Abstract
Pulmonary contrast enhanced magnetic resonance angiography (CE-MRA) is useful for the primary diagnosis of pulmonary embolism (PE). Many sites have chosen not to use CE-MRA as a first line of diagnostic tool for PE because of the speed and higher efficacy of computerized tomographic angiography (CTA). In this review, we discuss the strengths and weaknesses of CE-MRA and the appropriate imaging scenarios for the primary diagnosis of PE derived from our unique multi-institutional experience in this area. The optimal patient for this test has a low to intermediate suspicion for PE based on clinical decision rules. Patients in extremis are not candidates for this test. Younger women (< 35 years of age) and patients with iodinated contrast allergies are best served by using this modality We discuss the history of the use of this test, recent technical innovations, artifacts, direct and indirect findings for PE, ancillary findings, and the effectiveness (patient outcomes) of CE-MRA for the exclusion of PE. Current outcomes data shows that CE-MRA and NM V/Q scans are effective alternative tests to CTA for the primary diagnosis of PE.
Keywords: Female, Lung, Neoplasms, Hypersensitivity, Pulmonary embolism, Magnetic resonance angiography, Radiation induced, Outcome assessment (health care), Artifacts, Computerized tomography angiography
Core tip: Pulmonary contrast enhanced magnetic resonance angiography (CE-MRA) is an effective alternative test for the primary diagnosis of pulmonary embolism (PE). In outcomes studies the negative predictive value of CE-MRA at 6 mo was 99%, which is similar to the negative predictive value of multidetector computerized tomographic angiography. The optimal patient selection is for younger female patients with a low to intermediate risk of PE or those with iodinated contrast allergies.
INTRODUCTION
Acute pulmonary embolism (PE) affects 0.1% of the population annually and is associated with significant morbidity and mortality[1,2]. Common symptoms of PE are acute chest pain and dyspnea. When the patients have symptoms suspected PE, the first step is use of a clinical decision rules (CDR) such as the Wells’ score, the Pulmonary Embolism Rule-out Criteria (PERC), the Revised Geneva score, the Simplified Revised Geneva score and a D-dimer test[3-5].
CTA of the chest is the current gold standard for the diagnosis of PE. In the recently published American College of Radiology (ACR) Appropriateness Criteria[6] for “Acute chest pain-suspected PE of intermediate probability with a negative D-dimer or low pretest probability”, pulmonary magnetic resonance angiography (CE-MRA) is listed as 2 out of 10 for appropriateness (where 10/10 is the highest value for appropriateness), while multi-detector computed tomographic angiography (CTA) chest is listed at 5 out of 10 and a chest X-ray (CXR) is rated a 9 out 10. For “acute chest pain-suspected PE of intermediate probability with a positive D-dimer or high pretest probability”, the ACR appropriateness criteria rate CE-MRA as 6 out of 10, CTA chest is rated at 9 out of 10 and a CXR is rated at 9 out 10. It is important to remember that the use of D-dimer for inpatients is limited due to their many comorbities. Further imaging workup is unnecessary for the patients with a negative D-dimer and a low clinical risk profile to exclude PE because the negative predictive value (NPV) does not change with the addition of an imaging test[3,4].
EARLY WORK
The use of pulmonary MRA is playing an increasingly important role for the primary diagnosis of PE and other causes of acute chest pain[2,7]. Early studies in this area began in 1993 and demonstrated the efficacy for the non-contrast MRA for depiction of PE. However, there were limitations that hampered routine clinical use. In one of the early prospective studies (18 patients) of the efficacy of non-contrast enhanced MRA for the diagnosis of PE found the sensitivity of two-dimensional time-of-flight pulmonary breath-hold MRA for detection of acute PE was 85% and was much lower for chronic emboli at 42%[8]. This group also showed that the size of the emboli was important for CE-MRA detection (PE larger than 1 cm were found with more than 75% confidence)[8]. In another prospective study (20 patients) from 1993, two-dimensional time-of-flight MRA had a sensitivity of 92%-100% and specificity of 62% for the diagnosis of PE[9]. A major limitation of these studies was their low sensitivity and specificity for chronic emboli, which were smaller and located eccentrically within the pulmonary arteries (web-like). These limitations were due to low resolution, the artifacts from slow blood flow, and respiratory-motion artifacts (30-s breath-hold).
Since the 2000’s, several studies have evaluated the performance of traditional contrast CE-MRA methods, namely breathhold, non-time resolved, 3D Cartesian encoded and T1-weighted spoiled gradient echo acquisitions. These studies[10-17] were often performed without parallel imaging and did not employ time-resolved (e.g., 4 dimensional) k-space sampling which delineates the arterial and venous phases of the bolus passage thereby separating the pulmonary arteries from the pulmonary veins. In addition, 3D time-resolved CE-perfusion was not performed in conjunction with the routine CE-MRA in these works[10-17]. In comparison with the historic reference standard digital subtraction angiography (DSA), CE-MRA yielded a sensitivity and specificity for the detection of PE between 75% and 100% and 95% and 100%, respectively; with good interobserver agreement (k values of 0.57-0.83)[10-17].
In contrast to traditional CE-MRA, a study using time-resolved CE-MRA directly compared diagnostic performance with DSA, CTA and/or nuclear medicine ventilation/perfusion imaging, and showed better diagnostic performance than could be attained with DSA, CTA and/or nuclear medicine studies[14]. They showed a sensitivity and specificity of time-resolved CE-MRA of 83% and 97% on a per-vascular zone basis and 92% and 94% on a per-patient basis. In addition, the efficacy of time-resolved CE-MRA was higher than ventilation and perfusion scintigraphy (V/Q scan) on a per-patient basis. This study also showed that in comparison with CTA and VQ scans, time-resolved CE-MRA demonstrated equal to or higher sensitivity [92% vs 83% (CTA) and 67% (VQ scan)] and specificity [94% vs 94% (CTA) and 78% (VQ scan)] for the detection of PE[14].
Although time-resolved CE-MRA is useful for diagnosis of PE, dynamic first-pass CE-perfusion magnetic resonance imaging (MRI) is also helpful for disease severity assessment and outcome prediction in PE patients[17]. In this study, the acute pulmonary thromboembolism (APTE) index, which was defined as the ratio between the volume of perfusion defects and the total lung volume determined by means of dynamic first-pass CE-perfusion MRI, showed accuracy for the prediction of patient outcome similar to that of the right ventricular/left ventricular (RV/LV) diameter ratio[17]. In addition, the specificity and accuracy of the RV/LV diameter ratio and the APTE index determined by means of dynamic first-pass CE-perfusion MRI were significantly higher than those of APTE indexes obtained from embolic burdens and observed on CTACTA and time-resolved CE-MRA, although logistic regression analysis demonstrated that each index was a significant predictor[17]. Although quantitatively analyzable software was not commercially available, this study showed the potential utility of quantitatively assessed dynamic first-pass CE-perfusion MRI for patients with PE. Therefore, not only clinical researchers but also clinicians continue to urge companies in the field to provide appropriate MR systems, bolus injection protocols, MR sequences and software for clinical settings using this technique for suspected PE patients. Furthermore, there has been some work showing an advantage of MRI pulmonary perfusion assessment for patients suspected PE[17,18].
TEST EFFICACY
Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III) was a multicenter study designed to assess the efficacy of CE-MRA (without/with MR venography) for diagnosing PE and venous thromboembolism (VTE)[16]. They found that CE-MRA was technically inadequate in 25% of their studies and that CE-MRA had a sensitivity of 78%, and a specificity of 99%[16]. The two major reasons for this high rate of technical inadequacy were a strict definition for complete visualization of the subsegmental pulmonary arteries and the fact that some centers were just not as good as others in producing high quality studies[19]. They also found that using a combination of MRA and MR venography had a sensitivity of 92% and a specificity of 96%[16]. Their results showed limited efficacy of CE-MRA for the diagnosis of PE. Based on their findings, the authors recommended that CE-MRA should only be considered at those centers that had a sufficient technical expertise and in those patients for where standard tests were contraindicated[16].
The limitations of PIOPED III included a slightly lower resolution CE-MRA and a lack of consistent technical quality amongst the multiple centers[16]. This study mentioned that CE-MRA could detect PE in a main or lobar pulmonary artery with a sensitivity of 79%. They also showed a sensitivity of 50% for segmental PE and 0% for detecting subsegmental PE (SSPE)[16]. In PIOPED III, the proportion of technically inadequate examinations varied between centers and ranged from 11% up to 51%[16]. The reason why CE-MRA was technically inadequate was poor arterial opacification (67%), motion artifact (36%), wrap-around artifact (4%), and parallel imaging artifact (2%)[16]. Further retrospective analysis was by the PIOPED III investigators to identify the factors of the CE-MRA examination that were associated with poor technical quality; they found that the two most important elements influencing MRA interpretability were vascular opacification and motion artifact[20].
TREATMENT FOR SUBSEGMENTAL PE REMAINS A CONUNDRUM
The significance of detecting subsegmental PE (SSPE) has been an ongoing debate for more than a decade[21]. An isolated SSPE could be a symptom of a thrombotic state, and may require treatment[22]. Recently Mehta et al[23] have shown that withholding anticoagulation in patients with a single SSPE and negative bilateral lower extremity venous duplex ultrasound exams was a safe and effective strategy. However, systematic reviews demonstrated that no randomized controlled trial evidence exists to allow for a safe conclusion as to whether or not withholding anticoagulant therapy in isolated SSPE is safe[24,25]. Therefore, the detection of SSPE will remain an important issue. All interested parties should know that isolated SSPE are a problem for any diagnostic test, including the old gold (now bronze) reference method pulmonary angiography[26] and the new gold standard CTA[27]. Nevertheless, long-term follow-up studies after normal pulmonary angiography[28], normal perfusion scintigraphy[29] and normal CTA[30] have shown a low risk for recurrent disease after a single SSPE.
MITIGATION OF MEDICAL RADIATION
The reasons to continue to work on improving non-contrast and CE-MRA for diagnosing PE is the mitigation of medical radiation[31-34]. Ionizing radiation administered for medical imaging is of increasing clinical concern[3,35,36] and is a risk factor for the development of primary breast cancer[37,38]. The increased risk of breast cancer is associated with more imaging follow-up, higher cumulative radiation doses and exposure at a younger age[38]. CE-MRA is the only non-ionizing imaging modality with data supporting for the primary diagnosis of PE[39]. This modality is particularly useful for the follow up of previously diagnosed PE in younger individuals and the pediatric population to determine the efficacy of anticoagulation therapy, or the presence of new PE, as there is no incremental medical radiation. This strength of CE-MRA (no ionizing radiation) in younger patients helps to mitigate its lower efficacy for the detection of SSPE.
TEST EFFECTIVENESS
At University of Wisconsin-Madison over 2000 pulmonary CE-MRA examinations for the primary diagnosis of PE have been performed over the last ten years (2007-2017). The routine MRI protocol at UW-Madison is shown in Tables 1 and 2. We retrospectively reviewed the first 675 patients who underwent CE-MRA for the primary diagnosis of PE to determine the six-month adverse event rate following the use of CE-MRA[39]. For all these patients, the same 13-17 s breath hold contrast enhanced CE-MRA method was used, and the details of the MRA imaging protocol has been previously described[2]. We excluded 56 of 675 (8.3%) patients for the following reasons: on anti-coagulation, pre-existing IVC filter, or atrial fibrillation. Two of 675 (0.3%) were incomplete electronic medical record (EMR). Eventually, we included 617 (91.4%) patients to assess the effectiveness of CE-MRA[39]. Of the included cases, 500 (81%) were negative for PE, 17 (2.8%) were equivocal, 46 (7.5%) were positive for PE (Figure 1)[39]. The proportion of technically limited CE-MRA exams, as determined by the word “limited” in the final report, was 8.8%. This result is far lower than the 25% technical failure rate reported in the PIOPED III[16]. This improvement in technical success likely reflects the maturation of CE-MRA methodology since the time of the PIOPED III scans nearly a decade ago (2006-2008). In addition, this improvement may be related to the fact that lack of visualization of the subsegmental pulmonary arteries was not a criterion for determining the presence of a limited examination. Only three of 500 (0.6%) patients with a negative CE-MRA exam experienced a VTE within 6 mo of their exam. Thus, using just the rate of VTE, the NPV of CE-MRA was 99.4% in a data set that reflects the real world experience of this test[39]. This value is similar to the reported NPV for CTA (98.8%)[40]. At our single site, we have found that CE-MRA to be a safe and effective alternative to CTA for the primary diagnosis of PE.
Table 1.
Pulmonary contrast-enhanced magnetic resonance angiograph imaging protocol at UW-Madison after Nagle et al[76]
| Three-plane SSFSE localizers |
| Pre-contrast T1 weighted 3D SGRE |
| Pulmonary arterial phase T1-weighted 3D SGRE |
| Immediate post-contrast T1-weighted 3D SGRE |
| Low flip angle post-contrast T1-weighted 3D SGRE |
| T1-weighted 2D axial or 3D SGRE with fat saturation |
SGRE: Spoiled gradient recalled echo; MRA: Magnetic resonance angiograph; SSFSE: Single-shot fast spin-echo.
Table 2.
Pulmonary contrast-enhanced magnetic resonance angiograph pulse sequence parameters after Schiebler et al[2]
| Parameter | Value |
| FOV | 18-45 (to fit the patient) cm |
| Slice acquisition plane | Sagittal |
| Resolution1 | SI 0.7 × RL 0.7 × AP 1.0 mm3 |
| TR/TE | 2.9 ms/1.0 ms |
| Parallel imaging factor | 3.6 |
| Flip angle | 28° (15°for 2nd post-contrast “low flip angle” scan) |
| Bandwidth | ± 88 kHz/pixel |
| Time for Breath hold | 15-21 s |
Interpolated resolution in all three planes. TR: Time to repetition; TE: Time to echo; FOV: Field of view.
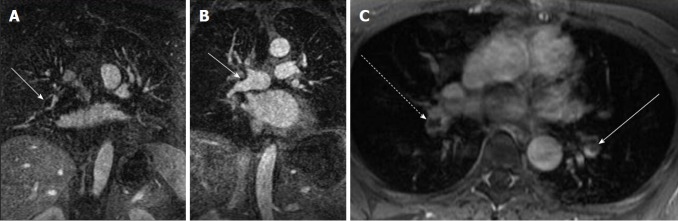
Figure 1.
Direct findings of pulmonary embolism. A: CE-MRA shows a filling defect in the interlobar artery (white arrow) consistent with the expected appearance of a pulmonary embolus (arrow); B: CE-MRA showing an eccentrically located pulmonary embolus that spans the truncus anterior and interlobar artery (arrow); C: Post gadolinium fat saturated breath hold axial spoiled gradient echo image showing bilateral filling defects in the lower lobe pulmonary arteries (interlobar PE-dashed arrow, left lower lobe pulmonary artery-arrow). CE-MRA: Contrast enhanced magnetic resonance angiograph; PE: Pulmonary embolism.
PATIENT SELECTION
For accurate diagnosis, it is important to understand its appropriate use in the patient population. CE-MRA for the primary diagnosis of PE is most effective when used in patients with the following criteria: (1) a low to intermediate pretest probability for venous thromboembolic disease; (2) patients with iodinated contrast allergies; (3) female subjects less than 35 years of age that are potentially at slightly higher risk from medical radiation; and (4) for patients with renal insufficiency (eGFR < 30) the use of ferumoxytol as an MRA contrast agent may be considered. The contraindications for CE-MRA are as follows: (1) MRI incompatible implants[41]; (2) claustrophobia; (3) critically ill patients with a high pretest probability for PE; (4) inability to hold their breath for > 13 s; and (5) patients with gadolinium and ferumoxytol contrast allergies[42,43]. Please be aware of the fact that MRI is not safe for suspected PE patients that are unstable. This is because cardiopulmonary resuscitation can only be performed after the patient is out of the magnet room.
OVERVIEW OF TECHNICAL METHODS FOR CE-MRA IN ACUTE PE
Imaging the lungs with CE-MRA is inherently challenging as the method of blood pool enhancement is predominantly a T1 weighted contrast that is also constrained by a heavily T2* weighted background signal of the air within the lungs (0.5 ms and 2 ms T2*; at 1.5T and 3T respectively)[44]. As such, delineation of the smaller vessels is difficult and this is made more challenging by the changes in susceptibility of the vessels with contrast passage due to the T2* blooming effect. This effect can be mitigated by employing the following: (1) short echo time gradient echo sequences[45], for better resolution of the underlying lung and vessel morphology; (2) scans at expiration; and (3) parallel imaging[46]. Due to the above reasons, CE-MRA is primarily performed at 1.5T. However, using methods like ultra-short echo time radial sampling[47] CE-MRA is also feasible at 3T. Incorporation of modest image acceleration factors using receiver array coils with auto calibrated parallel imaging and centric k-space encoding also helps in best capturing peak pulmonary arterial enhancement during bolus passage[2,48].
NON-CONTRAST PULMONARY MRA
Multiple approaches are available for non-contrast imaging of the pulmonary arteries, pulmonary veins and perfusion of the lung parenchyma (Figure 2). The general protocol recommendations for imaging acute PE includes fast MRI imaging sequences to increase sensitivity and specificity[15,49]. First, a steady state GRE sequence acquired in two or three planes during free breathing, may serve for early detection of large central emboli within the first five minutes of the examination - with a sensitivity of 90% and a specificity approaching 100% (Figure 3)[15,50,51]. Central embolism detected at this time can be directly referred to the intensive care unit for treatment, this is as efficient as diagnosis using CTA[49]. In many cases, such as in pregnancy when the administration of Gadolinium based contrast agents (GABA’s) is critical, using a non-contrast enhanced MRA can provide a reliable exclusion of a massive central PE. The further contrast-enhanced steps of the recommended examination protocol would contribute to confirm this result and increase sensitivity and specificity (Figure 3)[2,49]. The diagnostic accuracy of a non-contrast enhanced examination could be improved by additional non-contrast enhanced perfusion imaging, based either on arterial spin labeling or on Fourier decomposition (Figure 2). Both have been shown to be sensitive for lung perfusion deficits related to acute PE, but are still on an experimental level[52].
Figure 2.
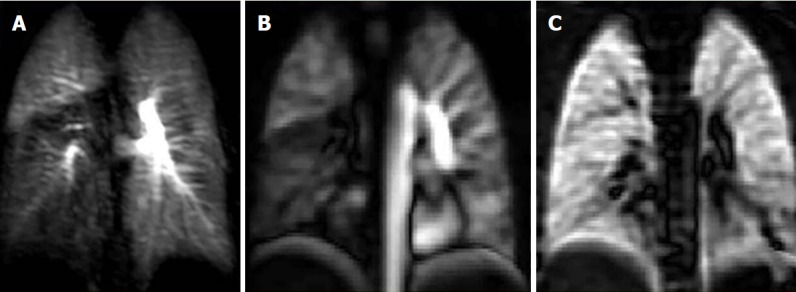
Case of pulmonary embolism to the right lower lobe. A: Coronal dynamic contrast MRI shows notable right lower lobe hypo-perfusion in a 25-year-old female with known acute pulmonary embolism one month ago; B: Corresponding (non-contrast) Fourier decomposition (FD) perfusion; C: Ventilation-weighted FD MR images also depict right lower lobe hypo-perfusion and normal ventilation (VQ mismatch). MRI: Magnetic resonance imaging.
Figure 3.
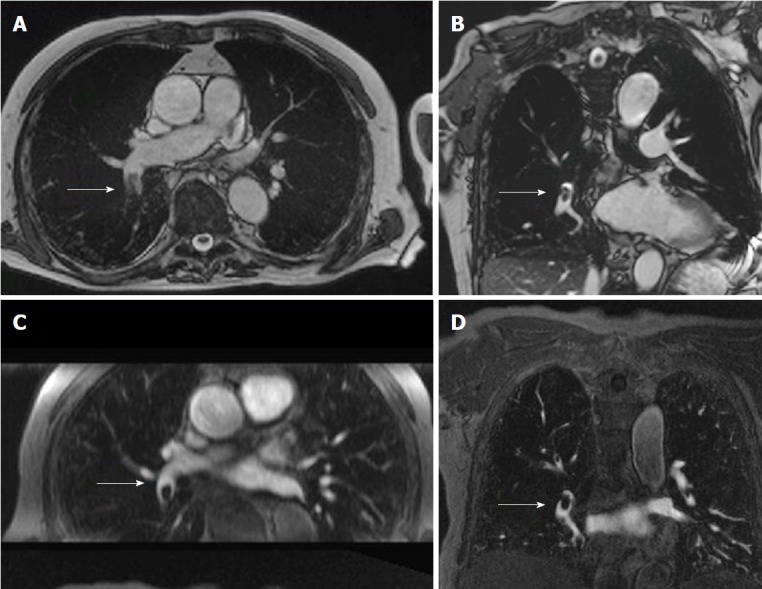
Non-contrast pulmonary magnetic resonance angiograph of an 82-year-old male with a history of san acute onset of dyspnea. A: Transverse non-contrast enhanced steady state GRE; B: Coronal oblique non-contrast enhanced steady-state GRE images of a fresh embolus in the right lower lobe artery; C: For comparison the transverse reformation; D: The original coronal images from the contrast-enhanced MRA (images courtesy of Heussel CP and Wielpuetz M, Thoraxklinik, Heidelberg, Germany). GRE: Gradient recalled echo; MRA: Magnetic resonance angiograph.
The high contrast to noise of the blood pool with a steady state free precession sequence means that it can serve as a non-contrast enhanced MR angiogram[53] when performed in 3D breathhold. Recent developments with 3D UTE SSFP and zero echo time sequences[54] also hold promise, however the lack of arterial and venous separation poses diagnostic limitations on this method when compared to state of the art CE MRA. Fourier decomposition (FD) MRI uses a continuously acquired two-dimensional steady-state free-precession (SSFP) or fast low angle shot (FLASH) acquisitions[55,56]. Since lung signal changes with inspiration depth (highest signal with lowest pulmonary air content in expiration) and cardiac motion (lowest signal with maximum blood flow in systole), both result in periodic changes of lung parenchymal signal that can be separated by means of Fourier decomposition[57]. Perfusion and ventilation-weighted images are generated from the high frequency oscillations related to the effects of pulsatile blood flow and the low-frequency lung signal oscillation related to respiration without contrast[55,58]. Further promising new developments of this technique using self-gated non-contrast-enhanced functional lung imaging (SENCEFUL) or phase-resolved functional lung (PREFUL) MRI have reported[59,60]. Principally, this technique has the potential to replace V/Q scans and has been already been validated against single-photon emission computed tomography (SPECT) perfusion and ventilation imaging[61], and against hyperpolarized 3He and perfusion MRI[62,63]. In a single center study, perfusion weighted FD MRI showed encouraging results for the diagnosis of PE in the non-acute clinical setting[64]. Arterial spin labeling (ASL) uses the intrinsic contrast of magnetized, inflowing blood into the imaging plane or volume without the need of contrast material injection[52,65]. In scientific applications, ASL has been used to study of the effects of inhaled oxygen concentration and physical exercise on ventilation-perfusion heterogeneity of the lungs in healthy human subjects[66-68]. However, although being principally suitable for the detection of lung perfusion deficits related to acute PE, neither arterial spin labeling nor Fourier decomposition MRI have been clinically implemented for the assessment of acute PE, mainly because of the lower robustness against artifacts, lower spatial resolution and inferior signal to noise compared to contrast enhanced dynamic perfusion imaging[52]. Therefore, currently only steady-state GRE sequences are used when a non-contrast enhanced MRI of the pulmonary vessels is required.
SAFETY OF GADOLINIUM BASED CONTRAST AGENTS
Currently GBCA’s are used for CE-MRA, even in those patients with borderline renal function[69]. A recent meta-analysis has shown that higher iconicity, protein binding and macrocyclic structures of GBCA’s are associated with an increased number of acute allergic reactions[42].
Recently work has shown gadolinium deposition in the brains, skin and bones of patients with normal renal function[70-72]. This can occur when linear or macrocyclic chelates GBCAs are used[73]. The association between the tissue deposition of gadolinium from GBCAs and any short or long-term clinical importance remains to be determined[74]. Due to concerns over brain Gadolinium deposition, macrocyclic agents such gadoterate meglumine and gadobutol are preferred[75].
We currently recommend injection of 0.1 mmol/kg of GBCA diluted to a total volume of 30 mL with saline injected at 1.5 mL/s at end-expiration[2,76]. The total length of time for the bolus administration of contrast material is important. Diluting the contrast with normal saline up to a volume of 30 mL allows administration of the entire length of the acquisition and thus helps to limit Maki artifacts (Figure 3). This artifact occurs when the scan acquisition starts before the bolus arrives, the effect is an edge-enhanced image and this can simulate PE[77].
RENAL FAILURE
An option for patients with renal failure or GBCA allergy is the off-label use of Ferumoxytol as a MRA contrast agent[78]. Ferumoxytol is an intravenously administered ultrasmall superparamagnetic iron oxide agent for treatment of anemia in adult patients. The standard intravenous dose is 3.0 mg/kg. There is a Food and Drug Administration “black box” warning against the rapid bolus administration of this agent, as it has been associated with hypotension and death. The rate of anaphylaxis is low at 0.02% to 1.3%[43]. Its T1 and T2 shortening effects, long blood-pool residence time and clearance through the reticuloendothelial system makes this a versatile MRI contrast agent[43,79]. Moreover, Ferumoxytol avoids any risk of Nephrogenic Systemic Fibrosis for patients with renal failure.
PREGNANCY
PE is one of the causes of death in the pregnancy[80,81]. The diagnosis of PE in these patients is challenging because of the necessity of keeping medical radiation exposure to a minimum. The American Thoracic Society and the Society of Thoracic Radiology have reached consensus in this clinical scenario[82]. They recommend ventilation-perfusion (V/Q) scintigraphy as a first line test to detect PE in pregnant patients with normal chest radiographs, with CTA reserved for those mothers with abnormal chest radiographs or indeterminate V/Q scans[82]. This remains an active area of research[83]. Non-contrast MRA using bright blood pulse sequences (bSSFP) and unenhanced Fourier decomposition lung perfusion are other options in this scenario that is non-ionizing and without contrast for the mother and fetus (Figure 2)[72,84]. Unfortunately, CE-MRA using GBCAs is limited because these agents cross the placenta to the fetus and there are reports of rheumatological, inflammatory, and infiltrative skin conditions in those exposed neonates[85]. Fortunately, there is another option for CE-MRA in this situation; the United States Food and Drug Administration has approved Ferumoxytol for use in pregnancy as a treatment for anemia, and we have used it (off-label) for CE-MRA in pregnant patients[86]. A recent Cochrane review assessed the value CTA, lung scintigraphy or MRA in pregnant patients with suspected PE[87]. No MRI examinations met the inclusion criteria for the study. The authors concluded that both CTA and lung scintigraphy are appropriate for exclusion of PE in pregnancy, however it was unclear which test had the higher accuracy. They emphasized the need for direct comparisons and the need to include MRI in prospective trials in this clinical scenario[87].
ARTIFACTS
Truncation artifact (Gibbs’’ ringing) is showed as a distinct central signal intensity drop within the pulmonary vasculature in pulmonary contrast-enhanced MRA[88]. Gibbs’ ringing may be misdiagnosed as PE (Figure 4), particularly by inexperienced MRA readers. The differentiation between Gibbs’ ringing and emboli is important. The signal intensity of this artifact is typically 50% or higher than the enhanced surrounding vessel lumen[88]. Errors approximation in the Fourier transformation from k-space to image space causes this artifact (Figure 3). Routine Cartesian reconstructions of k-space into image space performs better when used for estimating gradual transitions in tissue signal intensity, not sharp ones[88]. There are cases where a Gibbs’ artifact is not distinguishable from a small central non-occlusive PE[88]. In those cases, a confirmatory CTA exam is required for an accurate diagnosis. The Maki artifact[77] can also simulate a PE. This error in image interpretation is avoidable by using multiple contrast phases and extending the bolus so that contrast is always flowing into the pulmonary arteries during the first acquisition (Figure 3)[76].
Figure 4.
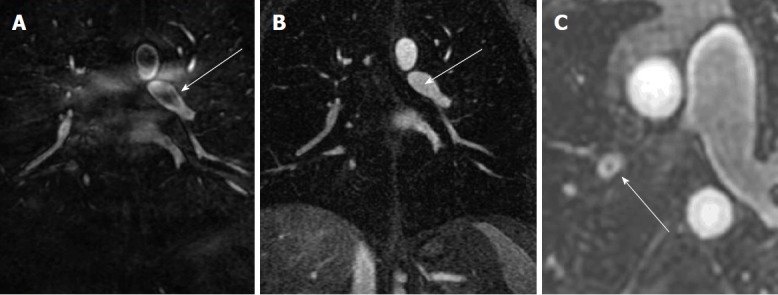
Artifacts: The Maki artifact. A: Acquisition of the central aspect of k-space was before the bolus of contrast agent filled the pulmonary artery causing a pseudo-clot within the left lower lobe pulmonary artery (arrow); B: Later phase acquisition from the same patient shows normal contrast enhancement of the Left lower lobe pulmonary artery (arrow); C: Gibbs’’ ringing artifact can simulate a central filling defect. Typically, the signal of emboli will be less than 50% of the signal intensity of the lumen.
ANCILLARY FINDINGS
The ancillary findings observed on CE-MRA exams in those patients without PE are similar to those of CTA (Figure 5)[89,90]. The field of view is larger and the soft tissue contrast is better on CE-MRA exams than CTA. In a recent study, the incidence of actionable findings (requiring follow up) from CE-MRA exams was 17% (pleural effusion, pneumonia, malignancy, ascending aortic aneurysm, aortic dissection, pericardial effusion, heart failure, septic emboli, lung abscess, trauma, and sarcoidosis). While the incidence of incidental findings (those findings not requiring follow up) was 36% (mild dependent atelectasis, small pleural effusion, normal vascular variant, simple cysts in liver or kidney and post-surgical changes)[89].
Figure 5.
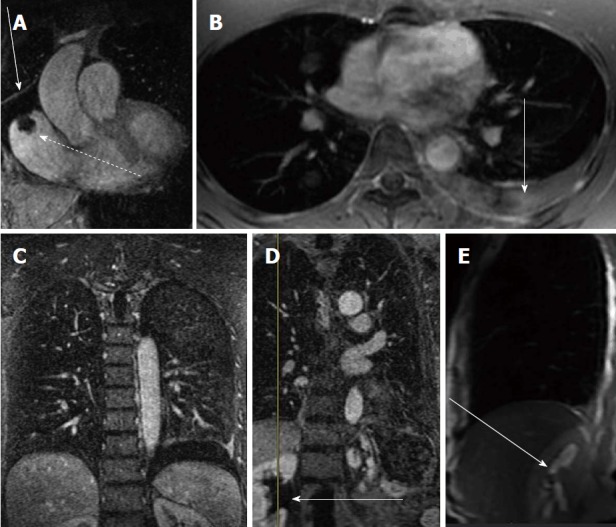
Ancillary findings on contrast enhanced magnetic resonance angiograph exams. A: Contrast enhanced MRA shows a right atrial thrombus from a long standing indwelling central venous catheter (dashed arrow) and a pericardial effusion (straight arrow); B: Post contrast breath hold fat saturated gradient echo showing a left pleural effusion (arrow); C: CE-MRA coronal image showing the same left pleural effusion ( arrow); D: CE-MRA showing right renal pelvis hydronephrosis (arrow); E: Fast spin echo scout sagittal image through the right renal pelvis showing the high signal intensity of the hydronephrosis (arrow). CE-MRA: Contrast enhanced magnetic resonance angiograph.
DIRECT AND INDIRECT FINDINGS OF PE
Just like multidetector computed tomographic angiography MDCT, PE are detected as a lumenal filling defect in the pulmonary arteries on CE-MRA (Figure 1). Other direct findings include that arterial cutoff sign, double bronchus sign, and high T1 signal intensity clots. The indirect findings include atelectasis, pleural effusion, high signal adjacent draining pulmonary vein, the “W-B-W” (white-black-white) sign, perfusion defects, enhancing visceral pleural surfaces, and an enlarged pulmonary trunk. Other findings indicative of right heart strain and elevated central venous pressure can help to estimate the degree of right heart dysfunction with larger clot burden[7,91].
FREE BREATHING PE-MRA
In the PIOPED III study, the sensitivity of PE detected by MRA in subsegmental artery was 0%[16]. The emboli in subsegmental vessels on MRA are difficult to distinguish from lung parenchyma or a nearby bronchus as this is a “black-on-black” perceptual event. Bannas et al[47] showed that free-breathing 3D radial ultra-short time to echo (UTE) imaging[45] can detect PE in subsegmental vessels. The reason for this is the high SNR of lung parenchyma in the UTE image. Their method used a free-breathing 3D radial UTE technique, which is quite advantageous in the setting of dyspnea, a common presenting symptom of PE.
OVERDIAGNOSIS OF PE USING MDCT
MDCT is the gold standard for the primary diagnosis of PE[6]. The SSPE, which are not detected with V/Q scintigraphy or earlier generation single detector CT, are now routinely diagnosed with MDCT[92]. Le Gal et al[92] in a review found that that MDCT found twice as many SSPE as the single detector CT scans. Sheh et al[93] First introduced the concept of “overdiagnosis” of PE due to the change from V/Q scintigraphy to MDCT and an increased diagnosis of a much less fatal spectrum of PE. In contrast to the idea that SSPE is benign, other authors have shown that the presence of SSPE remains important for the likelihood of future venous thromboembolism (VTE)[94-96]. Current clinical practice guidelines suggest that anticoagulation therapy of these SSPE should be tailored to the individual patient’s risks and benefits[96]. The American College of Chest Physicians 2016 guidelines now recommend withholding anticoagulation for SSPE in those patients with a low risk for recurrent thrombus and no concurrent deep vein thrombosis[97].
WEAKNESSES OF MRA
There are limitations for the use of MRA for the primary diagnosis of PE. First, this modality should not be used for unstable patients. Second, patients with allergies to gadolinium based contrast material should only be imaged if there is no access to MDCT or Ventilation Perfusion scanning, and only then after premedication with steroids for 24 h and Benadryl. Third, small children or adults who are unable to hold their breath, or hold still, for the 13-20 s MRA are poor candidates for this exam. Fourth, readers experienced with the interpretation of MRA for PE are needed to ensure that the correct diagnosis is reached in these exams. Fifth, up to date MRI hardware (high performance gradients and multicoils) and software (rapid k-space sampling and accelerated image acquisition) are needed to allow for the acquisition of 3D MRA exams with nearly isotropic voxels. There is noise associated with the rapid switching of the gradient coils that may bother some patients if there is not adequate hearing protection.
The costs of this test will vary depending on each country’s healthcare plan. In our experience, the cost of this procedure is similar to MSCT for PE. There can be access challenges for the emergent use of CE-MRI for PE from the Emergency Department. With effort, we have found that the time from order to final interpretation of these exams can be around one hour[76]. Experienced sites will not have difficulty starting a CE-MRA program for the primary diagnosis of PE, however we recognize that there are many medical centers that do not have access to these instruments and lack adequately trained medical/technical staff for the performance of these exams. In this low to intermediate risk patient population, there are many patients that do not need imaging; this is why the careful application of CDR’s is needed to screen all patients prior to ordering an exam for PE.
PERFORMANCE GAP: CE-MRA EFFECTIVENESS > EFFICACY
The most recent effectiveness data from UW-Madison showed a negative predictive value of 99% (95%CI: 97%-100%) in 500 patients[39]. The reader can easily surmise that this effectiveness value (NPV-99%) is quite different than the efficacy value (sensitivity -77%) reported in PIOPED III[16]. How do we reconcile this difference? Perhaps this relative “over-performance” of CE-MRA, using outcomes data as a surrogate for effectiveness, vs the lower efficacy can be explained by the following possibilities: (1) Better technical CE-MRA exams than were available for PIOPED III; (2) Readers experienced with the artifacts of this exam; and (3) Small PE in younger and healthy patients, that may be missed on CE-MRA or NM V/Q scanning are not important for survival or subsequent VTE. One reason for this may be that these isolated SSPE’s may indeed be “scrubbed” from the pulmonary arterial vasculature by the patient’s endogenous thrombolytic activity. Another possible reason is that there is a great deal of cardiopulmonary reserve and that sacrifice of a few subsegmental pulmonary arteries is not significant in the normal population. Please note that the situation of repeated SSPE may lead to chronic thromboembolic pulmonary hypertension[98].
Recently Cronin and Dwamena[99] have used the PIOPED II data to calculate likelihood ratios (LR) for PE in this cohort based on the pretest probability from CDR (e.g., Wells’ score, Geneva score and Pisa score). The use of LR is important in this age of outcomes driven research as these reflect the clinical utility of any given testing method. The numerator of the positive LR (+LR) is the sensitivity of the test for that disease. The denominator is 1-specificity of the test for that disease[99]. Their analysis showed that the use of CE-MRA for the diagnosis of PE had a higher LR+ than CTA[99].
LR+ = Sensitivity/(1-specificity)
CONCLUSION
Currently computed tomographic angiography is the study of choice for the diagnosis of PE. We have reviewed our experience using pulmonary CE-MRA as a first line diagnostic test for patients suspected of having PE. We have found equivalent six-month outcomes to computed tomographic angiography when using this test. We recommend using strict patient selection criteria for improving the likelihood for the technical success of this test. First, a low to intermediate pretest probability for venous thromboembolic disease by the formalized is used of CDR (Wells’ criteria, PERC or the Geneva score); Second, patients with iodinated contrast allergies can benefit from using this test; Third, female subjects less than 35 years of age to mitigate medical radiation exposure to the breast; Fourth, employing ferumoxytol as the MRA contrast agent in renal failure patients; Finally, yet importantly, ensuring that the patient is stable and can hold their breath for the 13-17 s CE-MRA. There is no overdiagnosis of PE when CE-MRA or NM V/Q scanning is used. In other words, these “less sensitive” tests may suffice for the primary diagnosis of PE. We are supportive of funding for randomized clinical trials to evaluate whether or not the clinical outcomes significantly vary between CTA and CE-MRA for the primary diagnosis of PE, as this remains an unmet need.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the research support of the Department of Radiology, UW-Madison and GE Healthcare.
Footnotes
Conflict-of-interest statement: There are no conflicts of interest related to this work. No financial support.
Manuscript source: Invited manuscript
Peer-review started: January 30, 2018
First decision: March 19, 2018
Article in press: May 30, 2018
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Aday AW, Qi X S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
Contributor Information
Nanae Tsuchiya, Department of Radiology, Graduate School of Medical Science, University of the Ryukyus, Okinawa 903-0215, Japan; Department of Radiology, University of Wisconsin-Madison, Madison, WI 53792, United States.
Edwin JR van Beek, Edinburgh Imaging, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh EH16 4TJ, United Kingdom.
Yoshiharu Ohno, Division of Functional and Diagnostic Imaging Research, Department of Radiology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan.
Hiroto Hatabu, Department of Radiology, Brigham and Women’s Hospital, Boston, MA 02115, United States.
Hans-Ulrich Kauczor, Department of Diagnostic and Interventional Radiology, University Hospital of Heidelberg, Heidelberg 69120, Germany.
Andrew Swift, Department of Radiology, Royal Hallamshire Hospital, University of Sheffield, Sheffield S10 2JF, United Kingdom.
Jens Vogel-Claussen, Department of Radiology, Carl-Neuberg Strasse 1, Hannover-Gr-Buchholz 30625, Germany.
Jürgen Biederer, Radiology Darmstadt, Gross-Gerau County Hospital, Gross-Gerau 64521, Germany.
James Wild, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield S10 2JF, United Kingdom.
Mark O Wielpütz, Department of Diagnostic and Interventional Radiology, University Hospital of Heidelberg, Heidelberg 69120, Germany.
Mark L Schiebler, Department of Radiology, University of Wisconsin-Madison, Madison, WI 53792, United States. mschiebler@uwhealth.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Schiebler ML, Nagle SK, François CJ, Repplinger MD, Hamedani AG, Vigen KK, Yarlagadda R, Grist TM, Reeder SB. Effectiveness of MR angiography for the primary diagnosis of acute pulmonary embolism: clinical outcomes at 3 months and 1 year. J Magn Reson Imaging. 2013;38:914–925. doi: 10.1002/jmri.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin D, Seo JB, Kiely DG, Hatabu H, Gefter W, van Beek EJ, Schiebler ML; 2013 International Workshop for Pulmonary Functional Imaging (IWPFI) Triage for suspected acute Pulmonary Embolism: Think before opening Pandora’s Box. Eur J Radiol. 2015;84:1202–1211. doi: 10.1016/j.ejrad.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Harringa JB, Bracken RL, Nagle SK, Schiebler ML, Pulia MS, Svenson JE, Repplinger MD. Negative D-dimer testing excludes pulmonary embolism in non-high risk patients in the emergency department. Emerg Radiol. 2017;24:273–280. doi: 10.1007/s10140-017-1478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sikkens JJ, Beekman DG, Thijs A, Bossuyt PM, Smulders YM. How Much Overtesting Is Needed to Safely Exclude a Diagnosis? A Different Perspective on Triage Testing Using Bayes’ Theorem. PLoS One. 2016;11:e0150891. doi: 10.1371/journal.pone.0150891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Expert Panels on Cardiac and Thoracic Imaging. Kirsch J, Brown RKJ, Henry TS, Javidan-Nejad C, Jokerst C, Julsrud PR, Kanne JP, Kramer CM, Leipsic JA, Panchal KK, Ravenel JG, Shah AB, Mohammed TL, Woodard PK, Abbara S. ACR Appropriateness Criteria® Acute Chest Pain-Suspected Pulmonary Embolism. J Am Coll Radiol. 2017;14:S2–S12. doi: 10.1016/j.jacr.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 7.François CJ, Hartung MP, Reeder SB, Nagle SK, Schiebler ML. MRI for acute chest pain: current state of the art. J Magn Reson Imaging. 2013;37:1290–1300. doi: 10.1002/jmri.24173. [DOI] [PubMed] [Google Scholar]
- 8.Schiebler ML, Holland GA, Hatabu H, Listerud J, Foo T, Palevsky H, Edmunds H, Gefter WB. Suspected pulmonary embolism: prospective evaluation with pulmonary MR angiography. Radiology. 1993;189:125–131. doi: 10.1148/radiology.189.1.8372181. [DOI] [PubMed] [Google Scholar]
- 9.Grist TM, Sostman HD, MacFall JR, Foo TK, Spritzer CE, Witty L, Newman GE, Debatin JF, Tapson V, Saltzman HA. Pulmonary angiography with MR imaging: preliminary clinical experience. Radiology. 1993;189:523–530. doi: 10.1148/radiology.189.2.8210385. [DOI] [PubMed] [Google Scholar]
- 10.Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med. 1997;336:1422–1427. doi: 10.1056/NEJM199705153362004. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Frazer CK, Ferguson JM, Kumar AB, Davis SJ, Fallon MJ, Morris IT, Drury PJ, Cala LA. Acute pulmonary embolism: diagnosis with MR angiography. Radiology. 1999;210:353–359. doi: 10.1148/radiology.210.2.r99fe53353. [DOI] [PubMed] [Google Scholar]
- 12.Oudkerk M, van Beek EJ, Wielopolski P, van Ooijen PM, Brouwers-Kuyper EM, Bongaerts AH, Berghout A. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet. 2002;359:1643–1647. doi: 10.1016/S0140-6736(02)08596-3. [DOI] [PubMed] [Google Scholar]
- 13.Ohno Y, Kawamitsu H, Higashino T, Takenaka D, Watanabe H, van Cauteren M, Fujii M, Hatabu H, Sugimura K. Time-resolved contrast-enhanced pulmonary MR angiography using sensitivity encoding (SENSE) J Magn Reson Imaging. 2003;17:330–336. doi: 10.1002/jmri.10261. [DOI] [PubMed] [Google Scholar]
- 14.Ohno Y, Higashino T, Takenaka D, Sugimoto K, Yoshikawa T, Kawai H, Fujii M, Hatabu H, Sugimura K. MR angiography with sensitivity encoding (SENSE) for suspected pulmonary embolism: comparison with MDCT and ventilation-perfusion scintigraphy. AJR Am J Roentgenol. 2004;183:91–98. doi: 10.2214/ajr.183.1.1830091. [DOI] [PubMed] [Google Scholar]
- 15.Kluge A, Luboldt W, Bachmann G. Acute pulmonary embolism to the subsegmental level: diagnostic accuracy of three MRI techniques compared with 16-MDCT. AJR Am J Roentgenol. 2006;187:W7–14. doi: 10.2214/AJR.04.1814. [DOI] [PubMed] [Google Scholar]
- 16.Stein PD, Chenevert TL, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Jablonski KA, Leeper KV Jr, Naidich DP, Sak DJ, Sostman HD, Tapson VF, Weg JG, Woodard PK; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III) Ann Intern Med. 2010;152:434–443, W142-W143. doi: 10.1059/0003-4819-152-7-201004060-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohno Y, Koyama H, Matsumoto K, Onishi Y, Nogami M, Takenaka D, Yoshikawa T, Matsumoto S, Sugimura K. Dynamic MR perfusion imaging: capability for quantitative assessment of disease extent and prediction of outcome for patients with acute pulmonary thromboembolism. J Magn Reson Imaging. 2010;31:1081–1090. doi: 10.1002/jmri.22146. [DOI] [PubMed] [Google Scholar]
- 18.Kalb B, Sharma P, Tigges S, Ray GL, Kitajima HD, Costello JR, Chen Z, Martin DR. MR imaging of pulmonary embolism: diagnostic accuracy of contrast-enhanced 3D MR pulmonary angiography, contrast-enhanced low-flip angle 3D GRE, and nonenhanced free-induction FISP sequences. Radiology. 2012;263:271–278. doi: 10.1148/radiol.12110224. [DOI] [PubMed] [Google Scholar]
- 19.Sostman HD, Jablonski KA, Woodard PK, Stein PD, Naidich DP, Chenevert TL, Weg JG, Hales CA, Hull RD, Goodman LR, et al. Factors in the technical quality of gadolinium enhanced magnetic resonance angiography for pulmonary embolism in PIOPED III. Int J Cardiovasc Imaging. 2012;28:303–312. doi: 10.1007/s10554-011-9820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodard PK, Chenevert TL, Sostman HD, Jablonski KA, Stein PD, Goodman LR, Londy FJ, Narra V, Hales CA, Hull RD, et al. Signal quality of single dose gadobenate dimeglumine pulmonary MRA examinations exceeds quality of MRA performed with double dose gadopentetate dimeglumine. Int J Cardiovasc Imaging. 2012;28:295–301. doi: 10.1007/s10554-011-9821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman LR. Small pulmonary emboli: what do we know? Radiology. 2005;234:654–658. doi: 10.1148/radiol.2343041326. [DOI] [PubMed] [Google Scholar]
- 22.Yoo HH, Queluz TH, El Dib R. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst Rev. 2016;(1):CD010222. doi: 10.1002/14651858.CD010222.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Mehta D, Barnett M, Zhou L, Woulfe T, Rolfe-Vyson V, Rowland V, Simpson D, Merriman E. Management and outcomes of single subsegmental pulmonary embolus: a retrospective audit at North Shore Hospital, New Zealand. Intern Med J. 2014;44:872–876. doi: 10.1111/imj.12507. [DOI] [PubMed] [Google Scholar]
- 24.Carrier M, Righini M, Wells PS, Perrier A, Anderson DR, Rodger MA, Pleasance S, Le Gal G. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010;8:1716–1722. doi: 10.1111/j.1538-7836.2010.03938.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoo HH, Queluz TH, El Dib R. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst Rev. 2014;(4):CD010222. doi: 10.1002/14651858.CD010222.pub2. [DOI] [PubMed] [Google Scholar]
- 26.van Beek EJ, Bakker AJ, Reekers JA. Pulmonary embolism: interobserver agreement in the interpretation of conventional angiographic and DSA images in patients with nondiagnostic lung scan results. Radiology. 1996;198:721–724. doi: 10.1148/radiology.198.3.8628860. [DOI] [PubMed] [Google Scholar]
- 27.Anderson DR, Kahn SR, Rodger MA, Kovacs MJ, Morris T, Hirsch A, Lang E, Stiell I, Kovacs G, Dreyer J, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298:2743–2753. doi: 10.1001/jama.298.23.2743. [DOI] [PubMed] [Google Scholar]
- 28.van Beek EJ, Brouwerst EM, Song B, Stein PD, Oudkerk M. Clinical validity of a normal pulmonary angiogram in patients with suspected pulmonary embolism-a critical review. Clin Radiol. 2001;56:838–842. doi: 10.1053/crad.2001.0778. [DOI] [PubMed] [Google Scholar]
- 29.van Beek EJ, Kuyer PM, Schenk BE, Brandjes DP, ten Cate JW, Büller HR. A normal perfusion lung scan in patients with clinically suspected pulmonary embolism. Frequency and clinical validity. Chest. 1995;108:170–173. doi: 10.1378/chest.108.1.170. [DOI] [PubMed] [Google Scholar]
- 30.van der Hulle T, van Es N, den Exter PL, van Es J, Mos ICM, Douma RA, Kruip MJHA, Hovens MMC, Ten Wolde M, Nijkeuter M, et al. Is a normal computed tomography pulmonary angiography safe to rule out acute pulmonary embolism in patients with a likely clinical probability? A patient-level meta-analysis. Thromb Haemost. 2017;117:1622–1629. doi: 10.1160/TH17-02-0076. [DOI] [PubMed] [Google Scholar]
- 31.Brenner DJ. What we know and what we don’t know about cancer risks associated with radiation doses from radiological imaging. Br J Radiol. 2014;87:20130629. doi: 10.1259/bjr.20130629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shuryak I, Brenner DJ. Mechanistic analysis of the contributions of DNA and protein damage to radiation-induced cell death. Radiat Res. 2012;178:17–24. doi: 10.1667/rr2877.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner DJ. Radiation and chest CT scans: are there problems? What should we do? Chest. 2012;142:549–550. doi: 10.1378/chest.12-0490. [DOI] [PubMed] [Google Scholar]
- 34.Brenner DJ. We can do better than effective dose for estimating or comparing low-dose radiation risks. Ann ICRP. 2012;41:124–128. doi: 10.1016/j.icrp.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Journy NMY, Dreuil S, Boddaert N, Chateil JF, Defez D, Ducou-le-Pointe H, Garcier JM, Guersen J, Habib Geryes B, Jahnen A, et al. Individual radiation exposure from computed tomography: a survey of paediatric practice in French university hospitals, 2010-2013. Eur Radiol. 2018;28:630–641. doi: 10.1007/s00330-017-5001-y. [DOI] [PubMed] [Google Scholar]
- 36.Journy NM, Lee C, Harbron RW, McHugh K, Pearce MS, Berrington de González A. Projected cancer risks potentially related to past, current, and future practices in paediatric CT in the United Kingdom, 1990-2020. Br J Cancer. 2017;116:109–116. doi: 10.1038/bjc.2016.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pijpe A, Andrieu N, Easton DF, Kesminiene A, Cardis E, Noguès C, Gauthier-Villars M, Lasset C, Fricker JP, Peock S, et al. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK) BMJ. 2012;345:e5660. doi: 10.1136/bmj.e5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drooger JC, Hooning MJ, Seynaeve CM, Baaijens MH, Obdeijn IM, Sleijfer S, Jager A. Diagnostic and therapeutic ionizing radiation and the risk of a first and second primary breast cancer, with special attention for BRCA1 and BRCA2 mutation carriers: a critical review of the literature. Cancer Treat Rev. 2015;41:187–196. doi: 10.1016/j.ctrv.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Schiebler M, Francois C, Repplinger M, Hamedani A, Lindholm C, Vigen K, Munoz del Rio A, Grist T, Reeder S, Nagle S. 2016. Effectiveness of Pulmonary Contrast Enhanced Magnetic Resonance Angiography for the primary workup of pulmonary embolism. ISMRM 24th Annual Meeting and Exhibition; pp. May 7–13; Singapore; Oral abstract presentation, No. 1074. [Google Scholar]
- 40.Mos IC, Klok FA, Kroft LJ, DE Roos A, Dekkers OM, Huisman MV. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491–1498. doi: 10.1111/j.1538-7836.2009.03518.x. [DOI] [PubMed] [Google Scholar]
- 41.Shellock FG. Magnetic resonance safety update 2002: implants and devices. J Magn Reson Imaging. 2002;16:485–496. doi: 10.1002/jmri.10196. [DOI] [PubMed] [Google Scholar]
- 42.Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate Allergic Reactions to Gadolinium-based Contrast Agents: A Systematic Review and Meta-Analysis. Radiology. 2018;286:731. doi: 10.1148/radiol.2017174037. [DOI] [PubMed] [Google Scholar]
- 43.Vasanawala SS, Nguyen KL, Hope MD, Bridges MD, Hope TA, Reeder SB, Bashir MR. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med. 2016;75:2107–2111. doi: 10.1002/mrm.26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alsop DC, Hatabu H, Bonnet M, Listerud J, Gefter W. Multi-slice, breathhold imaging of the lung with submillisecond echo times. Magn Reson Med. 1995;33:678–682. doi: 10.1002/mrm.1910330513. [DOI] [PubMed] [Google Scholar]
- 45.Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013;70:1241–1250. doi: 10.1002/mrm.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griswold MA, Blaimer M, Breuer F, Heidemann RM, Mueller M, Jakob PM. Parallel magnetic resonance imaging using the GRAPPA operator formalism. Magn Reson Med. 2005;54:1553–1556. doi: 10.1002/mrm.20722. [DOI] [PubMed] [Google Scholar]
- 47.Bannas P, Bell LC, Johnson KM, Schiebler ML, François CJ, Motosugi U, Consigny D, Reeder SB, Nagle SK. Pulmonary Embolism Detection with Three-dimensional Ultrashort Echo Time MR Imaging: Experimental Study in Canines. Radiology. 2016;278:413–421. doi: 10.1148/radiol.2015150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brau AC, Beatty PJ, Skare S, Bammer R. Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Magn Reson Med. 2008;59:382–395. doi: 10.1002/mrm.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biederer J, Beer M, Hirsch W, Wild J, Fabel M, Puderbach M, Van Beek EJ. MRI of the lung (2/3). Why … when … how? Insights Imaging. 2012;3:355–371. doi: 10.1007/s13244-011-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kluge A, Gerriets T, Stolz E, Dill T, Mueller KD, Mueller C, Bachmann G. Pulmonary perfusion in acute pulmonary embolism: agreement of MRI and SPECT for lobar, segmental and subsegmental perfusion defects. Acta Radiol. 2006;47:933–940. doi: 10.1080/02841850600885377. [DOI] [PubMed] [Google Scholar]
- 51.Kluge A, Gerriets T, Müller C, Ekinci O, Neumann T, Dill T, Bachmann G. [Thoracic real-time MRI: experience from 2200 examinations in acute and ill-defined thoracic diseases] Rofo. 2005;177:1513–1521. doi: 10.1055/s-2005-858688. [DOI] [PubMed] [Google Scholar]
- 52.Biederer J, Heussel CP, Puderbach M, Wielpuetz MO. Functional magnetic resonance imaging of the lung. Semin Respir Crit Care Med. 2014;35:74–82. doi: 10.1055/s-0033-1363453. [DOI] [PubMed] [Google Scholar]
- 53.Edelman RR, Silvers RI, Thakrar KH, Metzl MD, Nazari J, Giri S, Koktzoglou I. Nonenhanced MR angiography of the pulmonary arteries using single-shot radial quiescent-interval slice-selective (QISS): a technical feasibility study. J Cardiovasc Magn Reson. 2017;19:48. doi: 10.1186/s12968-017-0365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibiino F, Sacolick L, Menini A, Landini L, Wiesinger F. Free-breathing, zero-TE MR lung imaging. MAGMA. 2015;28:207–215. doi: 10.1007/s10334-014-0459-y. [DOI] [PubMed] [Google Scholar]
- 55.Bauman G, Puderbach M, Deimling M, Jellus V, Chefd’hotel C, Dinkel J, Hintze C, Kauczor HU, Schad LR. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of fourier decomposition in proton MRI. Magn Reson Med. 2009;62:656–664. doi: 10.1002/mrm.22031. [DOI] [PubMed] [Google Scholar]
- 56.Voskrebenzev A, Gutberlet M, Becker L, Wacker F, Vogel-Claussen J. Reproducibility of fractional ventilation derived by Fourier decomposition after adjusting for tidal volume with and without an MRI compatible spirometer. Magn Reson Med. 2016;76:1542–1550. doi: 10.1002/mrm.26047. [DOI] [PubMed] [Google Scholar]
- 57.Suga K, Ogasawara N, Okada M, Tsukuda T, Matsunaga N, Miyazaki M. Lung perfusion impairments in pulmonary embolic and airway obstruction with noncontrast MR imaging. J Appl Physiol (1985) 2002;92:2439–2451. doi: 10.1152/japplphysiol.00900.2001. [DOI] [PubMed] [Google Scholar]
- 58.Bauman G, Johnson KM, Bell LC, Velikina JV, Samsonov AA, Nagle SK, Fain SB. Three-dimensional pulmonary perfusion MRI with radial ultrashort echo time and spatial-temporal constrained reconstruction. Magn Reson Med. 2015;73:555–564. doi: 10.1002/mrm.25158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer A, Weick S, Ritter CO, Beer M, Wirth C, Hebestreit H, Jakob PM, Hahn D, Bley T, Köstler H. SElf-gated Non-Contrast-Enhanced FUnctional Lung imaging (SENCEFUL) using a quasi-random fast low-angle shot (FLASH) sequence and proton MRI. NMR Biomed. 2014;27:907–917. doi: 10.1002/nbm.3134. [DOI] [PubMed] [Google Scholar]
- 60.Voskrebenzev A, Gutberlet M, Klimeš F, Kaireit TF, Schönfeld C, Rotärmel A, Wacker F, Vogel-Claussen J. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med. 2018;79:2306–2314. doi: 10.1002/mrm.26893. [DOI] [PubMed] [Google Scholar]
- 61.Bauman G, Lützen U, Ullrich M, Gaass T, Dinkel J, Elke G, Meybohm P, Frerichs I, Hoffmann B, Borggrefe J, et al. Pulmonary functional imaging: qualitative comparison of Fourier decomposition MR imaging with SPECT/CT in porcine lung. Radiology. 2011;260:551–559. doi: 10.1148/radiol.11102313. [DOI] [PubMed] [Google Scholar]
- 62.Bauman G, Scholz A, Rivoire J, Terekhov M, Friedrich J, de Oliveira A, Semmler W, Schreiber LM, Puderbach M. Lung ventilation- and perfusion-weighted Fourier decomposition magnetic resonance imaging: in vivo validation with hyperpolarized 3He and dynamic contrast-enhanced MRI. Magn Reson Med. 2013;69:229–237. doi: 10.1002/mrm.24236. [DOI] [PubMed] [Google Scholar]
- 63.Bauman G, Bieri O. Matrix pencil decomposition of time-resolved proton MRI for robust and improved assessment of pulmonary ventilation and perfusion. Magn Reson Med. 2017;77:336–342. doi: 10.1002/mrm.26096. [DOI] [PubMed] [Google Scholar]
- 64.Schönfeld C, Cebotari S, Voskrebenzev A, Gutberlet M, Hinrichs J, Renne J, Hoeper MM, Olsson KM, Welte T, Wacker F, et al. Performance of perfusion-weighted Fourier decomposition MRI for detection of chronic pulmonary emboli. J Magn Reson Imaging. 2015;42:72–79. doi: 10.1002/jmri.24764. [DOI] [PubMed] [Google Scholar]
- 65.Keilholz SD, Mai VM, Berr SS, Fujiwara N, Hagspiel KD. Comparison of first-pass Gd-DOTA and FAIRER MR perfusion imaging in a rabbit model of pulmonary embolism. J Magn Reson Imaging. 2002;16:168–171. doi: 10.1002/jmri.10138. [DOI] [PubMed] [Google Scholar]
- 66.Arai TJ, Henderson AC, Dubowitz DJ, Levin DL, Friedman PJ, Buxton RB, Prisk GK, Hopkins SR. Hypoxic pulmonary vasoconstriction does not contribute to pulmonary blood flow heterogeneity in normoxia in normal supine humans. J Appl Physiol (1985) 2009;106:1057–1064. doi: 10.1152/japplphysiol.90759.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burnham KJ, Arai TJ, Dubowitz DJ, Henderson AC, Holverda S, Buxton RB, Prisk GK, Hopkins SR. Pulmonary perfusion heterogeneity is increased by sustained, heavy exercise in humans. J Appl Physiol (1985) 2009;107:1559–1568. doi: 10.1152/japplphysiol.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mai VM, Knight-Scott J, Berr SS. Improved visualization of the human lung in 1H MRI using multiple inversion recovery for simultaneous suppression of signal contributions from fat and muscle. Magn Reson Med. 1999;41:866–870. doi: 10.1002/(sici)1522-2594(199905)41:5<866::aid-mrm2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 69.Soulez G, Bloomgarden DC, Rofsky NM, Smith MP, Abujudeh HH, Morgan DE, Lichtenstein RJ, Schiebler ML, Wippold FJ 2nd, Russo C, Kuhn MJ, Mennitt KW, Maki JH, Stolpen A, Liou J, Semelka RC, Kirchin MA, Shen N, Pirovano G, Spinazzi A. Prospective Cohort Study of Nephrogenic Systemic Fibrosis in Patients With Stage 3-5 Chronic Kidney Disease Undergoing MRI With Injected Gadobenate Dimeglumine or Gadoteridol. AJR Am J Roentgenol. 2015;205:469–478. doi: 10.2214/AJR.14.14268. [DOI] [PubMed] [Google Scholar]
- 70.Prybylski JP, Semelka RC, Jay M. The stability of gadolinium-based contrast agents in human serum: A reanalysis of literature data and association with clinical outcomes. Magn Reson Imaging. 2017;38:145–151. doi: 10.1016/j.mri.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Huckle JE, Altun E, Jay M, Semelka RC. Gadolinium Deposition in Humans: When Did We Learn That Gadolinium Was Deposited In Vivo? Invest Radiol. 2016;51:236–240. doi: 10.1097/RLI.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 72.Herédia V, Altun E, Ramalho M, de Campos R, Azevedo R, Pamuklar E, Semelka RC. MRI of pregnant patients for suspected pulmonary embolism: steady-state free precession vs postgadolinium 3D-GRE. Acta Med Port. 2012;25:359–367. [PubMed] [Google Scholar]
- 73.Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T, Takeshita K, Furui S. High Signal Intensity in Dentate Nucleus on Unenhanced T1-weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration. Radiology. 2015;275:803–809. doi: 10.1148/radiol.14140364. [DOI] [PubMed] [Google Scholar]
- 74.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16:564–570. doi: 10.1016/S1474-4422(17)30158-8. [DOI] [PubMed] [Google Scholar]
- 75.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagle SK, Schiebler ML, Repplinger MD, François CJ, Vigen KK, Yarlagadda R, Grist TM, Reeder SB. Contrast enhanced pulmonary magnetic resonance angiography for pulmonary embolism: Building a successful program. Eur J Radiol. 2016;85:553–563. doi: 10.1016/j.ejrad.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maki JH, Prince MR, Londy FJ, Chenevert TL. The effects of time varying intravascular signal intensity and k-space acquisition order on three-dimensional MR angiography image quality. J Magn Reson Imaging. 1996;6:642–651. doi: 10.1002/jmri.1880060413. [DOI] [PubMed] [Google Scholar]
- 78.Finn JP, Nguyen KL, Hu P. Ferumoxytol vs. Gadolinium agents for contrast-enhanced MRI: Thoughts on evolving indications, risks, and benefits. J Magn Reson Imaging. 2017;46:919–923. doi: 10.1002/jmri.25580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ripley B, Wilson GJ, Lalwani N, Briller N, Neligan PC, Maki JH. Initial Clinical Experience with Dual-Agent Relaxation Contrast for Isolated Lymphatic Channel Mapping. Radiology. 2018;286:705–714. doi: 10.1148/radiol.2017170241. [DOI] [PubMed] [Google Scholar]
- 80.Skeith L, Rodger MA. Pulmonary Complications of Pregnancy: Venous Thromboembolism. Semin Respir Crit Care Med. 2017;38:135–147. doi: 10.1055/s-0037-1602241. [DOI] [PubMed] [Google Scholar]
- 81.Tromeur C, van der Pol LM, Klok FA, Couturaud F, Huisman MV. Pitfalls in the diagnostic management of pulmonary embolism in pregnancy. Thromb Res. 2017;151 Suppl 1:S86–S91. doi: 10.1016/S0049-3848(17)30075-0. [DOI] [PubMed] [Google Scholar]
- 82.Leung AN, Bull TM, Jaeschke R, Lockwood CJ, Boiselle PM, Hurwitz LM, James AH, McCullough LB, Menda Y, Paidas MJ, et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline--Evaluation of Suspected Pulmonary Embolism in Pregnancy. Radiology. 2012;262:635–646. doi: 10.1148/radiol.11114045. [DOI] [PubMed] [Google Scholar]
- 83.Wan T, Skeith L, Karovitch A, Rodger M, Le Gal G. Guidance for the diagnosis of pulmonary embolism during pregnancy: Consensus and controversies. Thromb Res. 2017;157:23–28. doi: 10.1016/j.thromres.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 84.Sommer G, Bauman G, Koenigkam-Santos M, Draenkow C, Heussel CP, Kauczor HU, Schlemmer HP, Puderbach M. Non-contrast-enhanced preoperative assessment of lung perfusion in patients with non-small-cell lung cancer using Fourier decomposition magnetic resonance imaging. Eur J Radiol. 2013;82:e879–e887. doi: 10.1016/j.ejrad.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 85.Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA. 2016;316:952–961. doi: 10.1001/jama.2016.12126. [DOI] [PubMed] [Google Scholar]
- 86.Johns CS, Schiebler ML, Swift AJ. Commentary on: Survey of UK imaging practice for the investigation of pulmonary embolism in pregnancy. Clin Radiol. 2017;72:702–703. doi: 10.1016/j.crad.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 87.van Mens TE, Scheres LJ, de Jong PG, Leeflang MM, Nijkeuter M, Middeldorp S. Imaging for the exclusion of pulmonary embolism in pregnancy. Cochrane Database Syst Rev. 2017;1:CD011053. doi: 10.1002/14651858.CD011053.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bannas P, Schiebler ML, Motosugi U, François CJ, Reeder SB, Nagle SK. Pulmonary MRA: differentiation of pulmonary embolism from truncation artefact. Eur Radiol. 2014;24:1942–1949. doi: 10.1007/s00330-014-3219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schiebler ML, Ahuja J, Repplinger MD, François CJ, Vigen KK, Grist TM, Hamedani AG, Reeder SB, Nagle SK. Incidence of actionable findings on contrast enhanced magnetic resonance angiography ordered for pulmonary embolism evaluation. Eur J Radiol. 2016;85:1383–1389. doi: 10.1016/j.ejrad.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richman PB, Courtney DM, Friese J, Matthews J, Field A, Petri R, Kline JA. Prevalence and significance of nonthromboembolic findings on chest computed tomography angiography performed to rule out pulmonary embolism: a multicenter study of 1,025 emergency department patients. Acad Emerg Med. 2004;11:642–647. [PubMed] [Google Scholar]
- 91.Benson DG, Schiebler ML, Nagle SK, François CJ. Magnetic Resonance Imaging for the Evaluation of Pulmonary Embolism. Top Magn Reson Imaging. 2017;26:145–151. doi: 10.1097/RMR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 92.Le Gal G, Righini M, Parent F, van Strijen M, Couturaud F. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724–731. doi: 10.1111/j.1538-7836.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- 93.Sheh SH, Bellin E, Freeman KD, Haramati LB. Pulmonary embolism diagnosis and mortality with pulmonary CT angiography versus ventilation-perfusion scintigraphy: evidence of overdiagnosis with CT? AJR Am J Roentgenol. 2012;198:1340–1345. doi: 10.2214/AJR.11.6426. [DOI] [PubMed] [Google Scholar]
- 94.Gómez-Sánchez MA. What is the clinical significance of isolated subsegmental pulmonary embolism? Rev Port Pneumol. 2014;20:179–180. doi: 10.1016/j.rppneu.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 95.den Exter PL, van Es J, Klok FA, Kroft LJ, Kruip MJ, Kamphuisen PW, Büller HR, Huisman MV. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood. 2013;122:1144–1149; quiz 1329. doi: 10.1182/blood-2013-04-497545. [DOI] [PubMed] [Google Scholar]
- 96.Carrier M, Klok FA. Symptomatic subsegmental pulmonary embolism: to treat or not to treat? Hematology Am Soc Hematol Educ Program. 2017;2017:237–241. doi: 10.1182/asheducation-2017.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 98.Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, Delcroix M, Pruszczyk P, Mairuhu AT, Huisman MV, Klok FA. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017;49 doi: 10.1183/13993003.01792-2016. [DOI] [PubMed] [Google Scholar]
- 99.Cronin P, Dwamena BA. A Clinically Meaningful Interpretation of the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) II and III Data. Acad Radiol. 2018;25:561–572. doi: 10.1016/j.acra.2017.11.014. [DOI] [PubMed] [Google Scholar]