Abstract
The TRAF3IP2 gene resides within one of at least 63 psoriasis susceptibility loci and encodes Act1, an adapter protein involved in IL-17 receptor and CD40 signaling pathways. TRAF3IP2 is distinctive (among <10% of candidate susceptibility genes) in that a strongly disease-associated variant encodes a missense SNP predicted to be functionally relevant (SNP rs33980500 C/T encoding Act1 pD10N). As assessed by flow cytometry, Act1 protein was expressed at the highest levels in monocytes, with lower levels in T-cells and B-cells. However, monocytes, T-cells and B-cells failed to respond to IL-17A stimulation of PBMC, as measured by flow cytometric determination of NF-κB phospho-p65. As an alternative stimulus, we treated PBMCs with trimerized recombinant human CD40L and assessed p65, p38 and Erk phosphorylation in CD19+ B-cells as a function of D10N genotype. The increase of phosphorylated p65, p38 and Erk was well-correlated across individuals, and CD40L-induced phosphorylation of p65, p38, and Erk was significantly attenuated in B-cells from Act1 D10N homozygotes, compared to heterozygotes and nullizygotes. Our results indicate that the Act1 D10N variant is a relevant genetic determinant of CD40L responsiveness in human B-cells, with the risk allele being associated with lower B-cell responses in an acute signaling context.
Keywords: psoriasis, psoriatic arthritis, immunology, genetics, functional genomics
INTRODUCTION
Psoriasis is a chronic immune-mediated inflammatory skin disease, affecting 1% to 2% of the Caucasian population.1 A pathogenic cross-talk between innate and adaptive cells, including keratinocytes, dendritic cells, and T-cells, underpins a dysregulated immune response leading to abnormal epidermal proliferation.2 Although psoriasis was initially considered as a Th1-mediated disease, mounting evidence from preclinical results, genetic data and clinical trials have demonstrated a key contribution of IL-17-producing T-cells to psoriasis in humans.3 Expression of IL-17A, IL-17F, and IL-17C is elevated in psoriatic lesional tissue compared with non-lesional tissue.4 IL-17A, IL-17F and IL-17C act directly on keratinocytes to stimulate the production of a number of molecules known to be elevated in psoriasis lesional tissue such as cytokines; antimicrobial peptides (AMPs); and chemokines such as IL-8, CCL20 and CCL2, thereby enabling IL-17 to bridge the innate and adaptive immune systems to sustain chronic inflammation.5, 6
The IL-17-signaling pathway utilizes a ubiquitin ligase signaling adaptor, Act1 (NF-κB activator 1), which is also known as CIKS (connection to IKK and SAPK/JNK), to propagate downstream signaling events.7–9 The binding of IL-17 to the heterodimeric IL-17 receptor (IL-17R) leads to Act1 recruitment through a homotypic SEFIR domain-dependent interaction. This results in incorporation of TNF receptor-associated factor 6 (TRAF6) into the signaling complex and the subsequent downstream activation of the NF-κB and MAPK pathways, leading to the induction of target genes in keratinocytes, epithelial cells, and fibroblasts stimulated with IL-17 family cytokines.9–11 The role of Act1 as a signal transducer is not confined to IL-17-responsive cells, as earlier studies found that Act1 is recruited to CD40 (TNF receptor superfamily member 5) and BAFFR (TNF receptor superfamily member 13C) upon stimulation of murine B-cells with CD40 ligand (CD40L, TNF superfamily member 5) and BAFF (tumor necrosis factor superfamily member 13b), respectively.12
One of the most robust genetic associations with psoriasis identified to date involves a variant in the TRAF3IP2 gene (rs33980500 C/T, which specifies a glutamic acid (D) to asparagine (N) change in Act1 (pD10N). This variant is unusual among complex disease susceptibility signals13 in that it specifies an amino acid change that appears to be functionally relevant. Moreover, this variant is associated with both cutaneous psoriasis and psoriatic arthritis14–17. Although this genetic association has been independently replicated, the manner in which the Act1 pD10N variant predisposes patients to psoriasis remains to be fully elucidated. An enigmatic feature of this association is that Act1 D10N appears to behave as a loss-of-function variant in acute signaling responses through IL-17R and CD40, whereas it appears to be pro-inflammatory in more broadly biological contexts. This enigma extends to mouse models in which Act1 has been silenced (see Discussion).
In order to better understand the functional consequences of the Act1 D10N variant, we have undertaken an approach in which individuals of known Act1 genotype obtained through our previous GWAS studies of psoriasis14–17 were re-contacted and asked to provide biological samples for further functional analysis of Act1 genetic variation. Short-term responses, such as signal transduction responses, are desirable for such an analysis, because longer-term cellular responses involve the interaction of increasing numbers of proteins, whose functional variants cannot easily be controlled for due to random segregation of unlinked genes. To this end, we profiled peripheral blood mononuclear cells (PBMC) for Act1 expression and responsiveness to IL-17, making use of phospho-flow cytometry to measure short-term signaling responses. However, although multiple PBMC subsets expressed Act1, we were unable to identify any robust signaling responses to IL-17. Because Act1 has also been implicated in signaling events downstream of CD4012, we assessed the impact of the Act1 D10N variant on CD40L-stimulated B-cell signaling events in PBMCs from individuals homozygous, heterozygous, or nullizygous for the Act1 D10N allele.
RESULTS
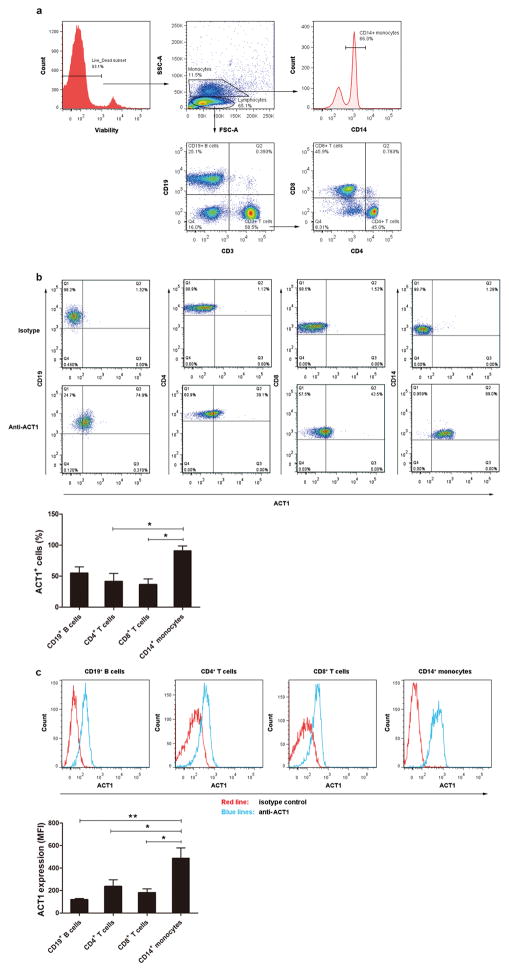
To identify candidate assays for functional genetic testing of the Act1 D10N variant, we assessed Act1 protein levels in different PBMC subsets by flow cytometry. CD19+ B-cells, CD3+CD4+ (“helper”) T-cells, CD3+CD8+ (“cytotoxic”) T-cells and CD14+ monocytes were gated as shown in Figure 1a. The percentages of Act1-positive cells were determined within each PBMC subset (Figure 1b). We found the highest percentage of Act1-positive cells in monocytes (90.9% of CD14+ cells), with lower percentages in T-cells and B-cells (41.5% of CD4+ T-cells, 36.3% of CD8+ T-cells, and 54.9% of CD19+ B-cells, Figure 1b). We also assessed Act1 expression levels by subtracting the median fluorescence intensity (MFI) of the isotype control mAb from that of the anti-Act1 Ab for each cell population (Figure 1c). As assessed by MFI, all cell types expressed Act1, with CD14+ monocytes again expressing the highest levels, followed by CD4+ T-cells, CD8+ T-cells, and B-cells (Figure 1c).
Figure 1.
Act1 is expressed in different subsets of PBMCs. PBMCs were stained with LIVE/DEAD Fixable Near-IR Dead Cell Stain, PE-Cy5-coupled anti-CD3, PE-coupled anti-CD19, V450-coupled anti-CD14, PE-Cy7-coupled anti-CD4 and V500-coupled anti-CD8 and eFluor 660-coupled anti-Act1 or eFluor 660-coupled isotype control. CD19+ B-cells, CD4+ T-cells, CD8+ T-cells and CD14+ monocytes were identified. (a) Cell subset distributions from a representative flow cytometry experiment (one of four experiments). (b) Act1+ cells percentages within each PBMC population from a representative flow cytometry experiment (one of four experiments). Mean Act1+ percentages from all experiments (n = 4). Bars represent mean ± SEM. *P < 0.05, as assessed by one-way analysis of variance followed by least significant difference post hoc test. (c) Act1 expression levels for each PBMC population from a representative experiment (one of four experiments). Median fluorescence intensities (MFI) from all experiments (n = 4). Error bars represent SEM. *P < 0.05, **P < 0.01, as assessed by one-way analysis of variance followed by least significant difference post hoc test.
The transcription factor NF-κB is a central mediator of immune and inflammatory responses.18 NF-κB is reported to be a downstream target of both IL-17R and CD40 signaling.12, 19, 20 To explore whether IL-17R and/or CD40 triggering leads to NF-κB activation in PBMC subsets, we combined surface marker phenotyping with intracellular assessment of phospho-NF-κB p65 (pp65) to assess IL-17A or CD40L-induced signaling by phospho-flow cytometry. As shown in the upper left panel of Figure 2, pp65 was increased after CD40L stimulation for 15 minutes in B-cells but not in T-cells or monocytes. In contrast, no increase in pp65 was observed after 15 min of stimulation with 100 ng/ml or 200 ng/ml IL-17A in any of the IL-17A-treated PBMC subsets that we analyzed (Figure 2, middle panels). In contrast, the levels of pp65 increased in all subsets when PBMCs were treated with phorbol myristate acetate (PMA) for 15 min as a positive control (Figure 2, lower panels).
Figure 2.
CD40L induces p65 phosphorylation in B-cells. Various PBMC subsets were assayed for phosphorylated p65 (pp65) after 15 minutes of stimulation with CD40L (100 ng/ml, upper panels), IL-17A (100 ng/ml, middle panels) or PMA (100 nM, lower panels). Results from one of four representative experiments are shown. Blue histograms demonstrate pp65 response after stimulation with CD40L, IL-17A, or PMA, as compared to unstimulated cells (red curves).
Previous studies in Act1-deficient mice demonstrated a general increase in the numbers of peripheral B-cells.12, 21 However, Act1-deficient mice on a different background did not show this phenotype.22 To ask whether the same phenomenon might be observed in humans as a function of the Act1 D10N variant, we assessed the percentage of B-cells in individuals homozygous, heterozygous, or nullizygous for the D10N allele. The frequency of CD19+ B-cells did not differ between the Act1 D10N homozygotes and nullizygotes, although it was modestly higher in Act1 D10N heterozygotes (Figure 3b). As shown in Figure 3a, the frequency of CD3+ T-cells also did not differ significantly as a function of Act1 D10N genotype.
Figure 3.
Percentages of T-cells and B-cells in PBMC, as a function of Act1 D10N status. PBMCs were collected from D10N homozygous (red), heterozygous (blue), and nullizygous (green) individuals. Percentages of CD3+ T-cells (a) and CD19+ B-cells (b) within the lymphocyte gate (defined by forward and side scatter) are shown. Each symbol represents three experiments averaged for one donor. Bars represent mean ± SEM. *P < 0.05, as assessed by one-way analysis of variance followed by least significant difference post hoc test.
We next investigated the functional consequences of the SNP-D10N on CD40 signaling in B-cells. The downstream pathways activated by CD40 triggering include not only NF-κB but also mitogen-activated protein kinases (MAPKs).23, 24 We thus sought to assay the phosphorylation state of the MAPKs p38 and Erk as potential functional measures of CD40 signaling. PBMCs from individuals homozygous, heterozygous, or nullizygous for the D10N allele were stimulated with CD40L for 15 minutes, and phosphorylation of p65, p38 and Erk was assayed by flow cytometry in the CD19+ B-cell subset. CD40L stimulation of PBMCs from all three genotype groups resulted in increased phosphorylation of p65, p38 and Erk in CD19+ B-cells (Figure 4a). We found that the increase of pp65, pp38 and pErk (expressed as fold-change in MFI relative to unstimulated cells) determined across individuals were significantly correlated with each other (Figure 4b). To determine the impact of the D10N variant on CD40 signaling in B-cells, we compared the increases of pp65, pp38 and pErk induced by CD40L among the three genotype groups. As shown in Figure 4c, CD40L-mediated increases in pp65, pp38 and pErk were significantly attenuated in CD19+ B-cells from Act1 D10N homozygotes, relative to D10N nullizygotes. Moreover, the pp38 and pErk responses were significantly smaller in D10N homozygotes, relative to D10N heterozygotes. While responses did not differ significantly in heterozygotes relative to nullizygotes, there was a significant linear trend for decreasing phosphorylation of p65, p38 and Erk as a function of decreasing SNP-D10N variant counts across genotypes (pp65: P < 0.01; pp38: P < 0.01; pErk: P < 0.01).
Figure 4.
D10N impairs CD40L-induced p65, p38 and Erk phosphorylation in PBMC-derived B-cells. PBMCs were cultured with (blue curves) or without (red shadow) 100 ng/ml CD40L for 15 minutes, followed by phospho-flow cytometric analysis of pp65, pp38 and pErk within the CD19+ gate. (a) Representative histograms (from one of two experiments performed for each genotype) are presented for PBMC-derived B-cells from Act1 D10N nullizygous (left panels), heterozygous (middle panels), and homozygous (right panels) individuals. (b) Correlations between MFI values for pp65, pp38 and pErk in stimulated CD19+ B-cells for all tested individuals (n = 63), Data are expressed as fold-change increases. Pearson correlations between pp65 and pp38, pp65 and pErk, pErk and pp38 were performed. Each symbol represents two experiments averaged for one donor (c) Phosphorylation of p65, p38 and Erk in B-cells from nullizygous, heterozygous, and homozygous groups in response to CD40L were expressed as fold MFI. Each symbol represents one individual donor. Summary data represented by horizontal lines represent mean ± SEM. *P < 0.05, **P < 0.01, as assessed by one-way analysis of variance followed by Dunnett’s post hoc test.
DISCUSSION
Genetic association studies of psoriasis have implicated a number of genes that function in NF-κB, IL-23R, and IL-17R signaling.14–16, 25–27 Act1, encoded by TRAF3IP2, is of particular interest due the fact that a disease-associated TRAF3IP2 SNP (rs33980500 C/T, encoding Act1 pD10N) appears to have functional impact15, 28, which may be due at least in part to its effect on the IL-17R signaling cascade.9–11 However, the direction of its effect seems paradoxical, in that the D10N mutation confers disease risk (i.e., is found at higher frequency in cases than in controls), yet it leads to loss of interaction with TRAF615 and behaves as a hypofunctional allele in short-term signaling and gene expression assays.28 Reminiscent of the D10N mutation, Act1 knockout mice manifest many of the short-term signaling defects in IL-17 responses that are seen in Act1 D10N mice,7 and yet have been reported to manifest a skin inflammation phenotype characterized by epidermal hyperplasia and cell infiltration.12
Reports considering the effects of the Act1 D10N variant in the context of human cells are limited, with one previous report finding that fibroblasts from a D10N homozygous individual responded weakly to IL-17A.29 In the present study, we assayed Act1 expression and signaling responses to IL-17 treatment in different PBMC subsets by flow cytometry. As measured by both percentages of positive cells and by MFI, Act1 was expressed at the highest levels in monocytes. Lower levels of Act1 expression were observed in CD4+ T-cells, CD8+ T-cells and B-cells, though the rank order of expression depended upon the assay utilized (Figure 1). However, despite the presence of Act1 in each cell type, PBMC-derived monocytes, as well as T-cells and B-cells, failed to respond to IL-17A stimulation of PBMC, as assessed by flow cytometric determination of NF-κB phospho-p65 (Figure 2).
This result was initially surprising, as a previous report indicated that human monocytes highly express both IL-17RA and IL-17RC, and that monocyte migration is stimulated by IL-17, in part due to PI3K and Erk-dependent induction of CCL2.30 However, that study involved longer-term assays using purified monocytes (a two-hour in vitro Boyden chamber chemotaxis assay, and a 24-hour in vivo sponge migration assay). While phosphorylation of p38 did increase after 15 and 30 minutes of IL-17 stimulation, three other IL-17-stimulated monocyte signaling events (pERK1/2, pJNK, and pAKT) were not significantly induced at 15 or 30 minutes, reaching maximal levels only after 60 to 180 minutes. In another paper, Krueger and colleagues31 reported gene expression profiling experiments in purified monocytes, which again involved longer IL-17 stimulation times (24 hours). In both of these studies, CD14+ monocytes were purified using magnetic beads, which might increase the sensitivity of monocytes to IL-17A treatment, whereas we utilized freshly-isolated PBMC. Future studies could utilize more sensitive detection methods, purified PBMC subsets, and/or assess longer-term responses to IL-17.
Based on the aforementioned outcomes, we turned to B-cells, in which we reproducibly observed robust short-term responses to CD40L stimulation as assessed by p65, p38, and ERK phosphorylation (Figures 2 and 4). In addition to its key role in IL-17R signaling, Act1 was identified as an important adaptor molecule for CD40 signaling in B-cells.12 Ligation of CD40 by CD40L expressed on activated T-cells stimulates B-cell survival, proliferation, differentiation, isotype switching, and upregulation of surface molecules contributing to antigen presentation.32, 33 The CD40 receptor utilizes adapter molecules (TNF receptor associated factors, or TRAFs) that recognize binding sites on the CD40 cytoplasmic tail upon binding of CD40L. TRAFs then recruit TRAF-interacting kinases and together influence a number of well-characterized signal transduction pathways, including NF-κB and MAPKs.34 In the process, Act1 is activated by its recruitment to CD40 through its interaction with TRAF proteins.12, 20, 24 It would be worthwhile to further examine the impact of D10N mutation on the physical interaction of Act1 with CD40 and TRAFs.
Engineering of the D10N variant into murine Act1 abolished its interaction with CD40 and BAFFR, suggesting that the D10N variant might phenocopy an Act1 knockout.28 However, previous studies in germ-line Act1 knockout mice revealed increased numbers of B-cells relative to WT littermates.12 These studies also found that activation of CD40 by treatment with a cross-linking anti-CD40 antibody increased survival of splenic B-cells, with stronger phosphorylation of IκBα and ERK.12 Taken together, these studies lead to the paradoxical conclusion that CD40 ligation activates short-term downstream signaling events in an Act1-dependent fashion, and yet in the long-term physiological context of transgenic animals, Act1 silencing increases overall B-cell numbers and function. A similar dichotomy has emerged from studies addressing the role of Act1 in IL-17 signaling in T-cells, as one strain of Act1 knockout mice develops inflammatory skin hyperplasia.28 It is possible that these paradoxical observations could be due to developmental influences and/or counter-regulatory feedback from other cell types, as the CD40L-dependent survival phenotype was much less evident in B-cells isolated from B-cell-targeted Act1−/− mice.12.
Physiologically, CD40L is expressed primarily by activated T lymphocytes, therefore, B-cell signaling following CD40L-CD40 ligation represents a crucial component of the process known as T-cell “help” for antibody production. However, in addition to their role as antibody-producing cells, it has become apparent that B-cells markedly influence immunity through the secretion of cytokines.35 In particular, a subset of IL-10 producing B-cells has been found to have anti-inflammatory activity by diminishing both acquired and innate immune responses.36, 37 In humans, evidence is accumulating that B-cells can exert similar suppressive functions.38 Among many potential explanations for the “Act1 paradox” that remain to be tested, it is possible that the Act1 D10N variant might compromise the CD40-mediated function of B regulatory cells.
In summary, we found that the Act1 D10N variant attenuated, rather than enhanced CD40L-dependent phosphorylation of NF-κB p65, p38, and Erk and MAPKs in B-cells (Figure 4). However, we did not find a significant effect of the D10N variant on overall B-cell numbers (Figure 3), as was previously observed in one strain of Act1 knockout mice.12 A limitation of our study is that our short-term signaling assays may not have probed more subtle levels of homeostatic regulation that are likely to be present in longer-term cellular responses and at the organismal level. In any case, our results establish a functional effect of the Act1 D10N variant in human cells for the first time, and as such, provide important albeit indirect evidence for the biological relevance of this variant to the immunopathogenesis of psoriasis.
MATERIALS AND METHODS
Study sample and genotyping
All human subjects provided written informed consent and were enrolled according to the protocols approved by the institutional review board of the University of Michigan Medical School, in adherence with the Declaration of Helsinki principles. Genomic DNA was isolated from peripheral blood by phenol-chloroform extraction followed by ethanol precipitation. Sixty-three individuals of Caucasian descent (21 males, 42 females; 8 psoriasis cases, 55 controls, mean age 44 years, range 19 – 83 years) were identified based on Act1 genotypes that were either measured directly or obtained from genome-wide or Immunochip-based association studies of psoriasis.17, 25, 27 and confirmed using a Taqman assay (Assay ID C_2473124_10, Applied Biosystems, Foster City, CA). The typing of these 63 subjects resulted in 13 Act1 D10N homozygotes, 20 Act D10N heterozygotes, and 30 Act1 D10N nullizygotes.
Isolation of PBMCs
For the isolation of PBMCs, anticoagulated blood was mixed with an equal volume of phosphate-buffered saline. The diluted blood was slowly layered over Histopaque®-1077 (Sigma-Aldrich, St. Louis, MO) solution by gently pipetting the diluted blood down the side of the tube, and was then centrifuged for 20 min at 800 g at 22°C, with no brake. The mononuclear cell layer was transferred into 10 ml phosphate-buffered saline and centrifuged for 10 min at 400 g at 4°C. After two washings with RPMI-1640, PBMCs were gently resuspended in freezing medium containing 90% FBS and 10% DMSO as described38 and cryopreserved with gradual cooling followed by storage in liquid nitrogen.
Flow cytometric analysis of ACT1 expression
PBMCs were thawed, washed by centrifugation, and 5 × 105 cells were aliquotted to each tube. Dead cells were excluded from the analysis by staining with LIVE/DEAD Fixable Near-IR Dead Cell Stain (Catalog#: L10119, Life Technologies, Waltham, MA). Antibodies to cell-surface markers were mixed and added to the cells, including PE-Cy5-coupled anti-CD3 (5 μl per 106 cells; Catalog#: 344808, BioLegend, San Diego, CA), PE-coupled anti-CD19 (Catalog#: 12-0199-41, eBioscience, Santa Clara, CA), V450-coupled anti-CD14 (Catalog#: 560349, BD Biosciences, San Jose, CA), PE-Cy7-coupled anti-CD4 (5 μl per 106 cells; Catalog#: 300512, BioLegend) and V500-coupled anti-CD8 (Catalog#: 560774, BD Biosciences). After incubation for 30 min at 4°C;, the cells were washed twice with BD Pharmingen™ Stain Buffer (Catalog#: 554656). The cells were then fixed and permeabilized for 60 min at 4°C; using the Fixation/Permeabilization diluent and concentrate (Catalog#: 00-5123-43 and 00-5223-56, eBioscience). Cells were then stained with eFluor 660-coupled anti-Act1 (Catalog#: 50-4040, eBioscience) or eFluor 660-coupled isotype control (eBioscience). The analysis was performed using flow cytometer (BD LSR II, BD Biosciences) and FlowJo 7.6.2 (TreeStar, Ashland, OR) software.
Flow cytometric analysis of phosphorylated proteins
For determination of phosphorylated proteins after CD40 stimulation, PBMCs were thawed and rested for 2 h and then either left unstimulated or stimulated with trimeric CD40L (100 ng/ml) (Enzo Life Sciences, Farmingdale, NY) for 15 min, performed concurrently with decoration with V450-coupled anti-CD19 (Catalog#: 560353, BD Biosciences). After incubation with BD Cytofix™ Fixation Buffer (Catalog#: 554655) for 10 minutes at 37°C;, followed by permeabilization in BD Phosflow™ Perm Buffer III (Catalog#: 558050) for 30 minutes on ice, cells were then stained with PE-Cy5-coupled anti-CD3 (BioLegend), FITC-coupled anti-pp38 (Catalog#: 4551, Cell Signaling Technology, Danvers, MA), PE-coupled anti-pp65 (Catalog#: 558423, BD Biosciences), or PE-coupled anti-pErk (Catalog#: 612566, BD Biosciences). The analysis was performed using an LSR II flow cytometer (BD Biosciences) and FlowJo 7.6.2 software (TreeStar, Ashland, OR).
Statistical analysis
For flow cytometry experiments, CD3+ T-cell and CD19+ B-cell percentages and MFI values obtained for each donor belonging to the three genetic groups were assessed for normal Gaussian distribution using the Kolmogorov-Smirnov test and then analyzed by one-way analysis of variance, followed by post hoc least significant difference (LSD) test or Dunnett’s test by using SPSS version 21.0 (IBM SPSS Statistics, New York). Pearson’s coefficient was used to assess two-way correlations between pp65 and pp38, pp65 and pErk, and pErk and pp38. All results are shown as mean and the standard error of the mean (mean ± SEM). In this exploratory analysis, a P value < 0.05 was considered to be significant.
Acknowledgments
We thank the volunteers who provided blood samples for this study, and Dr. Andrew Johnston for advice on flow cytometry. This research was supported by National Institutes of Health (NIH) R01 grants AR042742, AR050511, AR054966, AR062382, and AR065183 to James T. Elder. We also acknowledge generous support from the Dawn and Dudley Holmes Memorial Fund and the Babcock Endowment Fund to the Department of Dermatology at the University of Michigan. James T. Elder is supported by the Ann Arbor Veterans Affairs Hospital.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Gudjonsson JE, Elder JT. Psoriasis. In: Goldsmith L, Katz SI, Gilchrest BA, Paller AS, Woolf K, Leffell DJ, editors. Dermatology in General Medicine. 8. Vol. 1. McGraw-Hill; New York: 2012. pp. 197–231. [Google Scholar]
- 2.Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatologic clinics. 2015;33(1):13–23. doi: 10.1016/j.det.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol. 2013;133(1):17–26. doi: 10.1038/jid.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160(2):319–24. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 5.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181(10):7420–7. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol. 2013;190(5):2252–62. doi: 10.4049/jimmunol.1201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281(47):35603–7. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 8.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8(3):247–56. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 9.Sonder SU, Saret S, Tang W, Sturdevant DE, Porcella SF, Siebenlist U. IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J Biol Chem. 2011;286(15):12881–90. doi: 10.1074/jbc.M110.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal. 2009;2(92):ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Q, Sun Y, Liu W, Qian C, Jin B, Tao F, et al. A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J Immunol. 2013;191(10):4969–78. doi: 10.4049/jimmunol.1300832. [DOI] [PubMed] [Google Scholar]
- 12.Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, et al. Act1, a negative regulator in CD40- and BAFF-mediated B cell survival. Immunity. 2004;21(4):575–87. doi: 10.1016/j.immuni.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Edwards SL, Beesley J, French JD, Dunning AM. Beyond GWASs: illuminating the dark road from association to function. Am J Hum Genet. 2013;93(5):779–97. doi: 10.1016/j.ajhg.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellinghaus E, Ellinghaus D, Stuart PE, Nair RP, Debrus S, Raelson JV, et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet. 2010;42(11):991–5. doi: 10.1038/ng.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet. 2010;42(11):996–9. doi: 10.1038/ng.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genetic Analysis of Psoriasis C the Wellcome Trust Case Control C Strange A. Capon F, Spencer CC, Knight J, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42(11):985–90. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuart PE, Nair RP, Tsoi LC, Tejasvi T, Das S, Kang HM, et al. Genome-wide Association Analysis of Psoriatic Arthritis and Cutaneous Psoriasis Reveals Differences in Their Genetic Architecture. Am J Hum Genet. 2015;97(6):816–836. doi: 10.1016/j.ajhg.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh S, Hayden MS. Celebrating 25 years of NF-kappaB research. Immunol Rev. 2012;246(1):5–13. doi: 10.1111/j.1600-065X.2012.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayden MS, Ghosh S. Regulation of NF-kappaB by TNF family cytokines. Semin Immunol. 2014;26(3):253–66. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giltiay NV, Lu Y, Cullen JL, Jorgensen TN, Shlomchik MJ, Li X. Spontaneous loss of tolerance of autoreactive B cells in Act1-deficient rheumatoid factor transgenic mice. J Immunol. 2013;191(5):2155–63. doi: 10.4049/jimmunol.1300152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, et al. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182(3):1617–30. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craxton A, Shu G, Graves JD, Saklatvala J, Krebs EG, Clark EA. p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. J Immunol. 1998;161(7):3225–36. [PubMed] [Google Scholar]
- 24.Li YY, Baccam M, Waters SB, Pessin JE, Bishop GA, Koretzky GA. CD40 ligation results in protein kinase C-independent activation of ERK and JNK in resting murine splenic B cells. J Immunol. 1996;157(4):1440–7. [PubMed] [Google Scholar]
- 25.Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41(2):199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsoi LC, Spain SL, Ellinghaus E, Stuart PE, Capon F, Knight J, et al. Enhanced meta-analysis and replication studies identify five new psoriasis susceptibility loci. Nat Commun. 2015;6:7001. doi: 10.1038/ncomms8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44(12):1341–8. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Wu L, Bulek K, Martin BN, Zepp JA, Kang Z, et al. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nat Immunol. 2013;14(1):72–81. doi: 10.1038/ni.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39(4):676–86. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahrara S, Pickens SR, Mandelin AM, 2nd, Karpus WJ, Huang Q, Kolls JK, et al. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184(8):4479–87. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CQ, Suarez-Farinas M, Nograles KE, Mimoso CA, Shrom D, Dow ER, et al. IL-17 Induces Inflammation-Associated Gene Products in Blood Monocytes and Treatment with Ixekizumab Reduces their Expression in Psoriasis Patient Blood. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.268. [DOI] [PubMed] [Google Scholar]
- 32.Kehry MR. CD40-mediated signaling in B cells. Balancing cell survival, growth, and death. J Immunol. 1996;156(7):2345–8. [PubMed] [Google Scholar]
- 33.Wennhold K, Shimabukuro-Vornhagen A, Theurich S, von Bergwelt-Baildon M. CD40-activated B cells as antigen-presenting cells: the final sprint toward clinical application. Expert review of vaccines. 2013;12(6):631–7. doi: 10.1586/erv.13.39. [DOI] [PubMed] [Google Scholar]
- 34.Hobeika E, Nielsen PJ, Medgyesi D. Signaling mechanisms regulating B-lymphocyte activation and tolerance. J Mol Med (Berl) 2015;93(2):143–58. doi: 10.1007/s00109-015-1252-8. [DOI] [PubMed] [Google Scholar]
- 35.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–8. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cellular & molecular immunology. 2013;10(2):122–32. doi: 10.1038/cmi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilgenberg E, Shen P, Dang VD, Ries S, Sakwa I, Fillatreau S. Interleukin-10-producing B cells and the regulation of immunity. Current topics in microbiology and immunology. 2014;380:69–92. doi: 10.1007/978-3-662-43492-5_4. [DOI] [PubMed] [Google Scholar]
- 38.Austin ED, Rock MT, Mosse CA, Vnencak-Jones CL, Yoder SM, Robbins IM, et al. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respiratory medicine. 2010;104(3):454–62. doi: 10.1016/j.rmed.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]