Abstract
Objective
The choice between neoadjuvant chemotherapy (NACT) versus primary debulking surgery (PDS) for advanced epithelial ovarian cancer (AEOC) remains controversial in the U.S. Generalizability of existing trials has been criticized due to less aggressive debulking. Using data from a trial performing aggressive debulking, we investigated the cost-effectiveness of NACT versus PDS for AEOC.
Methods
A decision tree model was constructed to estimate differences in short-term outcomes and costs for a hypothetical cohort of 20,000 AEOC patients (the U.S. annual incidence of ovarian cancer) treated with NACT versus PDS over a 1-year time horizon from a Medicare payer perspective. Outcomes included costs per cancer-related death averted, life-years and quality-adjusted life-years (QALY) gained. Base-case probabilities, costs, and utilities were based on the SCORPION trial. Base-case analyses assumed equivalent survival; threshold analysis estimated the maximum survival difference that would result in NACT being cost-effective at $50,000/QALY and $100,000/QALY willingness-to-pay thresholds. Probabilistic sensitivity analysis (PSA) was used to characterize model uncertainty.
Results
Compared to PDS, NACT was associated with $189M in cost savings, 1,463 fewer AEOC-related deaths, and 1,807 life-years and 2,148 QALYs gained, making it the dominant treatment strategy for all outcomes. In PSA, NACT remained dominant in 99.3% of simulations. NACT remained cost-effective at $50,000/QALY and $100,000/QALY willingness-to-pay thresholds if survival differences were <2.7 and 1.4 months, respectively.
Conclusion
In the short term, NACT is cost-saving with improved outcomes. If PDS provides a longer-term survival advantage, it may be cost-effective. Future research is needed on the role of patient preferences in tradeoffs between survival and quality-of-life.
Keywords: cost-effectiveness analysis, neoadjuvant chemotherapy, advanced ovarian cancer
Introduction
Ovarian cancer is the fifth leading cause of cancer-related death among women, largely due to the majority of cases being diagnosed at advanced stage [1, 2]. The standard treatment for advanced ovarian cancer in the U.S. has traditionally been primary debulking surgery (PDS), which aims to remove the majority of tumor burden upfront, followed by adjuvant chemotherapy (ACT). PDS that results in minimal to no residual disease is an important predictor of survival [3–11]. However, achieving optimal cytoreduction with PDS often requires radical surgery that can be associated with substantial morbidity, including post-operative complications that can delay chemotherapy initiation and decrease overall survival [4, 12–14].
An alternative treatment approach is neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) and ACT. NACT aims to reduce the tumor burden prior to surgery, which can decrease the need for aggressive procedures. This reduces the risk of post-operative morbidity that can result in costly hospital readmissions and decrements to quality-of-life. Accordingly, NACT may be a cost-saving treatment alternative if patients treated with NACT have comparable overall survival to patients treated with PDS.
The comparative effectiveness of NACT versus PDS in terms of overall survival remains controversial in the U.S. [15–23]. Among women with AEOC, two Phase III randomized trials, conducted in the UK and Europe, observed non-inferior survival with NACT compared to PDS [24, 25]. However, the generalizability of these studies to U.S. practice has been questioned due to shorter median overall survival and lower rates of optimal debulking among PDS patients than typically seen in the large U.S. centers [15, 17, 19]. For instance, in the European Organization for Research and Treatment of Cancer (EORTC) 55971 trial, only 42% of PDS patients were optimally debulked, whereas optimal debulking rates of 75% are commonly reported at U.S. centers [15, 17]. These differences may result from more aggressive surgical approaches commonly used in the U.S. Without existing evidence from an aggressive surgical setting, the role of NACT for AEOC in the U.S. is widely disputed [15–23].
Currently, a Phase III superiority trial is being conducted in Italy (Surgical Complications Related to Primary or Interval Debulking in Ovarian Neoplasms [SCORPION]), comparing NACT to PDS among women with bulky AEOC. This trial is employing more aggressive surgery, with optimal debulking rates more comparable to those in the U.S. [26]. Although overall survival outcomes have not yet been reported, results of postoperative outcomes indicate that NACT patients experienced significantly fewer early and late postoperative complications and reported significantly better quality-of-life, compared to patients who received PDS. These results represent the first randomized trial data on short-term morbidity and mortality between NACT and PDS in a population that has undergone aggressive debulking surgery.
In the absence of conclusive evidence on the effectiveness of NACT versus PDS in terms of overall survival, cost-effectiveness analyses of short-term outcomes may help inform decision-making among women for whom the ideal treatment is unclear. No cost-effectiveness analyses have compared NACT and PDS for AEOC using an aggressive surgical paradigm comparable to U.S. To that end, the objectives of the present study were to: 1) estimate the cost-effectiveness and cost-utility of NACT versus PDS for stage IIIC-IV AEOC patients using an aggressive surgical approach, and 2) estimate the maximum difference in overall survival between NACT and PDS at which NACT would remain cost-effective at $50,000/quality-adjusted life-year (QALY) and $100,000/QALY willingness-to-pay (WTP) thresholds.
Methods
A decision tree model was constructed to estimate differences in Grade III-V peri- and post-operative complications and costs for a hypothetical cohort of 20,000 AEOC patients over a 1-year time horizon from a U.S. Medicare payer perspective (Figure 1). With nearly half of ovarian cancer cases occurring in women age 65 and over, Medicare represents a major U.S. payer in the treatment of this disease [2]. A cohort size of 20,000 was chosen based on the annual incidence of ovarian cancer in the U.S. [2] Women in the hypothetical cohort received either PDS followed by six rounds of adjuvant intravenous (IV) paclitaxel/carboplatin [27], or 3 rounds of neoadjuvant IV paclitaxel/carboplatin followed by interval debulking surgery (IDS) and an additional 3 rounds of adjuvant IV paclitaxel/carboplatin.
Figure 1. Decision tree comparing neoadjuvant chemotherapy to primary debulking surgery for bulky advanced ovarian cancer.
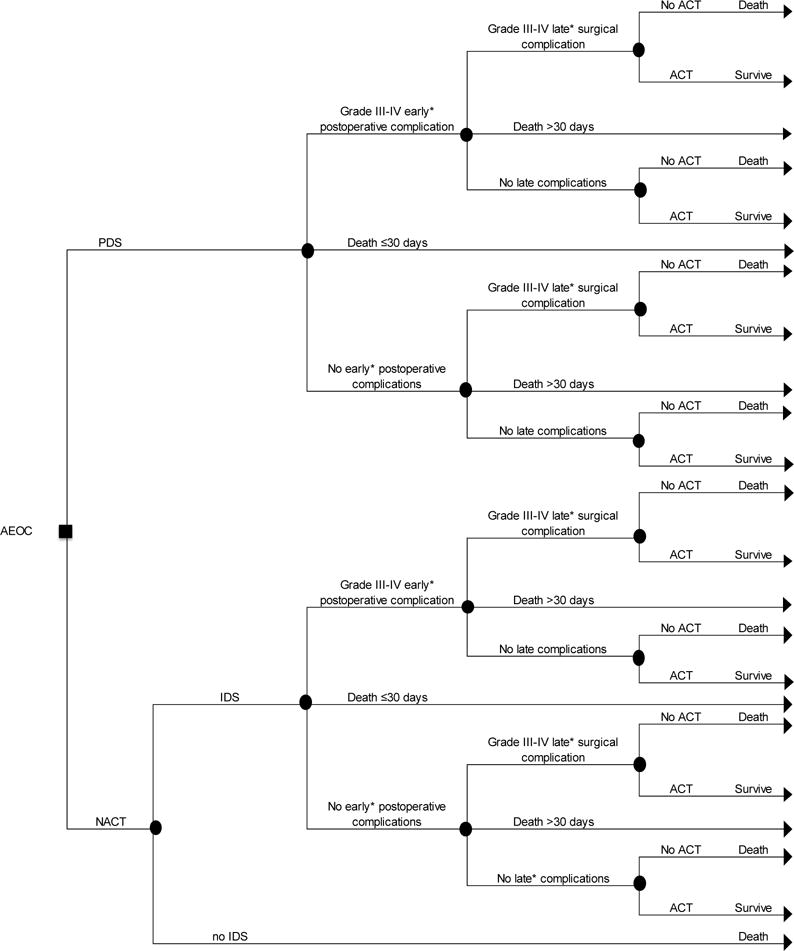
The decision tree presented here describes the potential experiences of bulky AEOC patients who receive either PDS followed by ACT or NACT followed by IDS. Patients move left to right through the tree. The square node represents the decision to treat patients with PDS or NACT. Circle nodes represent the chance of a given outcome; triangle nodes represent a terminal event.
Abbreviations: AEOC, advanced epithelial ovarian cancer; PDS, primary debulking surgery; NACT, neoadjuvant chemotherapy; IDS, interval debulking surgery; ACT, adjuvant chemotherapy
*Early complications include those occurring ≤30 days post-surgery; Late complications include those occurring 1-6 months post-surgery.
For each treatment alternative in the decision tree, we estimated the cohort-level treatment costs, number of ovarian cancer-related deaths, and survival time in life-years and quality-adjusted life-years (QALYs) that would be observed if all 20,000 women in the hypothetical cohort received a given treatment (NACT or PDS). Quality-adjusted life-years (QALYs) represent the time spent in a particular health state (for example, postoperative complications), weighted using utilities that reflect the quality-of-life associated with that health state. Utility weights range from 0 to 1, with 0 representing the worst health state (e.g., death) and 1 representing the utility associated with perfect health. Estimation of outcomes is described below. These outcomes were then used to calculate incremental cost-effectiveness ratios (ICERs), representing the cost per life-year gained, cost per QALY gained, and cost per short-term ovarian cancer-related death averted, associated with treatment with NACT relative to treatment with PDS.
Event probabilities
Base-case event probabilities were based on results reported in the SCORPION trial (Table 1) [26]. The SCORPION trial was selected as it is the currently available randomized trial in which the most aggressive surgical approach was taken. Patients treated with NACT who did not undergo IDS due to disease progression were estimated to have a life expectancy of 10.5 months from the start of treatment, based on existing literature [28]. Patients who did not receive ACT following debulking surgery were estimated to have a life expectancy of 2.7 months, based on prior trial evidence [24].
Table 1.
Event probabilities used in decision tree analysis of neoadjuvant chemotherapy versus primary debulking surgery for bulky advanced epithelial ovarian cancer
| Probability | ||
|---|---|---|
| Event | PDS | NACT |
| Debulking surgery | 1.00 | 0.95 |
| Neoadjuvant chemotherapy | – | 1.00 |
| Early post-operative complications (≤30 days post-surgery) | ||
| Grade III-IV | 0.49 | 0.06 |
| Death | 0.04 | 0.00 |
| Adjuvant chemotherapy | ||
| Among late complications | 0.81 | 1.00 |
| Among no late complications | 0.94 | 1.00 |
| Late post-operative complications (1-6 months post-surgery) | ||
| Grade III-IV | 0.13 | 0.00 |
| Death | 0.02 | 0.00 |
Abbreviations: PDS, primary debulking surgery; NACT, neoadjuvant chemotherapy
All probabilities were derived from Fagotti et al[26]
Costs
Costs associated with treating 20,000 women in the hypothetical cohort included the cost of the hospitalization associated with debulking surgery (with or without major complications) [29], the base cost of the debulking procedure plus a weighted average of the cost of additional procedures [30] (using procedure rates reported for each arm of SCORPION), the cost of major late (>30 days post-surgery) complications [29], and the cost of chemotherapy administered (Table 2) [31]. Administrative billing codes used to estimate costs are available in Supplemental Table 1. All costs were inflation-adjusted to 2015 US dollars using the medical care components of the Consumer Price Index from the Bureau of Labor Statistics [32].
Table 2.
Cost estimates for decision tree analysis of neoadjuvant chemotherapy versus primary debulking surgery for bulky advanced epithelial ovarian cancer
| Event | PDS | NACT | Source |
|---|---|---|---|
| Debulking surgery | $2,095 | $2,095 | 2015 Medicare Physician Fee Schedule[30] |
| Additional surgical procedures* | $924 | $3,907 | 2015 Medicare Physician Fee Schedule[30] |
| Surgical hospitalization | |||
| Without complications | $11,470 | $11,470 | HCUPnet[29] |
| With major complications | $27,030 | $27,030 | HCUPnet[29] |
| Readmission for late complication* | $5,152 | $5,152** | HCUPnet[29] |
| Death | $0 | $0 | |
| Chemotherapy (3 rounds) | $1,254 | $1,254 | 2015 ASP[31] + 6%, 2015 Medicare Physician Fee Schedule[30] |
Abbreviations: PDS, primary debulking surgery; NACT, neoadjuvant chemotherapy; HCUP, Hospital Cost and Utilization Project; ASP, average sales price
Cost was calculated as the weighted average of results reported in Fagotti et al[26]; see Table S1 for individual procedures and probabilities.
No late complications were observed among NACT patients in Fagotti et al[26]; the cost of late complications were assumed to be equal to those of PDS when varying this probability in sensitivity analysis.
Chemotherapy costs were calculated based on treatment with paclitaxel and carboplatin, administered every 21 days. Chemotherapy dosing was calculated as 175 mg/m2 for paclitaxel and an area under the curve (AUC) of 5 for carboplatin, based on treatment in the SCORPION trial and current guidelines (Table S1) [26, 33]. We assumed that all patients who initiated chemotherapy completed all cycles (3 cycles NACT + 3 cycles ACT among NACT patients; 6 cycles ACT among PDS patients). Because there were no statistically significant differences in the incidence of chemotherapy-related toxicities between groups in SCORPION, these events were not incorporated into our model.
Quality of life
To calculate average QALYs associated with each treatment alternative, EORTC Cancer Quality of Life Questionnaire (QLQ-C30) scores from SCORPION were converted to EuroQol five dimension (EQ-5D) utility weights using a published algorithm [34]. Utility weights from the published literature [35] were used for patients who did not undergo ACT following debulking surgery and patients who did not undergo IDS following NACT, since these patients did not complete quality-of-life assessments in SCORPION [26]. QALYs were calculated as a weighted average of utility weights for the time spent in each sub-branch of the decision tree (Table 3).
Table 3.
Utility weights used for quality-adjusted life-years in decision tree analysis of neoadjuvant chemotherapy versus primary debulking surgery in bulky advanced epithelial ovarian cancer
| Utility weight | Time frame (months) | |||
|---|---|---|---|---|
| PDS | NACT | Source | ||
| Neoadjuvant chemotherapy | – | 0.58 | 2.08 | Fagotti et al[26] |
| Post-surgery + 3 rounds ACT | 0.48 | 0.66 | 3.07 | Fagotti et al[26] |
| ACT (rounds 4-6) | 0.57 | – | 2.08 | Fagotti et al[26] |
| no ACT following PDS | 0.28 | – | 2.70 | Havrilesky et al[35] |
| no IDS following NACT | – | 0.16 | 8.42 | Havrilesky et al[35] |
| Death | 0.00 | 0.00 | varies | |
| >1 year post-treatment | 0.83 | 0.83 | varies* | Havrilesky et al[35] |
Abbreviations: ACT, adjuvant chemotherapy; PDS, primary debulking surgery; IDS, interval debulking surgery; NACT, neoadjuvant chemotherapy
Utility weight applied to all survival time beyond 1 year (threshold analysis)
Threshold analysis
In addition to the main analysis, a threshold analysis was performed to identify the overall survival among NACT patients that would result in cost-effectiveness at $50,000/QALY and $100,000/QALY WTP thresholds. The threshold analysis assumed that all time beyond the initial 1-year time horizon was associated with a utility weight of 0.83, taken from prior literature [35]. Costs incurred beyond the 1-year time horizon were assumed to be equal between treatment alternatives.
Sensitivity analyses
A probabilistic sensitivity analysis with 10,000 Monte Carlo simulations was performed using Crystal Ball (Oracle, Redwood City, CA) to characterize the uncertainty of our model. Factors varied in sensitivity analyses included event probabilities, costs, and utility weights, with distributions selected based on published guidance [36]. Costs were varied along a gamma distribution, with alpha and beta parameters estimated using the method of moments, using a standard error of 10% of the mean. Utilities were varied along a triangular distribution, using base-case utility weights as the most likely value and minimum and maximum values of ±0.05. Events probabilities were varied along a beta distribution, with alpha and beta parameters estimated using the incidence of events reported in the SCORPION trial [26].
Because no late complications were observed in the NACT group, we used the cost of late complications observed in the PDS group as the estimate for the cost of late complications in the NACT group to inform sensitivity analyses. Similarly, we assumed the probability of receiving ACT following IDS with late complications was equal to the probability observed in the PDS group.
In addition to the probabilistic sensitivity analysis, we conducted a scenario analysis replacing the utility weights calculated from SCORPION with literature-based utilities (Table S2) [35, 37, 38].
Results
In a hypothetical cohort of 20,000 AEOC cases, NACT was associated with $189 million in costs savings, 1,463 fewer AEOC-related deaths, 1,807 additional life-years, and 2,148 additional QALYs (Table 4). Because it was both cost-saving and produced superior outcomes relative to PDS, NACT was considered the dominant treatment strategy over the one-year time horizon. On average, treatment with NACT cost approximately $9,452 per patient less than treatment with PDS and was associated with a 7.3% lower risk of post-operative death.
Table 4.
Outcomes and incremental cost effectiveness ratios for decision tree analysis of neoadjuvant chemotherapy versus primary debulking surgery for bulky advanced epithelial ovarian cancer
| PDS | NACT | Difference (NACT minus PDS) | |
|---|---|---|---|
| Cost per 20,000 AEOC cases | $529M | $340M | −$189M |
| Cost per AEOC patient | $26,448 | $16,997 | −$9,452 |
| AEOC-related short-term deaths | 2,543 | 1,080 | −1,463 |
| Cost per short-term death averted | DOMINANT* | ||
| Life-years | 18,058 | 19,865 | 1,807 |
| Cost per life-year | DOMINANT* | ||
| Quality-adjusted life-years | 12,115 | 14,401 | 2,286 |
| Cost per quality-adjusted life-year | DOMINANT* | ||
Abbreviations: PDS, primary debulking surgery; IDS, interval debulking surgery; NACT, neoadjuvant chemotherapy; M, millions; AEOC, advanced epithelial ovarian cancer; QALY, quality-adjusted life-year; WTP, willingness-to-pay
A dominant treatment strategy costs less and provides superior outcomes compared to the treatment alternative.
The threshold analysis calculated the maximum difference in overall survival that would result in PDS being the cost-effective strategy at each WTP threshold, given the observed incremental cost-effectiveness ratios observed in the main analysis.
Our threshold analysis revealed that NACT would remain cost-effective at $50,000/QALY and $100,000/QALY WTP thresholds if overall survival among PDS patients was no more than 0.23 years (2.73 months) and 0.11 years (1.37 months) greater than that of NACT patients, respectively.
In probabilistic sensitivity analysis, NACT was the dominant strategy in 99.3% of 10,000 Monte Carlo simulations. Of the 10,000 simulations, 72 (0.7%) resulted in NACT being associated with lower costs and lower QALYs, compared to PDS (quadrant 3). The remaining simulations resulted in lower costs and higher QALYs associated with NACT, compared to PDS (quadrant 4), making NACT the dominant strategy (Figure 2).
Figure 2. Incremental cost-effectiveness ratio plane showing results of probabilistic sensitivity analysis of decision tree analysis of neoadjuvant chemotherapy versus primary debulking surgery for bulky advanced epithelial ovarian cancer.
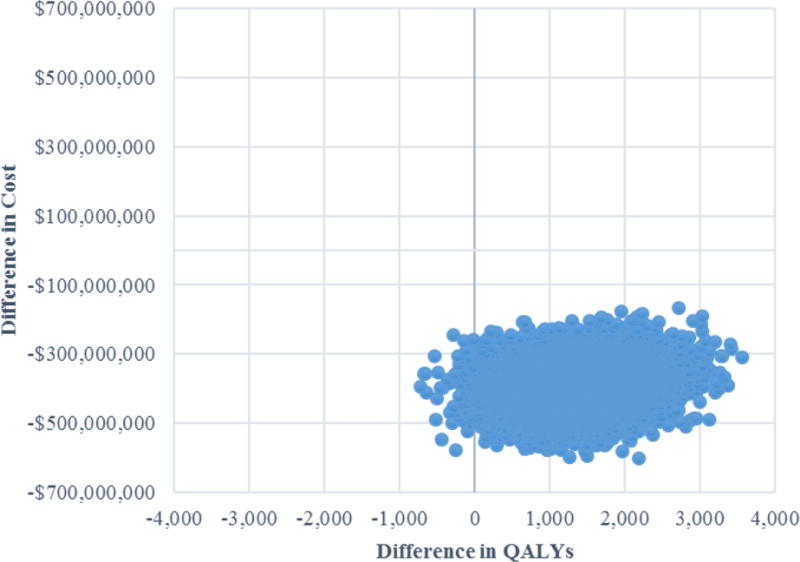
Abbreviations: QALY, quality-adjusted life-year
Results of the scenario analysis using literature-based utility weights did not meaningfully alter our results (Table S3).
Discussion
In this cost-effectiveness study of short-term outcomes associated with treatment with NACT versus PDS for bulky AEOC, NACT was associated with lower costs, fewer peri- and post-operative deaths, and increased life-years and QALYs, making it the dominant treatment strategy for all outcomes assessed.
To our knowledge, this is the first cost-effectiveness study to assess NACT versus PDS using data from an RCT conducted in an aggressive surgical setting, which may better reflect surgical practices in the U.S., compared to prior RCTs. Evaluating cost-effectiveness in an aggressive surgical setting is critical to obtaining estimates that are relevant to a U.S. payer, given concerns about generalizability of existing RCTs in terms of potential differences in upfront surgical effort. If debulking surgery in the U.S. is typically more aggressive, then cost-effectiveness studies using estimates from CHORUS or EORTC 55971 likely underestimate the true cost of PDS procedures and associated complications. Comparison of our results to those of a prior cost-effectiveness study using input data from EORTC 55971 support this hypothesis [37].
Concerns around lack of generalizability associated with existing RCTs make consideration of differences in overall survival an important component in evaluating the cost-effectiveness of NACT for bulky AEOC, since more aggressive upfront surgery which results in resection to minimal or no gross residual disease may be associated with a long-term survival advantage. In the absence of data on overall survival from the SCORPION trial, we attempted to address this point using a threshold analysis, which calculated the maximum survival difference for which NACT would remain cost-effective, given the cost savings we estimated for the one-year time horizon. We found that, although NACT was associated with cost savings and superior QALY outcomes under the assumption of no survival differences, if PDS provided a survival advantage of more than 1.37 months over NACT, PDS would be cost-effective at a $100,000/QALY WTP threshold. If PDS provided a survival advantage of more than 2.73 months, PDS would be cost-effective at a $50,000/QALY WTP threshold.
The survival differences estimated in our threshold analysis are smaller than differences observed in some U.S. non-randomized studies, which have observed 3-10 months of additional survival with PDS [39, 40]. However, it is important to note that unmeasured confounding present in these studies likely introduces bias that makes survival differences appear larger than they actually are. Additionally, results of the threshold analysis are driven by the low overall cost of treatment with NACT and PDS relative to our willingness to pay. Although treatment with PDS costs approximately 56% more than NACT, the absolute difference in cost per patient between the two treatments (roughly $9,500) is much lower than the $50,000 or $100,000 typically used as the willingness to pay to gain an additional year of life in perfect health (1 QALY). In this way, patient preferences with respect to tradeoffs between overall survival and quality-of-life may be a more important consideration than cost for treatment decisions, given the low absolute cost and low cost differential. This is an important area for future research.
This study is not without limitations. There are currently no ongoing randomized studies in the U.S. Therefore, our model relied on inputs derived from preliminary results of the Italian SCORPION trial. Trial patients may not be representative of the U.S.; however, SCORPION represents the only existing randomized comparison of NACT and PDS employing surgical practices similar to those in the United States. Data from a randomized setting is especially important when comparing outcomes between patients treated with NACT versus PDS because of the potential for strong confounding in observational studies. In practice, treatment with NACT is often selected for patients with more widely distributed tumors and those whose poor health status is a contraindication for upfront surgery [39–42], both of which are associated with poor prognosis. As a result, obtaining unbiased effect estimates for this comparison in a non-experimental setting is extremely difficult.
In addition to generalizability, the SCORPION trial is limited by its small sample size, resulting in a lack of precision in event probabilities. Additionally, some rare events may not have been captured. To address this, we performed a probabilistic sensitivity analysis, which suggested that our model was robust to this uncertainty in our inputs.
There are currently no published algorithms to convert EORTC QLQ-C30 scores to utility weights for patients with advanced ovarian cancer. In order to calculate utility weights from the EORTC QLQ-C30 scores reported in the SCORPRION trial, we used a published algorithm developed in a population of patients with non-small cell lung cancer [34]. The validity of this algorithm in an ovarian cancer population is unknown. As a scenario analysis, we used utility weights from published literature, with ovarian cancer-specific utilities used whenever possible [35, 37, 38]. The results of this analysis were very similar to the main analysis.
In conclusion, our findings indicate that in an aggressive surgical paradigm, NACT is the dominant treatment alternative to PDS if survival between the two treatments is equal. However, these conclusions are sensitive to differences in overall survival between treatments due to the low cost of both NACT and PDS relative to typical willingness-to-pay thresholds. Although NACT is more cost effective in the one-year time horizon, a 2.7-month increase in survival, relative to NACT, would result in PDS being cost-effective at typical willingness-to-pay thresholds. Future analyses will be important once data on overall survival become available from an RCT using an aggressive cytoreduction strategy.
Supplementary Material
Highlights.
NACT dominates PDS for short-term AEOC outcomes in an aggressive surgical setting
Compared to PDS, NACT saved $9,452/patient, resulted in fewer deaths, more QALYs
PDS is cost-effective at $50,000/QALY WTP if survival is >2.7 months more than NACT
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.SEER Stat Fact Sheets: Ovarian Cancer. National Cancer Institute Surveillance, Epidemiology, and End Results.
- 3.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 4.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197:676.e1–7. doi: 10.1016/j.ajog.2007.10.495. [DOI] [PubMed] [Google Scholar]
- 5.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 6.Winter WE, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:83–9. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 7.Bristow RE, Montz FJ, Lagasse LD, Leuchter RS, Karlan BY. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol. 1999;72:278–87. doi: 10.1006/gyno.1998.5145. [DOI] [PubMed] [Google Scholar]
- 8.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 9.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90:390–6. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 10.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–64. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 11.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103:1070–6. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Wright JD, Herzog TJ, Neugut AI, Burke WM, Lu YS, Lewin SN, et al. Effect of radical cytoreductive surgery on omission and delay of chemotherapy for advanced-stage ovarian cancer. Obstet Gynecol. 2012;120:871–81. doi: 10.1097/AOG.0b013e31826981de. [DOI] [PubMed] [Google Scholar]
- 13.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–41. doi: 10.1097/01.sla.0000179621.33268.83. discussion 41-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patankar S, Burke WM, Hou JY, Tergas AI, Huang Y, Ananth CV, et al. Risk stratification and outcomes of women undergoing surgery for ovarian cancer. Gynecol Oncol. 2015;138:62–9. doi: 10.1016/j.ygyno.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schorge JO, Clark RM, Lee SI, Penson RT. Primary debulking surgery for advanced ovarian cancer: are you a believer or a dissenter? Gynecol Oncol. 2014;135:595–605. doi: 10.1016/j.ygyno.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Chi DS, Bristow RE, Armstrong DK, Karlan BY. Is the easier way ever the better way? J Clin Oncol. 2011;29:4073–5. doi: 10.1200/JCO.2011.35.9935. [DOI] [PubMed] [Google Scholar]
- 17.Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, Leitao MM, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT) Gynecologic oncology. 2012;124:10–4. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Dewdney SB, Rimel BJ, Reinhart AJ, Kizer NT, Brooks RA, Massad LS, et al. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: survey results from members of the Society of Gynecologic Oncologists. Gynecol Oncol. 2010;119:18–21. doi: 10.1016/j.ygyno.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Mahner S, Trillsch F, Chi D, Harter P, Pfisterer J, Hilpert F, et al. Neoadjuvant chemotherapy in ovarian cancer revisited. Ann Oncol. 2016;27(Suppl 1):i30–i2. doi: 10.1093/annonc/mdw092. [DOI] [PubMed] [Google Scholar]
- 20.Vergote I, Amant F, Leunen K. Gynecol Oncol. United States: 2010. Neoadjuvant chemotherapy in advanced ovarian cancer: what kind of evidence is needed to convince US gynaecological oncologists? pp. 1–2. [DOI] [PubMed] [Google Scholar]
- 21.Vergote I, Tropé CG, Amant F, Ehlen T, Reed NS, Casado A. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J Clin Oncol. 2011;29:4076–8. doi: 10.1200/JCO.2011.36.9785. [DOI] [PubMed] [Google Scholar]
- 22.Vergote I, Leunen K, Amant F. Primary surgery or neoadjuvant chemotherapy in ovarian cancer: what is the value of comparing apples with oranges? Gynecol Oncol. 2012;124:1–2. doi: 10.1016/j.ygyno.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Vergote I, du Bois A, Amant F, Heitz F, Leunen K, Harter P. Neoadjuvant chemotherapy in advanced ovarian cancer: On what do we agree and disagree? Gynecol Oncol. 2013;128:6–11. doi: 10.1016/j.ygyno.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. The New England journal of medicine. 2010;363:943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 25.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–57. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 26.Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer. 2016;59:22–33. doi: 10.1016/j.ejca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 28.Davis A, Tinker AV, Friedlander M. “Platinum resistant” ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol Oncol. 2014;133:624–31. doi: 10.1016/j.ygyno.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 29.HCUPnet. Agency for Healthcare Quality and Research
- 30.Physician Fee Schedule. Center for Medicare and Medicaid Services.
- 31.Medicare Part B Drug Average Sales Price. Center for Medicare and Medicaid Services.
- 32.Consumer Price Index. Bureau of Labor Statistics.
- 33.Network NCC, editor. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Ovarian cancer: including fallopian tube cancer and primary peritoneal cancer. Fort Washington, PA2014: 2014. Version 3. [Google Scholar]
- 34.Jang RW, Isogai PK, Mittmann N, Bradbury PA, Shepherd FA, Feld R, et al. Derivation of utility values from European Organization for Research and Treatment of Cancer Quality of Life-Core 30 questionnaire values in lung cancer. J Thorac Oncol. 2010;5:1953–7. doi: 10.1097/jto.0b013e3181f77a6a. [DOI] [PubMed] [Google Scholar]
- 35.Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113:216–20. doi: 10.1016/j.ygyno.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford University Press; 2006. [Google Scholar]
- 37.Rowland MR, Lesnock JL, Farris C, Kelley JL, Krivak TC. Cost-utility comparison of neoadjuvant chemotherapy versus primary debulking surgery for treatment of advanced-stage ovarian cancer in patients 65 years old or older. American journal of obstetrics and gynecology. 2015;212:763.e1–8. doi: 10.1016/j.ajog.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 38.Ebm C, Cecconi M, Sutton L, Rhodes A. A cost-effectiveness analysis of postoperative goal-directed therapy for high-risk surgical patients. Crit Care Med. 2014;42:1194–203. doi: 10.1097/CCM.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 39.Meyer LA, Cronin AM, Sun CC, Bixel K, Bookman MA, Cristea MC, et al. Use and Effectiveness of Neoadjuvant Chemotherapy for Treatment of Ovarian Cancer. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rauh-Hain JA, Melamed A, Wright A, Gockley A, Clemmer JT, Schorge JO, et al. Overall Survival Following Neoadjuvant Chemotherapy vs Primary Cytoreductive Surgery in Women With Epithelial Ovarian Cancer: Analysis of the National Cancer Database. JAMA Oncol. 2017;3:76–82. doi: 10.1001/jamaoncol.2016.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauh-Hain JA, Rodriguez N, Growdon WB, Goodman AK, Boruta DM, 2nd, Horowitz NS, et al. Primary debulking surgery versus neoadjuvant chemotherapy in stage IV ovarian cancer. Ann Surg Oncol. 2012;19:959–65. doi: 10.1245/s10434-011-2100-x. [DOI] [PubMed] [Google Scholar]
- 42.Wright JD, Ananth CV, Tsui J, Glied SA, Burke WM, Lu YS, et al. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer. 2014;120:1246–54. doi: 10.1002/cncr.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


