Abstract
The medial ulnar collateral ligament complex of the elbow, which is comprised of the anterior bundle [AB, more formally referred to as the medial ulnar collateral ligament (MUCL)], posterior (PB), and transverse ligament, is commonly injured in overhead throwing athletes. Attenuation or rupture of the ligament results in valgus instability with variable clinical presentations. The AB or MUCL is the strongest component of the ligamentous complex and the primary restraint to valgus stress. It is also composed of two separate bands (anterior and posterior) that provide reciprocal function with the anterior band tight in extension, and the posterior band tight in flexion. In individuals who fail comprehensive non-operative treatment, surgical repair or reconstruction of the MUCL is commonly required to restore elbow function and stability. A comprehensive understanding of the anatomy and biomechanical properties of the MUCL is imperative to optimize reconstructive efforts, and to enhance clinical and radiographic outcomes. Our understanding of the native anatomy and biomechanics of the MUCL has evolved over time. The precise locations of the origin and insertion footprint centers guide surgeons in proper graft placement with relation to bony anatomic landmarks. In recent studies, the ulnar insertion of the MUCL is described as larger than previously thought, with the center of the footprint at varying distances relative to the ulnar ridge, joint line, or sublime tubercle. The purpose of this review is to consolidate and summarize the existing literature regarding the native anatomy, biomechanical, and clinical significance of the entire medial ulnar collateral ligament complex, including the MUCL (AB), PB, and transverse ligament.
Keywords: Elbow, Anterior bundle, Medial ulnar collateral ligament, Native anatomy, Biomechanics, Valgus stability
Core tip: The anterior bundle of the medial ulnar collateral ligament complex plays a crucial role in elbow stability, specifically as a valgus and rotational constraint. Based on recent studies and our own cadaveric dissections, the ulnar footprint has a broader insertion that is more tapered and elongated than previous considered. A comprehensive understanding of the anatomy and biomechanical properties of the medial ulnar collateral ligament is imperative to optimize reconstructive efforts, and to enhance clinical and radiographic outcomes.
INTRODUCTION
The medial ulnar collateral ligament [MUCL, also referred to as the ulnar collateral ligament (UCL), medial collateral ligament (MCL), and anterior bundle (AB)] is the primary restraint to valgus instability of the elbow[1-5]. The MUCL is one of three ligaments that comprise the “medial ulnar collateral ligament complex” of the elbow with the posterior bundle (PB) and transverse ligament (TL) being the other two (Figure 1). The MUCL, in particular, has been shown to be the primary stabilizer of the elbow during valgus stress, followed by the radial head and dynamic stabilizers of the elbow such as the flexor-pronator muscle mass[6-10]. The MUCL is composed of two separate bands (anterior and posterior) that provide reciprocal function with the anterior band tight in extension, and the posterior band tight in flexion.
Figure 1.
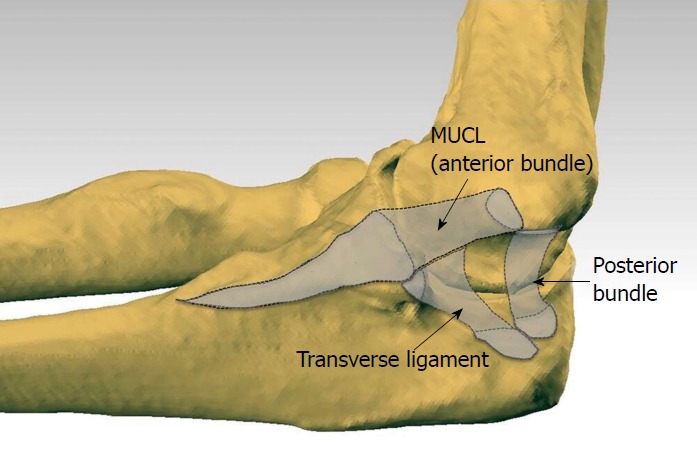
Medial ulnar collateral ligament complex of the elbow with outlined ligaments generated by co-registering the three dimensional digitized anatomy and computed tomography scan of a cadaveric elbow. Note the tapered and distally elongated insertion of the medial ulnar collateral ligament on the sublime tubercle and ulnar ridge. MUCL: Medial ulnar collateral ligament.
The MUCL is commonly injured in overhead throwing athletes when a valgus moment is placed on the elbow during the late cocking and early acceleration phases[11-15]. Incompetence or rupture of the ligament leads to valgus instability which has varying clinical presentations. Patients may complain of instability, however, most will report pain, reduced accuracy, and decreased velocity. Clinically significant pathology often requires surgical intervention. Ligament reconstruction relies on appropriate graft positioning at both the humeral origin and the ulnar insertion. A thorough understanding the native anatomy of the MUCL facilitates the surgeon’s ability to effectively restore stability and function.
In 1985, Morrey and An[10] published the first quantitative analysis of the medial ulnar collateral ligament complex. Based on 10 fresh frozen cadavers, they described the dimensions of the AB and PB at various degrees of elbow flexion. Since that time, multiple studies have assessed the anatomy and biomechanics of the AB[2,3,6,16,17]. Historically, general consensus held that the AB inserts solely and directly onto the sublime tubercle. However, recent studies have shown that the AB insertion is in fact broader, tapered, and significantly larger in terms of surface area than previously thought.
The purpose of this review is to consolidate and summarize the existing literature regarding the anatomy, biomechanical function, and clinical significance of the native (non-reconstruction) MUCL. It is our hope that this work may serve as a framework for better understanding valgus instability in the elbow and refining surgical techniques in order to optimize post-operative outcomes.
ANATOMY
As mentioned, the MUCL (AB) is the primary restraint to valgus instability of the elbow[1-5]. The PB is a soft tissue stabilizer of the elbow with contributions greatest during flexion[17]. It is generally thought that the TL does not provide a significant contribution to elbow stability[10]; however, recent study has revealed a direct insertion of the TL onto the AB that may potentially play a role in elbow stability[18]. The AB, in particular, has been shown to be the primary stabilizer of the elbow in valgus stress, with the radial head and dynamic stabilizers of the medial elbow also contributing[6-10]. It originates on the anterioinferior surface of the medial epicondyle and inserts onto the sublime tubercle of the ulna (Figure 1).
Origin
Surface area and footprint center: The origin of the AB is posterior to the elbow’s axis of rotation, on the anterior, inferior, and lateral aspect of the medial epicondyle[10]. The surface area of the humeral origin has been widely variable in the literature (Table 1). Dugas et al[19] showed that the AB origin was round with a mean surface area of 45.5 mm2 (range of 25.9-59.4 mm2) in 13 fresh frozen cadavers utilizing a three-dimensional (3-D) electromagnetic tracking and digitalizing device. Similar to the findings of Dugas et al[19], a recent study by Camp et al[18] found a mean origin surface area of 32.3 mm2. In contrast to these two studies, Frangiamore et al[20] found this measurement to be notably smaller at 17.0 mm2 (range of 14.9-19.1 mm2) through analysis of 10 fresh frozen cadaver specimens. Potentially due to differences in measurement techniques, these studies demonstrated variable results. Additionally, Frangiamore et al[20] and Dugas et al[19] described the center of the ligament origin in different terms, which is of clinical importance when determining the location of humeral tunnel placement during MUCL reconstruction surgery. Dugas et al[19] described the center of the origin as an area of tissue on a flat surface anterior and inferior to the medial epicondyle. The mean distance they measured was 13.4 mm from the center of the medial epicondyle to the center of the origin, with Camp et al[18] finding a similar mean distance of 11.7 mm. Rather than describing this as a linear distance with no specific angle, Frangiamore et al[20] described this measurement in terms of two separate measurements, reporting the center of the origin to be located, on average, 8.5 mm distal (inferior) and 7.8 mm lateral (anterior) to the medial epicondyle. The use of two measurements relative to a single point in the latter study may assist with better reproducibility.
Table 1.
Summary of anatomic studies describing the length, width, and surface area of the anterior bundle
| Ref. | Specimen | AB length (mm) | AB width (mm) | Origin surface area (mm2) | Insertion surface area (mm2) | AB surface area (mm2) |
| Alcid et al[2] 2004 | - | 27 | 4.5 | - | - | 121.5 |
| Beckett et al[23] 2000 | 39 | 26.7 | - | - | - | - |
| Camp et al[35] 2017 | 10 | - | 32.3 | 187.6 | 324.2 | |
| Dugas et al[19] 2007 | 13 | - | 6.8 | 45.5 | 127.8 | - |
| Eygendaal et al[9] 2002 | 5 | 26 | 5 | - | - | - |
| Farrow et al[22] 2014 | 10 | 53.9 | - | - | - | - |
| Farrow et al[21] 2011 | 12 | 51.7 | - | - | - | - |
| Floris et al[25] 1998 | 18 | - | 5.8 | - | - | - |
| Morrey et al[10] 1985 | 10 | 27.1 | 4.7 | - | - | - |
| Regan et al[17] | 8 | 21.1 | 7.6 | - | - | - |
| Safran et al[5] 2005 | 12 | - | 7.2 | - | - | - |
| Timmerman et al[26] 1994 | 10 | - | 6 | - | - | - |
| Frangiamore et al[20] 2018 | 10 | 21.5 | - | 17 | 66.4 | - |
AB: Anterior bundle.
Insertion
Surface area and footprint center: Historically, the ulnar footprint of the AB has generally been described as inserting solely onto the sublime tubercle, serving as the anatomical landmark for surgical repairs and reconstructions. In one such early report, the mean AB insertional surface area was 66.4 mm2[20].
Recently, authors have described the AB insertion as a longer, distally tapered area that follows the ulnar ridge. The surface area of this broader insertion has was reported by Dugas et al[19] to have a mean surface area of 127.8 mm2. In this study, length of the ulnar footprint measured an average of 29.2 mm. Others have found similar lengths when appreciating a tapered insertion with means of 30.2 mm and 29.2 mm[21,22]. Further study by Camp et al[18] identified a tapered insertion with a mean surface area of 187.6 mm2 and an ulnar footprint length averaging 29.7 mm.
Because the footprint center of a broader tapered insertion may not occur in the location previously assumed (apex of the sublime tubercle), the optimal position of the ulnar tunnel in reconstructive efforts may still need to be elucidated. Clinical relevance of the broader tapered ulnar insertion described in recent studies by Dugas et al[19] and Camp et al[18] is in need of further investigation.
One recent study of 10 cadaveric specimens has shown the mean proximal insertional width to be 9.4 mm which is greater than previously noted[18]. This discrepancy in widths leaves room for further investigation to ensure that the native anatomy of the AB is fully documented and understood (Figure 2).
Figure 2.
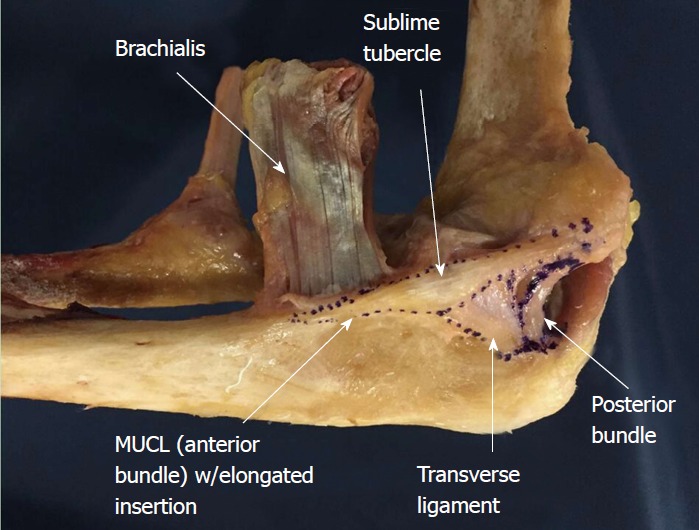
Cadaveric specimen outlining all ligaments of the medial ulnar collateral ligament complex including the medial ulnar collateral ligament/anterior bundle, posterior bundle, and transverse ligament. MUCL: Medial ulnar collateral ligament.
Overall ligament dimensions
Length: The AB is the longest ligament of the medial elbow, spanning the inferior aspect of the medial epicondyle to the sublime tubercle and extending distally along the ulnar ridge. The average length of AB has been reported between 21.1 mm and 31.4 mm in several older studies[2,9,10,17,20,23]. These lengths were measured from the center of the origin to center of sublime tubercle, based on a direct insertion onto the sublime tubercle without a distal extension. In contrast, more recent reports measured from the center of the humeral origin to the most distal point of tapered insertion reported mean lengths of 53.9 mm[21] and 51.7 mm[22]. The difference in length measured between a non-tapered sublime insertion and a tapered insertion calls for further study to evaluate native AB anatomy and the clinical relevance of different measurements. In particular, appropriate length of the ligament component has important implications for ligament reconstruction.
It is important to note that the AB is not an isometric soft tissue stabilizer but instead changes in length throughout flexion. Studies have shown the length of the AB changes by 18%, between 2.8 mm and 4.8 mm as the elbow moves from extension to flexion[6,10,24]. The dynamic length of the AB is an aspect of native biomechanics that must also be considered during reconstruction procedures.
Width: The width of the AB varies, increasing distally to its greatest width at the sublime tubercle before tapering to a point as it inserts distally along the ulnar ridge. Generally, there has been limited variability in the reported widths of the AB, ranging from 4.0 to 7.6 mm[2,5,10,17,25,26].
Surface area: The mean surface area of the AB has been reported between 108 mm2 to 135 mm2[2,10] in studies that did not take the full distal footprint into consideration. Given that Dugas et al[19] has shown the tapered ulnar footprint alone to have a mean surface area of 127.8 mm2, the overall surface area of the ligament will undoubtedly be significantly greater than previously assumed in historical reports. In contrast to these historical reports, and in support of the Dugas et al[19] findings, a more recent study published by Camp et al[18] identified the mean surface area of the entire AB to be 324.2 mm2 (Figure 3).
Figure 3.
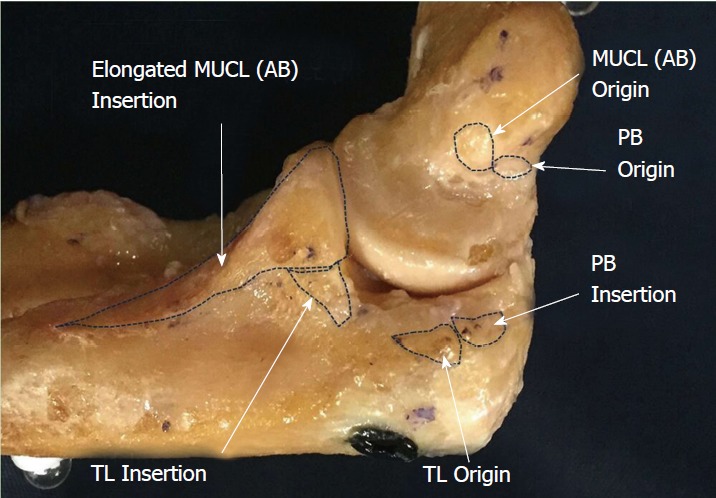
Cadaveric specimen showing origins and insertions of all ligaments of the medial ulnar collateral ligament complex including: Anterior bundle, posterior bundle and transverse ligament. AB: Anterior bundle; PB: Posterior bundle; TL: Transverse ligament.
BIOMECHANICS
Valgus instability
The MUCL provides a vital contribution to the stability of the elbow when a valgus stress is applied. With an intact radial head, a fresh frozen cadaveric model demonstrated that the MUCL contributes 31% and 54% to valgus restraint at 0° and 90° of flexion, respectively[1]. In this study, 4 fresh frozen cadavers had a varying valgus load from 0 to 3 nm applied at 0° and 90° of flexion. With the maximum force applied, there was 3° of valgus laxity in full extension and 2° of laxity in flexion. Several studies have performed similar biomechanical testing at various degrees of flexion and various amount of force[2,3,5,6,10,25,27]. The amount of valgus laxity varies from 2° to 8° with an intact MUCL (Table 2).
Table 2.
Maximum physiologic valgus demonstrated in various studies with noted elbow position and load applied during testing
At 30° and 90° of flexion, Callaway et al[3] showed valgus laxity of 3.6° under a 2nm load compared to an unloaded elbow. Safran et al[5] analyzed 12 cadaveric specimens with a 2Nm load applied at 30 degrees of elbow flexion, and showed a mean alignment of 10.7° of valgus with a neutral forearm rotation position. This study did not determine the inherent valgus alignment in an unloaded elbow, which affects the ability to compare these studies. Thus, with an intact MUCL and a 2 nm load applied, the amount of valgus laxity is generally greatest at 30° of flexion[5].
Other authors have evaluated the effect of transecting the AB on elbow stability (Table 3). When the AB is disrupted in cadaveric models, the amount of valgus instability increases until the point at which secondary osseous stabilizers such as the radial head impart stability. In the setting of AB deficiency, Callaway et al[3] demonstrated the greatest instability at 90° of flexion. The study reported a gain of 1.6, 2.8, 3.2, and 3.0 degrees of valgus motion at 30, 60, 90, and 120 degrees of flexion, respectively when compared to the intact state. Additionally, Mullen et al[28] showed that at 90 degrees of flexion, the transected AB increases valgus instability by 150%. Floris et al[25] and Søjbjerg et al[29] showed the greatest instability occurred at 70 degrees of flexion, with recorded valgus angles of 14.2˚and 11.8˚. Safran et al[5] produced a maximal gain of 6.3˚of laxity under 2 nm of valgus load with a transected AB at 50˚ of elbow flexion. Finally, Morrey et al[4] showed a gain of laxity over baseline ranging from 3.3˚ to 4.8˚ in cadaver specimens at 20 degrees of flexion under gravity. In summary, an intact AB is vital in maintaining valgus stability of the elbow throughout the entire range of flexion.
Table 3.
Maximum valgus reported when the anterior bundle is transected in various studies with noted elbow position and load applied during testing
| Ref. | Specimens | Maximum valgus gain (degrees) | Maximum absolute valgus | Elbow flexion (degrees) | Load (nm) |
| Ahmad et al[8] 2004 | 7 | 7.37 | - | 30 | 2 |
| Callaway et al[3] 1997 | 28 | 3.2 | - | 90 | 2 |
| Floris et al[25] 1998 | 18 | 5.7 | - | 30 | 0.75 |
| Morrey et al[4] 1991 | 6 | 3.9 | - | 20 | gravity |
| Safran et al[5] 2005 | 12 | 6.3 | - | 50 | 2 |
| Sojbjerg et al[29] 1987 | 12 | - | 11.8 | 70 | 1.5 |
Some studies reported gain in valgus from physiologic state, while others noted absolute valgus.
Rotational instability
Internal rotation of the forearm relative to the humerus is constrained by the soft tissues stabilizer of the medial elbow. While there is inherent internal rotation during flexion, the degree of rotation is limited between 2.8˚-6˚ in an uninjured elbow[4,6,25]. Transection of the AB permits internal rotation of the forearm to increase to 18.5˚ at 60˚ of joint flexion (Table 4)[25]. Correct understanding of these biomechanics is important in repair and reconstruction, as a rotatory moment is part of the mechanism of injury.
Table 4.
Summary of the studies evaluating the effect of the anterior bundle on relative internal rotation of the forearm in the native and pathologic state
| Ref. | Specimen | Intact AB internal rotation (degrees) | Cut AB internal rotation (degrees) | Gain of internal rotation with cut AB |
| Bryce et al[6] 2008 | - | 4 | - | - |
| Floris et al[25] 1998 | 18 | 6 | 18.5 | 12.5 |
| Morrey et al[4] 1991 | 6 | 2.8 | 7.8 | 5 |
AB: Anterior bundle.
Tissue strength
Despite being the most frequently injured ligament in the overhead throwing athlete, the AB or MUCL has been shown to have the most inherent strength and stiffness[6,17]. This fact emphasizes the significant loads placed on the medial side of the elbow during the late cocking and early acceleration phase[30,31]. In a cadaveric model with each soft tissue stabilizer evaluated under stress, the AB was the strongest with an average load to failure of 260.9 N[17]. During overhead throwing, the elbow experiences 64 nm of mean valgus torque and 290 N of tensile force on the medial side (which is greater than the threshold for failure of 260.9 N)[30,31]. Furthermore, a maximal mean valgus load of 90Nm has been reported[32-34]. A recent study of 81 professional baseball pitchers (MLB and MiLB) over 82000 throws showed a mean valgus torque of 60 nm with individual participant means ranging from 41 nm to 94 nm[35]. Thus, it is clearly evident why the AB fails in this subset of athletes based on the load to failure being below the force imparted on the elbow.
CONCLUSION
The AB of the medial ulnar collateral ligament complex plays a crucial role in elbow stability, specifically as a valgus and rotational constraint. The AB originates on the humerus and inserts onto the sublime tubercle of the ulna. Based on recent studies and our own cadaveric dissections, the ulnar footprint has a broader insertion that is more tapered and elongated than previous considered. The data regarding the centers of the ulnar and humeral footprints provides guidance for proper tunnel placement during reconstructive efforts. The width and stiffness of the AB is described and can be used to guide graft selection during recons ruction.
Lastly, although the ligament is quite strong, the amount of force placed across the elbow in elite overhead throwing athletes is routinely exceeds the ligaments average load to failure. Accordingly, it is not surprising why MUCL injuries are so common amongst baseball players and pitchers.
Understanding native anatomy and biomechanics of the AB/MUCL is clinically important to help understand the pathoanatomy and guide surgical techniques when treating MUCL injuries.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Manuscript source: Invited manuscript
Peer-review started: March 25, 2018
First decision: April 24, 2018
Article in press: May 10, 2018
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Georgiev GPP S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
Contributor Information
Joshua R Labott, Mayo Clinic School of Medicine, Mayo Clinic, Rochester, MN 55905, United States.
William R Aibinder, Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN 55905, United States.
Joshua S Dines, Sports Medicine and Shoulder Service, Hospital of Special Surgery, New York, NY 10021, Unites States.
Christopher L Camp, Department of Orthopedic Surgery and Sports Medicine, Mayo Clinic, Rochester, MN 55905, United States. camp.christopher@mayo.edu.
References
- 1.Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11:315–319. doi: 10.1177/036354658301100506. [DOI] [PubMed] [Google Scholar]
- 2.Alcid JG, Ahmad CS, Lee TQ. Elbow anatomy and structural biomechanics. Clin Sports Med. 2004;23:503–517, vii. doi: 10.1016/j.csm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Callaway GH, Field LD, Deng XH, Torzilli PA, O’Brien SJ, Altchek DW, Warren RF. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79:1223–1231. doi: 10.2106/00004623-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Morrey BF, Tanaka S, An KN. Valgus stability of the elbow. A definition of primary and secondary constraints. Clin Orthop Relat Res. 1991;265:187–195. [PubMed] [Google Scholar]
- 5.Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21:1381–1395. doi: 10.1016/j.arthro.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Bryce CD, Armstrong AD. Anatomy and biomechanics of the elbow. Orthop Clin North Am. 2008;39:141–154, v. doi: 10.1016/j.ocl.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS, Bruno RJ. The collateral ligaments of the elbow: anatomy and clinical correlation. Clin Orthop Relat Res. 2001;383:123–130. doi: 10.1097/00003086-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32:1607–1612. doi: 10.1177/0363546503263149. [DOI] [PubMed] [Google Scholar]
- 9.Eygendaal D, Valstar ER, Söjbjerg JO, Rozing PM. Biomechanical evaluation of the elbow using roentgen stereophotogrammetric analysis. Clin Orthop Relat Res. 2002;396:100–105. doi: 10.1097/00003086-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop Relat Res. 1985;201:84–90. [PubMed] [Google Scholar]
- 11.Bruce JR, Andrews JR. Ulnar collateral ligament injuries in the throwing athlete. J Am Acad Orthop Surg. 2014;22:315–325. doi: 10.5435/JAAOS-22-05-315. [DOI] [PubMed] [Google Scholar]
- 12.Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94:e49. doi: 10.2106/JBJS.K.01034. [DOI] [PubMed] [Google Scholar]
- 13.Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30:541–548. doi: 10.1177/03635465020300041401. [DOI] [PubMed] [Google Scholar]
- 14.Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74:67–83. [PubMed] [Google Scholar]
- 15.Singh H, Osbahr DC, Wickham MQ, Kirkendall DT, Speer KP. Valgus laxity of the ulnar collateral ligament of the elbow in collegiate athletes. Am J Sports Med. 2001;29:558–561. doi: 10.1177/03635465010290050601. [DOI] [PubMed] [Google Scholar]
- 16.O’Driscoll SW, Jaloszynski R, Morrey BF, An KN. Origin of the medial ulnar collateral ligament. J Hand Surg Am. 1992;17:164–168. doi: 10.1016/0363-5023(92)90135-c. [DOI] [PubMed] [Google Scholar]
- 17.Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170–179. [PubMed] [Google Scholar]
- 18.Camp CL, Jahandar H, Sinatro AM, Imhauser CW, Altchek DW, Dines JS. Quantitative Anatomic Analysis of the Medial Ulnar Collateral Ligament Complex of the Elbow. Orthop J Sports Med. 2018;6:2325967118762751. doi: 10.1177/2325967118762751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16:657–660. doi: 10.1016/j.jse.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Frangiamore SJ, Moatshe G, Kruckeberg BM, Civitarese DM, Muckenhirn KJ, Chahla J, Brady AW, Cinque ME, Oleson ML, Provencher MT, et al. Qualitative and Quantitative Analyses of the Dynamic and Static Stabilizers of the Medial Elbow: An Anatomic Study. Am J Sports Med. 2018;46:687–694. doi: 10.1177/0363546517743749. [DOI] [PubMed] [Google Scholar]
- 21.Farrow LD, Mahoney AJ, Stefancin JJ, Taljanovic MS, Sheppard JE, Schickendantz MS. Quantitative analysis of the medial ulnar collateral ligament ulnar footprint and its relationship to the ulnar sublime tubercle. Am J Sports Med. 2011;39:1936–1941. doi: 10.1177/0363546511406220. [DOI] [PubMed] [Google Scholar]
- 22.Farrow LD, Mahoney AP, Sheppard JE, Schickendantz MS, Taljanovic MS. Sonographic assessment of the medial ulnar collateral ligament distal ulnar attachment. J Ultrasound Med. 2014;33:1485–1490. doi: 10.7863/ultra.33.8.1485. [DOI] [PubMed] [Google Scholar]
- 23.Beckett KS, McConnell P, Lagopoulos M, Newman RJ. Variations in the normal anatomy of the collateral ligaments of the human elbow joint. J Anat. 2000;197 Pt 3:507–511. doi: 10.1046/j.1469-7580.2000.19730507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong AD, Ferreira LM, Dunning CE, Johnson JA, King GJ. The medial collateral ligament of the elbow is not isometric: an in vitro biomechanical study. Am J Sports Med. 2004;32:85–90. doi: 10.1177/0363546503258886. [DOI] [PubMed] [Google Scholar]
- 25.Floris S, Olsen BS, Dalstra M, Søjbjerg JO, Sneppen O. The medial collateral ligament of the elbow joint: anatomy and kinematics. J Shoulder Elbow Surg. 1998;7:345–351. doi: 10.1016/s1058-2746(98)90021-0. [DOI] [PubMed] [Google Scholar]
- 26.Timmerman LA, Andrews JR. Histology and arthroscopic anatomy of the ulnar collateral ligament of the elbow. Am J Sports Med. 1994;22:667–673. doi: 10.1177/036354659402200515. [DOI] [PubMed] [Google Scholar]
- 27.Rooker JC, Smith JRA, Amirfeyz R. Anatomy, surgical approaches and biomechanics of the elbow. Orthop Trauma. 2016;30:283–290. [Google Scholar]
- 28.Mullen DJ, Goradia VK, Parks BG, Matthews LS. A biomechanical study of stability of the elbow to valgus stress before and after reconstruction of the medial collateral ligament. J Shoulder Elbow Surg. 2002;11:259–264. doi: 10.1067/mse.2002.122622. [DOI] [PubMed] [Google Scholar]
- 29.Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186–190. [PubMed] [Google Scholar]
- 30.Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17:274–278. doi: 10.2519/jospt.1993.17.6.274. [DOI] [PubMed] [Google Scholar]
- 31.Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23:233–239. doi: 10.1177/036354659502300218. [DOI] [PubMed] [Google Scholar]
- 32.Fleisig GS, Kingsley DS, Loftice JW, Dinnen KP, Ranganathan R, Dun S, Escamilla RF, Andrews JR. Kinetic comparison among the fastball, curveball, change-up, and slider in collegiate baseball pitchers. Am J Sports Med. 2006;34:423–430. doi: 10.1177/0363546505280431. [DOI] [PubMed] [Google Scholar]
- 33.Fleisig GS, Escamilla RF. Biomechanics of the elbow in the throwing athlete. Oper Techn Sport Med. 1996;4:62–68. [Google Scholar]
- 34.Loftice J, Fleisig GS, Zheng N, Andrews JR. Biomechanics of the elbow in sports. Clin Sports Med. 2004;23:519–530, vii-viii. doi: 10.1016/j.csm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Camp CL, Tubbs TG, Fleisig GS, Dines JS, Dines DM, Altchek DW, Dowling B. The Relationship of Throwing Arm Mechanics and Elbow Varus Torque: Within-Subject Variation for Professional Baseball Pitchers Across 82,000 Throws. Am J Sports Med. 2017;45:3030–3035. doi: 10.1177/0363546517719047. [DOI] [PubMed] [Google Scholar]


