Abstract
Addiction to drugs such as cocaine is marked by cycles of compulsive drug-taking and drug-seeking behavior. Although the transition to addiction is thought to recruit neural processes in dorsal striatum, little is known regarding the role of dorsal striatal projections to the substantia nigra (i.e., the direct pathway) in regulating these behaviors. Combining a Cre-recombinase dependent chemogenetic approach with a cocaine self-administration paradigm that produces both low-risk and high-risk addiction phenotypes, we examined the effect of transiently decreasing direct pathway activity in the dorsomedial striatum on drug-taking and drug-seeking under conditions of normal and pathological drug use. Surprisingly, transient inhibition of direct pathway striatal neurons had no effect on several measures of addictive behavior during on-going drug use, including loss of control over drug intake, high motivation to obtain drug, and drug use despite negative consequences (i.e., drug use paired with foot shock). However, chemogenetic inhibition of these neurons during reinstatement reduced cue-induced drug-seeking, but only in the high-risk addiction phenotype group. Cue-induced reinstatement was relatively normal in the low-risk addiction phenotype group, as well as following reinstatement to cues associated with sucrose pellet consumption. These results demonstrate that dorsomedial direct pathway striatal neurons play a very specific role in addictive behaviors, which is to regulate the pathological drug-seeking that accompanies relapse.
INTRODUCTION
Drug addiction is a chronic disease characterized by compulsive drug-seeking and -taking, loss of control over drug intake, and a persistent craving for the drug. Although only a fraction of individuals that try drugs (~15–20% for cocaine) will develop these pathological behaviors, addiction remains a public health epidemic with far reaching economic and social consequences (Anthony et al., 1994; Penberthy et al., 2010). Currently little is known about what renders some individuals vulnerable to develop addiction and protects others against it. Furthermore, treatment options for addicts are limited, which is problematic given the high rates of recidivism. Thus, parsing the adaptations in neural circuits that regulate casual, controlled drug consumption from those that contribute to compulsive drug use and relapse are critical for developing effective preventative and therapeutic strategies for vulnerable individuals.
Psychostimulant drugs produce an abundance of cellular and molecular adaptations in the striatum, which serves as a key interface of the cortico-basal ganglia-thalamic circuit (Berke and Hyman, 2000; Nestler, 2001; Moussawi et al., 2011; Yager et al., 2015). However, the striatum is composed primarily of two intermixed populations of GABAergic medium spiny projection neurons (MSNs) that give rise to distinct output pathways. Direct pathway MSNs (dMSNs) project monosynaptically to the substantia nigra (SN) whereas indirect pathway MSNs (iMSNs) project to the SN via the globus pallidus external (GPe) and subthalamic nucleus (Gerfen and Surmeier, 2011). Classic studies have demonstrated that the direct and indirect pathways can mechanistically and functionally oppose one another (Albin et al., 1989; Gerfen and Surmeier, 2011; Calabresi et al., 2014), and it has been hypothesized that an imbalance between signaling in dMSNs and iMSNs may contribute to the development and persistence of addiction (Lobo et al., 2010; Smith et al., 2013; Volkow et al., 2013). Indeed, recent work has shown that dMSNs and iMSNs regulate both drug reward and the psychomotor activating effects of psychostimulant drugs in opposing ways (Lobo et al., 2010; Ferguson et al., 2011).
While these studies have led to a better understanding of the role of dMSNs and iMSNs in behavioral effects following drug exposure, they utilized experimenter-administered drug delivery paradigms. It is important to also determine the function of these pathways in models that can more readily mimic the hallmarks of human addiction. Only recently has the role of iMSNs been examined during cocaine self-administration, and it was found that optogenetic activation of NAc iMSNs suppressed drug-taking while inhibition of NAc iMSNs, via activation of Gi/o-coupled DREADDs (Designer Receptors Exclusively Activated by Designer Drugs; hM4Di), enhanced the motivation to take cocaine as assessed with a progressive ratio schedule of reinforcement (Bock et al., 2013). However, the function of dMSNs in key features of compulsive drug-use, as well as relapse, remains unknown.
To investigate this question, we utilized a Cre-recombinase (Cre-) dependent, intersectional viral vector approach to selectively express Gi/o-DREADDs in dMSNs. Activation of hM4Di increases Gi/o signaling in striatal cells, leading to a decrease in cAMP activity, stimulated c-Fos expression and action potential firing (Armbruster et al., 2007; Ferguson et al., 2011). This technique was used to examine how decreasing activity of dorsomedial striatum neurons that project to SN (i.e., dMSNs) impacts several measures of compulsive drug-use characteristic of addiction (loss of control over drug-seeking, motivation to take drugs, continued drug use despite negative consequences), as well as during cue-induced reinstatement of drug-seeking.
MATERIALS AND METHODS
Animals
All experiments were approved by the Seattle Children’s Research Institute Institutional Animal Use and Care Committee and adhered to NIH guidelines. Male Sprague-Dawley rats (n=60 Envigo) weighing 250–274g upon arrival were single- (cocaine self-administration experiment) or pair-housed (sucrose self-administration experiment and calcium imaging) in a temperature- and humidity-controlled vivarium on a 12 h light/dark cycle. Rats were acclimatized to the environment for at least 3 days prior to any experimental manipulations. Food was provided ad libitum until 5 days prior to the start of the experiment, after which rats were mildly food restricted and fed 16–20 g of chow per day. Water was available ad libitum. 20 rats were excluded from analysis because of catheter failure (n=2), inability to learn to self-administer (n=2), resistance to extinction (n=2), failure to reinstate (n=3), and virus localization (n=11).
Drugs
Clozapine-N-Oxide (CNO) was obtained from the National Institutes of Health as part of the Rapid Access to Investigate Drug Program funded by the NINDs. CNO was administered into the intraperitoneal cavity (ip) in a volume of 1 ml/kg at a dose of 5 mg/kg. CNO was dissolved in 100% DMSO, then diluted in sterile water for a final concentration of 6% DMSO. For calcium imaging experiments, CNO was dissolved in aCSF at a concentration of 10 μM. Vehicle injections consisted of 6% DMSO mixed in sterile water. Cocaine HCl was obtained from the National Institute of Drug Abuse and was dissolved in sterile 0.9% saline.
Viral vectors
A Cre-dependent hM4Di viral vector driven by the human synapsin promoter (AAV5-hSYN-DIO-hM4Di-mCherry) was packaged in adenoassociated virus serotype 5 (AAV5) at the University of North Carolina viral vector core and had a titer of ~5×1012 viral genomes/μL. A GCaMP6m viral vector driven by the human synapsin promoter (AAV5-hSYN-GCaMP6m-WPRE-SV40) was packaged in AAV5 at the University of Pennsylvania viral vector core and had a titer of ~1×109 viral genomes/μL. A Cre-dependent TdTomato viral vector driven by the chicken β actin promoter (AAV8-CAG-FLEX-TdTomato) was packaged in AAV8 at the University of North Carolina viral vector core and had a titer of ~4×1012 viral genomes/μL. Canine adenovirus expressing Cre (CAV2-Cre) had a titer of ~2.5×109 viral genomes/μL and was prepared as previously described (Kremer et al., 2000).
Surgical techniques
Rats were anesthetized with isoflurane (2–4% inhaled; Patterson Veterinary) during all surgical procedures. Rats received an injection of meloxicam (0.2 mg/kg SC; Patterson Veterinary) prior to surgery for analgesia. Rats were monitored for at least 3 days following each surgical procedure.
Standard stereotaxic procedures were followed to perform viral infusions. For cocaine and sucrose self-administration experiments, 33-gauge needles attached to gas-tight syringes (Hamilton Company) were placed above the regions of interest. 1.0 μl of hM4Di was bilaterally infused into the DMS (relative to bregma and from skull surface: A/P +0.4 mm, M/L ±2.4 mm, D/V −4.2 mm) and 0.75 μl of CAV2-Cre was bilaterally infused into the SN (relative to bregma and from skull surface: A/P −5.4 mm, M/L ±2.4 mm, D/V −8.0 mm) at a rate of 0.4 μl/min. For calcium imaging experiments, 27-gauge stainless steel injectors were placed above the regions of interest. 1.2 μl of hM4Di, GCaMP6m, and tdTomato (0.4μl each) was bilaterally infused into the DMS (relative to bregma and from skull surface: A/P +0.2 mm, M/L ±2.0 mm, D/V −4.1 mm) and 0.5 μl of CAV-Cre was bilaterally infused into the SN (relative to bregma and from skull surface: A/P −5.3 mm, M/L ±2.2 mm, D/V −7.6 mm) at a rate of 0.4 μl/min. Needles were left in place for an additional 5 minutes to allow for diffusion away from the injection site and through the injection tract.
For cocaine self-administration experiments, following a minimum 3-day recovery period, chronic indwelling catheters were placed into the right jugular vein and attached to a back-mounted port as previously described (Crombag et al., 2000). Following surgery, catheters were flushed daily with 0.2 ml of sterile saline containing 5 mg/ml gentamicin sulfate (Patterson Veterinary) to prevent occlusions and minimize infections. Catheter patency was tested prior to the first self-administration session, after the last self-administration session, and if any behavioral changes suggested loss of patency, by IV injection of 0.2 ml of methohexital sodium (10 mg/ml in sterile water; Patterson Veterinary). Rats that became ataxic within 5 s were considered to have patent catheters. If a catheter lost patency during the self-administration experiment, rats underwent an additional surgery to place a new jugular catheter into the left jugular vein.
Calcium imaging
Slice preparation
Animals (n=6) were anesthetized and perfused with a cold, high-sucrose aCSF solution (in mM: 210 sucrose, 5 KCl, 1.25 NaH2PO4•H2O, 3.5 MgSO4•7H2O, 0.5 CaCl2•2H2O, 26 NaHCO3, and 10 D-glucose, osmolarity ~300 mOsm). They were then decapitated and brains quickly removed and transferred to a chamber of cold, high-sucrose aCSF. 300 μM thick coronal slices were taken with a vibrating microtome while the brains were submerged in cold, high-sucrose aCSF. Slices were then transferred to a chamber filled with NMDG protective recovery aCSF (in mM: 93 N-methyl-D-glucamine, 93 HCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4•7H2O, and 0.5 CaCl2•2H2O, osmolarity ~300 mOsm) for less than 15 minutes. Finally, slices were transferred to a chamber filled with room temperature aCSF (in mM: 119 NaCl, 5 KCl, 1.3 MgSO4•7H2O, 2.5 CaCl2•2H2O, 1 NaH2PO4•H2O, 16.2 NaHCO3, 11 D-glucose, and 10 HEPES, osmolarity ~300 mOsm) and allowed to rest for ~1 hr. All solutions were bubbled with a 95% O2-5% CO2 mixture.
Image acquisition and processing
Slices were submerged in oxygenated aCSF (32°C), which was superfused throughout the experiments. Fluorescence images were captured at 5 Hz with an Olympus FV1000 upright microscope equipped with 25X 1.05NA Ultra-objectives driven by a femtosecond pulsed MaiTai DeepSee laser (Spectra Physics) tuned to 890nm excitation. After acquisition, images were processed through the use of FIJI and FluoroSNNAP (Patel et al., 2015). ROIs were identified from image stacks, which were motion corrected. The ΔF/F record was calculated by subtracting raw fluorescence values with the mean of the lower 50% of previous 10 sec values and dividing by the mean of the lower 50% of previous 10 sec values (Patel et al., 2015). This ΔF/F record was then used to infer the underlying spike probability using the algorithms described by Vogelstein et al. (2010).
Cocaine self-administration
Self-administration chambers
Behavioral testing was conducted in 20 standard operant self-administration chambers equipped with retractable levers, green and white stimulus lights, a house light, and a metal grid floor that was connected to a grid floor scrambler (MedAssociates). The front wall housed two white stimulus lights, one located above each lever, and a green stimulus light mounted above the white stimulus light above the active lever. The back wall contained a white house light. A syringe pump, located outside of the box, delivered cocaine via tubing attached to a swivel and the catheter backport. All tubing was attached to a suspended swivel, which allowed rats to move freely within the chambers.
Cocaine self-administration: FR training
At least 5 days following catheter implantation, rats were trained, during their light cycle, to lever press for cocaine on an FR5 schedule. This included 4 days of training on an FR1 schedule and 2 days of training on an FR3 schedule, after which an FR5 schedule of reinforcement was maintained for the duration of self-administration. The beginning of the session was indicated by insertion of the two levers into the chamber. Completion of the FR on the active lever resulted in a single cocaine infusion (0.4 mg/kg/infusion in 50 μl over 2.8 s) and illumination of the stimulus light above the active lever (4 s). Responses on the inactive lever had no programmed consequences (i.e., no cocaine and no light). Additional presses on the active lever during the cue light presentation were recorded, but did not yield any additional cocaine (i.e., 4 s time-out). The location of the active lever was counterbalanced across rats. The session ended after 3 hours or when rats received 20 cocaine infusions, whichever came first.
Experimental Design
Prolonged intermittent access self-administration (IntA SA)
Following completion of FR5 training, rats underwent 37 sessions of intermittent access to cocaine. This procedure was based upon self-administration methods presented in Deroche-Gamonet et al. (2004) and Zimmer et al. (2012). A single session consisted of 5 min access to cocaine (“cocaine available”) followed by a 25-min time-out period (“cocaine not available”). This cycle repeated for a total of 155 min (6 cocaine-available periods and 5 cocaine not available periods). Completion of an FR5 during the “cocaine available” periods resulted in a single cocaine infusion (0.4 mg/kg/infusion in 50 μl over 2.8 s) and illumination of the stimulus light above the active lever (4 s). The levers remained extended during the “cocaine not available” periods, and both the number of active and inactive responses made during this period was recorded, but resulted in no programmed consequences. Constant illumination of the white house light signaled the “cocaine not available” periods while extinction of the white house light signaled the “cocaine available” periods.
Addiction risk screening
After 37 days of IntA cocaine SA, 3 tests of addiction-like behavior were administered. All tests were separated by 3 sessions of IntA SA.
Persistence of drug-seeking behavior. During sessions 38, 39, and 40 of IntA SA, the number of active responses made during the “cocaine not available” periods were recorded.
Motivation to obtain drug. In order to measure motivation to obtain drug, the breakpoint in a progressive ratio schedule of reinforcement was measured. During the progressive ratio test, the number of responses required to earn each successive cocaine infusion increased using the following progression, as outlined in Deroche-Gamonet et al. (2004): 5, 10, 20, 30, 45, 65, 85, 115, 145, 185, 225, 275, 325, 385, 445, 515, 585, 665, 745, 835, 925, 1025, 1125, 1235, 1345, 1465, 1585. During the session the house light remained off to signal that drug was available. The session was terminated after 5 h or when 1 h elapsed since the previously completed ratio.
Continued drug-seeking despite adverse consequences. To measure continued drug use despite negative consequences, the animals’ responding for drug when drug delivery was associated with punishment was assessed. This session followed the same structure as daily IntA SA, except that drug infusions were paired with a brief foot shock (see Deroche-Gamonet et al. (2004)). During the “cocaine available periods”, the first active lever press (FR1) resulted in illumination of the green cue light, signaling the presence of shock. Completion of the fourth active lever press (FR4) resulted in an electric shock (0.2 mA, 1 s). Upon completion of the fifth lever press (FR5), rats received both an electric shock (0.2 mA, 1 s) and a cocaine infusion (0.4 mg/kg), along with presentation of the associated cocaine cue light. The green cue light was then extinguished. If after completion of the FR1, rats failed to complete the FR4 or FR5 within 1 min, the green cue light was extinguished and the sequence was reinitiated.
High- and low-risk phenotype classification
Rats were ranked for each of the addiction-like behaviors independently based on the classification system outlined in Deroche-Gamonet et al. (2004). The following measurements to rank rats for each behavior were used.
Persistence of drug-seeking behavior. To classify animals, the average number of responses during the no-drug available periods were averaged over sessions 38, 39, and 40 of IntA SA.
Motivation to obtain drug. The last ratio completed (“breakpoint”) was used as an index of motivation.
Continued drug-seeking despite adverse consequences. The change in the number of cocaine infusions earned when associated with an electric foot shock compared to the prior baseline IntA SA session (expressed as a percent change) was used.
If rats fell within the top 40% of the group for a behavior, they received a score of 1, and if they did not they received a score of 0. The scores were then summed, and rats that received a combined score of 2 or 3 were classified as having a “high-risk” addiction phenotype whereas rats the received a combined score of 0 or 1 were classified as having a “low-risk” addiction phenotype.
Addiction-risk testing
We next assessed the effect of transiently decreasing activity of dMSNs on the 3 measures of addiction-like behavior. Prior to the first test session, rats in the high- and low-risk groups were divided into CNO or Vehicle (VEH) pretreatment groups. Rats received the same treatment prior to each test session (i.e., rats in the CNO group received CNO prior to each test and rats in the VEH group received VEH prior to each test). Groups were matched based on their behavior during the baseline addiction-like tests. Twenty min prior to each test session, rats received an ip injection of either CNO (5mg/kg) or VEH. Injections were given prior to 2 sessions to assess persistence of drug-seeking behavior, 1 session to assess motivation to obtain drug, and 1 session to assess continued drug-seeking despite negative consequences. The structure of the sessions was identical to what is described above. All tests were separated by 3 sessions of IntA SA.
Extinction
Rats underwent extinction training at the conclusion of the addiction-risk tests. During these 60-min sessions, responses on the active lever had no programmed consequences (i.e., no cocaine delivery or illumination of the cue light). Rats underwent extinction training for at least 10 days, or until they pressed the active lever less than 25 times for 2 consecutive days.
Cue-induced reinstatement of cocaine-seeking
After rats reached extinction criteria, they underwent 2 tests of cue-induced reinstatement of cocaine-seeking. 20 min prior to the test session, rats received injections of VEH or CNO (5 mg/kg). The order of drug pre-treatment was counterbalanced across rats. Rats were then connected to the infusion line and placed into the chamber. The session began with extension of the levers into the chamber and illumination of the cue light above the active lever for 10 s. Subsequently, completion of an FR5 on the active lever resulted in 4 s presentation of the cocaine-associated cue light. Rats underwent additional extinction training between the cue-induced reinstatement sessions until they met criteria again (2 consecutive days of less than 25 lever presses).
Sucrose self-administration, extinction, and cue-induced reinstatement of sucrose-seeking
Sucrose self-administration was similar to FR training for cocaine self-administration. At least 5 days following viral infusions, rats were trained, during their light cycle, to lever press for sucrose pellets on an FR5 schedule. This included 4 days of training on an FR1 schedule and 2 days of training on an FR3 schedule, after which an FR5 schedule of reinforcement was maintained for the duration of self-administration. Rats then underwent 2 weeks of FR5 sucrose self-administration. The beginning of the session was indicated by insertion of the two levers into the chamber, which remained extended for the entire 60-min session. There was no limit to the number of pellets rats could earn during any session. Completion of the FR on the active lever resulted in delivery of a single sucrose pellet (Bio-Serv) and illumination of the stimulus light above the active lever (4 s). Responses on the inactive lever had no programmed consequences (i.e., no sucrose pellet and no light). Additional presses on the active lever during the cue light presentation were recorded, but did not yield any additional sucrose pellets (i.e., 4 s time-out). The location of the active lever was counterbalanced across rats. Rats were rank ordered by the total number of sucrose pellets that were consumed over the 2 weeks of FR5 self-administration. Rats that fell within the top 40% of the group for consumption were classified as “high-responders” and the rest of the rats were classified as “low-responders.” Rats next underwent extinction training for at least 4 days, or until rats pressed the active lever less than 25 times for 2 consecutive days. Cue-induced reinstatement sessions were identical to those described for reinstatement of cocaine-seeking.
Immunohistochemistry
Accuracy of injection coordinates was confirmed by visualization of mCherry immunofluorescence in DMS. Rats were anesthetized with Beuthanasia-D (Patterson Veterinary) and perfused transcardially with PBS (pH 7.4) followed by 4% paraformaldehyde (PFA). Brains were extracted, post-fixed overnight in 4% PFA, and sliced into 60 μm sections on a vibrating microtome. Floating sections were washed in 0.5% Triton-X/PBS for 10 min, blocked in 0.25% Triton-X/5% normal goat serum (NGS)/PBS for 2 h, and incubated in 0.25% Triton-X/2.5% NGS/PBS containing an antibody to mCherry (1:400, rabbit host; Clontech) with gentle agitation at room temperature overnight. Next, sections were rinsed 4 times in PBS and incubated in anti-rabbit Alexa 568 (red)-conjugated secondary antibody (1:400; Invitrogen) for 2 h. Sections were then washed 2 times in PBS, mounted on slides, and cover-slipped with Vectashield mounting medium with DAPI (Vector Labs). Z-stacks were captured using a Zeiss LSM 710 Confocal microscope, and images were processed using ImageJ software (National Institutes of Health).
Statistical analysis
GraphPad Prism 6 was used for all statistical analyses. Group differences between low- and high-risk phenotypes in the number of responses made during the “cocaine not available” periods, the final ratio (“breakpoint”) completed in the progressive ratio test, the change in the number of infusions earned when infusions were associated with punishment, and the total number of drug infusions earned across sessions were analyzed with unpaired t-tests. Two-way repeated measures ANOVA (risk phenotype x session) were used to analyze group differences in the number of responses made during the “cocaine not available” periods, the number of infusions or pellets earned, and extinction responses across sessions. A two-way repeated measures ANOVA (risk phenotype x pretreatment) was used to analyze differences in cocaine cue-induced reinstatement and sucrose cue-induced reinstatement (pretreatment x phase). A two-way ANOVA was used to assess the effects of CNO on addiction-like behaviors (risk phenotype x pretreatment). All ANOVA analyses were followed by Sidak’s multiple comparison’s tests as appropriate. A paired t-test was used to compare inferred spike activity rates before and after CNO application for calcium imaging experiments. For all comparisons, α ≤ 0.05. Data is graphed as mean ± SEM.
RESULTS
Differences in addiction-like behaviors between high- and low-risk phenotypes
A summary of the experimental design is shown in Figure 1A. All rats included in the final analysis (n=27) acquired and maintained stable levels of cocaine self-administration and learned to discriminate between the active and inactive levers (data not shown). High- (n=12, consisting of nine 2-criteria and three 3-criteria rats) and low- (n=15, consisting of six 1-criteria and nine 0-criteria rats) risk phenotypes differed on all measures of addiction-like behavior, confirming that response heterogeneity existed among each of the 3-criteria.
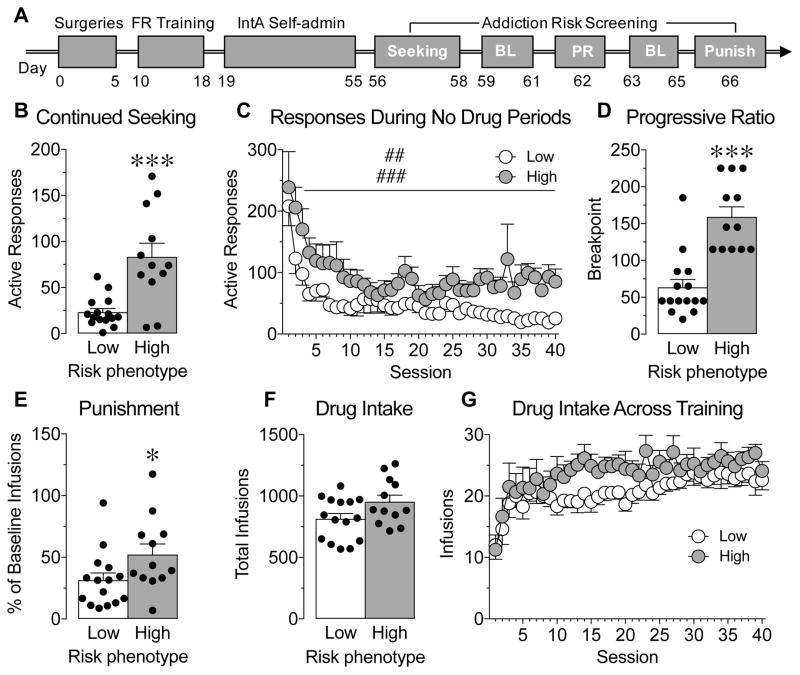
Figure 1.
Prolonged drug use produces low- and high-risk addiction phenotypes. A, Illustration of the experimental paradigm. Addiction risk screening occurred following 37 sessions of cocaine self-administration and included tests for loss of control over drug intake (Seeking), high motivation to obtain drug (Progressive ratio, PR) and drug use despite negative consequences (drug infusions paired with foot shock, Punish). Each test was separated by three baseline (BL) sessions. B–E, Low- and high-risk addiction phenotypes showed a significant difference in the number of active responses made during no drug periods (B, data averaged across the last 3 training sessions, ***p=0.001 vs. low-risk addiction phenotype and C, ##main effect of phenotype, p=0.004; ###main effect of session, p<0.001.), in the breakpoint reached on a PR schedule of reinforcement (D, ***p=0.001 vs. low-risk addiction phenotype), and in the percent of baseline infusions earned when infusions were paired with foot shock (E, *p<0.05 vs. low-risk addiction phenotype.) F,G, Total infusions (F) and the number of infusions earned across training (G) did not differ between low- and high-risk addiction phenotypes. White bars/symbols represent the low-risk addiction phenotype and grey bars/symbols represent the high-risk addiction phenotype. Black dots represent data points of individual animals. N=12–15/group.
Persistence of drug-seeking behavior
To determine the persistence of drug-seeking behavior, we recorded the number of active responses made during the “cocaine not available” periods of each session of IntA SA. During the final 3 sessions of IntA SA (averaged together), high-risk rats made more responses on the active lever during the “cocaine not available” periods than low-risk rats (Figure 1B; t-test, t25=4.29, P=0.0002). Additionally, this increased drug-seeking during signaled periods of drug was seen across days of training (Figure 1C; 2-way RM ANOVA, main effect of session F(39,975)=10.49, P<0.001; main effect of phenotype F(1,25)=9.96, P=0.004; no session x phenotype interaction F(39,975)=0.93, P=0.59).
Motivation to obtain drug
To determine the motivation to obtain drug, we measured the breakpoint in a progressive ratio schedule of reinforcement. High-risk rats reached higher breakpoints during the progressive ratio test than low-risk rats (Figure 1D; t-test, t25=5.59, P<0.0001).
Continued drug-seeking despite adverse consequences
To assess continued drug use despite negative consequences, we measured responding for drug when drug delivery was associated with punishment (brief, mild foot shock). While both high- and low-risk phenotypes reduced responding during this session, high-risk rats showed a lower reduction in the number of infusions earned during this session compared to baseline responding than low-risk rats (Figure 1E; t-test, t25=2.06, P=0.05).
Cocaine intake
While high- and low-risk phenotypes differed in the three measures of addiction-like behavior, they had minimal differences in the total amount of infusions earned (Figure 1F; t-test, t25=2.01, P=0.06). Furthermore, across days of training, both groups escalated the amount of infusions earned at a similar rate (Figure 1G; 2-way RM ANOVA, main effect of session F(39,975)=7.22, P<0.001; no main effect of phenotype F(1,25)=2.95, P=0.10; no session x phenotype interaction F(39,975)=0.82, P=0.78). These results suggest that although there was a trend toward increased consumption in the high-risk phenotype group, the amount of drug consumed was unlikely to be the sole factor for the differences in behavioral phenotype.
Intersectional viral vector approach results in transgene expression in dorsomedial striatal neurons that project to SN (i.e., dMSNs)
We used a Cre-dependent, intersectional viral vector approach to selectively express hM4Di receptors in dMSNs. Specifically, rats received bilateral injections of CAV2-Cre into the SN and a Cre-dependent AAV expressing hM4Di into the DMS (Figure 2A). This approach resulted in robust expression of hM4Di in cell bodies of dMSNs of the DMS as well as in axons/terminals in the SN (Figure 2B).
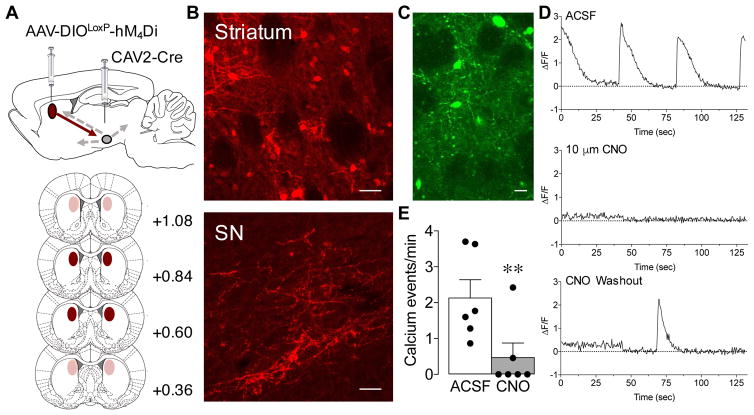
Figure 2.
Chemogenetic inhibition of direct pathway striatal neurons (dMSNs) reduces neuronal activity. A, (Top) Illustration of the intersectional viral vector approach: A Cre-recombinase (Cre)-dependent AAV vector expressing hM4Di was injected into the dorsomedial striatum and a retrograde CAV2-Cre virus was injected into the substantia nigra (SN). (Bottom) Viral spread is depicted in red. Dark shading indicates areas of expression common to all animals and light shading indicates areas of expression that varied across animals. B, Representative sections of mCherry-tagged hM4Di immunofluorescence in the striatum (top) and in terminals in the SN (bottom) at ~4 months following viral infusions. Scale bar, 50 μm C, Representative section of GCaMP6m fluorescent expression in dMSNs following viral infusion. Scale bar, 20 μm. D, Representative calcium events (measured as ΔF/F) in dMSNs at baseline (top), following 10 μm CNO (middle) and following CNO washout (bottom). E, Calcium events were significantly reduced in dMSNs following CNO exposure (grey bar) compared to ACSF (white bar). **p=0.01 vs. baseline. Black dots represent data points of individual animals. N=6.
Expression of hM4Di in dMSNs reduces their activity
There is abundant evidence that activation of hM4Di receptors by CNO reduces neuronal activity (for review see Sternson and Roth, 2014). This effect has been documented in dMSNs of both the DMS and the nucleus accumbens (Ferguson et al., 2011; Calipari et al., 2016). To verify that the DREADD manipulation was, in fact, reducing activity of dMSNs, we performed ex vivo calcium imaging in DMS slices that expressed both hM4Di and GCaMP6m in dMSNs (Figure 2C; cells identified by co-expression of green and red fluorescence). Application of CNO (10 μM) significantly reduced the spike activity rate inferred from the ΔF/F record in dMSNs of DMS (Figure 2E; paired t-test, t5=3.91, P=0.01), confirming that activation of hM4Di receptors in dMSNs is sufficient to reduce neuronal activity.
Decreasing activity of dMSNs does not alter addiction-like behaviors of high- and low-risk phenotypes
We next tested whether dMSN activity regulates the 3 measures of addiction-like behavior. Following completion of IntA SA and screening for addiction risk, rats received injections of either vehicle or CNO (5 mg/kg, ip) 20 minutes prior to each test session for addiction-like behaviors (Figure 3A).
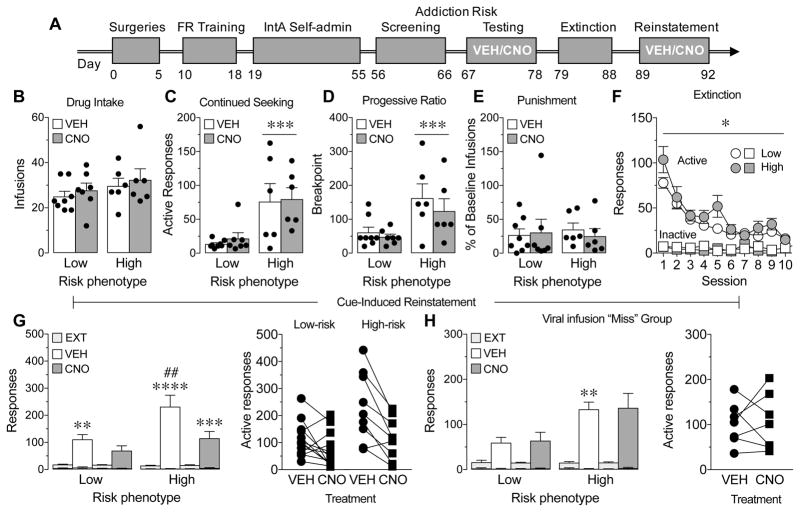
Figure 3.
Chemogenetic inhibition of direct pathway striatal neurons (dMSNs) reduces cue-induced reinstatement of drug-seeking only in the high-risk addiction phenotype. A, Illustration of the experimental paradigm. B–E, Low- and high-risk addiction phenotypes showed a significant difference in the number of active responses made during no drug periods (C, data averaged across 2 test sessions, ***main effect of phenotype, p=0.0004) and in the breakpoint reached on a PR schedule of reinforcement (D, main effect of phenotype, p=0.003) but not in the number of infusions earned averaged across 2 test session (B) or in the percent of baseline infusions earned when infusions were paired with foot shock (E). Decreasing dMSN activity had no effect on the number of earned infusions averaged across 2 test sessions (B), the number of active responses made during no drug periods averaged across 2 test sessions (C), the breakpoint reached on a PR schedule of reinforcement (D), or the percent of baseline infusions earned when infusions were paired with foot shock (E) in either low-risk or high-risk addiction phenotypes. F, Active (but not inactive) responses during extinction varied over session between low-risk and high-risk addiction phenotypes (*session x phenotype interaction, P=0.04), but both groups extinguished responding. G, Both low-risk and high-risk addiction phenotypes showed a significant increase in the number of active responses made during cue-induced reinstatement compared to extinction (**P=0.004, ****P<0.0001 vs. extinction); however, the high-risk addiction phenotype showed a greater level of cue-induced reinstatement compared to the low-risk phenotype (##P=0.004 vs. low-risk phenotype). Decreasing dMSN activity significantly reduced the number of active responses made during cue-induced reinstatement only in the high-risk addiction phenotype (***P=0.0004 vs. vehicle treatment in the high-risk phenotype). H, In the viral injection “miss” group, the high-risk addiction phenotype showed a significant increase in the number of active responses made during cue-induced reinstatement compared to the low-risk phenotype (**P=0.02 vs. low-risk phenotype). However, there were no differences in cue-induced reinstatement between vehicle and CNO treatment in either phenotype. Lower error bars indicate inactive presses, which did not differ across groups. White bars represent vehicle (VEH) treatment and dark grey bars represent CNO treatment except in F where white symbols represent the low-risk addiction phenotype and grey symbols represent the high-risk addiction phenotype. Circles represent active responses and squares represent inactive responses. Black circles and squares represent data points of individual animals. N=3–13/group.
Drug intake
We found that reducing activity of dMSNs during IntA SA had no effect on the number of infusions earned averaged across 2 test sessions (Figure 3B; 2-way RM ANOVA, no main effect of treatment F(1,23)=0.60 P=0.45; no main effect of phenotype F(1,23)=1.80, P=0.19; no treatment x phenotype interaction F(1,23)=0.00005, P=1.00).
Persistence of drug–seeking
Decreasing activity of dMSNs also had no effect on the number of responses made during the “cocaine not available” periods averaged across 2 test sessions (Figure 3C; 2-way RM ANOVA, no main effect of treatment F(1,23)=0.16, P=0.69; main effect of phenotype F(1,23)=16.80, P=0.0004; no treatment x phenotype interaction F(1,23)=0.02, P=0.88).
Motivation to obtain drug
Decreasing activity of dMSNs had no effect on the final breakpoint completed during the progressive ratio test (Figure 3D; 2-way RM ANOVA, no main effect of treatment F(1,23)=0.93, P=0.34; main effect of phenotype F(1,23)=10.98, P=0.003; no treatment x phenotype interaction F(1,23)=0.22, P=0.65).
Continued drug-seeking despite adverse consequences
In contrast to the initial screening test, there were no differences between low-risk and high-risk rats in the number of infusions earned when infusions were paired with the negative consequence of a brief, mild foot shock during a second test session (Figure 3E; 2-way RM ANOVA, no main effect of phenotype F(1,23)=0.01, P=0.92). Additionally, decreasing activity of dMSNs had no effect on this behavioral metric of addiction (Figure 3E; 2-way RM ANOVA, no main effect of treatment F(1,23)=0.05, P=0.82; no treatment x phenotype interaction F(1,23)=0.27, P=0.61).
Decreasing activity of dMSNs attenuates cocaine cue-induced reinstatement in the high risk-addiction phenotype but not in the low-risk addiction phenotype or in sucrose cue-induced reinstatement
Cocaine cue-induced reinstatement
We next explored whether dMSNs regulate relapse behavior, specifically reinstatement of drug-seeking behavior by a cocaine-associated cue (Figure 3A). Following completion of self-administration and the addiction risk tests, rats underwent extinction training. Only rats that met the extinction criteria, less than 25 responses on the active lever for 2 consecutive days, were included in the analysis (n=22). Both high- (n=9, consisting of six 2-criteria and three 3-criteria rats) and low- (n=13, consisting of eight 0-criteria and five 1-criteria rats) risk phenotypes decreased responding across sessions (Figure 3F; 2-way RM ANOVA, main effect of session F(9,180)=45.38, P<0.0001) and there were no group differences overall (Figure 3F; 2-way RM ANOVA, no main effect of phenotype F(1,20)=2.17, P=0.16). However, active responses across sessions varied as a function of phenotype (Figure 3F; 2-way RM ANOVA, session x phenotype interaction F(9,180)=2.00, P=0.04), which was due to lower responding in the low-risk group. These data suggest that low-risk rats were extinguishing at a faster rate than high-risk rats.
Following extinction training, rats received injections of vehicle or CNO (5 mg/kg, ip) in a counterbalanced order 20 min prior to two cue-induced reinstatement tests (additional extinction training was given between tests). We found that although both groups of rats increased their number of active lever presses during cue-induced reinstatement compared to extinction (Figure 3G, 2 way RM ANOVA, main effect of phase F(1,20)=58.31, P<0.0001; main effect of phenotype F(1,20)=7.91, P=0.01; phase x phenotype interaction F(1,20)=9.37, P=0.006), high-risk rats reinstated more than low-risk rats (P=0.004). In addition, decreasing activity by CNO treatment reduced reinstatement in the high-risk rats to the level observed in low-risk rats (Figure 3G; 2-way RM ANOVA, main effect of phenotype F(1,20)=6.78, P=0.10; main effect of treatment F(1,20)=22.87, P=0.0001; phenotype x treatment interaction F(1,20)=5.07, P=0.04). Of note, there were no differences in responding during either test between animals that had previously received repeated VEH treatments and those that received repeated CNO treatments (data not shown), suggesting that multiple CNO injections were not contributing to the observed effects on reinstatement. Finally, we also examined the effect of CNO on cue-induced reinstatement in rats that were excluded from the study due to absence of viral expression (i.e., the “miss” group). There was no effect of CNO on reinstatement in either low-risk or high-risk rats in this control group (Figure 3H; 2-way RM ANOVA, main effect of phenotype F(1,5)=13.14, P=0.02; no main effect of treatment F(1,5)=0.02, P=0.89; no phenotype x treatment interaction F(1,5)=0.0004, P=0.98), suggesting that CNO was not producing off-target effects on responding during cue-induced reinstatement.
Sucrose cue-induced reinstatement
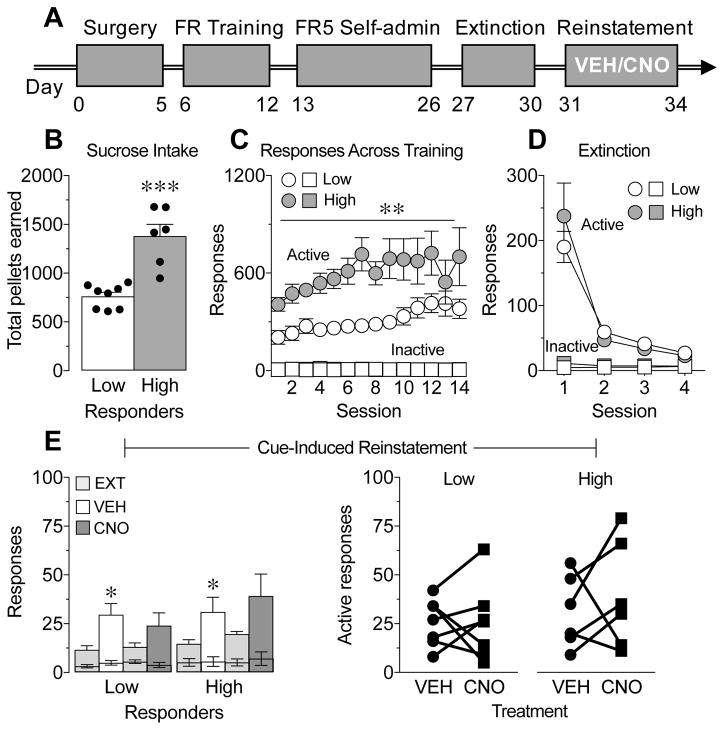
We next wanted to assess whether dMSNs specifically regulate motivation for cocaine-associated cues or if this effect extends to cues associated with a natural reward. To test this, an independent cohort of rats was trained to self-administer sucrose. A summary of the experimental design is shown in Figure 4A. Following completion of sucrose self-administration, rats were divided into low-responder (n=8) and high-responder (n=6) phenotypes based on their rank-ordered level of sucrose consumption. Although both groups increased the amount of pellets earned over training (Figure 4C; 2-way RM ANOVA, main effect of session F(13,156)=3.72, P<0.0001), the high-responder group had approximately twice the intake of the low-responder group (Figure 4B; t-test, t12=5.39, P=0.0002; Figure 4C; 2-way RM ANOVA, main effect of phenotype F(1,12)=17.08, P=0.001). Rats then underwent extinction training, and both groups rapidly extinguished responding across sessions (Figure 4D; 2-way RM ANOVA, main effect of session F(3,36)=46.8, P<0.001; no main effect of phenotype F(1,12)=0.16, P=0.70; no session x phenotype interaction F(3,36)=1.18, P=0.33). Following extinction training, rats received injections of vehicle or CNO (5 mg/kg, ip) in a counterbalanced order 20 min prior to two sucrose cue-induced reinstatement tests (rats received additional extinction training between tests). We found that both groups reinstated to cues associated with sucrose-seeking; however, there was no difference in the degree of reinstatement (Figure 4E; 2-way RM ANOVA, main effect of session F(1,12)=14.1, P=0.003; no main effect of phenotype F(1,12)=0.19, P=0.67; no session x phenotype interaction F(1,12)=0.03, P=0.86). In addition, decreasing activity of dMSNs had no effect on sucrose cue-induced reinstatement in either group (Figure 4E, F; 2-way RM ANOVA, no main effect of treatment F(1,12)=0.03, P=0.86; no main effect of phenotype F(1,12)=1.01, P=0.34; no treatment x phenotype interaction F(1,12)=0.94, P=0.35), suggesting that dMSNs do not modulate reactivity to all classes of reward-associated cues.
Figure 4.
Chemogenetic inhibition of direct pathway striatal neurons (dMSNs) has no effect on cue-induced reinstatement to sucrose-seeking across phenotype A, Illustration of the experimental paradigm. B,C, Low- (white) and high- (grey) responder phenotypes showed a significant difference in the total number of pellets earned (B, ***p=0.0002 and C, **main effect of phenotype, p=0.001). D, Active (grey circles) and inactive (white circles) responses during 4 sessions of extinction training; extinction responding did not differ between groups. E, The number of active responses were significantly increased in both phenotypes during cue-induced reinstatement (*p<0.05 vs. extinction (EXT)). Decreasing dMSN activity had no effect on the number of active responses in either phenotype during cue-induced reinstatement (#p<0.05 vs. vehicle (VEH)). The number of inactive responses (lower error bars) did not differ across groups. Light grey represents EXT, white represents VEH, dark grey represents CNO. Black circles and squares represent data points of individual animals. N=16.
DISCUSSION
In order to examine the role of dMSNS in patterns of drug use that are characteristic of addiction, we used a prolonged, intermittent access cocaine self-administration paradigm. Of note, we found that animals that were classified as having a high-risk addiction phenotype (i.e., displayed loss of control over drug-seeking, high motivation to obtain drug, continued drug-seeking despite negative consequences) had higher levels of cue-induced reinstatement relative to animals that were classified as having a low-risk phenotype, suggesting they were more susceptible to relapse. This result is consistent with previous work that characterized animals based on their level of drug prime-induced reinstatement, and reported that rats with the highest levels also had greater amounts of cue-induced reinstatement as well as higher levels of the three addiction criteria measured in the present study (Deroche-Gamonet et al., 2004). In addition, these behavioral differences manifested despite the fact that the overall pattern of drug use and amount of drug intake were largely the same across animals. Thus, this model afforded the opportunity to investigate the contribution of dMSNs in both controlled and pathological drug-taking and drug-seeking behaviors.
Given that chronic exposure to cocaine produces a host of cellular and molecular adaptations in dMSNs and these neurons are part of the pathway that, when activated by dopamine, is thought to initiate motivated behaviors (Berke and Hyman, 2000; Nestler, 2001; Moussawi et al., 2011; Yager et al., 2015), we hypothesized that chemogenetic inhibition of dMSNs would decrease drug-taking and drug-seeking behaviors across groups. Surprisingly, we found that manipulating activity of dMSNs had no effect on drug intake nor on persistence of drug-seeking, motivation to obtain drug or drug-seeking during punishment. This lack of behavioral modulation was seen in both the high-risk and low-risk addiction phenotypes. While these findings may seem contradictory to previous work that demonstrated a role for dMSNs in the reinforcing and sensitizing effects of psychostimulant drugs, there are a number of important distinctions between studies that must be considered. For example, previous work using cell-specific targeting methods to modulate activity utilized behavioral paradigms (conditioned place preference, locomotor sensitization) that assessed different aspects of addiction and studied the role of dMSNs in the acquisition of these behaviors (Hikida et al., 2010; Lobo et al., 2010; Ferguson et al., 2011). In the present study, we examined the effect of cellular inhibition following protracted cocaine self-administration during drug use. Although pharmacological studies have shown that dopamine D1 receptors in the striatum (which are expressed primarily on dMSNs) can modulate drug intake and motivation for drugs during self-administration (Self, 2010), D1 receptor ligands lack sufficient specificity to conclude that the reported effects were only a result of direct pathway modulation.
In addition, the vast majority of studies examining the role of dMSNs have focused on the nucleus accumbens (Lobo and Nestler, 2011; Yager et al., 2015), so it is unknown whether the results would extend to the dorsal striatum. We chose to study dMSNs in dorsomedial striatum in the present set of experiments because the neural substrates that underlie drug-taking and drug-seeking are thought to shift from the nucleus accumbens to the dorsal striatum over time (Everitt and Robbins, 2005, 2016), and we used a lengthy drug self-administration paradigm in order to produce compulsive-like drug use in a subset of rats (i.e., the high-risk addiction phenotype). Thus, it remains to be determined whether dMSNs in other striatal regions can regulate motivation and drug-seeking during controlled or compulsive drug use.
Nonetheless, we did find that dMSNs in dorsal striatum play a critical role in reinstatement of drug-seeking following extinction of cocaine self-administration. Specifically, decreasing activity of these neurons during cue-induced reinstatement reduced drug-seeking in the high-risk phenotype group without altering drug-seeking in the low-risk phenotype group. In addition, chemogenetic inhibition of dMSNs had no effect on reinstatement to cues associated with sucrose self-administration in both low and high consumers of sucrose. Taken together, the results from the current experiments suggest that dMSNs in dorsomedial striatum play a very specific role following prolonged drug use, which is to regulate pathological, cue-driven drug-seeking in the absence of drug use. Only one study has previously examined the contribution of dMSNs in relapse, and that study reported that optical inhibition of dMSN projections from the nucleus accumbens core to the substantia nigra had no effect on reinstatement of drug-seeking induced by cocaine + cues (Stefanik et al., 2013). Although there are numerous procedural differences between that study and ours that can account for the disparate results, including variation in the targeted striatal sub-regions, self-administration and reinstatement protocols and methods for cellular modulation, their data are consistent with the lack of effect we observed in the low-risk phenotype. In addition, given that they used a fairly limited self-administration schedule (~12 sessions), it is unlikely that the animals in that study displayed a compulsive drug-seeking/taking phenotype.
It should be noted that recent work has highlighted potential off-target behavioral effects of CNO that could be mediated through conversion of CNO to clozapine (Gomez et al., 2017; MacLaren et al., 2016). In the present study, we used a vehicle DREADD control in order to obtain baseline measures of behavior and use a within-subject design for the reinstatement tests. We did not find differences in drug intake, in the three addiction criteria, or in reinstatement to cues associated with sucrose self-administration between CNO and vehicle groups, suggesting that 5 mg/kg CNO does not produce non-DREADD mediated behavioral effects on these measures. In addition, we did not find effects of CNO on cue-induced reinstatement in rats that had failed viral injections (i.e., no DREADD receptor expression). Finally, we and others have previously shown that CNO has no effect on cue-induced or drug-primed reinstatement of cocaine-seeking in GFP-injected or no virus control rats (Kerstetter et al., 2016; Mahler et al., 2014) or in rats with DREADD expression in subsets of thalamic projection neurons (Wunsch et al., 2017). Thus, it is unlikely that the effects on cue-induced reinstatement to cocaine-seeking observed in the present work are a result of off-target CNO actions.
The direct and indirect pathways have long been proposed to mechanistically and functionally oppose one another to modulate addiction-related behaviors (Albin et al., 1989; Gerfen and Surmeier, 2011; Calabresi et al., 2014). It is worth noting, therefore, that recent work examining the role of iMSNs in cocaine self-administration reported that chemogenetic inhibition of iMSNs in the nucleus accumbens - but not the dorsal striatum - enhances the motivation for cocaine (Bock et al., 2013). In addition, cocaine + cue-induced reinstatement of drug-seeking was blocked by optical inhibition of iMSNs projections from the nucleus accumbens core to the ventral pallidum (Stefanik et al., 2013). Although these studies involved fairly limited cocaine self-administration experience; together with our results, they challenge the notion that a simple balance in activity between the direct and indirect striatal pathways accounts for the regulation of all aspects of drug use. Instead, it appears that direct and indirect pathway modulation of drug addiction is more nuanced, such that each striatal pathway may govern distinct aspects of drug-related behaviors, or perhaps in some cases, concomitant activation of both pathways is necessary, as has recently been shown to be the case for modulation of movement (Calabresi et al., 2014). Although dMSNs collateralize to iMSNs, we have previously shown that activation of hM4Di receptors in dMSNs does not alter activity in neighboring hM4Di-negative cells (Ferguson et al., 2011). Nonetheless, given that a subset of dMSNs are also known to collateralize to the GPe (Parent et al., 2000), it is possible that dMSN-mediated inhibition of both pathways contributed to the dampening of cue-induced reinstatement seen in the high-risk phenotype.
In summary, the present work identifies a novel and specific role for dMSNs in dorsomedial striatum in regulating cue-induced reinstatement of drug-seeking behavior only in rats exhibiting a high-risk addiction phenotype. A critical aspect of any successful pharmacological treatment for drug addiction involves a therapeutic agent that can dampen drug-craving in addicts without altering normal motivational processes. The findings from these experiments have important implications for addressing this critical treatment requirement, as they suggest that reducing activity of dorsomedial striatum dMSNs is capable of reducing pathological drug urges without altering normal cue-driven associations and motivated behavior. Thus, selective targeting of dMSN neuronal activity may prove to be a highly effective treatment for reducing relapse in recovering addicts.
SIGNIFICANCE STATEMENT.
Drug use and abuse continue to pose a major public health crisis that has profound medical consequences to individuals, as well as serious social and economic impacts on our society. Unfortunately, treatment options remain limited, in part due to our incomplete understanding of the cells and circuits that regulate recreational drug use as well as those that underlie drug addiction and relapse. The present set of experiments sought to address the role of striatal cells that project to the substantia nigra (i.e., the direct/’go’ pathway) in casual and pathological drug use. The findings from this work reveal a novel and specific contribution of direct pathway cells in addiction, which is to regulate drug-seeking following compulsive drug use.
Acknowledgments
We thank Dr. John Neumaier and Dr. Michelle Kelly for providing the CAV2-Cre virus for this work, and Ms. Anna Surowiecki for assistance in running the behavioral assays. This work was supported by grants from the National Institute on Drug Abuse (T32DA007278 to LMF and AFG and R01DA036582 to SMF).
CITATIONS
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244. [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Pena CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci U S A. 2016;113:2726–2731. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogentics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Wunsch AM, Nakata KG, Donckels E, Neumaier JF, Ferguson SM. Corticostriatal afferents modulate responsiveness to psychostimulant drugs and drug-associated stimuli. Neuropsychopharm. 2016;41(4):1128–37. doi: 10.1038/npp.2015.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer EJ, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J Virol. 2000;74:505–512. doi: 10.1128/jvi.74.1.505-512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD. Clozapine N-oxide administration produces behavioral effects in long-evans rats: implications for designing DREADD experiments. eNeuro. 2016;3(5):1–14. doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones GJ. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17(4):577–85. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Parent A, Sato F, Wu Y, Gauthier J, Levesque M, Parent M. Organization of the basal ganglia: The importance of axon collateralization. Trends Neurosci. 2000;23:S20–S27. doi: 10.1016/s1471-1931(00)00022-7. [DOI] [PubMed] [Google Scholar]
- Patel TP, Man K, Firestein BL, Meaney DF. Automated quantification of neuronal networks and single-cell calcium dynamics using calcium imaging. J Neurosci Methods. 2015;243:26–38. doi: 10.1016/j.jneumeth.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy JK, Ait-Daoud N, Vaughan M, Fanning T. Review of treatment for cocaine dependence. Curr Drug Abuse Rev. 2010;3:49–62. doi: 10.2174/1874473711003010049. [DOI] [PubMed] [Google Scholar]
- Self DW. Dopamine Receptor Subtypes in Reward and Relapse. In: Neve KA, editor. The Dopamine Receptors. Totowa, NJ: Humana Press; 2010. pp. 479–524. [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23:546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J Neurosci. 2013;33:13654–13662. doi: 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Vogelstein JT, Packer AM, Machado TA, Sippy T, Babadi B, Yuste R, Paninski L. Fast nonnegative deconvolution for spike train inference from population calcium imaging. J Neurophysiol. 2010;104:3691–3704. doi: 10.1152/jn.01073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol. 2013;23:639–648. doi: 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunsch AM, Yager LM, Donckels EA, Le CT, Neumaier JF, Ferguson SM. Chemogenetic inhibition reveals midline thalamic nuclei and thalamo-accumbens projections mediate cocaine-seeking in rats. Eur J Neurosci. 2017;46(3):1850–1862. doi: 10.1111/ejn.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: role in drug addiction. Neuroscience. 2015;301:529–541. doi: 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DC. The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology. 2012;37:1901–1910. doi: 10.1038/npp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]