), before successful inference (Mcorrect = 0.52, SE = 0.03; t(23) < 1, p > .25, mean difference = 0.02, 95% CI = [−0.04, 0.07], d = 0.12) or before unsuccessful inference (Mincorrect = 0.50, SE = 0.04; t(23) < 1, p > .25, mean difference = −0.001, 95% CI = [−0.09, 0.09], d = 0.007).
False Value Transfer to unrewarded ‘A’ and ‘C’ items
To examine the effects of flexible recombination mechanisms during associative inference on subsequent false value transfer across event boundaries, we examined the proportion of value memory questions for which the participant chose the correct value associated with the overlapping ‘A’ (or ‘C’) item and attributed the value to the currently cued unrewarded ‘C’ (or ‘A’) item with a paired samples t-test comparing trials for which participants got the inference correct to incorrect inference trials. Critically, the value memory test for Experiment 3 differs from Experiment 2 in that all of the value memory questions were tested after all of the directly learned and associative inference trials. Consistent with results from Experiment 2, participants falsely attributed value more often to the overlapping event after successful inference retrieval (Mcorrect = 0.28, SE = 0.02) than after unsuccessful inference retrieval (Mincorrect = 0.21, SE = 0.03; t(23) = 2.52, p = .019, mean difference = 0.07, 95% CI = [0.01, 0.13], d = 0.51), suggesting that recombination processes underlying successful inference at retrieval can also lead to false transfer of value to unrewarded items.
False Value Transfer to unrewarded ‘B’ items
To examine the effects of flexible recombination mechanisms during associative inference on subsequent false value transfer within associative pairs, we examined the proportion of value memory questions for which the participant chose the correct value associated with the overlapping ‘A’ (or ‘C’) item and attributed the value to the currently cued unrewarded ‘B’ item with a paired samples t-test comparing trials for which participants got the inference correct to incorrect inference trials. Results revealed no significant difference between false value transfer scores for unrewarded ‘B’ items following successful inference retrieval (Mcorrect = 0.30, SE = 0.03) compared to after unsuccessful inference retrieval (Mincorrect = 0.25, SE = 0.03; t(23) = 1.50, p = .16, mean difference = 0.04, 95% CI = [0.02, 0.10], d = 0.30).
True Value Memory
To examine the effects of flexible recombination mechanisms during retrieval on subsequent true value memory, we examined correct responses on the value memory questions with a paired-samples t-test. Consistent with results from Experiment 2, results revealed no significant difference in the proportion of correct responses on the value memory test for correct (Mcorrect = 0.40, SE = 0.02) as compared to incorrect inference trials (Mincorrect = 0.41, SE = 0.02; t(23) < 1, p > .25, mean difference = 0.007, 95% CI = [−0.03, 0.05], d = 0.08).
Discussion
The results of Experiment 3 replicate and extend the false value transfer results of Experiments 1 and 2. Critically, results of the decision bias test from Experiment 3 support the role of recombination during retrieval in both successful associative inference and biasing implicit decisions. That is, participants’ implicit decision bias scores were significantly higher following correct than incorrect inference trials, but only when the decision bias test was given after the associative inference test during which the overlapping AB and BC associations are reactivated and flexibly recombined in order to infer the relationship between ‘A’ and ‘C.’ Results revealed no significant difference in decision bias scores for correct as compared to incorrect inference when the decision bias test was given before inferential retrieval. In sum, the current results support the role of a recombination-related retrieval mechanism in both the explicit transfer of reward-context to a previously unrewarded item that was indirectly linked to a rewarded item (Experiments 1 and 2) and biasing subsequent value-based decision-making when participants are required to choose between two previously unrewarded items (Experiment 3).
General Discussion
The three experiments reported here provide evidence that the same flexible retrieval mechanism that supports successful inferential retrieval also allows for the systematic updating of reward-contexts that bias novel value-based decisions. Experiments 1 and 2 provided evidence that flexible retrieval processes required for successful associative inference also produce increases in false value transfer scores when the value memory test is given after the test of directly learned and associative inference trials. Experiment 2 replicated and extended the results of Experiment 1 by providing evidence that the specific value (i.e., high- and low-value) associated with an item is more frequently transferred to an overlapping non-valued item only following successful inference. The results of Experiment 3 extend those of Experiments 1 and 2 by providing evidence that recombination-related retrieval processes underlying successful inference also contribute to the implicit biasing of participants’ future decisions when they are asked to choose between two previously unrewarded items that were indirectly tied to rewarded items of differing value (i.e., indirectly associated with a high- or low-value item). Thus, whereas Experiment 1 showed that general reward-context (i.e., valued vs. no-value) can be falsely transferred and biases novel decisions following successful inference as compared to unsuccessful inference, Experiments 2 and 3 provide evidence that these effects can also occur for a specific reward-context (i.e., high- vs. low-value reward). That is, only following successful inference, participants more frequently transferred the specific reward-context from the rewarded item to the unrewarded item (e.g., high-value ‘A’ item results in an unrewarded ‘C’ item as being remembered as a high-value ‘C’ item; Experiment 2). Further, when choosing between two unrewarded items where one is indirectly linked to a high-value item and the other is indirectly linked to a low-value item (Experiment 3), participants more frequently chose the unrewarded item that was indirectly linked to a high-value item following successful inference than unsuccessful inference.
These data provide direct experimental support for the idea that a mechanism that flexibly retrieves and recombines elements from distinct events in order to make novel connections that have not been directly experienced also supports the biased updating of specific value representations across distinct contexts, particularly when the original events have only been experienced once. More generally, our results add to the growing evidence supporting the role of episodic memory processes in novel value-based decision-making (Murty et al., 2016; Palombo, Keane &Verfaellie, 2015; Schacter et al., 2015; Shohamy & Daw, 2015; Wimmer & Büchel, 2016; Wolosin, Zeithamova, & Preston, 2012, 2013).
Flexible Retrieval and Integrative Encoding
As noted in the Introduction, previous research suggests that both integrative encoding processes and flexible recombination mechanisms during retrieval play a role in successful associative inference (Shohamy & Wagner, 2008; Zeithamova & Preston, 2010). If integrative encoding is responsible for false value transfer or biased value-based decision-making in our current paradigm, then there should be higher false value transfer and decision bias scores for successful than unsuccessful inferential retrieval before the associative inference test; however, our results revealed such effects only when the value memory and decision bias tests were given after the associative inference test. Previous research identifying the role of integration during encoding on successful associative inference has focused on instances where participants are able to learn the associations over multiple repetitions, thus providing participants with more opportunities to bind the overlapping AB and BC associations during the encoding phase (Shohamy & Wagner, 2008; Zeithamova, Dominick & Preston, 2012). Critically, our paradigm utilizes single-trial learning that targets flexible recombination mechanisms during successful inferential retrieval (Carpenter & Schacter, 2017). While our results provide evidence for the link between recombination-related retrieval mechanisms and subsequent false value transfer and decision bias, they do not rule out a similar link to integrative encoding under different experimental conditions that more strongly target integrative encoding processes. Thus, it is possible that under conditions where associations (AB and BC) are learned over multiple repetitions during the encoding phase, integrative encoding may contribute to the type of false value transfer across distinct events and value-based decision bias that we observed here.
Moreover, even under the current set of experimental conditions, it is theoretically possible that integrative encoding contributes to, and is perhaps even necessary for, increased false value transfer and decision bias for successful associative inference. By this view, false value transfer and decision bias scores are increased for successful vs. unsuccessful inferences only after the inference test is given because the inference test serves to remind participants of a novel connection previously established via integrative encoding; increased false value transfer and decision bias for successful inference are not observed prior to the inference test because participants “forget” the across-episode link established by integrative encoding and require a test-based reminder to retrieve that link. It is well known that test trials can serve as powerful promoters of encoding and subsequent retention (Karpicke, 2012; Roediger & Karpicke, 2006), and we believe that our findings can be viewed as another example of a kind of “testing effect”. However, it is unclear why participants would forget the across-episode link established by integrative encoding and require a reminder of it during testing. Thus, while we cannot rule out that integrative encoding played some role in our key findings, we believe that our proposal that flexible recombination during the associative inference test produces the observed false value transfer and decision bias effects is more parsimonious than an account that seeks to attribute the effects to a combination of integrative encoding and test-based reminding. Even though this issue cannot be settled definitively, the current results highlight that successful associative inference results in a more integrated representation, and that a byproduct of this flexible integration or recombination is that elements of one event may be mistakenly bound to the overlapping event. This is true whether 1) integration occurs during encoding and is further strengthened by the retrieval of the integrated representation, or 2) the recombination occurs specifically during retrieval. Nonetheless, future research should attempt to examine the role of integrative encoding mechanisms on both subsequent false value transfer and value-based decision-making.
More generally, in line with arguments made by Carpenter and Schacter (2017), we do not think that the present results should be conceived within a simple encoding-retrieval dichotomy. Memory researchers have known for decades that encoding processes involve retrieval and vice versa (e.g., Brainerd, Howe, & Desrochers, 1982; Howe, 1988; Schacter, 2001a). Thus in the present paradigm integrative encoding requires some amount of retrieval (i.e., during study of BC, participants retrieve an overlapping AB pair in order to encode an integrated ABC representation), and flexible retrieval results in some degree of encoding that produces subsequent false value transfer and decision bias. Still, our data reveal clear differences in patterns of false value transfer and decision bias before and after the associative inference test.
Implications for Reward-Related Learning
Although we are not aware of any prior studies that have specifically linked successful associative inference with false value transfer and value-based decision-making, as noted earlier previous research has shown that specific reward-contexts can affect the value attributed to unrewarded items that were previously tied to the rewarded item (Wimmer & Shohamy, 2012). Importantly, there are several differences between the current experiments and previous research by Wimmer and Shohamy (2012). Namely, past research required participants to learn the direct relationships between two items implicitly over many repeated trials, and thus they were not required to recombine elements in order to infer the relationship between two distinct episodes (Wimmer & Shohamy, 2012). By contrast, in the current experiments participants were only exposed to the paired items once and were explicitly told to learn both the reward-context (i.e., no-value, low-value or high-value) and the direct relationship between two simultaneously presented items (AB or BC). Further, in our experiments participants were aware that they would be tested on the indirect relationship between ‘A’ and ‘C’, which required them to infer the relationship between the two overlapping yet distinct episodes. Thus, while results from Wimmer and Shohamy (2012) provide strong support for an additional mechanism that is responsible for the updating of value representations during the learning phase, thus bypassing the need for effortful recombination at the time of retrieval or decision (for review see Shohamy & Daw, 2015), the current experiments more specifically target the role of a recombination-related retrieval mechanism on subsequent reward-context memory and value-based decision-making. Accordingly, although our results do not indicate generalization or spreading of the reward-context across items in the ABC triad during encoding, they do not preclude the possibility that similar patterns of reward-context spreading within and potentially across overlapping associations would emerge under experimental conditions more similar to those used by Wimmer and Shohamy (2012) (i.e., utilizing an implicit encoding paradigm and targeting integrative encoding mechanisms across multiple repetitions). It is likely that the explicit encoding instructions and single-trial learning conditions used here are not sufficient to support such generalized spreading of reward-contexts because participants are not given multiple opportunities to strongly bind the item and its associate to the specific reward-context.
How does successful inferential retrieval both support the transfer of reward-context across event boundaries and bias novel value-based decisions? We suggest that the pattern of results found here reflects the operation of two distinct mechanisms: reward-related learning (Murty et al., 2016; Wimmer & Büchel, 2016; Wimmer & Shohamy, 2012; Wolosin, Zeithamova & Preston, 2012, 2013) and flexible recombination-related retrieval processes (Carpenter & Schacter, 2017; Hupbach, Gomez, Hardt, & Nadel, 2007; Schacter & Addis, 2007a, 2007b; St. Jacques & Schacter, 2013). Reward-related learning processes allow one to encode the relationship between a specific reward-context and a currently cued item, thereby linking a specific item to its potential future value (Murty et al., 2016; Wimmer & Büchel, 2016; Wimmer & Shohamy, 2012; Wolosin, Zeithamova & Preston, 2012, 2013). Thus, representations of previously experienced events include the specific information identifying the reward-context in which the information was encoded, which affects subsequent value-based decision-making. That is, participants are more likely to choose an option if it was previously associated (either directly or indirectly) with a positive outcome (Murty et al., 2016; Wimmer & Büchel, 2016; Wimmer & Shohamy, 2012). Further, previous research suggests that the reward-context tied to a previously learned item can spread to an associated item, indirectly linking the unrewarded associate to the reward-context itself (Wimmer & Shohamy, 2012). Thus, reward-related learning mechanisms may allow for the generalization or spreading of value within associative pairs (Wimmer & Shohamy, 2012; for a discussion of how this spreading of value affects memory see Loh, Deacon, de Boer, Dolan & Duzel, 2016).
However, reward-related learning and the generalization of reward-context within associative pairs alone cannot account for the key findings of our experiments – that increased false value transfer and implicit decision bias scores were observed for correct inference trials, but only when the value memory and decision bias tests were given after the associative inference tests. In order for reward-contexts to be transferred across distinct event boundaries, our results implicate retrieval-mediated recombination processes where elements of both overlapping events (i.e., AB and BC) are reactivated and recombined in order to link items ‘A’ and ‘C’ based on their shared relationship with item ‘B.’ That is, when people make the correct inference about the relationship between elements from overlapping yet distinct episodes (i.e., AC), they may more fully bind the reward-context associated with item ‘A’ to their representation of the unrewarded item ‘C.’ Thus, previously unrewarded items may gain a positive value simply by way of co-activating and recombining elements of previously distinct experiences (for a similar mechanistic account see Carpenter & Schacter, 2017, and see Bridge & Voss, 2014a, 2014b, for additional discussion of cross-episode binding and memory distortion). These findings fit well with previous research showing that reactivating and recombining elements of distinct past events in order to simulate possible future experiences allows one to link the prospective scenario to experienced past and expected future rewards (Benoit, Gilbert & Burgess, 2011; Benoit et al., 2014; Schacter, et al., 2015). From this perspective, in our experimental paradigm false value transfer and value-based decision bias arise when overlapping AB and BC relationships (along with their specific reward-context) are reactivated and flexibly recombined in order to encode the novel inference between the previously unrelated ‘A’ and ‘C’ items. Consistent with our current results, findings from Benoit et al. (2014) suggest that integrating elements from distinct autobiographical memories in order to imagine a possible future event allows one to process the affective quality of the future episode. Therefore, previous evidence supports the ideas that 1) reward-contexts can transfer affective value within previously learned associations and 2) simulating potential future episodes allows one to link elements of the simulated future episode to a previously experienced reward. The present studies add to these findings by providing novel evidence that the same flexible recombination mechanisms that support the ability to link together elements from two distinct events, such as associative inference, also increases false value transfer of a specific reward-context to a previously unrewarded item and further biases novel value-based decision-making.
Additional Implications
While all three experiments reported here focus on recombination-related retrieval processes that occurred during the associative inference test for successful inference (AC) trials, participants were also tested for AB and BC items that did appear together previously. Thus, it is possible that the increases in false value transfer and decision bias scores are linked to retrieval of directly learned associations. However, two key features of our data speak strongly against this possibility. First, if retrieval of directly learned associations were responsible for the increase in false value transfer and decision bias scores, then scores after the associative inference test should have been similar for both successful and unsuccessful inferential retrieval. Second, if testing of directly learned pairs were responsible for the increase in false value transfer and decision bias scores after as compared to before the associative inference test, then these effects should have been found for unsuccessful inference trials because only triads for which participants were able to remember the directly learned associations were included in the subsequent analyses. Importantly, in neither experiment did we find significant differences in false value transfer or decision bias scores before as compared to after unsuccessful inferential retrieval. Moreover, our previous research (Carpenter & Schacter, 2017, Experiment 4) experimentally assessed the effects of testing directly learned associations on subsequent recombination-related source memory errors by testing the directly learned associations only after both sets of detail and source monitoring questions were completed. We still found an increase in source memory errors only after successful inferential retrieval, thereby providing evidence that testing of directly learned pairs during the associative inference test was not responsible for the increase in source memory errors. In sum, in view of the aforementioned evidence from the present experiments as well as our earlier work, it is highly unlikely that the current results linking successful inferential retrieval to false value transfer and decision bias are attributable to direct retrieval of previously studied pairs.
Throughout the current set of experiments we have proposed that flexible recombination during retrieval both supports successful inference and allows for the systematic updating of value representations that further bias novel value-based choice. Neuroimaging studies have implicated the hippocampus in flexible retrieval processes that support associative inference (e.g., Zeithamova & Preston, 2010) and constructing episodic simulations of hypothetical events based on recombining elements of past experiences (for a recent review, see Schacter, Addis, & Szpunar, 2017). In addition, neuroimaging evidence also links the hippocampus with the retrieval of the specific reward-context in which the information was originally learned, specifically when its activity is coupled with reward-related regions such as the ventral tegmental area and the nucleus accumbens (Wolosin et al., 2012, 2013). However, it is not currently known what role, if any, the hippocampus or reward-related regions play in the kinds of recombination-related false memories and false preferences documented here. Future research should attempt to delineate the contributions of the hippocampus and reward-related regions in recombination-related retrieval processes that support the systematic updating of value representations in memory.
Finally, it should be noted that the current experiments add to the growing body of evidence conceptualizing various kinds of memory errors and distortions as products of adaptive cognitive processes (cf., Bridge & Voss, 2014a; Howe, Wilkinson, Garner, & Ball, 2016; Hupbach et al., 2007; Lew & Howe, 2017; Schacter, 2001b; Schacter, Guerin, & St. Jacques, 2011; St. Jacques et al., 2013). Here we have extended the range of such distortions to the domain of value-based decision-making. Although we have provided novel evidence linking such adaptive processes as retrieval-related recombination and associative inference with false value transfer and implicit decision bias, we should point out that similar adaptive constructive processes (Schacter, 2012) played an important role in classic studies of judgment and decision-making by Tversky and Kahneman (1974). They had the important insight that memory-based heuristics that people commonly use under conditions of uncertainty “are quite useful, but sometimes they lead to severe and systematic errors” (1974, p. 1174). We believe that further investigations of the relations among flexible episodic retrieval processes, memory errors and biases, and value-based decision-making are likely to yield important new insights into the nature of constructive memory and cognition.
Context of the Research
Over the past decade there has been a large influx of literature that examines how memory-based processes affect the value associated with a specific event (or related events) and further how these memory mechanisms bias participants’ future choices. The ideas for the current set of experiments originated as an extension of the authors’ previous work evaluating the role of recombination during retrieval on subsequent source memory errors, where contextual details of one event are mistakenly bound to an overlapping event following successful inferential retrieval (Carpenter & Schacter, 2017). The current set of three experiments evaluate whether the reward-context associated with a single event may similarly be mistakenly bound to an overlapping event following successful inferential retrieval. The findings of the current experiments in concert with the authors’ past work fit within a broader research program evaluating the flexibility of the episodic memory system and further how such adaptive constructive processes may result in memory error as an unintended consequence. The authors plan to extend the current work by utilizing neuroimaging (fMRI) techniques to evaluate the neural correlates associated with associative inference and false memory. Additionally, the authors plan to utilize the current paradigm to examine age-related changes in associative inference and subsequent biases in future value-based decisions.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Mental Health grant MH060941 and National Institute on Aging grant AG08441 to Daniel L. Schacter. We thank Aleea Devitt, Ciara Greene, Helen Jing, and Preston Thakral for helpful comments on an earlier draft of the manuscript. Data from the current three experiments are original and have not previously been disseminated.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. Journal of Neuroscience. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Szpunar KK, Schacter DL. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. PNAS. 2014;111:16550–16555. doi: 10.1073/pnas.1419274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein AM, Norman KA. Reinstated episodic context guides sampling-based decisions for reward. Nature Neuroscience. 2017;20(7):997–1003. doi: 10.1038/nn.4573. [DOI] [PubMed] [Google Scholar]
- Bornstein AM, Khaw MW, Shohamy D, Daw ND. Reminders of past choices bias decisions for reward in humans. Nature Communications. 2017;8:15958. doi: 10.1038/ncomms15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. Evolutionary economics of mental time travel? Trends in Cognitive Sciences. 2008;12:219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Howe ML, Desrochers A. The general theory of two-stage learning: A mathematical review with illustrations from memory development. Psychological Bulletin. 1982;91:634–665. doi: 10.1037/0033-2909.91.3.634. [DOI] [Google Scholar]
- Bridge DJ, Voss JL. Hippocampal binding of novel information with dominant memory traces can support both memory stability and change. Journal of Neuroscience. 2014a;34:2203–13. doi: 10.1523/JNEUROSCI.3819-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge DJ, Voss JL. Active retrieval facilitates across-episode binding by modulating the content of memory. Neuropsychologia. 2014b;63:154–64. doi: 10.1016/j.neuropsychologia.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AC, Schacter DL. Flexible retrieval: When true inferences produce false memories. Journal of Experimental Psychology: Learning, Memory & Cognition. 2017;43:335–349. doi: 10.1037/xlm0000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Shohamy D, Daw ND. Multiple memory systems as substrates for multiple decision systems. Neurobiology of Learning and Memory. 2015;117:4–13. doi: 10.1016/j.nlm.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KD, Shohamy D. Memory states influence value-based decisions. Journal of Experimental Psychology: General. 2016;145:1420–1426. doi: 10.1037/xge0000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Gluth SS, Sommer T, Reiskamp J, Büchel C. Effective connectivity between the hippocampus and ventromedial prefrontal cortex controls preferential choices from memory. Neuron. 2015;86:1078–1090. doi: 10.1016/j.neuron.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Hennessee JP, Castel AD, Knowlton BJ. Recognizing what matters: Value improves recognition by selectively enhancing recollection. Journal of Memory and Language. 2017;94:195–205. doi: 10.1016/j.jml.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe ML. Measuring memory development in adulthood: A model-based approach to disentangling storage-retrieval contributions. In: Howe ML, Brainerd CJ, editors. Cognitive development in adulthood: Progress in cognitive development research. New York: Springer-Verlag; 1988. pp. 39–64. [Google Scholar]
- Howe ML, Wilkinson S, Garner SR, Ball LJ. On the adaptive function of children’s and adults’ false memories. Memory. 2016;24(8):1062–77. doi: 10.1080/09658211.2015.1068335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learning and Memory. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpicke JD. Retrieval-Based Learning. Current Directions in Psychological Science. 2012;21(3):157–163. doi: 10.1177/0963721412443552. [DOI] [Google Scholar]
- Lew AR, Howe ML. Out of place, out of mind: Schema-driven false memory effects for object-location bindings. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2017;43:404–421. doi: 10.1037/xlm0000317. [DOI] [PubMed] [Google Scholar]
- Loh E, Deacon M, de Boer L, Dolan RJ, Duzel E. Sharing a context with other rewarding events increases the probability that neutral events will be recollected. Frontiers in Human Neuroscience. 2016;9:683. doi: 10.3389/fnhum.2015.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JE, Mack ML, Gelman BD, Preston AR. Knowledge of social affiliations biases economic decisions. PLoS ONE. 2016;11(7):1–14. doi: 10.1371/journal.pone.0159918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, FeldmanHall O, Hunter LE, Phelps EA, Davachi L. Episodic memories predict adaptive value-based decision-making. Journal of Experimental Psychology: General. 2016;145:548–558. doi: 10.1037/xge0000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo JD, Keane MM, Verfaellie M. How does the hippocampus shape decisions? Neurobiology of Learning and Memory. 2015;125:93–97. doi: 10.1016/j.nlm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14:148–52. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Karpicke JD. Test-enhanced learning: Taking memory tests improves long-term retention. Psychological Science. 2006;17:249–255. doi: 10.1111/j.1467-9280.2006.01693.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Forgotten ideas, neglected pioneers: Richard Semon and the story of memory. Philadelphia: Psychology Press; 2001a. [Google Scholar]
- Schacter DL. The seven sins of memory: How the mind forgets and remembers. Boston and New York: Houghton Mifflin; 2001b. [Google Scholar]
- Schacter DL. Adaptive constructive processes and the future of memory. The American Psychologist. 2012;67 doi: 10.1037/a0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007a;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The ghosts of past and future. Nature. 2007b;445:27. doi: 10.1038/445027a. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Benoit RG, Szpunar KK. Episodic future thinking: Mechanisms and functions. Current Opinion in Behavioral Sciences. 2017;17:41–50. doi: 10.1016/j.cobeha.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Guerin SA, St Jacques PL. Memory distortion: An adaptive perspective. Trends in Cognitive Sciences. 2011;15:467–474. doi: 10.1016/j.tics.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Benoit RG, De Brigard F, Szpunar KK. Episodic future thinking and episodic counterfactual thinking: Intersections between memory and decisions. Neurobiology of Learning and Memory. 2015;117:14–21. doi: 10.1016/j.nlm.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Szpunar KK. Escaping the past: Contributions of the hippocampus to future thinking and imagination. In: Hannula DE, Duff MC, editors. The hippocampus from cells to systems: Structure, connectivity, and functional contributions to memory and flexible cognition. New York: Springer; 2017. pp. 439–465. [Google Scholar]
- Schlichting ML, Preston AR. Memory integration: Neural mechanisms and implicatioms for behavior. Current Opinion in Behavioral Sciences. 2015;1:1–8. doi: 10.1016/j.cobeha.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Daw ND. Integrating memories to guide decisions. Current Opinion in Behavioral Sciences. 2015;5:85–90. doi: 10.1016/j.cobeha.2015.08.010. [DOI] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: Hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–89. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Olm C, Schacter DL. Neural mechanisms of reactivation-induced updating that enhance and distort memory. Proceedings of the National Academy of Sciences. 2013;110:19671–19678. doi: 10.1073/pnas.1319630110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Schacter DL. Modifying memory: Selectively enhancing and updating personal memories for a museum tour by reactivating them. Psychological Science. 2013;24:537–543. doi: 10.1177/0956797612457377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Judgment under uncertainty: Heuristics and biases. Science. 1974;185:1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- Wimmer GE, Büchel C. Reactivation of reward-related patterns from single past episodes supports memory-based decision-making. The Journal of Neuroscience. 2016;36:2868–2880. doi: 10.1523/JNEUROSCI.3433-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: How memory mechanisms in the hippocampus bias decisions. Science. 2012;338:270–273. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Reward modulation of hippocampal subfield activation during successful associative encoding and retrieval. Journal of Cognitive Neuroscience. 2012;24:1532–1547. doi: 10.1162/jocn_a_00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Distributed hippocampal patterns that discriminate reward context are associated with enhanced associative binding. Journal of Experimental Psychology: General. 2013;142:1246–1276. doi: 10.1037/a0033609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–79. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Preston AR. Flexible memories: Differential roles for medial temporal lobe and prefrontal cortex in cross-episode binding. Journal of Neuroscience. 2010;30:14676–84. doi: 10.1523/JNEUROSCI.3250-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Schlichting ML, Preston AR. The hippocampus and inferential reasoning: Building memories to navigate future decisions. Frontiers in Human Neuroscience. 2012;6:70. doi: 10.3389/fnhum.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


