Abstract
Current approaches to determine the cause of acute kidney injury (AKI) in patients with cirrhosis are suboptimal. The aim of this study was to determine the utility of fractional excretion of urea (FEUrea) for the differential diagnosis of AKI in cirrhotic patients. A retrospective analysis was performed in patients (n=50) with cirrhosis and ascites admitted with AKI. Using adjudicated etiology assessment as the reference standard, receiver operating curves (ROC) and optimal cutoff, sensitivity (Sn) and specificity (Sp) for the diagnosis of prerenal azotemia (PRA), type 1 hepatorenal syndrome (HRS) and acute tubular necrosis (ATN) was derived. Validation was performed in an independent cohort (n=50) and by bootstrap analysis. The causes of AKI (derivation:validation cohorts) were: PRA 21:21, HRS 18:15, ATN: 11:14. Median FEUrea were statistically different across all etiologies of AKI in the derivation cohort (PRA 30.1 vs HRS 20.2 vs ATN 43.6, p=<0.001) and validation cohort (PRA 23.1 vs HRS 13.3 vs ATN 44.7, p=<0.001). The AUC (cutoff, Sn/Sp) for FEUrea was 0.96 (33.4, 85/100) for ATN vs non-ATN, 0.87 (28.7, 75/83) for HRS vs non-HRS, and 0.81 (21.6, 90/61) for PRA vs HRS. When applied to the validation cohort, the Sn/Sp were maintained for ATN vs non-ATN (93/97), HRS vs non-HRS (100/63), and for PRA vs HRS (67/80). After bootstrapping, the Sn/Sp for FEUrea in the ATN vs non-ATN, HRS vs non-HRS, and PRA vs HRS was 88/96, 63/97, and 55/87 respectively. Conclusions: FEUrea is a promising tool for the differential diagnosis of AKI in patients with cirrhosis.
Keywords: ascites, hepatorenal syndrome, acute tubular necrosis, pre-renal azotemia, cirrhosis
INTRODUCTION
Acute kidney injury (AKI) is a common complication in patients with cirrhosis, especially in those with ascites1. It occurs in about 20% of cirrhotic patients admitted to the hospital2 and is associated with increased short-term mortality3,4,5. The principal causes of AKI in this setting includes: (i) pre-renal azotemia (PRA) that results from decreases in intravascular volume (e.g. aggressive diuretic treatment, diarrhea); (ii) hepatorenal syndrome type 1 (HRS), AKI that is unresponsive to albumin infusion and withdrawal of diuretics in the absence of identifiable causes6; and (iii) acute tubular necrosis (ATN) that results from intrinsic damage.
AKI is associated with a high mortality in those with cirrhosis; it is therefore imperative to diagnose and identify the mechanism underlying AKI quickly and institute therapy quickly to maximize the potential for reversal. Early adjudication is often attempted via assessment of the clinical scenario, laboratory tests and a challenge of albumin infusion. Historically, the fractional excretion of sodium (FENa) was used to distinguish prerenal and HRS from ATN; its use is however confounded by the use of diuretics7 and sepsis8 and its clinical utility has diminished considerably9. In usual clinical practice, in the absence of obvious granular casts in the urinary sediment, a volume challenge is given with albumin and if the creatinine does not improve the differential diagnosis is narrowed to HRS versus ATN. This is suboptimal because renal function can deteriorate during this period before the correct diagnosis is made and appropriate therapy initiated. Increasing creatinine, and thus progression of AKI has been linked to increased mortality10. Other biomarkers, such as neutrophil gelatinase-associated lipocalin11, are research tools, expensive, and unavailable to a practicing clinician. These underscore the need to develop additional clinical tools to distinguish between functional AKI (i.e. HRS and PRA) from intrinsic AKI (i.e. ATN).
Urea is filtered in the glomerulus and then largely reabsorbed in the proximal tubule and also in the distal tubule12,13. The reabsorption of urea is increased by vasopressin and the renin-angiotensin-aldosterone system12,13. The fractional excretion of urea under conditions of decreased renal perfusion and increased vasopressin and RAAS, such as that seen in cirrhosis with PRA or HRS type 1, should therefore decrease. Conversely, renal tubular injury should impair reabsorption and increase its fractional excretion. Since urea absorption is largely modulated in the proximal tubules, it is not affected by diuretics acting more distally7,12. We therefore hypothesized that the fractional excretion of urea (FEUrea) could serve as a clinical aid in making an early distinction between ATN versus PRA and HRS type 1 in patients with cirrhosis and ascites presenting with AKI. The current study was designed to test this hypothesis.
The aim of this study was to evaluate the diagnostic performance of FEUrea for the differential diagnosis of AKI in patients with cirrhosis and ascites presenting to a tertiary care hospital. Specifically, the ability of FEUrea to distinguish between (1) ATN versus PRA and HRS, and (2) PRA versus HRS type 1 was assessed. An initial study cohort was used to develop the diagnostic model and thresholds which were then validated in a separate cohort of subjects. The overall design was aligned with a TRIPOD type 3 validation study and the approach conformed with TRIPOD guidance14.
PATIENTS AND METHODS
STUDY DESIGN
This was a retrospective study that was carried out at Virginia Commonwealth University Medical Center which is a tertiary care academic center. Potential patients were identified by screening all cirrhotic patients who were admitted for AKI (see “definitions of AKI”) to a specialized hepatology inpatient unit. Those who met inclusion criteria (see “inclusion criteria”) were included for analysis. The derivation and validation of FEUrea was designed according to the TRIPOD guidelines14. The protocol was approved by the institutional review board at our center.
INCLUSION CRITERIA
Liver cirrhosis of any etiology diagnosed by clinical parameters involving laboratory tests, endoscopic or radiologic evidence of cirrhosis, history of decompensation (hepatic encephalopathy, ascites, variceal bleeding, jaundice), and liver biopsy if available
Age greater than 18 years
Presence of moderate or severe ascites15
Use of either loop diuretics and/or distal diuretics until the time of admission
Availability of a baseline serum creatinine as defined by the ICA6
Availability of the following urine and laboratory studies within 24 hours of admission: urine sodium, urine creatinine, urine urea, urine analysis with microscopy, complete blood counts, basic metabolic profile, hepatic panel, and prothrombin time/international normalized ratio
Patients excluded from analysis were those who did not meet inclusion criteria as well as the following: prior liver or kidney transplant, advanced chronic kidney disease defined as serum creatinine greater than 4 mg/dL16, patients on acute or chronic renal replacement therapy, ambiguous diagnosis of AKI and phenotype of AKI (see Definitions section below), and patients with hepatocellular carcinoma.
DERIVATION COHORT
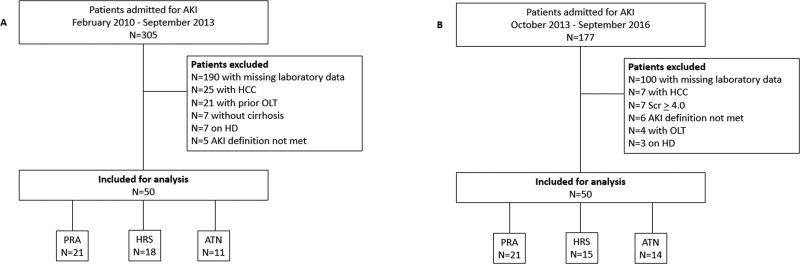
Subjects admitted with cirrhosis and ascites with AKI between February 2010 and September 2013 were screened for eligibility (Figure 1). In those that met eligibility, data were collected on the etiology of cirrhosis, demographics, mean arterial pressure (MAP), body mass index (BMI), admission laboratory data (complete blood count, metabolic panel, hepatic panel, and urinary indices mentioned above), medications (use of diuretics, nonsteroidal anti-inflammatory drugs, and beta blockers), the presence of diabetes/hypertension, a concurrent diagnosis on admission (overt hepatic encephalopathy, gastrointestinal bleed, and infections), and presence of 2 or more systemic inflammatory response syndrome (SIRS) criteria17. The severity of cirrhosis was recorded on admission through the calculation of the Model for Endstage Liver Disease Sodium (MELD-Na)18 and Child-Turcotte-Pugh (CTP)19 scores.
Figure 1.
A: Derivation Cohort; B: Validation Cohort; AKI: acute kidney injury; HCC: hepatocellular carcinoma; OLT: orthotopic liver transplant; HD: hemodialysis; PRA: pre-renal azotemia; HRS: hepatorenal syndrome; ATN: acute tubular necrosis; Scr: serum
The etiology of cirrhosis was categorized into viral hepatitis C (HCV), alcohol, non-alcoholic steatohepatitis (NASH), autoimmune hepatitis, and other (primary biliary cholangitis, etc). The cause of AKI (diuretic use, infections, gastrointestinal bleeding, and other) was recorded. In addition, response to therapy (see definitions below), use of midodrine, albumin infusions, octreotide, normal saline infusions, and renal replacement therapy were recorded.
VALIDATION COHORT
177 patients were screened from October 2013 to September 2016. A total of 50 consecutive patients who met inclusion criteria were used for the validation study (Figure 1). Data collected was analogous to the derivation cohort (see above).
DEFINITIONS OF AKI and ADJUDICATION OF AKI
The Acute Kidney Injury Network criteria20, which have been endorsed by the ICA and Acute Dialysis Quality Initiative21 for patients with cirrhosis, were applied to identify patients with AKI. Since urine output documentation can be unreliable, only the rise of serum creatinine ≥ 0.3 or ≥ 1.5 times baseline was utilized. Response to therapy (full, partial, and none) were defined by the ICA criteria6 (Supplementary Table 1).
REFERENCE STANDARD
The reference standard for assessment of FEUrea was an adjudicated diagnosis of the cause of AKI as has been used in previous publications10,11,22. All cases for the phenotype of AKI were evaluated by a hepatologist with a focused interest in cirrhosis-related renal disease and a nephrologist. HRS and ATN diagnoses required agreement amongst both services. The criteria used for the adjudication included the ICA22 and KDIGO (Kidney Disease Improving Global Outcomes) clinical practice guidelines23. These adjudications were performed by the hepatologist and nephrologist without any knowledge about the FEUrea.
CONTEXT OF USE
The current studies were performed to evaluate the diagnostic performance of FEUrea in patients with cirrhosis 0and ascites admitted to a tertiary care hospital with AKI. The testing was performed to distinguish between (1) ATN versus PRA and HRS type 1, and (2) PRA and HRS type 1. The potential decisions to be made based on such distinctions would be volume replacement for PRA, volume correction with vasoconstrictor therapy for HRS type 1 and renal replacement therapy as needed for ATN.
CALCULATION OF FEUREA
Using admission values of serum urea, serum creatinine, spot measurement of urine creatinine, and spot measurement of urine urea, FeUrea was calculated as follows:
STATISTICAL ANALYSIS
The distribution of demographic variables, etiology of cirrhosis, presence of diabetes/hypertension, medications (nonsteroidal anti-inflammatory and beta blockers), BMI, severity of liver cirrhosis (MELD-Na and CTP), baseline serum creatinine (Scr), baseline serum blood urea nitrogen (BUN), admission Scr, admission BUN, admission MAP, serum sodium (Na), urine Na, urine creatinine, urine urea, SIRS, FEUrea, and response to therapy was described. Continuous variables were presented as mean ± standard deviation (s.d.) and median interquartile range where deemed appropriate. Categorical variables were presented as percentages. Differences across groups with respect to categorical variables were analyzed using chi-square and Fishers Exact tests, whereas continuous variables were analyzed using the nonparametric Kruskal-Wallis test. A nominal p-value of less than or equal to 0.05 was considered significant.
To evaluate the diagnostic accuracy of FEUrea, the area under receiver operating characteristic (AUROC) was constructed for the following diagnoses: (1) ATN vs non-ATN, (2) HRS type 1 vs non HRS, (3) PRA vs HRS type 1. The Youden index was used to determine the optimal cut-offs for each group. Using this optimal cutoff, sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), negative likelihood ratio (NLR), and positive likelihood ratio (PLR) were calculated. Performances of cut-off values at a fixed sensitivity of 90% and a specificity 90% were also investigated. The optimal cut-offs identified in the derivation cohort were then applied to the validation cohort to determine sensitivity, specificity, NPV, PPV, NLR, and PLR for the aforementioned diagnostic studies. The entire cohort was then also bootstrapped for internal validation by resampling of the entire cohort. Average accuracy statistics (sensitivity, specificity, NPV, PPV, NLR, and PLR) across 1000 bootstrap repetitions were calculated. Statistical analysis was performed using SPSS software for Windows, version 24 (SPSS, Inc, Chicago, IL) and SAS 9.4 (SAS, Cary, NC).
RESULTS
PATIENT CHARACTERISTICS
AKI phenotype in the derivation:validation cohorts (n:n) was adjudicated as follows: PRA 21:21, HRS 18:15, ATN: 11:14 (Table 1 and Table 2). The clinical characteristics of both cohorts are summarized in Table 1 and 2. There were no statistical differences found between the derivation cohort and validation cohort with respect to demographic and clinical variables (Supplementary Table 3). Patients in derivation and validation cohorts had advanced liver disease with mean MELD-Na scores of 27.41 ± 7.65 and 29.28 ± 7.77 respectively. MELD-Na scores were found to be statistically different between all 3 AKI phenotypes in the derivation cohort (p=0.010) and in the validation cohort (p=0.045). Median urine sodium, urine creatinine, and urine urea were significantly different across all phenotypes of AKI in both cohorts as well (Table 1 and Table 2). Similarly, median FeUrea were statistically different across all phenotypes of AKI in the derivation cohort (PRA 30.1 vs HRS 20.2 vs ATN 43.6, p=<0.001) and the validation cohort (PRA 23.1 vs HRS 13.3 vs ATN 44.7, p=<0.001).
Table 1.
Derivation Cohort Baseline Clinical Characteristics
| Pre-Renal Azotemia (N=21) |
HRS Type 1 (N=18) |
ATN (N=11) |
p-value | |
|---|---|---|---|---|
|
| ||||
| Age | 55.76 ± 6.45 | 55.72 ± 9.42 | 61.00 ± 12.02 | 0.644 |
|
| ||||
| Gender, n (%) | ||||
| Male | 15 (71) | 14 (78) | 5 (45) | 0.176 |
| Female | 6 (29) | 4 (22) | 6 (55) | |
|
| ||||
| Etiology of Cirrhosis, n (%) | ||||
| HCV | 8 (38) | 8 (44) | 4 (36) | 0.887 |
| NASH | 5 (23) | 2 (11) | 4 (36) | 0.272 |
| Alcohol | 6 (29) | 7 (39) | 2 (18) | 0.489 |
| Autoimmune | 1 (5) | 0 (0) | 0 (0) | 0.494 |
| Other | 1 (5) | 1 (6) | 1 (9) | 0.883 |
|
| ||||
| Body Mass Index (kg/m2) | 29.92 ± 7.00 | 31.33 ± 7.46 | 30.89 ± 5.31 | 0.911 |
|
| ||||
| MELD-Na | 23.71 ± 5.63 | 30.56 ± 6.81 | 29.50 ± 9.79 | 0.010 |
|
| ||||
| CTP | 10.33 ± 1.96 | 11.61 ± 1.82 | 10.60 ± 1.84 | 0.096 |
|
| ||||
| NSAIDS, n (%) | 3 (14) | 0 (0) | 1 (9) | 0.258 |
|
| ||||
| NSBB, n (%) | 8 (38) | 9 (50) | 3 (27) | 0.467 |
|
| ||||
| Diabetes, n (%) | 10 (48) | 5 (19) | 3 (27) | 0.346 |
|
| ||||
| Hypertension, n (%) | 5 (24) | 3 (17) | 1 (9) | 0.579 |
|
| ||||
| Baseline Scr (mg/dL) | 1.22 ± 0.70 | 1.34 ± 0.48 | 1.22 ± 0.58 | 0.412 |
|
| ||||
| Baseline BUN (mg/dL) | 21.95 ± 16.28 | 24.00 ± 15.39 | 20.73 ± 12.08 | 0.724 |
|
| ||||
| Admission Scr (mg/dL) | 2.24 ± 1.15 | 2.93 ± 1.18 | 4.17 ± 2.66 | 0.019 |
|
| ||||
| Admission BUN (mg/dL) | 38.33 ± 16.20 | 51.67 ± 27.95 | 55.73 ± 32.74 | 0.283 |
|
| ||||
| Mean Arterial Pressure (mmHg) | 80.14 ± 14.50 | 79.83 ± 14.70 | 81.91 ± 14.57 | 0.913 |
|
| ||||
| Serum Sodium (mmol/L) | 133.62 ± 5.69 | 127.72 ± 11.80 | 135.27 ± 4.94 | 0.071 |
|
| ||||
| Urine Sodium, median (IQR) | 24.0 (10.0 – 68.0) | 16.0 (10.0 – 82.0) | 70.5 (11.0 – 112.0) | 0.002 |
|
| ||||
| Urine Urea, median (IQR) | 608.0 (310.0 – 1053.0) | 440.5 (66.0 – 771.0) | 227.0 (48.0 – 1085.0) | 0.002 |
|
| ||||
| Urine Scr, median (IQR) | 105.0 (43.0 – 304.0) | 135.0 (63.0 – 366.0) | 43.8 (11.0 – 115.0) | <0.001 |
|
| ||||
| SIRS, n (%) | 2 (9) | 2 (11) | 4 (36) | 0.113 |
|
| ||||
| FEUrea %, median (IQR) | 30.14 (17.75 – 42.05) | 20.24 (4.63 – 33.10) | 43.61 (33.41 – 60.10) | <0.001 |
|
| ||||
| Response to therapy, n (%) | ||||
| No response | 1 (5) | 17 (94) | 5 (45) | <0.001 |
| Partial response | 5 (24) | 0 (0) | 6 (55) | 0.003 |
| Full response | 15 (71) | 1 (6) | 0 (0) | <0.001 |
HCV: hepatitis C; NASH: non-alcoholic steatohepatitis; BMI: body mass index; MELD-Na: Model for Endstage Liver Disease Sodium; CTP: Child-Turcotte-Pugh; NSAIDS: non-steroidal anti-inflammatory drugs; NSBB: non-selective beta blocker; Scr: serum creatinine; BUN: blood urea nitrogen; eGFR: estimate glomerular filtration rate
Table 2.
Validation Cohort Baseline Clinical Characteristics
| Pre-Renal Azotemia (N=21) |
HRS Type 1 (N=15) |
ATN (N=14) |
p-value | |
|---|---|---|---|---|
|
| ||||
| Age | 57.86 ± 7.72 | 56.67 ± 9.80 | 59.14 ± 11.70 | 0.891 |
|
| ||||
| Gender, n (%) | ||||
| Male | 14 (67) | 10 (67) | 7 (50) | 0.552 |
| Female | 7 (33) | 5 (33) | 7 (50) | |
|
| ||||
| Etiology of Cirrhosis, n (%) | ||||
| HCV | 9 (43) | 3 (20) | 2 (14) | 0.130 |
| NASH | 3 (14) | 5 (33) | 5 (36) | 0.272 |
| Alcohol | 6 (29) | 7 (47) | 3 (21) | 0.314 |
| Autoimmune | 1 (5) | 0 (0) | 1 (7) | 0.601 |
| Other | 2 (9) | 0 (0) | 3 (21) | 0.157 |
|
| ||||
| Body Mass Index (kg/m2) | 29.72 ± 5.84 | 31.27 ± 7.10 | 31.06 ± 6.11 | 0.678 |
|
| ||||
| MELD-Na | 26.38 ± 7.93 | 32.80 ± 6.82 | 29.86 ± 7.24 | 0.045 |
|
| ||||
| CTP | 10.52 ± 2.23 | 11.40 ± 1.84 | 10.64 ± 1.74 | 0.373 |
|
| ||||
| NSAIDS, n (%) | 0 (0) | 0 (0) | 0 (0) | 0.999 |
|
| ||||
| NSBB, n (%) | 9 (43) | 7 (47) | 5 (36) | 0.832 |
|
| ||||
| DM, n (%) | 6 (29) | 4 (27) | 6 (43) | 0.586 |
|
| ||||
| HTN, n (%) | 4 (19) | 3 (20) | 4 (29) | 0.781 |
|
| ||||
| Baseline Scr (mg/dL) | 1.17 ± 0.29 | 1.55 ± 0.94 | 1.26 ± 0.40 | 0.766 |
|
| ||||
| Baseline BUN (mg/dL) | 26.65 ± 15.30 | 28.92 ± 17.16 | 22.57 ± 11.24 | 0.552 |
|
| ||||
| Admission Scr (mg/dL) | 2.07 ± 0.59 | 3.06 ± 1.75 | 2.66 ± 1.26 | 0.282 |
|
| ||||
| Admission BUN (mg/dL) | 43.00 ± 18.24 | 51.53 ± 23.47 | 52.21 ± 32.50 | 0.654 |
|
| ||||
| Mean Arterial Pressure (mmHg) | 82.60 ± 13.62 | 75.40 ± 11.42 | 85.91 ± 16.08 | 0.187 |
|
| ||||
| Serum Sodium (mmol/L) | 134.33 ± 4.78 | 130.40 ± 5.64 | 135.86 ± 6.19 | 0.048 |
|
| ||||
| Urine Sodium, median (IQR) | 20.0 (10.0 – 109.0) | 20.0 (10.0 – 40.0) | 54.5 (10.0 – 105.0) | 0.003 |
|
| ||||
| Urine Urea, median (IQR) | 708.0 (294.0 – 1143.0) | 391.0 (191.0 – 719.0) | 471.50 (193.0 – 938.0) | 0.002 |
|
| ||||
| Urine Scr, median (IQR) | 149.0 (79.0 – 205.0) | 157.0 (89.0 – 464.0) | 59.0 (19.0 – 124.0) | <0.001 |
|
| ||||
| SIRS n (%) | 3 (14) | 2 (13) | 3 (21) | 0.519 |
|
| ||||
| FEUrea %, median (IQR) | 23.1 (13.49 – 33.26) | 13.35 (4.79 – 25.01) | 44.17 (32.54 – 58.46) | <0.001 |
|
| ||||
| Response to therapy, n (%) | ||||
| No response | 3 (14) | 12 (80) | 7 (50) | <0.001 |
| Partial response | 7 (33) | 2 (13) | 6 (43) | 0.202 |
| Full response | 11 (52) | 1 (7) | 1 (7) | 0.001 |
HCV: hepatitis C; NASH: non-alcoholic steatohepatitis; MELD-Na: Model for Endstage Liver Disease Sodium; CTP: Child-Turcotte-Pugh; NSAIDS: non-steroidal anti-inflammatory drugs; NSBB: non-selective beta blocker; Scr: serum creatinine; BUN: blood urea nitrogen; eGFR: estimate glomerular filtration rate
HOSPITALIZATION DETAILS
A concurrent diagnosis of overt hepatic encephalopathy (n=15) and infection (n=20) were present on admission in the derivation cohort. This was similar in the validation cohort (n=16 and n=23 respectively). Furthermore, infections were also reported to be the most frequent identifiable cause of AKI in both cohorts, followed by diuretic-induced volume depletion (n=14 in both). Concurrent diagnosis of gastrointestinal bleeding was negligible in both cohorts (n=2 and n=2 respectively).
DIAGNOSTIC ACCURACY OF FEUREA
Derivation Cohort
ATN vs non-ATN
The AUROC for FEUrea was 0.96 (95% CI 0.91, 1.00). Using the Youden index, the optimal cut-off was determined to be 33.41%. A value greater than 33.41% predicted ATN with 100% sensitivity and 85% specificity (Table 3). When specificity was fixed at 90%, the sensitivity of FEUrea was 91% (optimal cut-off 36.20%, NPV 97%, PPV 71%). Similarly, when sensitivity was fixed at 90%, the specificity was preserved at 93% (optimal cut-off 37.70%, NPV 97%, PPV 77%) (Supplemental table 4).
Table 3.
Diagnostic Accuracy of FEUrea – Derivation Cohort
| AUC (95% CI) | Cutoff FEUrea % |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
PLR | NLR | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ATN vs non-ATN | 0.96 (0.91, 1.00) | 33.41 | 100 | 85 | 65 | 100 | 6.50 | 0.00 |
| <33.41: non-ATN | ||||||||
| ≥33.41: ATN | ||||||||
|
| ||||||||
| HRS vs non-HRS | 0.87 (0.78, 0.97) | 28.16 | 75 | 83 | 89 | 65 | 4.50 | 0.30 |
| <28.16: HRS | ||||||||
| ≥28.16: non-HRS | ||||||||
|
| ||||||||
| PRA vs HRS | 0.81 (0.67, 0.95) | 21.35 | 91 | 61 | 73 | 85 | 2.33 | 0.16 |
| <21.35: HRS | ||||||||
| ≥21:35: PRA | ||||||||
ATN: acute tubular necrosis; HRS: hepatorenal syndrome type 1; PRA: pre-renal azotemia; PPV: positive predictive value; NPV: negative predictive value; PLR: positive likelihood ratio; NLR: negative likelihood ratio
HRS vs non-HRS
The AUROC for FEUrea was 0.87 (95% CI 0.78, 0.97) and the optimal cut-off point was 28.16%. A value greater than 28.16% predicted non-HRS with a sensitivity of 75% and specificity of 83% (Table 3). When specificity was fixed at 90% the sensitivity decreased to 53% (cut-off 32.86%, NPV 51%, PPV 89%), and similarly when sensitivity was fixed at 90%, the specificity decreased to 61% (cut-off 21.40%, NPV 79%, PPV 81%).
PRA vs HRS
The AUROC for FEUrea was 0.81 (95% CI 0.67, 0.95) and the optimal cut-off point was determined to be 21.35% (sensitivity of 91% and specificity of 61%). Thus, if the FEUrea is less than 21.35%, the diagnoses of HRS is likely vs. PRA when greater than 21.35%. At a fixed specificity of 90%, there was a significant drop in sensitivity to 29% (cut-off 32.86%, NPV 51%, PPV 75%). The specificity, NPV, and PPV was similar to the optimal cut-off point when sensitivity was fixed at 90% (Supplementary table 4).
Validation Cohort
Using the optimal cutoffs identified in the derivation cohort, diagnostic accuracy was maintained for ATN vs non-ATN (sensitivity 93% and specificity of 97%), HRS vs non-HRS (sensitivity 63% and specificity 100%), and PRA vs HRS (sensitivity 68% and specificity 80%) (Table 4).
Table 4.
Diagnostic Accuracy of FEUrea – Validation Cohort
| Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
PLR | NLR | |
|---|---|---|---|---|---|---|
| ATN vs non-ATN | 93 | 97 | 93 | 97 | 33.43 | 0.07 |
| HRS vs non-HRS | 63 | 100 | 100 | 54 | Inf | 0.37 |
| PRA vs HRS | 67 | 80 | 83 | 63 | 3.33 | 0.42 |
ATN: acute tubular necrosis; HRS: hepatorenal syndrome type 1; PRA: pre-renal azotemia; PPV: positive predictive value; NPV: negative predictive value; PLR: positive likelihood ratio; NLR: negative likelihood ratio; inf: infinity
Internal Validation
Applying the optimal cutoffs identified in the derivation cohort, diagnostic accuracy was calculated for FEUrea across the entire cohort (test and validation) using 1000 bootstrap repetitions. The sensitivity and specificity was found to be preserved for ATN vs non-ATN. The sensitivity was found to be slightly decreased in the HRS vs non-HRS and PRA vs HRS groups, however this was accompanied with a concurrent rise in specificity (Table 5).
Table 5.
Internal Validation of FEUrea – Entire cohort
| Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
PLR | NLR | |
|---|---|---|---|---|---|---|
| ATN vs non-ATN | 88 | 86 | 96 | 96 | Inf | 0.17 |
| HRS vs non-HRS | 63 | 96 | 97 | 54 | Inf | 0.39 |
| PRA vs HRS | 55 | 87 | 88 | 59 | Inf | 0.15 |
ATN: acute tubular necrosis; HRS: hepatorenal syndrome type 1; PRA: pre-renal azotemia; PPV: positive predictive value; NPV: negative predictive value; PLR: positive likelihood ratio; NLR: negative likelihood ratio; inf: infinity
DISCUSSION
In this study, we have demonstrated that FEUrea has excellent diagnostic ability in differentiating structural AKI (ATN) from functional AKI (HRS and PRA) within 24 hours of admission in patients with decompensated cirrhosis and ascites. The diagnostic utility of FEUrea was further validated in an independent cohort of patients and showed high diagnostic accuracy with a sensitivity and specificity exceeding >90%. Furthermore, we were able to demonstrate its ability in separating HRS from non-HRS and PRA with good accuracy.
Urea is a primary osmolyte in urine and more than half of the urinary osmolality is supplied by urea when concentrated urine is formed13. The majority of its filtered load is absorbed in the proximal tubule and distally in the inner medulla collecting ducts through urea transporters that are influenced by vasopressin and aldosterone13. During states of antidiuresis, water is osmotically absorbed in the proximal tubule causing a progressive increase in urea concentration downstream towards the collecting duct. Consequently, when urea reaches the inner medullary collecting duct, urea exits via urea transporters (urea transporter A1 and A3) towards the inner medullary interstitium and gets trapped because of the low effective blood flow from the countercurrent exchange that is supplied by the vasa-recta24,25. In the presence of vasopressin, urea permeability is significantly higher allowing urea to accumulate in interstitium at high concentrations in an effort to equilibrate the high urea concentration in the collecting duct lumen13.
As a result of these physiological mechanisms of urea handling, FEUrea is dependent on the structural integrity of the tubules, vasopressin/aldosterone’s absorptive influence, but also, to a great extent, on the filtration fraction of urea and urine output26. For example, when the filtration fraction of urea is severely reduced, as in ATN, less urea is filtered, resulting in a lower urine urea concentration. In contrast, in pre-renal AKI, the filtration fraction is increased which leads to a much higher urea concentration in the urine as compared to ATN7,12,26. Concurrently, when decreased urine output is the result of avid water re-absorption, the level of urinary creatinine increases inversely to urine output7,27. Thus, a high urine creatinine concentration identifies if oliguria is the result of avid water re-absorption, as in PRA and to a greater extent HRS vs. loss of function (i.e. ATN) where the urinary creatinine concentration is much lower. Therefore, these biological considerations support our findings of a higher FEUrea cut-off for ATN (>33%) compared to lower cut-off values for PRA (<33% and >21%) and HRS (<21%) (Table 3). Furthermore, we found that our cutoff values for FEUrea were much lower compared to non-cirrhotics7. This is likely attributed to the increased secretion of vasopressin and underproduction of urea that is prominent in cirrhosis.
Interestingly, we found that urine urea concentration was much lower in HRS when compared to PRA. The reasons for this are probably multifactorial. For example, to a certain degree, the filtration fraction has been found to be reduced in HRS28,29, suggesting an element of tubular damage. This finding corroborates with prior studies11,30 proposing that there is likely overlap between HRS and milder forms of ATN. Moreover, coupled with avid water absorption (indicated by a high urine creatinine concentration, Table 1 and 2), and perhaps increased urea absorption in the proximal and distal tubule (via vasopressin), could explain why FEUrea levels were much lower than PRA levels.
Early adjudication between the etiologies of AKI in decompensated cirrhosis is imperative as it has management and prognostic implications2,31,32. This is especially challenging in cases of differentiating between functional AKI (HRS and PRA) and structural AKI (ATN) as features of all three major types of AKI can be present. In this clinical setting, FeUrea can be a valuable “biomarker” given its high diagnostic accuracy (Table 3 and 4). FEUrea, could therefore be an informative tool to a clinician in determining the therapeutic approach early. This is likely to be particularly relevant for those with type 1 HRS where exclusion of ATN with accuracy can allow institution of vasoconstrictor therapy within 24 hours along with albumin infusion6. The clinical utility of rapid differential diagnosis of AKI now awaits prospective validation.
There are certain situations which may affect the interpretation of FEUrea. In these situations, such as consumption of a recent high protein meal and hypercatobolism, the plasma concentration of urea rises disproportionally to serum creatinine. This increases the filtered load of urea which consequently increases urine urea concentration mirroring pre-renal states33. However, in such situations, prior studies have shown that the differentiation of high urea producing states from pre-renal states could be determined biochemically by a high urine urea/serum creatinine ratio. Here a ratio much greater than 10 is observed in high urea producing conditions7,12. In our cohort, none of patients with PRA (or HRS) had a urine urea/urine creatinine ratio greater than 10 suggesting that the determined cut-offs of FEUrea are appropriate. Furthermore, it is well accepted that patients with advanced liver disease are malnourished34 which advocates that the clinical utility of FEUrea may be ideal in this patient population.
It is important to note that the FEUrea is a simple and widely available tool whose final place in the clinical management of AKI in cirrhosis will need to be defined in additional prospective studies. It is not meant to replace the use of new renal biomarkers such as neutrophil gelatinase-associated lipocalin, etc. but may allow more selective use of these more expensive analyses. As with any diagnostic test, there are however boundaries within which its use must be considered.
In our study, we could not determine if the presence and/or severity of sarcopenia affects the current diagnostic cut offs for FEUrea. As such, the effect of sarcopenia would need to be explored in future studies. Second, because of our rigorous inclusion and exclusion criteria, we were unable to evaluate the diagnostic ability of FEUrea in those with PRA who did not respond to therapy (n=4). This scenario is often stressful and challenging with regards to clinical management, and thus this subgroup of patients should be evaluated in future studies. Furthermore, even with our extensive adjudication for the type of AKI, there is a possibility of misdiagnosis as we were unable to compare our findings to kidney biopsy which is considered the gold standard. Although a prior study showed that kidney biopsy is safe and supportive in the right clinical setting9, they are rarely performed given the concern for bleeding and high risk of complications from an operator’s standpoint. Lastly, we were unable to track changes in FEUrea with response to therapy or worsening of AKI as most patients did not have urinary chemistries on subsequent days of hospital admission. This could be a direct result of anuria or physician practice methods.
In conclusion, in this adjudicated AKI cohort study, FEUrea was found to be an excellent simple tool for the differential diagnosis of AKI in patients with decompensated cirrhosis and ascites. In our study, FEUrea has also proven to be useful “tubular injury” marker12 by differentiating ATN from non-ATN with high diagnostic accuracy. However, future studies are needed to compare the non-inferiority of FEUrea to other known kidney injury biomarkers to substantiate its role as a useful clinical biomarker. Further prospective studies are also needed to validate its predictive value for AKI progression and to evaluate response to treatment. Ultimately, studies will be needed to demonstrate if a FEUrea-based early diagnosis alters clinical outcomes.
Supplementary Material
Acknowledgments
Grant Support: 5T32 DK07150-40 and UO1 AA 021891 awarded to Dr. Sanyal; NIDDK RO1DK087013 and VA Merit Review CX10076 awarded to Jasmohan Bajaj.
Listing of Abbreviations
- AKI
acute kidney injury
- PRA
pre-renal azotemia
- HRS
hepatorenal syndrome type 1
- ATN
acute tubular necrosis
- FENa
fractional excretion of sodium
- RAAS
renin–angiotensin–aldosterone system
- FEUrea
fractional excretion of urea
- ICA
International Club of Ascites
- MAP
mean arterial pressure
- BMI
body mas index
- SIRS
systemic inflammatory response syndrome
- MELD-Na
Model for Endstage Liver Disease Sodium
- CTP
Child-Turcotte-Pugh
- HCV
viral hepatitis C
- NASH
non-alcoholic steatohepatitis
- BUN
blood urea nitrogen
- Scr
serum creatinine
- Na
sodium
- AUC
area underneath the curve
- CI
confidence interval
- NPV
negative predictive value
- PPV
positive predictive value
- NLR
negative likelihood ratio
- PLR
positive likelihood ratio
- s.d.
standard deviation
Footnotes
Disclosures: None for KRP, LK, JSB, DC
Conflicts of Interests: None for all authors.
AUTHOR CONTRIBUTIONS
Study Concept and Design: KRP and AJS
Data Analysis: KRP, LK, and AJS
Manuscript Preparation: KRP, LK, DC, and AJS
Manuscript Review: All authors
Contributor Information
Kavish R. Patidar, Division of Gastroenterology, Hepatology and Nutrition, Virginia Commonwealth University, Richmond Virginia, USA
Le Kang, Department of Biostatistics, Virginia Commonwealth University, Richmond Virginia, USA.
Jasmohan S. Bajaj, Division of Gastroenterology, Hepatology and Nutrition, Virginia Commonwealth University, Richmond Virginia, USA
Daniel Carl, Division of Nephrology, Virginia Commonwealth University, Richmond Virginia, USA.
Arun J. Sanyal, Division of Gastroenterology, Hepatology and Nutrition, Virginia Commonwealth University, Richmond Virginia, USA
References
- 1.Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, Morando F, et al. Evaluation of the acute kidney injury network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59(3):482–489. doi: 10.1016/j.jhep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48(6):2064–2077. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 3.Cardenas A, Gines P, Uriz J, Bessa X, Salmeron JM, Mas A, Ortega R, et al. Renal failure after upper gastrointestinal bleeding in cirrhosis: Incidence, clinical course, predictive factors, and short-term prognosis. Hepatology. 2001;34(4 Pt 1):671–676. doi: 10.1053/jhep.2001.27830. [DOI] [PubMed] [Google Scholar]
- 4.Tandon P, Garcia-Tsao G. Renal dysfunction is the most important independent predictor of mortality in cirrhotic patients with spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2011;9(3):260–265. doi: 10.1016/j.cgh.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, et al. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57(2):753–762. doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the international club of ascites. J Hepatol. 2015;62(4):968–974. doi: 10.1016/j.jhep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62(6):2223–2229. doi: 10.1046/j.1523-1755.2002.00683.x. [DOI] [PubMed] [Google Scholar]
- 8.Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med. 1985;145(1):108–112. [PubMed] [Google Scholar]
- 9.Wadei HM, Geiger XJ, Cortese C, Mai ML, Kramer DJ, Rosser BG, Keaveny AP, et al. Kidney allocation to liver transplant candidates with renal failure of undetermined etiology: Role of percutaneous renal biopsy. Am J Transplant. 2008;8(12):2618–2626. doi: 10.1111/j.1600-6143.2008.02426.x. [DOI] [PubMed] [Google Scholar]
- 10.Belcher JM, Garcia-Tsao G, Sanyal AJ, Thiessen-Philbrook H, Peixoto AJ, Perazella MA, Ansari N, et al. Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol. 2014;9(11):1857–1867. doi: 10.2215/CJN.09430913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60(2):622–632. doi: 10.1002/hep.26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein MH, Lenz PR, Levitt MF. Effect of urine flow rate on urea reabsorption in man: Urea as a "tubular marker". J Appl Physiol. 1969;26(5):594–599. doi: 10.1152/jappl.1969.26.5.594. [DOI] [PubMed] [Google Scholar]
- 13.Fenton RA, Knepper MA. Urea and renal function in the 21st century: Insights from knockout mice. J Am Soc Nephrol. 2007;18(3):679–688. doi: 10.1681/ASN.2006101108. [DOI] [PubMed] [Google Scholar]
- 14.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 15.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55(Suppl 6):vi1–12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;17:1–138. [Google Scholar]
- 17.American college of chest Physicians/Society of critical care medicine consensus conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 18.Elwir S, Lake J. Current status of liver allocation in the united states. Gastroenterol Hepatol (N Y) 2016;12(3):166–170. [PMC free article] [PubMed] [Google Scholar]
- 19.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, et al. Working party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60(5):702–709. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 22.Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, et al. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57(2):753–762. doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;17:1–138. [Google Scholar]
- 24.Berliner RW, Bennett CM. Concentration of urine in the mammalian kidney. Am J Med. 1967;42(5):777–789. doi: 10.1016/0002-9343(67)90095-2. [DOI] [PubMed] [Google Scholar]
- 25.Jamison RL, Bennett CM, Berliner RW. Countercurrent multiplication by the thin loops of henle. Am J Physiol. 1967;212(2):357–366. doi: 10.1152/ajplegacy.1967.212.2.357. [DOI] [PubMed] [Google Scholar]
- 26.Chesley LC. Urea excretion at low urine volumes. the calculation of "minimal" urea clearances. J Clin Invest. 1938;17(2):119–123. doi: 10.1172/JCI100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller TR, Anderson RJ, Linas SL, Henrich WL, Berns AS, Gabow PA, Schrier RW. Urinary diagnostic indices in acute renal failure: A prospective study. Ann Intern Med. 1978;89(1):47–50. doi: 10.7326/0003-4819-89-1-47. [DOI] [PubMed] [Google Scholar]
- 28.Dagher L, Moore K. The hepatorenal syndrome. Gut. 2001;49(5):729–737. doi: 10.1136/gut.49.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mindikoglu AL, Dowling TC, Wong-You-Cheong JJ, Christenson RH, Magder LS, Hutson WR, Seliger SL, et al. A pilot study to evaluate renal hemodynamics in cirrhosis by simultaneous glomerular filtration rate, renal plasma flow, renal resistive indices and biomarkers measurements. Am J Nephrol. 2014;39(6):543–552. doi: 10.1159/000363584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deshpande P, Rausa K, Turner J, Johnson M, Golestaneh L. Acute kidney injury as a causal factor in mortality associated with hepatorenal syndrome. Hepatol Int. 2011;5(3):751–758. doi: 10.1007/s12072-011-9269-8. [DOI] [PubMed] [Google Scholar]
- 31.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35(5):1179–1185. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 32.Parajuli S, Foley D, Djamali A, Mandelbrot D. Renal function and transplantation in liver disease. Transplantation. 2015;99(9):1756–1764. doi: 10.1097/TP.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 33.Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol. 2015;10(8):1444–1458. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juakiem W, Torres DM, Harrison SA. Nutrition in cirrhosis and chronic liver disease. Clin Liver Dis. 2014;18(1):179–190. doi: 10.1016/j.cld.2013.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.