Abstract
Context
Acute postoperative pain remains inadequately assessed and managed. A valid instrument that assesses acute pain in sedated postanesthesia care unit (PACU) patients is needed.
Objectives
Two behavioral pain assessment instruments, the nonverbal pain scale revised (NVPS-R) and critical care pain observation tool (CPOT), were used to determine whether these instruments adequately assess acute pain in the PACU.
Methods
A crossover study design was used. The study was conducted in the Medical Services Administration at the Puerto Rico Medical Center. Upon PACU arrival, patient sedation levels were evaluated using the Richmond Agitation Sedation Scale. Acute pain was assessed using the CPOT (scored, 0 to 8) and the NVPS-R (scored, 0 to 10) at time points 0, 15, 30, 45, 60, 90, and 120 minutes. Descriptive statistics and mixed model regression analysis were used to compare pain score assessment between instruments.
Results
Clinically significant increases in vital signs and respiratory indicators using the NVPS-R were not seen in patients with significant pain at time 0, 15, and 120 minutes. The CPOT vocalization indicator was more frequent in patients with significant pain.
Conclusions
Findings suggest that NVPS-R and CPOT can assess acute pain in sedated PACU patients. In patients with significant pain, the CPOT vocalization indicator was more consistent than physiological and respiratory indicators in detecting acute pain. Thus, our data do not support the exclusive use of vital sign indicators to assess acute pain, suggesting the superiority of the CPOT for the assessment of acute pain in sedated PACU patients.
Keywords: acute pain assessment, behavioral pain scales, critical care pain observation tool (CPOT), nonverbal pain scale revised (NVPS-R), postanesthesia care unit (PACU)
Acute pain represents a significant concern for surgical postanesthesia care unit (PACU) patients during the early postoperative period and remains inadequately assessed and managed.1 The American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists (ASA) Committee on Regional Anesthesia reported a gap in the assessment of acute pain in postoperative sedated patients unable to self-report pain.1 The most commonly used instrument for the assessment of acute pain is the numeric rating scale, considered the gold standard for pain assessment. It is designed to evaluate pain intensity in conscious patients who can report their pain.2 The absence of specific recommendations from professional societies and health institutes for the use of specific instruments and/or protocols for pain assessment in sedated PACU patients after general anesthesia, could affect the early assessment and adequate management of acute postoperative pain.
According to data reported by the Centers for Disease Control and Prevention, ∼100 million surgical procedures are performed in the United States each year; of these, 40% are in an inpatient setting.3 Data suggest that 80% of those receiving inpatient surgery experience pain postoperatively.4
Several studies examined the efficacy of the management of postoperative pain in surgical inpatients. In 2003, Apfelbaum et al5 published a retrospective study of postoperative pain in patients who received inpatient or outpatient surgery. Pain reported by participants varied by whether their surgeries occurred within the last year or within the past 2 to 5 years. Those with surgeries in the last year reported lower incidences of acute pain overall. Participants reported an 80% to 84% incidence of acute pain. Of those, 44% to 51% reported moderate pain, and 38% to 42% reported severe to extreme pain. Similarly, in a recent retrospective study, 91.8% of those who received inpatient surgery (N=146) experienced pain, with 47% of these reporting moderate postoperative pain, and 32% of these reporting severe to extreme pain. In contrast, in a recent prospective study of surgical inpatients (N=441),4 Buvanendran et al6 reported a 66% incidence of pain at discharge, with 54% of participants reporting moderate pain, and only 12% reporting severe to extreme pain. Despite differences in methodology, taken together, these studies suggest a gradual decrease in postoperative pain intensity over time. However, the incidence of acute postoperative pain remains a significant issue.
Evidence-based recommendations are needed to identify an optimal behavioral pain instrument for acute pain assessment in postoperative adult patients who cannot self-report their pain.1 In some instances the assessment of acute pain begins with a subjective scale, after patients are able to self-report pain. Although patient safety must always be considered in the use of sedation and analgesia, judicious use in unconscious patients is warranted. Some studies, including a functional brain imaging study, reported patterns of nervous system activation in unconscious patients similar to those of conscious controls, suggesting that pain perception may be intact in unconscious patients, and indicate stimulation of the pain regions of the brain.7 “The inability to communicate verbally does not negate the possibility that an individual is experiencing pain and is in need of appropriate pain-relieving treatment.”8
The use of behavioral instruments, for the assessment of acute pain in sedated adult patients unable to self-report is recommended by the Critical Care Medicine Association, the American Society of Perianesthesia Nurses, the ASA, and the American Pain Society, among others. Although they have not been examined in sedated PACU patients, behavioral instruments studied in patients with similar characteristics include the critical care pain observation tool (CPOT) and the nonverbal pain scale revised (NVPS-R).9–12 The main differences between the 2 instruments are the presence of the vocalization indicator for the CPOT and the physiological and respiratory indicators for the NVPS-R. The CPOT is described as the most psychometrically sound behavioral pain instrument for monitoring pain in medical, postoperative cardiac, and trauma intensive care unit adult patients who are unable to self-report.13
Although it is recommended that clinicians use a validated behavioral pain assessment instrument, there is inadequate evidence to guide recommendations for an optimal instrument for use in PACU. The purpose of this study was to evaluate and compare pain scores on 2 behavioral pain assessment instruments, the NVPS-R and CPOT, to determine whether either of these instruments is superior in adequately assessing the presence of acute pain in sedated patients in the PACU.
Study aims were: (1) to describe the relationships and change in pain scores over time obtained using the CPOT and NVPS-R assessment instruments; (2) to assess differences in pain scores between CPOT and NVPS-R assessment instruments and; (3) to explore the contribution of vocalization (CPOT) and physiological pain indicators (NVPS-R) in patients with significant pain.
METHODS
Design
A crossover design was used for this study. The study protocol was approved by the University of Massachusetts-Amherst Human Research Protection Office (HRPO) (Protocol #20152603) and University of Puerto Rico Medical Sciences Campus Institutional Review Board (IRB) (Protocol #A5570115).
Study Participant Recruitment
To generate baseline information that allowed us to explore this topic in-depth in a prospective study, we used a convenience sample of 40 patients. The information generated with this study allowed us to formulate information about the functionality of the CPOT and NVPS-R instrument in a group of Hispanic patients undergoing abdominal, pelvic, gastrointestinal, or gynecologic surgeries between October 20 and December 2, 2015 at the Medical Services Administration (ASEM) in the Puerto Rico Medical Center. Patients were included in the study if they: (1) were adults 21 years or older; (2) were able to give informed consent; (3) were under general anesthesia during surgery; (4) were unable to self-report pain using the traditional verbal scale upon arrival in the PACU; (5) were able to breathe spontaneously, and (6) had a Richmond Agitation Sedation Scale =–4 to –2 (indicating deep sedation [–4], moderate sedation [–3], or light sedation [–2]).14 Patients were excluded if the surgery was canceled, were under spinal or epidural anesthesia, remained verbal after the surgical procedure, or if they had cognitive impairment (ie, diagnosis of dementia or Alzheimer disease).10
Behavioral Pain Assessment Instruments
Acute pain in sedated patients unable to self-report was assessed using both NVPS-R and CPOT. The NVPS-R includes 3 behavioral indicators (facial expression, activity, and guarding), 1 physiological parameter; heart rate (HR) and blood pressure (BP), and 1 respiratory parameter; respiratory rate (RR) and pulse oxygen saturation (SpO2). The CPOT includes 4 behavioral pain indicators (facial expression, body movements, muscle tension, and compliance with the ventilator for intubated patients or vocalization for nonintubated patients). In this study, the vocalization indicator was used because only patients able to breathe spontaneously were included in the study. For both scales, each category of indicators is rated by behavioral or physiological severity from 0 to 2 to generate a total possible score of 10 for NVPS-R11 and a total possible score of 8 for CPOT.12 The presence of significant acute pain, for both instruments, was defined as a total score of ≥3.10,12 Therefore, those patients with a total score from 0 to 2 were categorized as not having significant pain.
Demographic and Clinical Data Acquisition
Patient medical records were used to collect demographic and clinical data that included sex, age, education, ASA physical status classification, surgery category, primary diagnoses, and preoperative and intraoperative pain medications administered (Table 1).
TABLE 1.
Characteristics of Study Participants (n=40)
| Characteristics | Total (n [%]) |
|---|---|
| Variables | |
| Sex | |
| Male | 11 (27.5) |
| Female | 29 (72.5) |
| Age (y) | |
| 49.3 (17.1) | |
| 22.0-87.0 | |
| American Society of Anesthesiologists Physical Status Classification System | |
| (1) Healthy patient | 4 (10.0) |
| (2) Mild systemic disease | 27 (67.5) |
| (3) Severe systemic disease | 8 (20.0) |
| (4) Incapacitating systemic disease | 1 (2.5) |
| Preoperative analgesic/medications treatment | |
| Yes | 17 (42.5) |
| No | 23 (57.5) |
| Preoperative analgesic/medications | |
| Acetaminophen | 6.0 (15.0) |
| Other pain medication | 13.0 (32.5) |
| No medication | 23.0 (57.5) |
| Surgery category | |
| Abdominal-pelvic | 7 (17.5) |
| Gastrointestinal | 15 (37.5) |
| Gynecologic | 18 (45) |
| Primary diagnoses | |
| Uterine myoma | 5 (12.5) |
| Hernia | 5 (12.5) |
| Gynecologic carcinoma | 10 (25) |
| Colon carcinoma | 6 (15.0) |
| Ureter carcinoma | 1 (2.5) |
| Other diagnoses | 13 (32.5) |
| Intraoperative opioids* | |
| Fentanyl | 40 (100.0) |
| Morphine | 21 (52.5) |
| Other | 0 (0.0) |
Not mutually exclusive.
Study Procedures
Preoperative Data Collection
Data collection began during the preoperative visit, 0 to 7 days before the scheduled surgery. Potential participants, who met inclusion criteria, were informed of the study purpose, risks, benefits, and confidentiality. Those who agreed to participate were asked to sign a consent form. Before surgery, sociodemographic information and vital signs (BP, HR, RR, and SpO2) were obtained from patient medical records to establish baseline physiological measures.
Postoperative Data Collection
After surgery, when the participant arrived at PACU (timepoint, 0 min), the baseline level of sedation was measured using the Richmond Agitation Sedation Scale. If the participant scored between –2 and –4, then both nonverbal pain scales, NVPS-R11 and CPOT,12 were administered using a randomized order. These scales were subsequently administered at timepoints 15, 30, 45, 60, 90, and 120 minutes until the patient was able to self-report in the PACU.
Statistical Analysis
The study cohort was described in terms of their sociodemographics, medical history, type of surgery, and pain variables using absolute frequencies and proportions (Table 1). For continuous variables, we used means (±SDs), or medians (interquartile range). Parameters of normality and homogeneity of variance were obtained. The nature and strength of the relationships between CPOT and NVPS-R total pain scores, at time 0 and 120 minutes, were explored using the Pearson correlations, scatter plots, and linear regression models.
To assess whether there were differences among the change in pain scores over time (at 0, 15, 30, 45, 60, 90, and 120 minutes after arrival in the PACU) between CPOT and NVPS-R we performed a paired t test analysis. A Bonferroni correction test was performed to adjust the P-value for the multiple comparison tests. In addition, a violin plot was generated to depict these results.
A multilevel linear regression model was performed to access the fixed and random effect that CPOT and NVPS-R have on the mean pain scores of patients across time. For this model, we evaluated the different pain behavior scores assessment instruments (CPOT, NVPS-R), and the 7 different timepoints (Table 2).
TABLE 2.
Linear Mixed Regression Model for Predicting the Mean Pain Scores Among Sedated Patients Unable to Self-Report at Postanesthesia Care Unit
| Estimated Coefficient | 95% Confidence Interval | P | |
|---|---|---|---|
| Fixed part | |||
| Intercept | 2.52 | 1.99-3.06 | ≤0.001* |
| Pain behavior assessment tool | |||
| CPOT | 0.00 | — | — |
| NVPS | −0.15 | −0.78 to 0.48 | 0.637 |
| Postoperative time (min) | |||
| 0 | 0.00 | — | — |
| 15 | 0.36 | −0.28 to 1.00 | 0.259 |
| 30 | 0.19 | −0.45 to 0.84 | 0.554 |
| 45 | 0.13 | −0.51 to 0.77 | 0.683 |
| 60 | −0.26 | −0.91 to 0.39 | 0.422 |
| 90 | −0.61 | −1.28 to 0.06 | 0.069 |
| 120 | −0.93 | −1.67 to −0.19 | 0.012* |
| Random effect | |||
| Intercept variance | 0.83 | 0.49-1.40 | — |
| Intercept residual | 2.02 | 1.78-2.31 | — |
CPOT for the groups and minute 0 for the time were considered as reference for this assessment.
No interaction was found between the pain behavior assessment tools and the postoperative time variables.
Statistically significant P-value (P<0.05).
CPOT indicates critical care pain observation tool; NVPS-R, nonverbal pain scale revised.
Additional analyses, using a Kruskal-Wallis test, compared CPOT and NVPS-R in terms of selected vital sign indicators: HR, mean arterial pressure (MAP), RR, and SpO2 at different timepoints (Tables 4–6). All the data for this study were stored in REDCap15 and was analyzed using Stata version 14 (StataCorp LP, College Station, TX).
TABLE 4.
Summary Statistics of Vital Signs and Respiratory Indicators by Pain Behavior Groups at Postanesthesia Care Unit: Time 0
| Group A (n=23) | Group B (n=3) | Group C (n=14) | P* | |
|---|---|---|---|---|
| Heart rate (L/min) | 0.11 | |||
| Median (IQR) | 77 (17) | 99 (12) | 82 (30) | |
| Range | 60-113 | 90-102 | 56-102 | |
| MAP (mm Hg) | 0.89 | |||
| Median (IQR) | 92.7 (20) | 97.7 (67.3) | 97.5 (18) | |
| Range | 73-117.7 | 68-135.3 | 68.7-119 | |
| SpO2 (%) | 0.21 | |||
| Median (IQR) | 100 (2) | 100 (0) | 99 (2) | |
| Range | 95-100 | 100-100 | 92.0-100 | |
| RR (r/min) | 0.74 | |||
| Median (IQR) | 18 (5) | 18 (1) | 16.5 (8) | |
| Range | 9-28 | 18-19 | 9-36 |
Group A, both CPOT and NVPS-R total pain scores were ≤2; group B, CPOT or NVPS-R total pain scores (but not both) were ≥3; group C, both CPOT and NVPS-R total pain scores were ≥3.
A Kruskal-Wallis test was performed to assess association between vital signs and pain behavior groups.
CPOT indicates critical care pain observation tool; MAP, mean arterial pressure; NVPS-R, nonverbal pain scale revised; RR, respiratory rate.
TABLE 6.
Summary Statistics of Vital Signs and Respiratory Indicators by Pain Behavior Groups at Postanesthesia Care Unit: Time 120
| Group A (n=23) | Group B (n=3) | Group C (n=14) | P* | |
|---|---|---|---|---|
| Heart rate (L/min) | 0.85 | |||
| Median (IQR) | 76 (16.5) | 84.5 (40) | 75 (15) | |
| Range | 53-110 | 58-115 | 66-88 | |
| MAP (mm Hg) | 0.32 | |||
| Median (IQR) | 95.3 (13) | 104.7 (13.3) | 100 (25.8) | |
| Range | 69.7-112.7 | 97.3-112 | 66.3-109.3 | |
| SpO2 (%) | 0.44 | |||
| Median (IQR) | 100 (0.5) | 100 (0) | 99.5 (4.5) | |
| Range | 98-100 | 100-100 | 92-100 | |
| RR (r/min) | 0.16 | |||
| Median (IQR) | 17.5 (6) | 13.5 (6.5) | 18 (4) | |
| Range | 8-27 | 10-18 | 18-26 |
Group A, both CPOT and NVPS-R total pain scores were ≤2; group B, CPOT or NVPS-R total pain scores (but not both) were ≥3; group C, both CPOT and NVPS-R total pain scores were ≥3.
A Kruskal-Wallis test was performed to assess association between vital signs and pain behavior groups.
CPOT indicates critical care pain observation tool; MAP, mean arterial pressure; NVPS-R, nonverbal pain scale revised; RR, respiratory rate.
RESULTS
Initially, 59 patients scheduled for abdominal, pelvic, gynecologic, or gastrointestinal surgery consented to participate in this study. Of these, a total of 19 patients were excluded due to chronic cognitive impairment (n=1), canceled surgeries (n=8), use of regional anesthesia during surgery (n=5), or that they were verbal upon arrival to the PACU (n=5). Our final convenience sample consisted of 40 patients with a mean age of 49.3±17.1 (Table 1). The majority of participants (72.5%) were female and all participants (100%) were of Hispanic origin. Most participants had mild systemic disease (67.5%) and did not receive preoperative pain medication. The surgical categories included in the study were 45% gynecologic, 37.5% gastrointestinal, and 17.5% abdominal-pelvic surgery. Most of the participants had a cancer diagnosis (42.5%); of these 25% were gynecologic carcinoma, 15% were colon carcinoma, and 2.5% were ureter carcinoma (Table 1).
A total of 246 assessments were obtained using CPOT and NVPS-R instruments in the 40 patients in this study. The positive linear relationship among the pain total scores at timepoint 0 when comparing CPOT and NVPS-R (r=0.88; P≤0.05) is shown in Figure 1. CPOT scores explained 77% of the variance observed in the NVPS-R scores. At the 120-minute timepoint, CPOT scores explained 80% of the variance in the NVPS-R scores (Fig. 2). Both results were statistically significant (P≤0.05).
FIGURE 1.
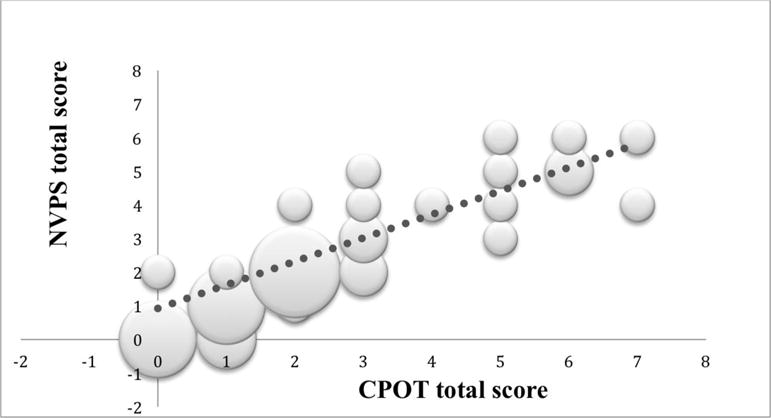
Scatter plot of CPOT versus NVPS-R total scores at 0-minute timepoint. The size of the dot visually represents the amount of possible (x, y) pairs in the same coordinate. The bigger the dot the more cases that reported the same scores on both scales. CPOT indicates critical care pain observation tool; NVPS-R, nonverbal pain scale revised.
(The size of the dot visually represents the amount of possible (x, y) pairs in the same coordinate. The bigger the dot the more cases that reported the same scores on both scales).
FIGURE 2.
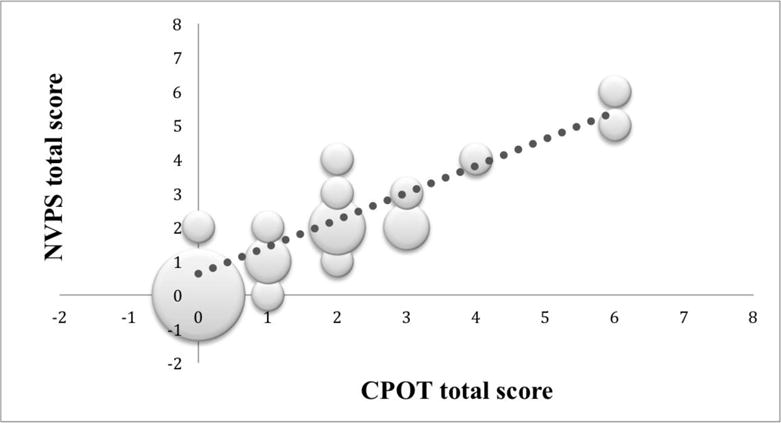
Scatter plot of CPOT versus NVPS-R total scores at 120-minute timepoint. The size of the dot visually represents the amount of possible (x, y) pairs in the same coordinate. The bigger the dot the more cases that reported the same scores on both scales. CPOT indicates critical care pain observation tool; NVPS-R, nonverbal pain scale revised.
(The size of the dot visually represents the amount of possible (x,y) pairs in the same coordinate. The bigger the dot the more cases that reported the same scores on both scales
To observe changes in pain scores over time, we evaluated the CPOT and NVPS-R total scores at 6 timepoints (0, 30, 45, 60, 90, and 120 min). In Figure 3 we show, in a violin plot, the distribution of the median change in scores across different timepoints. After the Bonferroni correction (whose significant P-value was set to be P≤0.007), we did not find any significant differences in the change in pain behavior scores obtained by CPOT and NVPS-R at different timepoints.
FIGURE 3.
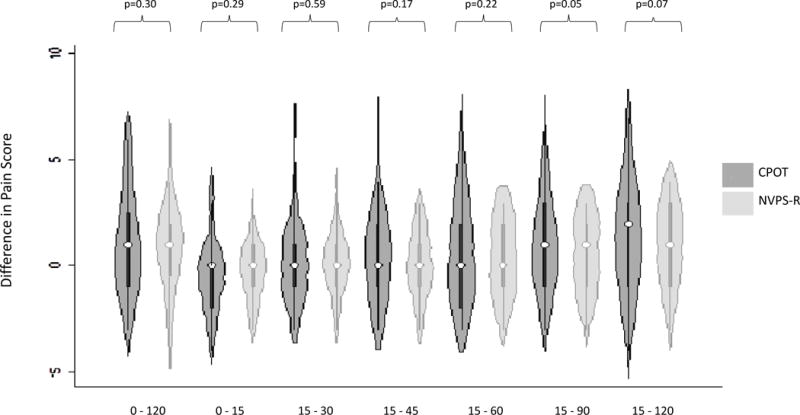
Violin plot showing difference in pain score between CPOT and NVPS-R across time. For the change in pain score behavior through time, P-values were calculated using paired t test analysis. A Bonferroni correction test was used and the statistical significance value was set to be (P≤0.007). CPOT indicates critical care pain observation tool; NVPS-R, nonverbal pain scale revised.
8For the change in pain score behavior through time, P-values were calculated using paired t-test analysis.
**A Bonferroni correction test was used and the statistical significance value was set to be (p ≤ 0.007)
In the multilevel mixed regression model we observed that the overall mean pain score of sedated patients unable to self-report in the PACU was ∼2.52 (95% confidence interval, 1.99-3.06), after adjusting for pain behavior assessment instruments and the multiple postoperative time in minutes. There was no statistically significant difference in the mean pain scores calculated by 2 pain assessment instruments (NVPS-R and CPOT). When we evaluated pain scores over the follow-up time, there were no statistically significant differences between the mean pain scores obtained at minutes 15 through 90, and those obtained at baseline (minute 0). However, the mean pain score was significantly lower at minute 120, when compared with that at minute 0, with the adjusted difference of (Δadj) of −0.93 (95% confidence interval, −1.67 to −0.19). The estimated intraclass correlation coefficient for this model was 0.29; indicating that ∼29% of the variance in pain scores can be attributed to differences between patients (Table 2).
Role of Vocalization and Physiological Indicators in Pain Assessment
An evaluation was made to explore in-depth indicators that were found to be different between the 2 pain instruments (ie, vocalization in CPOT and physiological and respiratory parameters in NVPS-R) (Table 3). Only patients with scores indicating significant pain (CPOT or NVPS-R≥3) were included. Results showed that CPOT vocalization was consistently frequent in patients with significant pain (from 74% to 100%), whereas the frequency of physiological indicators on the NVPS-R varied among timepoints in patients with significant pain. In addition, changes in the RR on the NVPS-R scale, were hardly found among patients with significant pain. As shown in Table 3, vocalization was more frequent at each timepoint when compared with the physiological and respiratory indicators. These findings do not confirm the original observation that NVPS-R vital signs correlate with behavioral indicators of pain. And they suggest that medicating based on acute vital signs alone may be dangerous in the sedated patient.
TABLE 3.
Patients With Significant Pain that Exhibit CPOT Vocalization, NVPS-R Physiological, and Respiratory Indicators ≥1
| CPOT | NVPS-R | ||||
|---|---|---|---|---|---|
| Timepoints (min) | CPOT ≥3 Frequency* | Vocalization ≥1 Frequency (n [%]) | NVPS-R≥3 Frequency* | Physiological ≥1 Frequency (Blood Pressure, Heart Rate) (n [%]) | Respiratory ≥1 Frequency (SpO2, Respiratory Rate) (n [%]) |
| 0 | 16 | 14 (88) | 15 | 5 (33) | 2 (13) |
| 15 | 22 | 19 (86) | 20 | 5 (25) | 0 (0) |
| 30 | 19 | 18 (95) | 15 | 7 (47) | 2 (13) |
| 45 | 19 | 14 (74) | 17 | 4 (24) | 2 (12) |
| 60 | 15 | 12 (80) | 12 | 3 (25) | 0 (0) |
| 90 | 10 | 9 (90) | 11 | 6 (55) | 3 (27) |
| 120 | 6 | 6 (100) | 6 | 4 (67) | 0 (0) |
Total patients with significant pain per timepoints using CPOT and NVPS-R.
CPOT indicates critical care pain observation tool; NVPS-R, nonverbal pain scale revised.
Ancillary analyses were used to evaluate the relationships between selected vital sign indicators of the CPOT and NVPS-R. These included HR, MAP, RR, and SpO2. Vital signs at the 0, 15, and 120-minute timepoints were evaluated using the Kruskal-Wallis test; none were significantly different (P>0.05). Another evaluation to assess differences among specific vital signs was made by classifying our patients into 3 groups considered clinically different (group A CPOT total ≤2 and NVPS-R total ≤2); (group B one of CPOT total ≥3 or NVPS-R total ≥3); and (group C CPOT total ≥3 and NVPS-R total ≥3) at the 0-minute (Table 4), 15-minute (Table 5), and 120-minute (Table 6) timepoints. All 3 groups were similar in terms of their median (IQR).
TABLE 5.
Summary Statistics of Vital Signs and Respiratory Indicators by Pain Behavior Groups at Postanesthesia Care Unit: Time 15
| Group A (n=23) | Group B (n=3) | Group C (n=14) | P* | |
|---|---|---|---|---|
| Heart rate (L/min) | 0.14 | |||
| Median (IQR) | 75 (15) | 91 (9) | 82 (28) | |
| Range | 56-92 | 80-94 | 59-120 | |
| MAP (mm Hg) | 0.69 | |||
| Median (IQR) | 94 (13.3) | 99.3 (50) | 95.5 (23) | |
| Range | 73-111 | 85-123 | 67.3-122.7 | |
| SpO2 (%) | 0.30 | |||
| Median (IQR) | 100 (1) | 100 (0) | 99.5 (2) | |
| Range | 96-100 | 100-100 | 83-100 | |
| RR (r/min) | ||||
| Median (IQR) | 17 (5) | 16 (4) | 17 (8) | 0.99 |
| Range | 11-26 | 14-20 | 10-25 | |
Group A, both CPOT and NVPS-R total pain scores were ≤2; group B, CPOT or NVPS-R total pain scores (but not both) were ≥3; group C, both CPOT and NVPS-R total pain scores were ≥3.
A Kruskal-Wallis test was performed to assess association between vital signs and pain behavior groups.
CPOT indicates critical care pain observation tool; MAP, mean arterial pressure; NVPS-R, nonverbal pain scale revised; RR, respiratory rate.
DISCUSSION
Postoperative patients first become aware of acute pain in the PACU; thus, one of the most important goals in the postoperative period is pain management. The consequences of severe postoperative acute pain can contribute to the development of several multisystem effects that may adversely affect postoperative outcomes. Patients may present with different physiological stress responses to surgery, and fluid retention initiated by neural stimuli.16 They are at risk for increases in physiological parameters such as HR and BP,17 atelectasis,18 blood clots, pneumonia, vasoconstriction, decreased tissue oxygen partial pressure,19 hypermetabolism resulting in hyperglycemia, fluid retention or elimination,16 delayed wound healing, risk of wound infection,20 reduced mobility, impaired physical function, disturbed sleep, and immune impairment, among others.21 In the United States, it has been reported that patients only have 1 in 4 chance of receiving adequate pain relief after surgery.22
In 2016, the American Pain Society reported that there was insufficient evidence to recommend specific pain assessment instruments to track responses to postoperative pain treatments to adjust personalized pain management plans.1 Clinicians must be able to reliably detect pain, using pain assessment methods adapted for patients unable to self-report pain. These include patients with diminished levels of consciousness (sedated).9 Behavioral pain scales have been studied and recommended for pain assessment in patients unable to self-report pain in contexts similar to PACU. Patients’ behavioral reactions can be used as surrogate measures of pain, as long as their motor function is intact.10 Therefore, this study examined, for the first time, the use of 2 behavioral pain assessment instruments, the CPOT and the NVPS-R, for the assessment of acute pain in sedated PACU patients unable to self-report. The use of objective measures by nurses and other health care professionals could reduce the underestimation of pain in postoperative patients, improve postoperative outcomes, and increase pain relief in the PACU.
Findings of this study provide evidence to support relationships between the CPOT and NVPS-R in certain behavioral indicators including facial expressions, muscle tension, and body movements. Pain scores on both instruments, based on behavioral indicators, were strongly correlated. Other studies with sedated, critical care patients established the reliability of behavioral pain indicators, which supports the observed correlations between CPOT and NVPS-R in this study.26,27 However, despite observed associations between total pain scores on both scales, findings reported here suggest that vital signs, as measured by the NVPS-R, are not consistent indicators of significant acute pain in sedated PACU patients. These findings were supported by other authors, who have indicated that changes in vital signs might not be specific to pain, and physiological indicators lack sensitivity in assessing acute pain.28,29
In contrast, the finding that the frequency of vocalization, as measured by the CPOT, is a consistent indicator of significant pain, warrants further investigation. The main differences observed between the 2 behavioral pain assessment instruments was that the vocalization indicator of the CPOT, in patients with significant pain, was the most frequent pain indicator in comparison with the physiological and respiratory indicators of the NVPS-R (Table 6). This suggests the superiority of the CPOT for the assessment of acute pain in sedated PACU patients.
There is an absence of studies to support and recommend a specific pain assessment instrument for sedated patients unable to self-report pain in the PACU. This can lead to inaccurate assessment and undertreatment of acute pain, and increase the gap in the standard of care for pain assessment and management. Sedated patients who are accurately assessed and adequately treated for acute pain in the PACU may have lower rates of postoperative complications.1,27
In summary, the results of this clinical study suggest that the vocalization indicator of the CPOT was superior at assessing acute postoperative pain as compared with the physiological and respiratory indicators of the NVPS-R. Significant change in the vital sign and respiratory indicators of the NVPS-R did not occur, even in the presence of significant pain. And, despite an overall high correlation between total pain scores of the NVPS-R and CPOT, findings suggest that physiological and respiratory pain indicators of the NVPS-R, measured over time in sedated patients with acute pain presence, are not consistent in their results. Identification of valid, reliable instruments will lead to the development of institutional policies and procedures for effective postoperative pain assessment and management. Future study of the CPOT, specifically the vocalization indicator, must continue to define and establish a valid, reliable, and consistent measure to assess acute pain in sedated PACU patients unable to self-report pain.
The strengths of this study include the randomization used to reduce the bias for order effect, and the use of 2 tested and validated objective instruments for the assessment of pain in a previously unstudied population: sedated PACU patients unable to self-report. Limitations of the study included the progressive attrition of patients throughout the study, as they could self-report pain they were no longer eligible for the evaluation, the selection of patients with specific type of surgeries, which limits the generalization of results to other populations with different characteristics or types of surgeries, and the small sample size. Finally, female sex could be a potential source of bias, because of the high proportion of gynecologic procedures, which comprised 45% of the total of surgical procedures in the study.
Supplementary Material
Acknowledgments
Supported by the University of Puerto Rico (UPR) Medical Sciences Campus, the Center for Research and Evidence-Based Practice at the UPR School of Nursing, the Puerto Rico Clinical and Translational Research Consortium (2U54MD007587) and the Medical Services Administration at the Medical Center of Puerto Rico.
Footnotes
The authors declare no conflict of interest.
References
- 1.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Commi. J Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Puntillo K, Miaskowski C. A review of objective pain measures for use with critical care adult patients unable to self-report. J Pain. 2008;9:2–10. doi: 10.1016/j.jpain.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Fast Stats Inpatient surgery. 2010 Available at: www.cdc.gov/nchs/fastats/insurg.htm. Accessed December 16, 2010.
- 4.Ghan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30:149–160. doi: 10.1185/03007995.2013.860019. [DOI] [PubMed] [Google Scholar]
- 5.Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 6.Buvanendran A, Fiala J, Patel KA, et al. The incidence and severity of postoperative pain following inpatient surgery. Pain Med. 2015;16:2277–2283. doi: 10.1111/pme.12751. [DOI] [PubMed] [Google Scholar]
- 7.Boly M, Faymonville ME, Schnakers C, et al. Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol. 2008;7:1013–1020. doi: 10.1016/S1474-4422(08)70219-9. [DOI] [PubMed] [Google Scholar]
- 8.International Association for the Study of Pain. Pain terms: a list with definitions and notes on usage. Pain. 2010;6:147. [Google Scholar]
- 9.Stites M. Observational pain scales in critically ill adults. Pain Manag. 2013;33:68–79. doi: 10.4037/ccn2013804. [DOI] [PubMed] [Google Scholar]
- 10.Barr J, Fraser G, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 11.Odhner M, Wegman D, Freeland N, et al. Assessing pain control in nonverbal critically ill adults. Dimens Crit Care Nurs. 2004;22:260–267. doi: 10.1097/00003465-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Gélinas C, Fillion L, Puntillo KA, et al. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;4:420–427. [PubMed] [Google Scholar]
- 13.Marmo L, Fowler S. Pain assessment tool in the critically ill post-open heart surgery patient population. Pain Man Nurs. 2010;11:134–140. doi: 10.1016/j.pmn.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 15.Harris P, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holte K. Pathophysiology and clinical implications of perioperative fluid management in elective surgery. Dan Med Bull. 2010;57:B4156. [PubMed] [Google Scholar]
- 17.Payen JF, Bru O, Bosson JL, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29:2258–2263. doi: 10.1097/00003246-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Puntillo KA, Weiss SJ. Pain: its mediators and associated morbidity in critically ill cardiovascular surgical patients. Nurs Res. 1994;43:31–36. [PubMed] [Google Scholar]
- 19.Akca O, Melischek M, Scheck T, et al. Postoperative pain and subcutaneous oxygen tension. Lancet. 1999;354:41–42. doi: 10.1016/S0140-6736(99)00874-0. [DOI] [PubMed] [Google Scholar]
- 20.McGuire L, Heffner K, Glaser R, et al. Pain and wound healing in surgical patients. Ann Behav Med. 2006;31:165–172. doi: 10.1207/s15324796abm3102_8. [DOI] [PubMed] [Google Scholar]
- 21.Leavitt SB. Postsurgical pain undertreated in most patients. 2011 [Google Scholar]
- 22.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215–2225. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- 23.Payen J, Bosson J, Chanques G, et al. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit. Anesthesiology. 2009;111:1308–1316. doi: 10.1097/ALN.0b013e3181c0d4f0. [DOI] [PubMed] [Google Scholar]
- 24.Anand KJ, Craig KD. New perspectives on the definition of pain. Pain. 1996;67:3–6. doi: 10.1016/0304-3959(96)03135-1. [DOI] [PubMed] [Google Scholar]
- 25.Cade CH. Clinical tools for the assessment of pain in sedated critically ill adults. Nurs Crit Care. 2008;13:288–297. doi: 10.1111/j.1478-5153.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- 26.Kabes AM, Graves JK, Norris J. Further validation of the nonverbal pain scale in intensive care patients. Crit Care Nurs. 2009;29:59–66. doi: 10.4037/ccn2009992. [DOI] [PubMed] [Google Scholar]
- 27.Kapoustina O, Echegaray-Benites C, Gélinas C. Fluctuations in vital signs and behavioural responses of brain surgery patients in the intensive care unit: are they valid indicators of pain? J Adv Nurs. 2014;70:2562–2576. doi: 10.1111/jan.12409. [DOI] [PubMed] [Google Scholar]
- 28.Gélinas C, Johnson C. Pain assessment in the criticallly ill ventilated adult: validation of the Critical-Care Pain Observation Instrument and physiological indicators. Clin J Pain. 2007;23:497–505. doi: 10.1097/AJP.0b013e31806a23fb. [DOI] [PubMed] [Google Scholar]
- 29.Hossein AP. Association between acute pain and hemodinamic parameters in a postoperative surgical intensive care unit. AORN J. 2017;6:571–578. doi: 10.1016/j.aorn.2017.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


