Abstract
PURPOSE
MRI or CT imaging can be used to identify the esophageal location prior to left atrial ablation but the esophagus may move making the location unreliable when ablating to minimize esophageal injury. The aim of this study was to evaluate esophageal position and movement based on serial MRI imaging with the goal of identifying imaging and clinical characteristics that can predict the esophageal movement.
METHODS
Fifty patients undergoing 190 MRI scans were analyzed. The relative position of the esophagus in each MRI along with clinical and imaging characteristics were quantified, including the gap between the LA and the vertebral body (GAP), an anatomic space in which the esophagus can move.
RESULTS
A mean of 3.8 MRIs were analyzed per patient. Sixteen patients (32.0%) experienced significant lateral esophageal movement of more than 10 mm. In the significant movement group, body mass index (BMI) was higher (33.0 ± 6.5 vs 28.8 ± 5.3, p=0.02) and the GAP was significantly larger (7.1 ± 2.5 vs 4.8 ± 5.1 mm, p=0.04). Multivariate logistic regression analysis revealed that the GAP ≤ 4.5 mm was the only independent predictor of the esophagus not moving (Odds Ratio=9.25, 95% confidence interval=1.72 to 49.67, p=0.0095).
CONCLUSION
A GAP of less than 4.5 mm between the LA and the vertebral body is associated with lack of esophageal movement (< 10 mm). This suggests that the measurement of GAP < 4.5 mm may be used to predict the esophageal location in patients undergoing atrial ablation.
Keywords: Esophageal movement, Atrial Fibrillation, Catheter Ablation, Magnetic Resonance Imaging
Introduction
Atrio-esophageal fistula is a rare but potentially devastating complication of catheter ablation for atrial fibrillation (AF)[1–3]. Esophageal injury is sometimes seen in radiofrequency- and cryo-ablation and results from collateral injury to the esophagus while ablating in the left atrium (LA). Such esophageal injury is due to the close physical proximity of the esophagus in relation to the posterior wall of the LA[4, 5]. One strategy for avoiding this complication is to not ablate in areas in the LA that are in contact with the esophagus[6, 7]; however, it is difficult to identify the esophageal location during the ablation procedure.
Cardiac CT or cardiac MRI is commonly performed in preparation for LA ablation[8–10]. These imaging exams show the esophageal location in relation to the left atrium; however, previous studies have shown that the esophagus can move easily in the anatomic space behind the left atrium[9, 11], making it difficult to reliably know the esophageal location from pre-procedure imaging. The goal of this study was to use serial cardiac MRIs to identify clinical and imaging markers that can predict esophageal mobility, or lack thereof, so that one may predict the likelihood of esophageal motion from imaging done prior to ablation to help guide the ablation procedure so that esophageal injury is avoided.
Methods
Patients and study design
This retrospective observational study reviewed cardiac imaging in patients with atrial fibrillation who underwent catheter ablation at the University of Utah. The study was approved by the Institutional IRB. Inclusion criteria for this study were patients who underwent cardiac MRI 3 or more times before or after the ablation. Baseline patient demographics, comorbidities, previous medical histories and pertinent laboratory tests and imaging were recorded.
MRI
All patients underwent LGE-MRI sequence as previously described[12] prior to catheter ablation, as well as three months post ablation and followed by other cardiac imaging as needed including evaluation of pulmonary vein stenosis, cardiac function or in planning for repeat ablation.
All MRI studies were performed on 1.5 or 3 Tesla clinical MR scanners (Siemens Healthcare, Erlangen, Germany). Each LGE-MRI scan was acquired about 15 minutes after contrast agent injection (0.1 mmol/kg, Multihance, Bracco Diagnostic Inc., Princeton, NJ) using a 3D inversion recovery prepared, respiration navigated, electrocardiogram (ECG)-gated, gradient echo pulse sequence using a standard institutional protocol. Typical acquisition parameters were: free-breathing using respiratory and ECG navigation, and an imaging volume with acquired voxel size = 1.25 × 1.25 × 2.5 mm (reconstructed to 0.625 × 0.625 × 1.25 mm). The other imaging parameters were optimized for the respective field strength of scanner to improve post-ablation scar visibility and simultaneously keep the scan duration acceptable for patients (< 15 minutes). For scans performed on 1.5 Tesla scanners, the parameters were set as follows: repetition time = 5.4 ms, echo time = 2.3 ms, and flip angle = 20°. For scans performed on the 3 Tesla scanner, parameters were: repetition time = 3.1 ms, echo time = 1.4 ms, flip angle = 14°. Depending on the subject’s respiration pattern, typical scan time for a LGE-MRI study on a 1.5 Tesla scanner was 8–12 minutes, and at 3 Tesla scan time was 5–9 minutes.
Measurement of LA volume
Two expert observers manually segmented the LA volumes using Seg3D image processing software (SCI Institute, University of Utah) to analyze the LGE-MRI images.
The protocol for segmentation was as follows:
The endocardial border of the LA was manually defined by tracing the LA-PV blood pool boundary in each slice of the LGE-MRI volume. This included defining the extent of the pulmonary vein (PV) sleeves extending out of the LA, as well as defining the left atrial appendage in each slice. The LA volume data was then automatically calculated for the whole LA.
Esophageal Position
We selected three axial slices through the LA to identify the position and relative motion of the esophagus in relation to the left atrium and the vertebral body. The three axial slices that were selected were at the level of a) the roof of the LA (superior area), b) the floor of the LA at the level of the coronary sinus (inferior area), and c) the mid point between the superior and inferior slices (middle area) as shown in Figure 1A. In each of the three slices, we measured the anterior-posterior extent (vertical) and the left to right (horizontal) extent of the esophagus from the outer edge to the outer edge as shown in Figure 1B. The diameter of the descending aorta was measured from the outer edge to the outer edge. The area of the descending aorta was measured by fitting a circle to the outer edge of the descending aorta. The distance of the esophagus from the vertebral body, or the GAP, was measured as the distance between the outer edge of the LA and the outer edge of vertebral body as shown in Figure 1B. The distance between the esophagus and the vertebral body was defined as the horizontal distance from the middle of the vertebral body to the middle of the esophagus for that slice. The GAP was defined as the minimum space between the LA body and the vertebral body for that slice.
Figure 1.
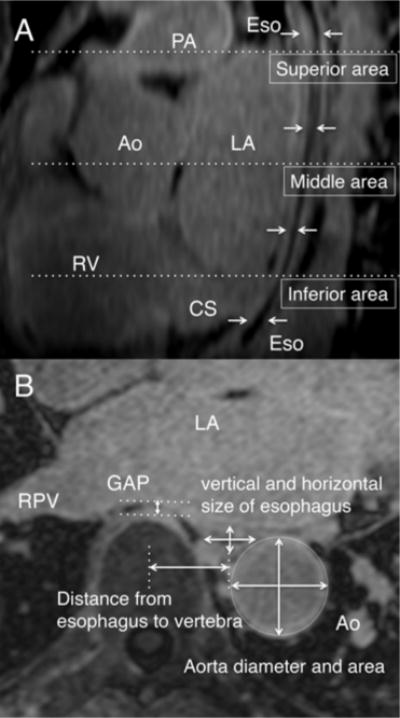
(A) The esophageal position was determined at the three locations at the top, middle and bottom of the LA. The arrows point to the Esophageal course behind the LA. (B) Axial slices at each level (top, bottom and the middle) were used for making the different measurements.
PA=pulmonary artery, Ao=Aorta, LA=left atrium, RPV=right pulmonary vein, RV=right ventricle, CS=coronary sinus, Eso=esophagus
The baseline position of the esophagus was classified into 3 groups for each of the slices as shown in Figure 2. The three groups were the left, right and the middle. The esophageal position was marked to be in the left or right side if the center of the esophagus was more than 10 mm to the left or right, respectively, on the vertical line from the center of the vertebral body. If the esophagus was within the 10 mm limit of the vertebral body, then it was classified to be in the middle. We defined the change in esophageal location to be significant if the esophagus moved by more than 10 mm laterally, irrespective of the direction or whether it crossed the boundary of left, middle or right side to another or not.
Figure 2.
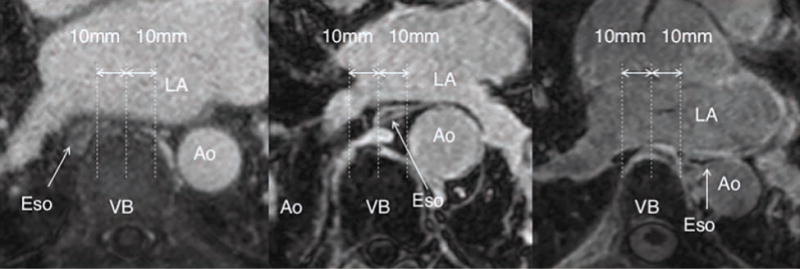
Definition of esophageal position at baseline. Going from left to right the panels show the esophageal location at the right, middle and left of the vertebral body.
Ao=Aorta, LA=left atrium, VB=vertebral body, Eso=esophagus
Statistical Analysis
Statistical analyses were performed with JMP 13 software (SAS Institute Inc., Cary, NC, USA). Kappa statistics for agreement of two reviewers were calculated. Categorical data were expressed as frequencies and were compared using chi-square or Fisher’ s exact tests. Continuous data were presented as mean ± standard deviation (SD) if normal or as median 〔minimum-maximum〕otherwise. Comparison of normally distributed variables between groups was performed by an independent-sample and paired t-test, as appropriate.
Non-normally distributed variables were compared by using the Mann-Whitney U-test. Comparison between variables and the GAP was done with univariate linear regression analysis. To assess the association between variables and esophageal movement, a multivariable logistic regression analysis was performed after adjusting for possible factors. The receiver-operator characteristic (ROC) curve was built to set the best threshold for dichotomizing the GAP by maximizing the Youden index and then the area under curve was calculated.
All the independent variables with a univariate p-value less than 0.25 were included in the model. A stepwise logistic regression model was used. A probability (p) value < 0.05 (two-tailed) was accepted as indicating statistical significance.
Result
We screened 117 patients undergoing ablation at University of Utah. A total of 67 patients in the screened cohort had less than 3 MRIs and hence were not included in this study, as they did not meet the inclusion criterion of having at least 3 images. Seventeen MRIs were excluded because of inadequate image quality. As a result, this retrospective observational study assessed 50 consecutive patients with 190 good quality MRI images that satisfied the inclusion/exclusion criteria.
In this study, in 16 patients (32.0%) the esophagus moved, defined as more than 10 mm movement of the esophagus and they form the Movement group. The remaining patients that showed less than 10 mm movement are in the Without Movement group (n=34). Figure 3 shows an example of one case in each group across multiple time points. The baseline clinical characteristics of the two groups are displayed in Table 1. Body Mass Index (BMI) was significantly higher in the Movement group, but there was no other significant difference between the two groups in terms of the age, sex or prevalence of Paroxysmal/Persistent atrial fibrillation.
Figure 3.
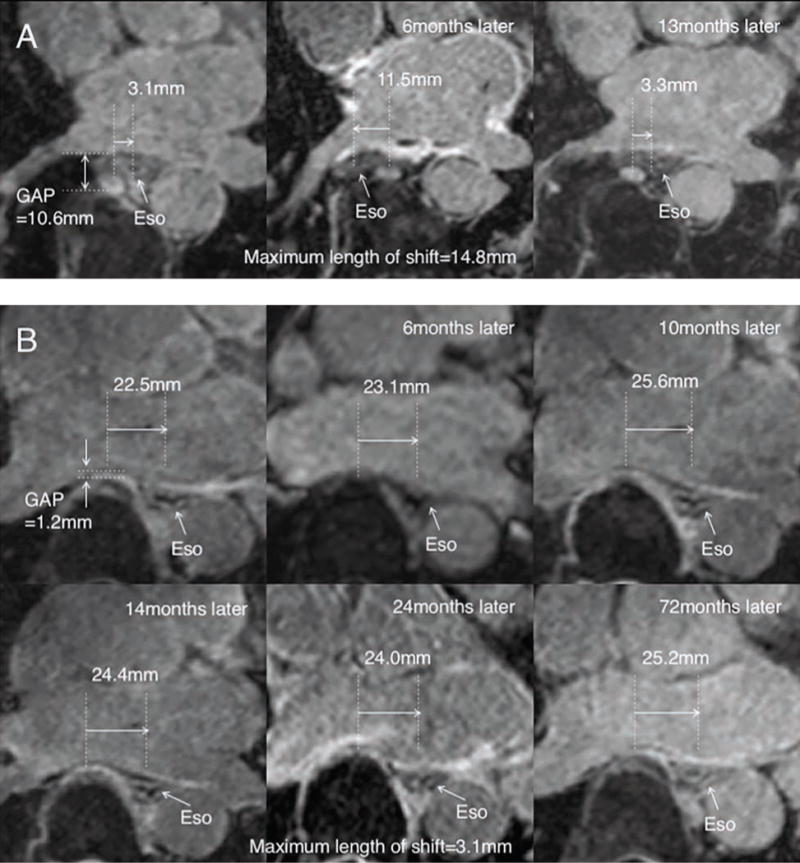
Example of a patient in (A) in the Movement group and (B) Without Movement group showing the esophageal position over time in serial scans.
Ao=Aorta, LA=left atrium, VB=vertebral body, Eso=esophagus
Table 1.
Comparison of clinical characteristics
| Movement group n=16 |
Without movement group n=34 |
p | |
|---|---|---|---|
| Age, years | 59.6 ± 8.8 | 64.1 ± 11.3 | 0.17 |
| Male, sex | 10 (62.5) | 24 (70.6) | 0.57 |
| Height, cm | 176.0 ± 10.4 | 177.7 ± 11.6 | 0.62 |
| Weight, kg | 102.2 ± 21.1 | 91.3 ± 20.8 | 0.09 |
| BMI, kg/m2 | 33.0 ± 6.5 | 28.8 ± 5.3 | 0.02 |
| BSA, m2 | 2.2 ± 0.2 | 2.1 ± 0.3 | 0.4 |
| Paroxysmal AF/Persistent | 11 (68.8)/5 (31.3) | 23 (67.7)/11 (32.4) | 0.94 |
| Coronary Artery Disease | 3 (18.8) | 10 (29.4) | 0.42 |
| Chronic Heart Failure | 3 (18.8) | 8 (23.5) | 0.7 |
| Hypertension | 12 (75.0) | 25 (73.5) | 0.91 |
| Diabetes mellitus | 5 (31.3) | 4 (11.8) | 0.09 |
| Stroke | 1 (6.3) | 2 (5.9) | 0.96 |
Numbers are expressed as mean ± SD or numbers of patients (%)
BMI=body mass index, BSA=body surface area, AF=Atrial Fibrillation, LA=left atrium
The baseline MRI data for the esophagus location, size, and left atrial volume is shown in Table 2. None of the patients had the esophagus on the right side at the inferior location. Also, at the inferior location more patients had the esophagus in the middle position in the movement group, but it was not statistically significant. The GAP was significantly larger in the movement group (7.1 ± 2.5 mm) as compared to the without movement group (4.8 ± 5.1 mm), p=0.04. Moreover, we also calculated the variation of the GAP in serial MRI scans for each patient and found that the mean difference of the GAP between the different MRIs was not significantly different in either of the two groups (0.4 ± 0.2 mm in the Movement group vs 0.3 ± 0.2 mm in the Without Movement group, p=0.39), also shown in Table 2. Table 2 also shows the maximum and the mean change for each area across the different MRIs for each patient in the two groups. As the GAP was significantly different between the two groups, we next found the most discriminant cut-off value for the GAP by maximizing the Youden Index as shown in Figure 4 and found it to be 4.5 mm. We also assessed the correlation of LA volume and the GAP. However, it showed no correlation with an R2 of 0.03 with p = 0.26.
Table 2.
Comparison of MRI findings and esophageal movement at each area
| Movement group n=16 |
Without movement group n=34 |
p | |
|---|---|---|---|
| Number of MRI studies | 3 [3–6] | 4 [3–7] | 0.41 |
| Days between the first and last MRI | 374.8 ± 580.9 | 660.4 ± 815.5 | 0.22 |
| Mean time interval between MRIs, days | 90.2 ± 100.5 | 161.6 ± 205.5 | 0.20 |
| LA volume, ml | 111.0 ± 40.7 | 111.0 ± 36.9 | 0.99 |
| LA volume index, ml/m2 | 51.9 ± 21.2 | 54.2 ± 19.3 | 0.70 |
| Mean Descending Aorta area, cm2 | 4.6 ± 0.7 | 5.0 ± 1.0 | 0.28 |
| Mean Descending Aorta diameter, mm | 24.1 ± 2.1 | 24.7 ± 2.3 | 0.38 |
| Mean vertical size of eso, mm | 7.5 ± 1.6 | 7.6 ± 1.9 | 0.90 |
| Mean horizontal size of eso, mm | 15.7 ± 3.1 | 15.3 ± 2.8 | 0.64 |
| Superior area | |||
| Right/Middle/Left side1 | 1 (6.3)/8 (50.0)/7 (43.8) |
2 (5.9)/11 (32.4)/21 (61.8) |
0.47 |
| Vertical size of eso, mm | 7.4 ± 1.6 | 7.7 ± 2.4 | 0.62 |
| Horizontal size of eso, mm | 14.9 ± 3.1 | 14.8 ± 3.8 | 0.98 |
| Middle area | |||
| Right/Middle/Left side1 | 5 (31.3)/4 (25.0)/7 (43.8) |
4 (11.8)/9 (26.5)/21 (61.8) |
0.23 |
| Vertical size of eso, mm | 7.1 ± 1.9 | 7.2 ± 2.3 | 0.82 |
| Horizontal size of eso, mm | 16.1 ± 4.6 | 14.8 ± 2.7 | 0.21 |
| Inferior area | |||
| Right/Middle/Left side1 | 0 (0)/12 (75.0)/4 (25.0) | 0 (0)/16 (47.1)/18 (52.9) |
0.06 |
| Vertical size of eso, mm | 8.1 ± 3.5 | 7.8 ± 2.1 | 0.74 |
| Horizontal size of eso, mm | 16.1 ± 3.2 | 16.1 ± 3.5 | 0.93 |
| Minimum GAP, mm | 7.1 ± 2.5 | 4.8 ± 5.1 | 0.04 |
| Variance of minimum GAP2,mm | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.39 |
| Maximum esophageal displacement, mm | |||
| Superior area | 13.8 ± 8.3 | 4.3 ± 2.3 | <.0001 |
| Middle area | 17.9 ± 12.2 | 4.7 ± 2.6 | <.0001 |
| Inferior area | 16.3 ± 9.5 | 3.9 ± 2.3 | <.0001 |
| Mean esophageal displacement, mm | |||
| Superior area | 8.9 ± 5.6 | 2.6 ± 1.5 | <.0001 |
| Middle area | 11.6 ± 7.7 | 2.6 ± 1.6 | <.0001 |
| Inferior area | 10.3 ± 7.1 | 2.1 ± 1.4 | <.0001 |
Numbers are expressed as mean ± SD or median 〔minimum-maximum〕 or numbers of patients (%)
Shows the location of the Eso in that slice.
Shows the difference in the measured distance between the LA and vertebral body across the different MRIs for the same patient.
Mean followup time is the time interval between the first and the last scan.
MRI=magnetic resonance imaging, LA=left atrium, Eso=esophagus, GAP=gap between the LA and the vertebral body
Figure 4.
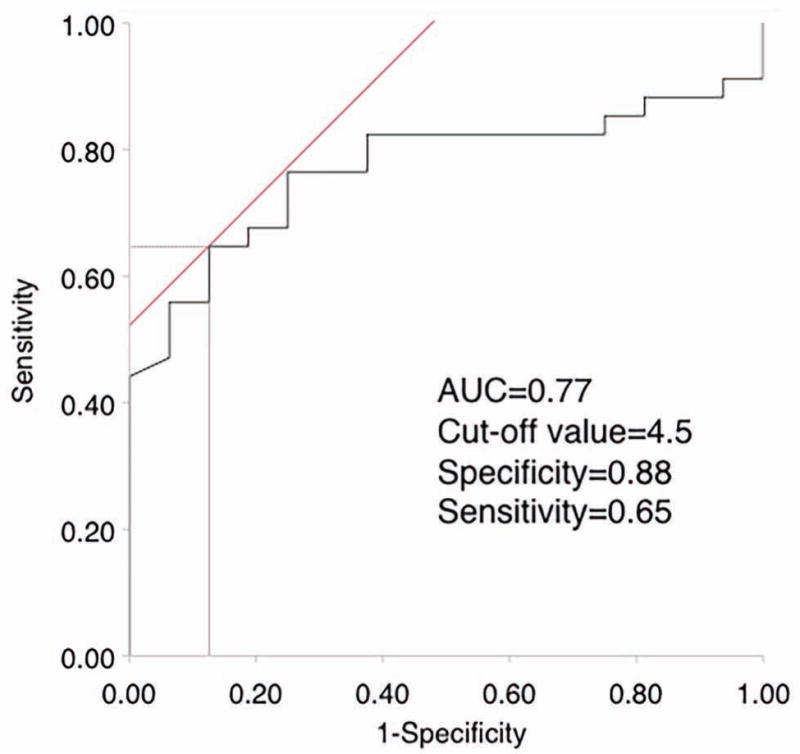
ROC curve for the distance between LA and vertebral body for predicting significant esophageal movement.
LA=left atrium
Results of univariate and multivariate analysis based on this GAP of 4.5 mm between the LA and the vertebral body is shown in Table 3. In Univariate analysis the lower BMI and the GAP ≤ 4.5mm were significant predictors of no esophageal movement but the OR of 11.31 for the GAP was much higher than 0.88 for BMI. Multivariate analysis showed that the only significant predictor was the GAP ≤ 4.5 mm with an OR of 9.25 with a 95% CI of 1.72 to 49.67 with p = 0.0095.
Table 3.
Univariate and multivariate analysis between significant esophageal movement and several clinical factors
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p | OR | 95% CI | p |
| BMI, kg/m2 | 0.88 | 0.79 – 0.99 | 0.02 | 0.91 | 0.81 – 1.03 | 0.12 |
| Male, sex | 1.44 | 0.41 – 5.04 | 0.57 | |||
| LA volume index, ml/m2 | 1.01 | 0.98 – 1.04 | 0.70 | |||
| Age, years | 1.04 | 0.98 – 1.10 | 0.16 | 1.01 | 0.95 – 1.08 | 0.75 |
| Number of MRI | 1.32 | 0.69 – 2.54 | 0.38 | |||
| Mean time interval between | ||||||
| MRIs, days | 1.06 | 0.74 – 5.04 | 0.68 | |||
| Aorta area, cm2 | 1.51 | 0.73 – 3.12 | 0.26 | |||
| Mean vertical size of eso, mm | 1.02 | 0.73 – 1.43 | 0.90 | |||
| Mean horizontal size of eso, mm | 0.95 | 0.77 – 1.17 | 0.63 | |||
| Minumum GAP ≤ 4.5mm | 11.31 | 2.2 – 58.01 | 0.0006 | 9.25 | 1.72 – 49.67 | 0.0095 |
OR=odds ratio, CI=confidence interval, BMI=body mass index, LA=left atrium, MRI=magnetic resonance imaging, eso=esophagus, GAP=gap between the LA and the vertebral body
In our series, there were 3 cases (case i, ii, and iii) that showed significant esophageal movement more than once. In case i, the esophagus moved 2 times in 3 MRIs (Figure 3A). In case ii, the esophagus also moved 2 times in 4 MRIs. In case iii, the esophagus moved 3 times in 5 MRIs. In these cases, the mean number of MRIs done for each patient was 4, and the mean GAP was 9.6 mm. On the other hand, Figure 3B is an example of a patient with multiple serial MRIs that showed no movement.
We also assessed for inter-observer variability between the two observers. Kappa statistics was 0.788 for esophageal location between the two observers.
There were two anatomical rare cases in this study (Figure 5) where the esophagus was on the left of the aorta. Significant esophageal movement was observed in both of these cases (31.6 mm and 33.9 mm), where the esophagus moved from one side of the aorta to the other but did not cross over the vertebral body.
Figure 5.
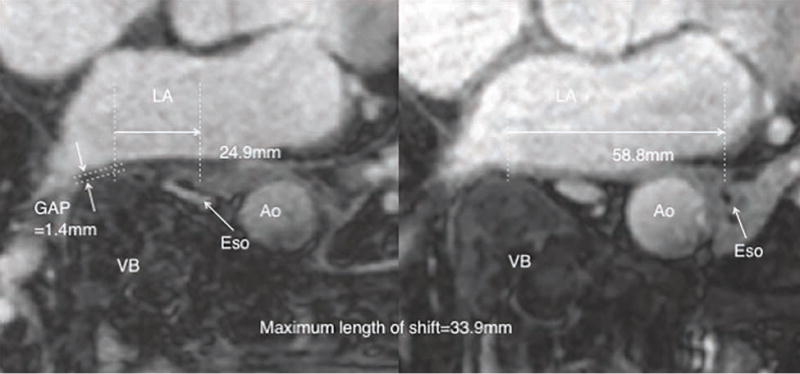
Anatomical rare case with the esophagus moving from one side of the aorta to other without crossing over the vertebral body.
Ao=Aorta, LA=left atrium, VB=vertebral body, Eso=esophagus
Discussion
In this study we analyzed the movement of the esophagus over time based on serial MRIs and we show that the esophagus moved in 32.0% of cases. More importantly, the biggest predictor of lack of esophageal movement is the GAP between the LA and the vertebral body. With GAP < 4.5 mm the OR of the esophagus not moving was 9.25. It is fairly common to perform CT or MRI imaging for the purpose of evaluating the anatomy of the LA and pulmonary veins before catheter ablation. It is relatively easy to measure the GAP and based on our study, this can be used as a guide for esophageal movement. For patients with a gap larger than 4.5 mm, one would need to plan for a larger likelihood of esophageal movement greater than 10 mm. For patients with a much smaller GAP, one might be able to use the esophageal position from the imaging study as a starting point for the esophageal location during the ablation.
The esophagus is a mobile structure and hence predicting the esophageal position based on an imaging study alone, can be inaccurate. The mobility of the esophagus has been well described in previous studies, including an autopsy study which reported that the esophagus can move laterally between 2.7–5.5 cm by using the stylet probe extending to the gastro-esophageal (GE) junction[13]. Other studies have tried using this mobility to move the esophagus during ablation by moving the esophagus with a catheter or echo probe[14, 15]. Kobza et al.[16] reported in a study of 18 patients that the esophagus moved more than 10 mm in 6 (33%) patients within 24 hrs. For that study a gastric tube was placed in the esophagus for the first CT based measurement and esophageal mapping was done for the second measurement. Given the mobility of the esophagus, simply trying to determine the location could result in esophageal movement. Another study reported the esophageal position based on two ablation procedures done 7 months apart in 42 patients. The esophageal location was identified with a barium swallow, and they reported that in 80% of the case the esophagus was in the same location, and in only 17% of cases the esophagus shifted by more than 10 mm[9]. In our study, 32% of patients experienced more than 10 mm of esophageal movement. We did image an average of 3.8 times per patients, so we are likely to find more movement but once again in patients with smaller GAP the esophageal position in the imaging studies can be used to predict the esophageal location. A prospective observational study is needed to firmly establish how useful the GAP data before ablation could be useful to predict the esophageal position.
A recent MRI based study on cadavers and patients has reported the presence of peri-esophageal connective tissue layers coursing from the aorta to the esophagus as well as a layer connecting esophagus and aorta referred to as the “aorto-esophageal ligament”[17]. These connective tissue planes can provide planes for the esophagus to slide from side to side, facilitated by a large gap between the LA and the vertebral body. The amount of peri-esophageal connective tissue might be related to higher BMI. In our univariate analysis, higher BMI was also predictive of esophageal movement though it was not significant in the multivariate analysis.
Barium paste[18–20] and an esophageal temperature monitoring probe[21, 22] have been used to locate the esophageal position and have been useful during the ablation procedure. However, the fluoroscopic location and temperature of the probe do not provide precise information of the outer surface of the esophageal muscle and the distance from LA to esophagus. If the location of the esophagus is unchanged based on the temperate probe or barium swallow during the procedure, we can use the entire esophageal location based on the imaging study as a guide for esophageal location during the study. In our study the horizontal esophageal dimension was almost 15 mm, so just using the temperature probe might not be sufficient to monitor the entire esophageal extent. Moreover, Müller et al.[23] reported that esophageal temperature probe use was related to endoscopically detected esophageal lesions. Based on these results, it may be a good option to perform the ablation without real-time esophageal monitoring in cases where the GAP was less than the cut-off value.
We also looked at the variability in the GAP across the serial images done for each patient to assess the measurement’s reliability. The variability in this measured GAP was small in the range of 0.3 to 0.4 mm in the two groups across all the images, indicating that this measurement can be used quite reliably for predicting the esophageal movement.
There were two anatomical rare cases in our study not reported in previous esophageal movement studies related to atrial fibrillation. In these cases, the esophagus was located on the left side of the descending aorta and esophagus moved from left side of aorta to right side of aorta, which was more than 10 mm laterally. The space between the LA and vertebral body should not affect this esophageal lateral movement around the aorta and this study’s results should not be applied to these cases, as the esophagus did not cross the vertebral body.
Limitations and Future Research
This is a retrospective study showing that the GAP of < 4.5 mm between the vertebral body and the LA is associated with a lack of esophageal movement more than 10 mm and the usual limitations of any retrospective study including patient selection from a single center apply. A prospective study using this GAP measurement as predictive of esophageal location will be useful in further evaluating how this can be used to minimize esophageal injury.
Conclusion
In 68.0% of the subjects there was less than 10 mm of esophageal movement. The biggest predictor of esophageal non-movement is the minimum GAP between the LA and the vertebral body, which can be easily measured and can be used when planning for ablation procedure as a guide to esophageal position.
Acknowledgments
Disclosures
Ravi Ranjan is supported by NIH grant K23 HL115084 and R56 HL128674 and also has research grants from Medtronic, St. Jude Medical and Biosense Webster and is a consultant to St. Jude Medical.
References
- 1.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53(19):179–03. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros De Vasconcelos JT, Filho S, Atie J, Maciel W, De Souza OF, Saad EB, et al. Atrial-oesophageal fistula following percutaneous radiofrequency catheter ablation of atrial fibrillation: the risk still persists. Europace. 2017;19(2):250–8. doi: 10.1093/europace/euw284. [DOI] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John RM, Kapur S, Ellenbogen KA, Koneru JN. Atrioesophageal fistula formation with cryoballoon ablation is most commonly related to the left inferior pulmonary vein. Heart Rhythm. 2017;14(2):184–9. doi: 10.1016/j.hrthm.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki R, Gauri A, Elmouchi D, Duggal M, Bhan A. Atrioesophageal fistula complicating cryoballoon pulmonary vein isolation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25(7):787–92. doi: 10.1111/jce.12426. [DOI] [PubMed] [Google Scholar]
- 6.Chugh A, Rubenstein J, Good E, Ebinger M, Jongnarangsin K, Fortino J, et al. Mechanical displacement of the esophagus in patients undergoing left atrial ablation of atrial fibrillation. Heart Rhythm. 2009;6(3):319–22. doi: 10.1016/j.hrthm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Black-Maier E, Pokorney SD, Barnett AS, Zeitler EP, Sun AY, Jackson KP, et al. Risk of atrioesophageal fistula formation with contact force-sensing catheters. Heart Rhythm. 2017;14(9):1328–33. doi: 10.1016/j.hrthm.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Kenigsberg DN, Lee BP, Grizzard JD, Ellenbogen KA, Wood MA. Accuracy of intracardiac echocardiography for assessing the esophageal course along the posterior left atrium: a comparison to magnetic resonance imaging. J Cardiovasc Electrophysiol. 2007;18(2):169–73. doi: 10.1111/j.1540-8167.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy R, Good E, Oral H, Huether E, Bogun F, Pelosi F, et al. Temporal stability of the location of the esophagus in patients undergoing a repeat left atrial ablation procedure for atrial fibrillation or flutter. J Cardiovasc Electrophysiol. 2008;19(4):351–5. doi: 10.1111/j.1540-8167.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 10.Parmar BR, Jarrett TR, Kholmovski EG, Hu N, Parker D, MacLeod RS, et al. Poor scar formation after ablation is associated with atrial fibrillation recurrence. J Interv Card Electrophysiol. 2015;44(3):247–56. doi: 10.1007/s10840-015-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan SC, Salazar M, Narula N. Anatomical basis for the mobility of the esophagus: implications for catheter ablation of atrial fibrillation. Indian Pacing Electrophysiol J. 2008;8(1):66–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Parmar BR, Jarrett TR, Burgon NS, Kholmovski EG, Akoum NW, Hu N, et al. Comparison of left atrial area marked ablated in electroanatomical maps with scar in MRI. J Cardiovasc Electrophysiol. 2014;25(5):457–63. doi: 10.1111/jce.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marar D, Muthusamy V, Krishnan SC. Avoiding oesophageal injury during cardiac ablation: insights gained from mediastinal anatomy. Europace. 2017 doi: 10.1093/europace/eux024. [DOI] [PubMed] [Google Scholar]
- 14.Mateos JC, Mateos EI, Pena TG, Lobo TJ, Mateos JC, Vargas RN, et al. Simplified method for esophagus protection during radiofrequency catheter ablation of atrial fibrillation–prospective study of 704 cases. Rev Bras Cir Cardiovasc. 2015;30(2):139–47. doi: 10.5935/1678-9741.20150009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koruth JS, Reddy VY, Miller MA, Patel KK, Coffey JO, Fischer A, et al. Mechanical esophageal displacement during catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23(2):147–54. doi: 10.1111/j.1540-8167.2011.02162.x. [DOI] [PubMed] [Google Scholar]
- 16.Kobza R, Schoenenberger AW, Erne P. Esophagus imaging for catheter ablation of atrial fibrillation: comparison of two methods with showing of esophageal movement. J Interv Card Electrophysiol. 2009;26(3):159–64. doi: 10.1007/s10840-009-9434-3. [DOI] [PubMed] [Google Scholar]
- 17.Weijs TJ, Goense L, van Rossum PS, Meijer GJ, van Lier AL, Wessels FJ, et al. The peri-esophageal connective tissue layers and related compartments: visualization by histology and magnetic resonance imaging. J Anat. 2017;230(2):262–71. doi: 10.1111/joa.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good E, Oral H, Lemola K, Han J, Tamirisa K, Igic P, et al. Movement of the esophagus during left atrial catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2005;46(11):2107–10. doi: 10.1016/j.jacc.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Pollak SJ, Monir G, Chernoby MS, Elenberger CD. Novel imaging techniques of the esophagus enhancing safety of left atrial ablation. J Cardiovasc Electrophysiol. 2005;16(3):244–8. doi: 10.1046/j.1540-8167.2005.40560.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamane T, Matsuo S, Date T, Mochizuki S. Visualization of the esophagus throughout left atrial catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(1):105. doi: 10.1111/j.1540-8167.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- 21.Redfearn DP, Trim GM, Skanes AC, Petrellis B, Krahn AD, Yee R, et al. Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16(6):589–93. doi: 10.1111/j.1540-8167.2005.40825.x. [DOI] [PubMed] [Google Scholar]
- 22.Perzanowski C, Teplitsky L, Hranitzky PM, Bahnson TD. Real-time monitoring of luminal esophageal temperature during left atrial radiofrequency catheter ablation for atrial fibrillation: observations about esophageal heating during ablation at the pulmonary vein ostia and posterior left atrium. J Cardiovasc Electrophysiol. 2006;17(2):166–70. doi: 10.1111/j.1540-8167.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 23.Muller P, Dietrich JW, Halbfass P, Abouarab A, Fochler F, Szollosi A, et al. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm. 2015;12(7):1464–9. doi: 10.1016/j.hrthm.2015.04.005. [DOI] [PubMed] [Google Scholar]


