Abstract
Background
Rheumatoid arthritis (RA) is a morbid, mortal and costly condition without a cure. Treatments for RA have expanded over the last two decades and direct medical costs may differ by types of treatments. There has not been a systematic literature review since the introduction of new RA treatments, including biologic disease modifying anti-rheumatic drugs (bDMARDs).
Methods
We conducted a systematic literature review with meta-analysis of direct medical costs associated with RA cared for in the US since the marketing of the first bDMARD. Standard search strategies and sources were used and data were extracted independently by two reviewers. The methods and quality of included studies were assessed. Total direct medical costs as well as RA-specific costs were calculated using random effects meta-analysis. Subgroups of interest included Medicare patients and those using bDMARDs.
Results
We found 541 potentially relevant studies and 12 papers met the selection criteria. The quality of studies varied: 1/3 were poor, 1/3 were fair, and 1/3 were good. Total direct medical costs were estimated at $12,509 (95% CI $7,451-21,001) for all RA patients using any treatment regimen and $36,053 (95% CI $32,138-40,445) for bDMARD users. RA-specific costs were $3,723 (95% CI $2,408-5,762) for all RA patients using any treatment regimen and $20,262 (95% CI $17,480-23,487) for bDMARD users.
Conclusions
The total and disease-specific direct medical costs of patients with RA is substantial. Among bDMARD users, cost of RA care is over half of all direct medical costs.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune systemic disease affecting roughly 0.7% of the population. (1) Treatment for RA was transformed in the late-1990s with the advent of biologic disease modifying anti-rheumatic drugs (bDMARDs) targeting specific immunologic pathways. As a disease with early adoption of bDMARDs, RA serves as a model for biologic drug use, where the treatment costs vary substantially based on therapeutic strategy.
Biologic DMARDs offer alternatives for patients unresponsive to traditional synthetic DMARDs, but the drugs carry an increased financial burden, with annual costs between $25,000 – $40,000. Thus, a detailed understanding of the cost of care for patients with RA since the advent of bDMARDs will be of importance to policy makers, administrators, and physicians; the high cost of RA treatments impacts the use of limited medical resources.
There is a body of primary literature examining the cost of RA since the introduction of bDMARDs, but a current critical review of these studies is lacking. The most recent reviews on cost-of-care research for RA in the US were both published before the advent of biologics. (2) (3) (4) Several more recent reviews have been published looking at costs outside of the US, (5) (6) but these studies have limited relevance to the US given the difficulties of comparing costs across health care systems with different payment structures and social priorities.
The question of direct medical costs for patients with RA is further complicated by the complexities and lack of standardization in methodology of cost-of-care analyses. When conducting a cost-of-care analysis, researchers must make decisions about the best source of data, the case definition for RA, the financial measure to refer to as “costs,” the elements of medical costs to be included, and the assignment of costs to RA versus other concurrent morbid diseases. Given the need for better understanding of cost of care for RA in the US and the need for standardization in cost-of-care analysis, we undertook a systematic literature review and meta-analysis with the following PICOS assignments: P (patients) – RA patients in the US since 1999; I (interventions) – any treatment regimen for RA; C (comparator) – no comparison group was included; O (outcomes) – direct medical costs; and S (study design) – all study types were included. We also examined the methodology employed by relevant studies.
METHODS
Study Selection
Studies were identified through a search of Medline using the following MeSH search terms: cost of illness; health care costs; expenditures; expenditures, direct; expenditures, health; expenditures, indirect; rheumatoid arthritis; and arthritis, rheumatoid (full search strategy available in Supplement 1). Because this analysis was meant to consider only studies assessing costs after the introduction of biologic DMARDs in 1999, the search was limited to studies published in 2000 and after. The last search we performed was on June 16, 2016.
Citations were screened by one reviewer for eligibility criteria to select articles for full text review. Criteria for inclusion were English language, focus on a US population, analysis of post-1999 data, and consideration of total direct costs of treatment for RA from a provider, insurer, or societal perspective. Studies were excluded if they were non-English language, focused on musculoskeletal or rheumatologic disorders other than RA, did not analyze cost as an outcome, were review papers or conference abstracts, focused on a population outside the US, focused on indirect costs, failed to analyze key elements of total direct cost, studied a non-generalizable population (e.g. only patients with a specific comorbidity), were economic evaluations of specific drugs or therapies, relied on pre-1999 data, or provided an insufficient description of their cost-analysis methodology. Reference lists of studies deemed potentially relevant were examined for papers not identified by the Medline search. Full text review of potentially relevant studies was performed independently by two reviewers (AH and DHS) to confirm eligibility.
Data Abstraction and Quality Rating
Two independent reviewers abstracted data from included studies using a standardized form (see Supplement 2). Information abstracted from each study included: the characteristics of study participants, such as age, gender, comorbidities, and health insurance coverage; the study’s inclusion and exclusion criteria; cost-analysis methodology; and cost of care findings. Specific aspects of the cost-analysis examined were the definition of RA, methods for comparisons with costs of non-RA patients, the overall costing methodology, and adjustment for inflation. Regarding costing methodology, some papers used the frequency of utilization of specific services and multiplied this by a pre-defined dollar amount; we defined this as the “utilization × standardized cost.” All abstracted costs were converted to 2015 dollars using the consumer price index medical care component.
Additionally, we developed a quality rating form based on the Newcastle-Ottawa Quality Assessment Form for Cohort Studies (7), modified to facilitate assessment of RA cost-of-illness analyses (see Supplement 3). The quality rating included assessment of the representativeness of the studied cohort to assess for risk of selection bias. The same two reviewers who abstracted data also independently rated the quality of included studies. Disagreements regarding data abstraction or quality ratings were resolved by discussion between the two reviewing authors.
Meta-Analysis
The description of the meta-analysis followed the PRISMA checklist (see Supplement 4). The analysis of costs utilized a pooled estimate from random-effects models. The variance of the pooled estimates was stabilized by the double arcsine transformation. This method was found to produce less bias and mean squared error than the traditional log transformation. (8) We assessed heterogeneity among studies using the Cochran Q test and quantified inconsistencies across studies and their impact on the analysis by using the I2 statistic (28). All analyses were done by MetaXL 1.4 package (http://www.epigear.com).
RESULTS
Study Selection
The Medline search yielded 541 citations and all abstracts were reviewed. Of these, 523 were discarded because they did not meet inclusion criteria (see Figure 1). Of those citations excluded based on abstracts, 364 were excluded because they were not cost-of-illness analyses, 68 because they examined non-US data, 62 because they focused on indirect costs or only a subset of total costs, 18 because they used data from years prior to 1999, and 11 because they focused on a population that could not be generalized to a national sample, such as only patients with a specific comorbidity or using a specific drug. After full text review of the remaining 18 articles, 6 did not meet the inclusion criteria: 2 were excluded because they did not describe their costing methodology sufficiently for comparison to other included articles, 2 because their populations could not be generalized, and 2 because on further inspection their sample included data from years prior to 1999. A total of 12 studies were identified for inclusion in the review.
Figure 1.
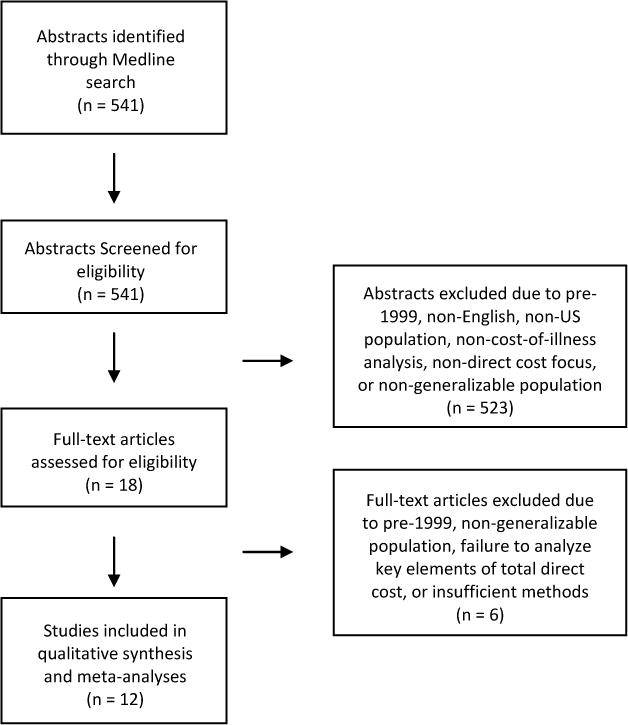
shows the assembly of literature for the systematic literature review.
Data Sources, Populations, and Quality Assessment
Table 1 displays the data sources and patient characteristics of the 12 studies included in final analyses. The included studies used a total of 16 different sources of patient data for their cost analyses. Only one database, the Medical Expenditure Panel Survey, was used in more than one of the included studies. The included studies analyzed costs within a number of different medical insurance settings; 6 studies reported costs for privately insured patients (9) (10) (11) (12) (13) (14), 3 studies reported costs for patients enrolled in Medicaid programs (15) (16) (11), and 1 study reported costs for patients enrolled in Medicare (17). Additionally, 3 studies utilized patient data from large, national databases and used weighting to generalize their findings to be representative of the entire US population (18) (19) (20). One paper (11) incorporated costs from multiple insurance settings in the same set of analyses.
Table 1.
Included Studies and Populations
| Author, Year | Data Source | Population | Patients, N | Mean Age, years | Female, % |
|---|---|---|---|---|---|
| Michaud, 2003 | National Databank for Rheumatic Diseases | RA patients enrolled by rheumatologists across the US | 7527 | 62 | 76.8 |
| Weycker, 2005 | Constella COMPASS database, Ingenix LabRx database | TNF-antagonist biologic users age 65 and older in private health plans across the US | Constella: 166 Ingenix: 114 |
71 | 76.0 |
| Khanna, 2007 | West Virginia Medicaid claims | Medicaid enrollees with RA in West Virginia |
1157 | 47 | 77.4 |
| Wu, 2007 | Private insurance claims database | TNF-antagonist biologic users among employees, spouses, and dependents from 31 large employers across the US | 997 | 50 | 73.9 |
| Johnson, 2008 | Arizona HealthQuery (AZHQ) database | Medicaid enrollees in Maricopa County, AZ |
1492 | 56 | 83.3 |
| Birnbaum, 2010 | Ingenix Enployer Database, Medicare SAF, Florida Medicaid claims | Beneficiaries from 37 large employers across the US, 5% sample of Medicare enrollees, Medicaid enrollees in Florida | Private: 14317 Medicaid: 6415 |
Private: 50 Medicaid: 45 |
Private: 70.4 Medicaid: 76.6 |
| McBride, 2011 | Private insurance claims database | Employees of 40 large employers across the US |
2,545 | 50 | 74.7 |
| Birnbaum, 2012 | Private insurance claims database | Employees of 40 large employers across the US |
837 | 50 | 31.9 |
| Harrold, 2012 | Medicare Current Beneficiary Survey | Medicare enrollees | 2000: 225 2002: 260 2004: 253 2006: 247 |
70 | 77.8 |
| Kawatkar, 2012 | Medical Expenditure Panel Survey | Sample of US population | 5.8 mil | 62 | 61.1 |
| Simons, 2012 | Medical Expenditure Panel Survey | Sample of US population | 2004: 34,403 2005: 33,645 2006: 34,145 |
2004: 57 2005: 58 2006: 59 |
77.0 |
| Baser, 2015 | Truven Health MarketScan Commercial Claims Database | TNF-antagonist biologic users who switched to a new TNF-antagonist or a non-TNF biologic | 3497 | 54 | 80.5 |
Identification of ICD-9-CM codes in the 714.xx series in medical claims was the most common method for identifying RA patients in claims databases; only one study (18) did not rely on ICD-9-CM codes in some capacity for patient identification. However, there was considerable variability in which codes were used and the number of instances of an RA code required for inclusion. Of the 11 studies using ICD-9-CM codes, 4 identified a narrow subset of codes to define RA (9) (15) (16) (14), while the rest accepted any code within the 714.xx series (10) (11) (12) (13) (17) (19) (20). Eight (73%) studies required only one instance of an RA code for inclusion; the remaining 3 (27%) required more than one instance of an RA code, or a combination of one RA code with either a claim for at least one DMARD or self-reported RA diagnosis.
Most studies included all patients with RA in their chosen database; however, 4 studies limited their population to only those patients using bDMARD medications. All studies, by design, analyzed costs incurred after 1999, but none analyzed costs later than 2010. There was an even distribution in the quality of included studies according to our established quality assessment framework – 4 studies were judged to be of poor quality, 4 were fair quality, and 4 were good quality. However, in all studies the observed cohort was deemed to be adequately representative of a national sample, indicating little risk of selection bias.
Cost-of-Illness Analysis Methods
Table 2 displays details on the cost analysis methodologies used by the included studies. The vast majority of studies based their cost analysis on actual reimbursements paid by an insurer for medical services or drugs as reported in a claims database. Michaud et al. (18) and Weycker et al. (9) instead employed a utilization-based method in which they applied a consistent standardized dollar amount to each instance of utilization of a given medical service or drug as reported in a claims database. For a second database, Weycker et al. based their cost analysis on charges billed by a health care institution for medical services and drugs, as opposed to the actual reimbursed amount. Weycker et al.’s findings yielded the highest cost estimates in our dataset.
Table 2.
Cost Analysis Methods
| Author, year | Definition of RA Cases | Comparison Group | Costing Method(s) | Inflation Adjusted | Analyzed RA Costs | Method for Determining RA Costs |
|---|---|---|---|---|---|---|
| Michaud, 2003 | Diagnosed by rheumatologist | No | Utilization × Standard cost | Yes | No | N/A |
| Weycker, 2005 | ≥1 claim with dx of ICD-9-CM 714.0, 714.1, 714.2, or 714.81 | No | Constella: Utilization × standard costs Ingenix: Charges | No | Yes | Sum of costs in claims with Dx of ICD-9-CM 714.xx in any position, or drug on list of RA-related medications |
| Khanna, 2007 | ≥1 claim with primary dx of ICD-9-CM 714.00 | No | Reimbursements, Utilization × standard costs | N/A | Yes | Population limited to patients with RA |
| Wu, 2007 | ≥1 claim with primary or secondary dx of ICD-9-CM 714.xx | No | Reimbursements | Yes | Yes | Sum of costs in claims with Dx of ICD-9-CM 714.xx in any position, or drug on list of RA-related medications |
| Johnson, 2008 | ≥1 claim with primary or secondary dx of ICD-9-CM 714.0 | No | Reimbursements | N/A | No | N/A |
| Birnbaum, 2010 | Dx of ICD-9-CM 714.xx | Yes | Reimbursements | Yes | Yes | Incremental costs of RA subjects vs comparison group |
| McBride, 2011 | ≥2 claims with dx of ICD-9-CM 714.xx | No | Reimbursements | Yes | Yes | Sum of costs in claims with Dx of ICD-9-CM 714.xx in any position, or drug on list of RA-related medications |
| Birnbaum, 2012 | ≥1 claim with dx of ICD-9-CM 714.xx | Yes | Reimbursements | N/A | Yes | Sum of costs in claims with Dx of ICD-9-CM 714.xx in any position, or drug on list of RA-related medications |
| Harrold, 2012 | 1) ≥2 dx of ICD-9-CM 714.xx OR 2) Self-reported RA dx and 1 dx of ICD 9-CM 714.xx OR 3) Use of biologic DMARD and self-reported RA dx or 1 dx of ICD-9-CM 714.xx | No | Reimbursements | Yes | Yes | Sum of costs for list of RA-related medications |
| Kawatkar, 2012 | 1) Dx of ICD-9-CM 714.xx OR 2) MEPS clinical classification code of 202 | Yes | Reimbursements | Yes | Yes | Incremental costs of RA subjects vs comparison group |
| Simons, 2012 | Dx of ICD-9-CM 714.xx | Yes | Reimbursements | No | Yes | Regression analysis |
| Baser, 2015 | ≥2 dx of ICD-9-CM 714.0x at least 2 months apart | No | Reimbursements | Yes | Yes | Sum of costs in claims with Dx of ICD-9-CM 714.0x in any position, or drug on list of RA-related medications |
Abbreviations: RA, rheumatoid arthritis; Dx, diagnosis; ICD, international classification of diseases; MEPS, Medical Expenditure Panel Survey
Of the 12 included studies, 10 reported at least one element of cost – such as costs of hospitalizations, ambulatory care, or prescription drugs – specific to care for RA as opposed to other comorbid diagnoses. Of those, 8 reported RA-specific costs across three cost domains and were included in the meta-analysis of total RA costs.
Cost of Care Findings
Among the 8 studies including all RA patients (and not only those using a biologic DMARD) in their analyses (18) (15) (16) (11) (13) (17) (19) (20), findings for annual total cost of care across all conditions ranged from $3,266 to $25,260, with the lowest estimate being in a population of Medicaid enrollees and the highest in a Medicare population (see Table 3). It is worth noting that the low estimate of $3,266 (16) is much lower than any other finding, being only 28% of the next lowest-estimate. Meta-analysis of studies including all RA patients found annual total cost of care to be $12,509 (95% CI $7,451-21,001) (see Figure 2A). Removing the low estimate did not substantially change the total cost of care ($12,458, 95% CI 7,381-21,025). Among studies restricted to RA patients using bDMARDs, annual total cost of care ranged from $26,469 to $52,837, with the lower estimate comprising privately insured working-age adults and the higher estimate privately insured adults over age 65. Meta-analysis found annual total cost of care among patients using biologics to be $36,053 (95% CI $32,138-40,445) (see Figure 2B).
Table 3.
Reported Cost of Care by Study
| Author | Year of Publication | Year(s) of Reported Costs | Total Cost of Care (2015 $) | Cost of RA-Specific Care (2015 $) |
|---|---|---|---|---|
| Overall US Population | ||||
| Michaud | 2003 | 1999-2001 | 15,189 | NR |
| Simmons | 2012 | 2004 | 17,159 | 6,208 |
| Simmons | 2012 | 2005 | 15,791 | 3,909 |
| Simmons | 2012 | 2006 | 12,192 | 2,437 |
| Kawatkar | 2012 | 2008 | 15,558 | 2,493 |
| Privately Insured Patients | ||||
| Birnbaum | 2010 | 1999-2005 | 12,904 | 7,006 |
| Birnbaum | 2012 | 2006 | 11,554 | 2,914 |
| Medicare | ||||
| Harrold | 2012 | 2000 | 21,445 | NR |
| Harrold | 2012 | 2002 | 23,707 | NR |
| Harrold | 2012 | 2004 | 23,024 | NR |
| Harrold | 2012 | 2006 | 25,260 | NR |
| Medicaid | ||||
| Khanna | 2007 | 2003 | NR | 3,486 |
| Johnson | 2008 | 2003 | 3,266 | NR |
| Birnbaum | 2010 | 1999-2005 | 19,519 | 7,849 |
| Biologic DMARD Users Only | ||||
| Weycker* | 2005 | 1999-2002 | 46,567 | NR |
| Weycker† | 2005 | 1999-2002 | 52,837 | NR |
| Wu | 2007 | 2003-2004 | 33,308 | 22,358 |
| McBride | 2011 | 1999-2007 | 27,373 | 16,716 |
| Baser | 2015 | 2005-2010 | 26,469 | 22,445 |
Constella COMPASS database, Util. x Std. Cost method
Ingenix LabRx database, Charges method
NR, not reported
Figure 2.
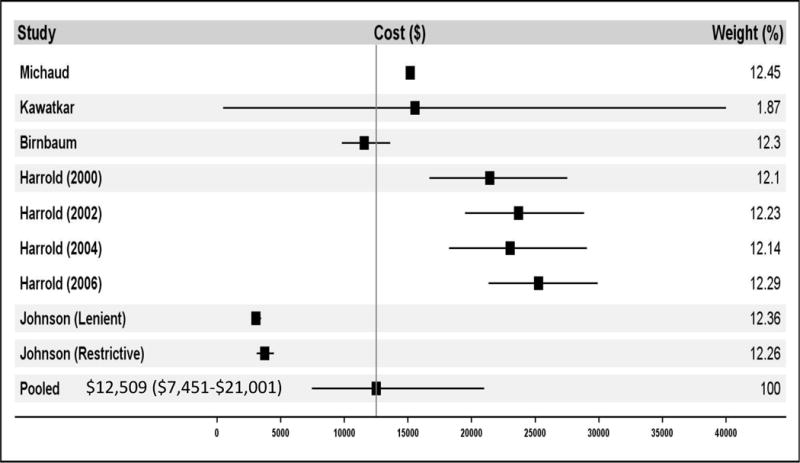
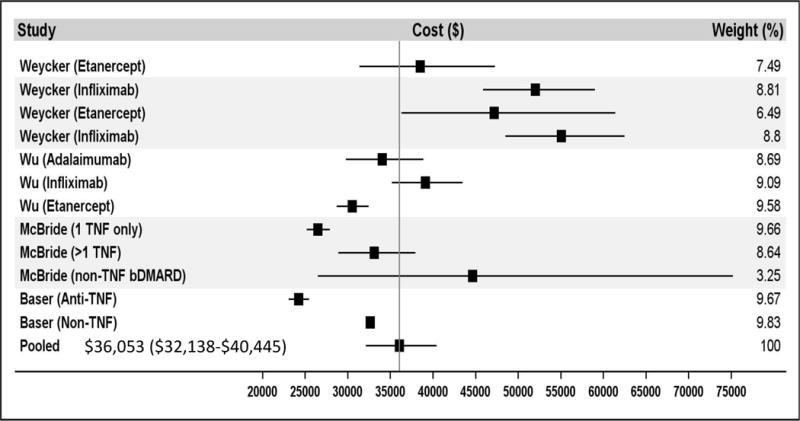
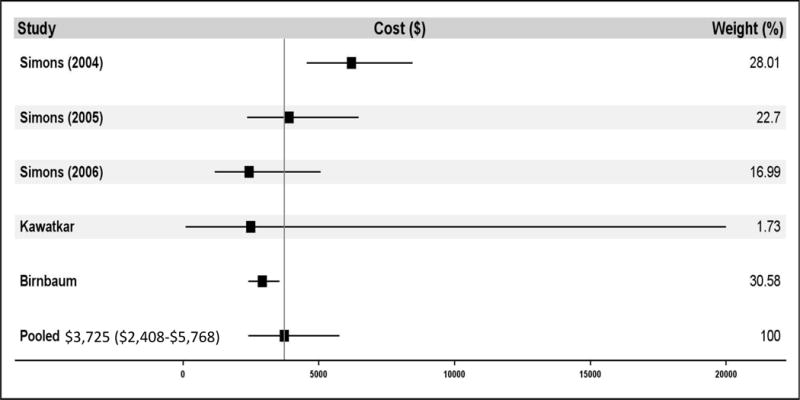
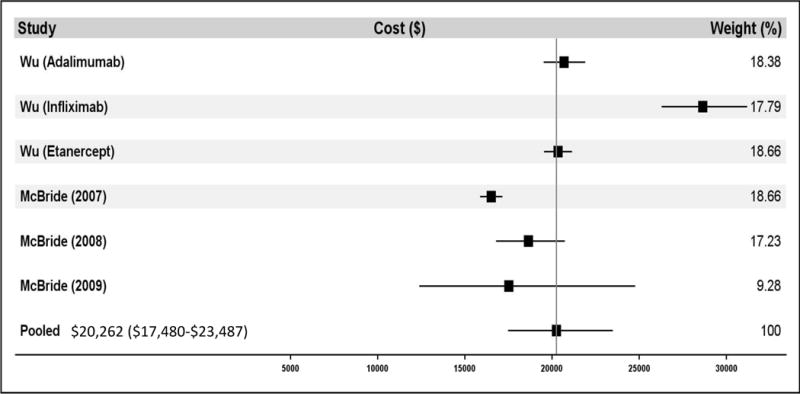
shows the meta-analysis results for the direct medical costs. Panel A demonstrates the total cost of care for all patients. Panel B demonstrates the total cost of care for biologic DMARD users only. Panel C demonstrates rheumatoid arthritis-specific costs of care for all patients. Panel D demonstrates rheumatoid arthritis-specific costs of care for biologic DMARD users only. Multiple papers included in these meta-analyses reported cost of care findings for several distinct patient populations. These distinct populations are each treated as individual contributors to the meta-analysis and are listed with the identifying characteristic of the patient population studied (e.g. individual year or specific drug treatment regimen).
Estimates for annual cost for RA-specific care ranged considerably. Estimates in studies including patients using any treatment regimen (i.e. not limiting to bDMARD users only) ranged from $2,437 to $7,849 (See Table 3), with the lower estimate representing a population modeling the general US population and the higher being comprised of Medicaid enrollees. Meta-analysis determined annual cost for RA-specific care to be $3,723 (95% CI $2,408-5,762) when accounting for patients using any treatment regimen (see Figure 2C), representing 30% of total costs for all care. Estimates of RA-specific costs in studies limiting their population to patients using bDMARDs ranged from $16,716 to $22,445, with both estimates being based on different claims databases comprising privately insured working-age adults. Meta-analysis found annual RA-specific cost within this population to be $20,262 (95% CI $17,480-23,487) (see Figure 2D), representing 56% of total costs for all care.
DISCUSSION
Rheumatoid arthritis is a morbid, mortal, and costly illness. The cost of treating RA has increased over the last two decades with the advent of bDMARDs, however this has not been well studied. We conducted a systematic literature review and analyzed prior cost-of-care studies for RA; the cost of direct medical care for a patient with RA was $12,509 and the costs attributable to RA were $3,725 or 30% of the total costs. Among patients using bDMARDs, total direct medical costs were $36,053 and costs attributable to RA were $20,262, or 56% of the total.
These findings suggest that costs associated with RA are in line with those for other prominent chronic diseases. Recent studies have reported annual total direct cost of care for diabetes patients as $14,732 (21), multiple sclerosis patients as $23,195 (22), ulcerative colitis between $4,032 and $13,722 (23), and COPD between $1,681 and $10,812 (24) using 2015 dollars. Our findings also suggest that the burden of RA patients on the US health care system may become outsized compared to the disease’s relatively small prevalence and compared to patients with these other chronic conditions as more patients use bDMARDs in the future.
In considering the observed costs, it is interesting to note that patients that use bDMARD's had increased cost over typical RA patients. Additionally, bDMARD use had a larger incremental effect on RA-specific costs (444% increase) than on total direct medical costs (188% increase). However in both cases the increment was below the total cost of bDMARDs themselves. This suggests that either the use of bDMARD's may be associated with lower total non-drug direct medical costs or that the patients who receive bDMDARDs have fewer comorbid conditions. Research comparing the characteristics of bDMARD users versus regular DMARD users has shown differences in the demographic and clinical characteristics of bDMARD users compared to regular DMARD users, (25) (26) but this topic is worthy of further examination.
While the cost of RA is extremely important to the healthcare system, the methodologies observed and used across the included prior studies varied substantially. The definition of RA differed, what was considered costs and their calculation was not standardized, and the attempt to partition RA costs from non-RA costs differed by study. Two predominant methods were used to identify RA-specific costs: 1) Identification of claims with an RA-related ICD-9-CM diagnosis code and 2) determination of the incremental costs between a population of RA patients versus a population of controls. Both methods have their limitations. Identification of claims with RA-related codes fails to address the possibility that claims may be misclassified as being RA-related or not based on a coding error. Likewise, assessment of incremental cost is subject to error in the nuance of defining a non-RA control population, especially in the consideration of which comorbidities may or may not be related to the pathophysiology of RA. In addition, the quality of reporting in the included studies was inconsistent.
The methods for studying direct medical costs need further standardization. Standardized methods would facilitate comparison across studies with less concern for heterogeneity and would also allow for better temporal trend analyses, ensuring “apples to apples” comparisons. Ideal methods would account for all inpatient, outpatient, prescription medication, and post-acute care costs, rely on actual reimbursement amounts reported in claims as the basis of analysis, define RA patients by requiring multiple instances of an RA-related diagnosis code in claims, assess both total cost for all medical care and RA-specific care, and compare costs between RA patients and similar non-RA patients from the same database.
The current meta-analysis is limited by the literature we included. As noted, the methods across studies were not consistent and this increases the uncertainty of our summarized results. Since we did not have individual patient-level data, we could not examine the associations between individual patient characteristics and cost. As well, the methods for partitioning RA costs were inconsistent making the RA-specific cost analyses more difficult to interpret. Finally, we did not include studies of “indirect” costs of RA, such as work lost and caregiver costs. While indirect costs are substantial (27) (28) (29), the methodologic variability is also significant; thus, we worried about introducing even more heterogeneity in this meta-analysis.
In light of these limitations, we conclude that the direct medical costs of patients with RA are significant. Clearly, medication costs comprise a substantial portion of these costs, especially for patients using bDMARDs. Without considering the health effects, benefits and risks, the current analysis cannot comment on whether specific treatments are of value. Cost-effectiveness analyses comparing different treatment strategies for RA are ongoing and will provide useful information. The studies included in this meta-analysis do not include assessments of RA-outcomes, but our findings may be a valuable resource for future cost-effectiveness analyses that will further understanding of the relative benefits of treatment options available to RA patients. As standards of care evolve in RA, the standards for studying cost of care in RA must also mature and become codified. This will facilitate better comparisons across treatments and across time.
Supplementary Material
SIGNIFICANCE AND INNOVATION.
We conducted a systematic literature review with meta-analysis of direct medical costs associated with RA cared for in the US since the marketing of the first bDMARD.
The 12 papers that met the selection criteria demonstrated that total direct medical costs were $12,509 (95% CI $7,451-21,001) for all RA patients using any treatment regimen and $36,053 (95% CI $32,138-40,445) for bDMARD users.
RA-specific costs were $3,723 (95% CI $2,408-5,762) for all RA patients using any treatment regimen and $20,262 (95% CI $17,480-23,487) for bDMARD users.
Among bDMARD users, cost of RA care is over half of all direct medical costs.
Acknowledgments
FUNDING
Funding for this research was supported through NIH grant AR K24 055989. Mr. Hresko was supported by the Harrold Williams Research Fellowship from the Tufts University School of Medicine.
Support: Tufts University School of Medicine Harrold Williams Research Fellowship; NIH AR K24 055989
Footnotes
DR. TZU-CHIEH LIN (Orcid ID : 0000-0002-8333-7666)
Potential Conflicts: Dr. Solomon receives research support from grants to his hospital from Amgen, Pfizer, Eli Lilly, AstraZeneca, Genentech, Bristol Myers Squibb, and Corrona. Dr. Lin is now an employee of Amgen but not at the time of writing this paper.
References
- 1.Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res. 2010;62:460–464. doi: 10.1002/acr.20041. [DOI] [PubMed] [Google Scholar]
- 2.Cooper NJ. Economic burden of rheumatoid arthritis. Rheumatology. 2000;39:28–33. doi: 10.1093/rheumatology/39.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Lubeck DP. A review of the direct costs of rheumatoid arthritis. Pharmacoeconomics. 2001;19:811–18. doi: 10.2165/00019053-200119080-00003. [DOI] [PubMed] [Google Scholar]
- 4.Rat CA, Boisser MC. Rheumatoid arthritis: direct and indirect costs. Joint Bone Spine. 2004;71:518–24. doi: 10.1016/j.jbspin.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Fautrel B, Verstappen SM, Boonen A. Economic consequences and potential benefits. Best Pract Clin Rheumatol. 2011;25:607–24. doi: 10.1016/j.berh.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Furneri G, Mantovani LG, Belisari A, Mosca M, Cristiani M, Bellelli S, et al. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin Exp Rheumatol. 2012;30:S72–S84. [PubMed] [Google Scholar]
- 7.Wells GA, Shea B, O’Connel D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses [Online] 2009 Feb 1; http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf.
- 8.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–8. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 9.Weycker D, Yu EB, Woolley JM, Oster G. Retrospective study of the costs of care during the first year of therapy with etanercept or inflizimab among patients aged >65 years with rheumatoid arthritis. Clin Ther. 2005;27:646–56. doi: 10.1016/s0149-2918(05)00090-1. [DOI] [PubMed] [Google Scholar]
- 10.Wu E, Chen L, Birnbaum H, Yang E, Cifaldi M. Cost of care for patients with rheumatoid arthritis receiving TNF-antagonist therapy using claims data. Curr Med Res Opin. 2007;23:1749–59. doi: 10.1185/030079907X210615. [DOI] [PubMed] [Google Scholar]
- 11.Birnbaum H, Pike C, Kaufman R, Maynchencko M, Kidolezi, Cifaldi M. Societal cost of rheumatoid arthritis pateints in the US. Curr Med Res Opin. 2010;26:77–90. doi: 10.1185/03007990903422307. [DOI] [PubMed] [Google Scholar]
- 12.McBride S, Sarsour K, White LA, Nelson DR, Chawla AJ, Johnston JA. Biologic disease-modifying drug treatment patterns and associate costs for patients with rheumatoid arthritis. J Rheumatol. 2011;38:2141–49. doi: 10.3899/jrheum.101195. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum HG, Pike C, Banerjee R, Waldman T, Cifaldi M. Changes in utilization and costs for patients with rheumatoid arthritis, 1997 to 2006. Pharmacoeconomics. 2012;30:323–36. doi: 10.2165/11589470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Baser O, Ganguli A, Roy S, Xie L, Cifaldi M. Impact of switching from an initial tumor necrosis factor inhibitor on health care resource utilization and costs among patients with rheumatoid arthritis. Clin Ther. 2015;37:1454–65. doi: 10.1016/j.clinthera.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Khanna R, Smith MJ. Utilization and costs of medical services and prescription medications for rheumatoid arthritis among recipients covered by a state Medicaid program. Clin Ther. 2007;29:2456–67. doi: 10.1016/j.clinthera.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Johnson TJ, Stahl-Moncada S. Medicaid prescription formulary restrictions and arthritis costs. Am J Public Health. 2008;98:1300–5. doi: 10.2105/AJPH.2007.118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrold LR, Peterson D, Beard AJ, Gurwitz JH, Briesacher BA. Time trends in medication use and expenditures in older rheumatoid arthritis patients. Am J Med. 2012;125:937.e9–15. doi: 10.1016/j.amjmed.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaud K, Messer J, Choi HK, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:2750–62. doi: 10.1002/art.11439. [DOI] [PubMed] [Google Scholar]
- 19.Kawatkar AA, Jacobsen SJ, Levy GD, Medhekar SS, Venkatasubramaniam KV, Herrinton LJ. Direct medical expenditure associated with rheumatoid arthritis in a nationally representative sample from the Medical Expenditure Panel Survey. Arthritis Care Res. 2012;64:1649–56. doi: 10.1002/acr.21755. [DOI] [PubMed] [Google Scholar]
- 20.Simons WR, Rosenblatt LC, Trivedi DN. The economic consequences of rheumatoid arthritis. J Occup Environ Med. 2012;54:48–55. doi: 10.1097/JOM.0b013e31823c13e7. [DOI] [PubMed] [Google Scholar]
- 21.Association, American Diabetes. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adelman G, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United States: a systematic review of the literature. J Med Econ. 2013;16:639–47. doi: 10.3111/13696998.2013.778268. [DOI] [PubMed] [Google Scholar]
- 23.Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31:693–707. doi: 10.1111/j.1365-2036.2010.04234.x. [DOI] [PubMed] [Google Scholar]
- 24.Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–45. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg JD, Kremer JM, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576–582. doi: 10.1136/ard.2010.129916. [DOI] [PubMed] [Google Scholar]
- 26.Kim G, Barner JC, Rascati K, Richards K. Factors associated with inititation of biologic disease-modifying drugs in Texas Medicaid patients with rheumatoid arthritis. J Manag Spec Pharm. 2015;21:401–7. doi: 10.18553/jmcp.2015.21.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfe F, Michaud K. Out-of-pocket expenses and their burden in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61:1563–70. doi: 10.1002/art.24724. [DOI] [PubMed] [Google Scholar]
- 28.Neovius M, Simard JF, Askling J, ARTHIS study group How large are the productivity losses in contemporary patients with RA, and how soon in relation to diagnosis do they develop? Ann Rheum Dis. 2011;70:1010–15. doi: 10.1136/ard.2010.136812. [DOI] [PubMed] [Google Scholar]
- 29.Raciborski F, Kłak A, Kwiatkowska B. Indirect costs of rheumatoid arthritis. Reumatologia. 2015;53:268–75. doi: 10.5114/reum.2015.55830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


