Abstract
Traumatic injury of the central nervous system (CNS) including brain and spinal cord remains a leading cause of morbidity and disability in the world. Delineating the mechanisms underlying the secondary and persistent injury versus the primary and transient injury has been drawing extensive attention for study during the past few decades. The sterile neuroinflammation during the secondary phase of injury has been frequently identified substrate underlying CNS injury, but as of now, no conclusive studies have determined whether this is a beneficial or detrimental role in the context of repair. Recent pioneering studies have demonstrated the key roles for the innate and adaptive immune responses in regulating sterile neuroinflammation and CNS repair. Some promising immunotherapeutic strategies have been recently developed for the treatment of CNS injury. This review updates the recent progress on elucidating the roles of the innate and adaptive immune responses in the context of CNS injury, the development and characterization of potential immunotherapeutics, as well as outstanding questions in this field.
Keywords: Brain injury, Spinal cord injury, Innate and adaptive immunity, Inflammatory response, Immunotherapy, Secondary injury
Abbreviations: APC, antigen-presenting cells; ASC, apoptosis-associated speck-like protein containing a carboxy-terminal CARD; BBB, blood-brain barrier; CNS, central nervous system; CSF, cerebral spinal fluid; DAMP, danger-associated molecular patterns; ECM, extracellular matrix; HDAC, histone deacetylase; HMGB1, high-motility group box 1; Hsp, heat shock proteins; IL, interleukin; IVIG, intravenous immunoglobulin G; MHC, major histocompatibility complex; NFκB, nuclear factor κB; NLR, nucleotide-binding domain leucine-rich repeats; PAMP, pathogen-associated molecular patterns; RAGE, receptor for advanced glycation end products; SCI, spinal cord injury; TBI, traumatic brain injury; Th, T helper cells; TLR, Toll-like receptors
Introduction
Traumatic brain injury (TBI) remains a major cause for death and disability worldwide. In the United States alone, TBI contributes to the deaths of nearly 50,000 people each year, with more than 282,000 hospitalizations and 2.5 million emergency department visits in 2013.1, 2 In Europe, TBI caused about 82,000 deaths with 2.1 million hospitalizations in 2012.3 In China, TBI caused 194,850 deaths (12.99/100,000 population).4 These figures may underestimate the scope of the TBI epidemic because mild TBI often goes unreported. Those who survive a TBI can face temporary or permanent disabilities such as impaired cognitive function, movement, sensation (e.g., vision or hearing), or emotional functioning (e.g., personality changes, depression). These issues not only affect patients, but can have lasting effects on families and communities. In addition, TBI has linked to post-traumatic stress disorders, chronic traumatic encephalopathy, and chronic neuroinflammation.
Additionally, traumatic spinal cord injury (SCI) has emerged as a significant economic burden for society, with direct costs ranging from 500,000 to 2 million US dollars accrued over the life-time for one patient. Around the world, approximately 250,000–500,000 people suffer from SCI each year.5 In the United States, around 17,000 new cases of SCI occur each year and 240,000–337,000 people live with SCI.6 About 52% of SCI survivors are paraplegic, while 47% are considered quadriplegic. The average age at injury has increased from 29 years in the 1970's to 42 years in 2016.7
Both TBI and SCI affect individuals of all ages and genders. Both of these disorders cause significant morbidity and mortality, with initial mechanical primary injuries and persistent secondary injuries. The secondary injury contributes largely to the neurological impairment seen in patients. Several mechanisms underlying the secondary injury have been identified: excitotoxicity caused by impaired glutamate homeostasis,8, 9 free radicals/oxidative stress/lipid peroxidation,10, 11 calcium overload,12, 13 autophagy,14 and sterile neuroinflammation.15 Recently, the sequential activation of resident and recruited immune cells has been extensively investigated for their participation in the secondary inflammatory/immune responses after CNS injury. Many treatments for TBI and SCI have been developed, including early neuroprotective therapies such as surgical decompression, methylprednisolone, and blood pressure augmentation, as well as recently developed neuroprotective interventions such as Riluzole, Minocycline, magnesium, therapeutic hypothermia, and cerebral spinal fluid (CSF) drainage.16 Some promising clinical trials are also under way for several drug targets such as Minocycline, Cethrin™, anti-NOGO antibody, cell-based approaches, and bioengineered biomaterials.17 In the past years, immunomodulatory strategies have been showing great potential for success. In this review, we will emphasize the importance of the immune response in the secondary injury and explore the future potential of immunotherapies for the treatment of both TBI and SCI.
The paradigm shifts of CNS immunity
The CNS has been long viewed as an immune-privileged organ due to its tolerance to antigen-induced immune responses. This mutated or slow innate immune response may result mainly from the presence of the blood–brain barrier (BBB), which relative impermeability is maintained by the tight junctions and basal lamina of brain endothelial cells, and the end feet processes of perivascular astrocytes. In addition, the lack of classical lymphatic drainage and antigen-presenting cells (APC) like dendritic cells within the brain parenchyma, as well as the low levels of major histocompatibility complex (MHC) class I and II molecules may contribute to the limited immune responses within the CNS. Therefore, the presence of any immune cells with the CNS parenchyma was traditionally perceived as a hallmark of pathology and the immune response plays a detrimental role after CNS injury. However, this paradigm has been changed over the last two decades.18, 19 Increasing sets of evidence demonstrate that the immune system keeps up a constant state of immunosurveillance, scouting for signals not only from external pathogens, but also from damaged tissue, particularly in the case of sterile injuries such as TBI, SCI, stroke, etc.20 The immune system plays key role in tissue homeostasis under healthy (physiological) conditions in addition to the pathogenic aggravation of sterile inflammatory responses after CNS injury.18
It is known that the resident microglia and astrocytes within the CNS participate the innate immune responses. Under physiological conditions, there are millions of immune cells circulating in the CSF, and populating the meninges and the epithelium of the choroid plexus. These immune cells include T cells, B cells, monocyte/macrophages, dendritic cells, and neutrophils. The meningeal lymphatic vessels within the CNS have been discovered recently, and function by draining meningeal immune cells and macromolecules from the CSF into the deep cervical lymph nodes.21, 22, 23, 24 This is supported by the observation that CNS-derived antigens induce an immune response in the deep cervical lymph nodes.25, 26, 27 Interestingly, this response is skewed towards B-cells for a humoral response, possibly to avoid a more detrimental inflammatory T-cell response. Dendritic cells from CSF have been found to migrate to B-cell follicles of the cervical lymph nodes.28, 29
The paradigm changes for CNS immune-privilege provide a new understanding of CNS immunity (immunosurveillance and tolerance) under healthy/physiological conditions. Additionally, the preexisting immune system within the CNS may account for the predominant role of the readily-available immune response in initiating or mediating the secondary injury after CNS primary injury. Both resident immune cells (microglia, astrocytes) and infiltrating immune cells (T cells, B cells, monocytes, and macrophages) play beneficial and detrimental roles in CNS injury. Such duality in the roles of the innate/adaptive immune responses relies on the timing, types and interventions of the injury.30 For neurodegenerative diseases such as Multiple Sclerosis or Alzheimer's disease, immunotherapies blocking immune/inflammatory responses has shown considerable efficacy.31, 32 However, the innate and adaptive immunity after TBI or SCI may help clear detrimental extracellular debris such as aggregated or misfolded proteins at certain time points after injury.33, 34, 35
Innate immune response after CNS injury
Activation of innate immune cells
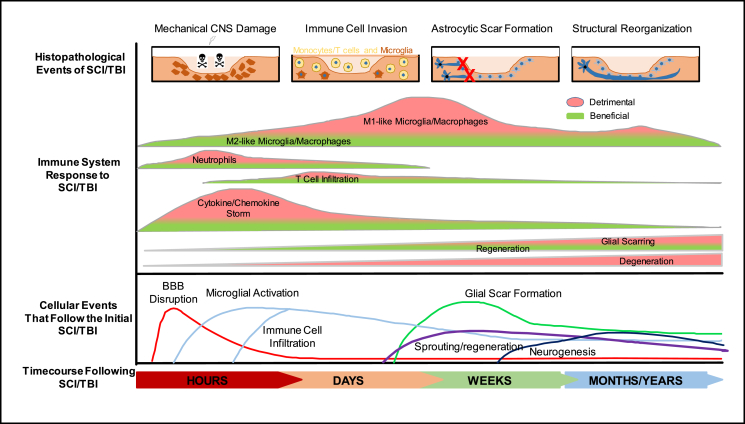
The initial inflammatory response to CNS injury can be a mechanism for the innate immune response, and is characterized by the initial generation of danger-associated molecular patterns (DAMPs), the production of inflammatory cytokines/chemokines by the resident innate immune cells (microglia and astrocytes), and the subsequent recruitment of infiltrating innate immune cells (Fig. 1). These infiltrating innate immune cells include monocytes (which subsequently differentiate into macrophages), mast cells, granulocytes (basophils, eosinophils and neutrophils), dendritic cells and natural killer cells. Within minutes after CNS injury, alarmins such as interleukin (IL)-33, ATP, heat shock proteins (Hsps), and high-motility group box 1 (HMGB1) are promptly released from the damaged meninges, glial limitans, and parenchymal white matter.36, 37 IL-33 is highly expressed in CNS, particularly in oligodendrocytes and astrocytes,38, 39 and plays a crucial role in CNS injury by recruiting microglia/macrophages.39, 40 These immediate alarmins bind to the pathogen-associated molecular patterns (PAMPs) and DAMP sensors such as Toll-like receptors (TLRs) and purinergic receptors in the innate immune cells, inducing the subsequent activation of nuclear factor κB (NFκB) signaling and stimulation of inflammatory gene expression or cytokine release.41, 42, 43 These inflammatory alarmins also induce complement activation and the recruitment of neutrophils, monocytes and T cells to the injury site.15, 39
Fig. 1.
Time course and dual roles of innate and adaptive immune responses after CNS injury. Within hours, primary mechanical damage of CNS may cause disruption of cell membrane, vasculature and BBB, leading to prompt release of alarmins and activation of resident immune cells, followed by secondary inflammatory/immune responses. Over the next days/weeks, continuous infiltration and subtype conversion of immune cells cause beneficial/detrimental effects on neural regeneration, and astrocytic activation induces glial scarring and regenerative failure. Limited neurogenesis and neural regeneration occur over months/years after CNS injury. Adapted from.2, 243
HMGB1 is a nuclear-localized DNA-binding transcription factor ubiquitously expressed. HMGB1 expression is increased after TBI and SCI.36, 44 It is released by damaged cells after CNS injury and also actively secreted by inflammatory macrophages.45 It is a potent inflammatory stimulus via its receptors such as TLR4 and the receptor for advanced glycation end products (RAGE).36 Both TLR4 and RAGE participate in the innate immune/inflammatory responses via NFκB activation.46 HMGB1 promotes the development of macrophages with a neurotoxic phenotype both in vitro and in vivo.36
Monocytes are the major types of the initial infiltrating innate immune cells after CNS injury and contribute to the propagation of the sterile inflammation.47, 48 Monocytes are categorized as CD115+Ly6ChiCD62+CCR2hi classical, CD115+Ly6CloCD62−CCR2lo non-classical and an intermediate subset that expresses a varying spectrum of these markers.49, 50 Classical monocytes infiltrate into the injured sites to generate tissue macrophages.51 Non-classical monocytes, known as patrolling monocytes, survey vascular endothelium such as the BBB and only extravasate under pathological conditions.52 The non-classical/patrolling monocytes express high levels of the CX3CR1 fractalkine receptor that allow their migration to CX3CRL1-expressing cells after TBI.53 The roles of monocytes appear to be significant, as global monocyte depletion in CCR2 knockout mice is associated with significant improvements in brain edema, motor coordination, and working memory.54, 55 Additionally, targeting CCR2+ macrophages with CCX872, a novel Phase I CCR2 selective antagonist, significantly reduced TBI-induced inflammatory response and improved hippocampal-dependent neurocognitive function.56 However, selective depletion of classical monocytes alone had no effect on neutrophil recruitment to the site of injury.47 Selective deletion of patrolling monocytes in CX3CR1 knockout mice significantly reduces neutrophil infiltration after TBI47 and SCI,57 implying the important role of patrolling monocytes in mediating CNS injury. Therefore, targeting of CX3CR1 signaling may represent a selective immunotherapy for CNS injury. However, patrolling monocytes are redundent in the progression and recovery of stroke,58 or exhibit neuroprotective function in sterile excitotoxicity model.59
The infiltrating neutrophils are highly migratory and possess high phagocytic ability to clean the damaged elements within the brain parenchyma after CNS injury.60, 61 Neutrophils also participate in BBB breakdown and subsequent brain edema formation.62, 63 The infiltrating dendritic cells can be activated by the damaged cells and present antigens to T cells, leading to adaptive immune response.64, 65
Mast cells are myeloid cells, mainly participating in the pathogenesis of allergic reactions. In addition to the extensive presence within tissues exposed to the external environment, such as the skin, gut, and respiratory tract, mast cells have been observed in the CNS, particularly the leptomeninges66 and perivascular space.67, 68 Mast cells respond to CNS injury by releasing inflammatory mediators including proteases and vasoactive amines such as GnRH, tryptase, and histamine. These inflammatory mediators induce microglia activation and neuroinflammation. The meningeal mast cells also damage the BBB and recruit neutrophils and activated T cells to the injury site. Therefore, inhibition of mast cells may prove to be a neuroprotective therapy for CNS injury.69, 70
Key role of inflammasomes in the secondary injury of CNS
The inflammasome is a multiprotein intracellular complex serving as an innate immune responder to pathogenic microorganisms and sterile damaging/stress signals. It regulates the activation of caspases, particularly caspase-1, leading to the generation of highly pro-inflammatory cytokines IL-1β and IL-18. Inflammasome also induces an inflammasome-specific form of cell death named pyroptosis.41, 43 PAMPs or DAMPs are recognized by the pattern recognition receptors including TLRs, nucleotide-binding domain leucine-rich repeats (NLRs), C-type lectins and membrane-bound, retinoic acid-inducible gene-I-like receptors. These pattern recognition receptors are mainly expressed in innate immune cells such as microglia, and astrocytes. Activation of TLRs induces the priming of inflammasomes through NFκB signaling while NLRs stimulate the assembly of inflammasome in most cell types.41, 42, 43 Different type of TLRs detects various DAMPs and PAMPs.71 Thirteen TLRs have been identified, although TLR11, TLR12 and TLR13 have yet to be discovered in humans.71 TLR3, TLR7 and TLR9 recognize cellular and microbial nucleic acids. TLR2 and TLR4 detect cellular Hsp60 and Hsp70. TLR5 detects bacterial flagella. NLRs can be activated by endogenous cellular products such as uric acid crystals and aggregated peptides. NLRs also detect cytosolic ion fluxes induced by ATP-stimulated activation of purinergic receptors.
Several inflammasome complexes are identified in CNS, among which, NLRP1 and NLRP3 are the most studied.72, 73, 74 The inflammasome complexes exist in a pre-assembled state prior to their activation, allowing a rapid activation of the innate immune system after CNS injury.75 Each inflammasome complex contains a cytosolic sensor (i.e. NLR), an adaptor protein (i.e. apoptosis-associated speck-like protein containing a carboxy-terminal CARD, ASC) and an effector caspase.76 The exact compositions of each inflammasome relies on environmental niche factors and injury types.77 Activation of these inflammasomes produces the mature/active form of caspase-1 leading to the generation of proinflammatory cytokines IL-1β and IL-18, which contribute to the secondary inflammatory response after CNS injury.78
Dual roles of activated microglia/macrophages and astrocytes after CNS injury
Microglia play a key role in the innate immune response within CNS. Microglia are phenotypically dynamic in both morphology and function, ranging from resting ramified steady state (M0), to the activated non-phagocytic (M1) or a phagocytic (M2) polarized state.79 M1 microglia act like APCs and are stimulated by IFN-γ, IL-6 and TNFα. The M2 microglia state has recently been further categorized into M2a, M2b and M2c states based on different stimulation, antigenic marker and function. The M2a state is stimulated by IL-4 and IL-13, and works to suppress inflammation.80, 81 The M2b state is stimulated by TLRs, while M2c state is stimulated by TGF-β, IL-10, and apoptotic cells.82, 83
Microglia not only survey the CNS, but also exhibit fundamental roles in regulating neurogenesis,84 neuronal polarization and synaptic remodeling/plasticity.85 Microglia within the non-pathological CNS monitor their environmental niche by extending and retracting their motile processes. Microglia determine neuronal differentiation and maturation by releasing various growth factors. Microglia support the formation and maturation of synapses. Under pathological conditions, microglia are very reactive, undergoing rapid activation, proliferation, and structural changes. They are the first line of immune defense against invading pathogens and/or local damaging signals. They can sense various types of inflammatory mediators such as cytokines/chemokines, glycolipids, lipoproteins, peptides, nucleotides, misfolded protein aggregates, and other abnormally processed proteins. They also produce various types of pro- and anti-inflammatory cytokines/chemokines, growth factors, matrix molecules and others. These inflammatory mediators induce phenotypic changes in these microglia and mediate their possible detrimental or beneficial functions after CNS injury.86, 87, 88
Astrocytes not only provide trophic support for neurons, but also play integral roles in many processes such as synaptic pruning, neurotoxin removal, neurogenic stimulation, blood flow regulation, and potassium/sodium buffering. In response to TLR and NLR signals, astrocytes produce the majority of innate immune/inflammatory mediators, including cytokines such as IL-1β, IL-6, chemokines such as CCL2, CXCL1, CXCL10, and CXCL12 and several complement components.89, 90 Astrocytes can be activated by various pathological factors including pro-inflammatory cytokines such as IL-1β. Reactive microglia also activate astrocytes.91, 92 Astrocyte-specific inflammatory signaling plays a key role in the secondary injury after SCI and TBI.93, 94, 95 NFκB signaling has been well known to participate the inflammatory response during the secondary CNS injury. It plays a dual role in the pathogenesis and functional recovery after CNS injury.96 Astroglial NFκB inhibition in a transgenic mouse model significantly improved functional recovery, increased white matter preservation and axonal sparing after SCI97, 98 and ischemic brain injury.99 This protective effect may result from the reduction of various inflammatory cytokines/chemokines such as CXCL10, CCL2, and TGF-β97 and the promotion of oligodendrogenesis.100 These reactive astrocytes also form a glial scar that produces axonal growth inhibitors and prevents axonal regeneration.101 Chondroitin sulfate proteoglycans are the key component of astroglial scarring and are significantly down-regulated by the astroglial NFκB inhibition.97 Therapeutic modulation of the chondroitin sulfate proteoglycans is an attractive approach to improve neuroregeneration and functional recovery after CNS injury.102
Adaptive immune response after CNS injury
The adaptive immune response occurs after CNS injury, most prominently within the deep cervical lymph nodes, the meningeal spaces (including the CSF), and the local injury site (Fig. 1). The T cell responses may be specific to CNS-restricted antigens. Recent studies showed that immune T cells enter CSF and meningeal spaces via meningeal blood vessels.23 The mechanisms underlying the entry of the meningeal T cells into the parenchyma remain largely unknown. It is possible that meningeal T cells infiltrate the parenchyma through some chemokines.103 Several evidences showed that T cells enter the meninges and the CSF through leptomeningeal and dura blood vessels or through the choroid plexus.104, 105
Upon CNS injury, T cells undergo extravasation through a chemokine gradient and adhesion molecule upregulation on the luminal surface of the vascular endothelium.103 The binding of very late antigen 4 (VLA4, also named Integrin α4β1) to vascular cell adhesion molecule 1 (VCAM1) is important for T cell extravasation and homing into the CNS.106, 107 Inhibition of this interaction by a neutralizing antibody against VLA4 attenuates T cell extravasation.107, 108, 109
T cells recognize antigens through their surface-bound T cell receptor, and are generally classified into CD8+ cytotoxic T cells and CD4+ helper T cells (Th). CD8+ T cells detect antigens presented by MHC class I (MHC I) molecules while CD4+ T cells recognize MHC II antigens primarily presented by APCs like dendritic cells, macrophages and B cells.110 Upon activation by their specific peptides, CD4+ T cells proliferate and, when exposed to certain secondary stimuli, differentiate to combat the specific threat.111 Upon various stimulation, Th cells differentiate into several subtypes including Th1, Th2, Th9, Th17, T regulatory and T follicular helper cells. The subtypes are characterized largely by the lineage-specific cytokines.112 For example, Th1 cells generate IL-2, and INFγ, Th2 cells produce IL-4, IL-5 and IL13, Th9 cells generate IL-9, IL-10 and IL-21, while Th17 cells produce IL-17 and IL-23. T follicular helper cells express CD40L and secrete IL-21 and IL-4.113 Under physiological conditions, the lymphocytes (mainly T cells) patrol the border surrounding the CNS, such as meninges, CSF and choroid plexus, and regulate neurobehavioral function.114 For example, genetic depletion of meningeal T cells or pharmacological trapping of T cells in the deep cervical lymph nodes impairs neurocognitive function in animal models.115, 116 However, the molecular mechanism underlying the patrolling role of immune cells remains largely unknown.
The adaptive immunity response also involves B cells, which express unique antigen-specific receptors via genome rearrangement and produce antibodies. Both TBI and SCI stimulated B cells to generate pathogenic antibodies, which subsequently contribute to the secondary tissue damage and neurological dysfunction after SCI.117, 118
Immunotherapy at the innate immune response after CNS injury
The innate immune response plays a major role in the sterile inflammation and neuroregenerative failure after CNS injury. The general immunosuppression regimens using broad steroids such as methylprednisolone have been extensively employed to treat patients with SCI or TBI for decades. Unfortunately, these immunosuppression regimens remain unsuccessful because they suppress both the pro- and anti-inflammatory activities of the innate immune response.119, 120 Recently, selective immunomodulatory approaches have been developed to ameliorate the pro-inflammatory M1-like response and promote the anti-inflammatory M2-like positive tissue remodeling. Macrophages/microglia play a key role in the innate immune response. Thus, immunomodulatory therapy targeting macrophages/microglia is becoming promising in treatment of CNS injury.
Minocycline targeting microglia after TBI and SCI
Minocycline is a clinically available tetracycline-class antibiotic that exhibits extensive neuroprotective effects at multiple stages after CNS injury.121 Most mechanisms involve the capability of suppressing innate immune responses after CNS injury, including inhibiting microglia activation,122, 123, 124 attenuating HMGB1 translocation,122 suppressing caspase-1125 and downregulating the release of pro-inflammatory mediators such as IL-1β, TNFα, and Cox2 after CNS injury.121 Several preclinical animal studies presented highly promising therapeutic benefits of minocycline in promoting neuroregeneration and improving neurobehavioral outcomes after SCI121, 126, 127 and TBI.123, 128 Additionally, Phase II Clinical trials showed positive results in improving motor score after SCI.129 As a result, a Phase III clinical trial entitled ‘Minocycline in Acute Spinal Cord Injury’ has been started.130
Extracellular matrix (ECM) and exosomes at innate immune cells after CNS injury
The xenogeneic ECM bioscaffolds have been extensively employed to decrease scar formation and promote tissue repair.131 The autologous microglia/macrophages can be pre-polarized with ECM or other immunomodulatory factors ex vivo to acquire an M2-like phenotype before transplantation back into the patients.132, 133, 134 Additionally, cell therapies using autologous macrophages have been shown to improve TBI and SCI.135 Due to potential cell-related side effects or injection difficulties for macrophage or stem cell injection, exosomes have been developed to replace the cell therapy because of their multifaceted benefits: low immunogenicity, high efficacy, efficient BBB permeability, active cellular communications, and microglial phagocytosis stimulation.136, 137, 138 The exosomes deliver various types of RNA, proteins, cytokines, lipids and other signaling molecules. After TBI or SCI, the CSF harbors increased number of exosomes that contain cytoskeletal proteins, neurite-outgrowth related proteins, and synaptic proteins, ECM proteins, and complement protein C1q subcomponent subunit B.139, 140, 141 By altering the encapsulated components via ECM-polarization or genetic engineering, exosomes can be selectively produced to modulate the beneficial immune response after CNS injury.137, 142, 143 For example, exosomes loaded with ASC siRNA can cross the injured BBB in vivo and reduce the ASC protein levels in the spinal cord after injury, leading to a significant decrease in caspase-1 activation and IL-1β production.144 NLRP1 inflammasome proteins exist in exosomes derived from CSF of SCI patients144 and TBI patients.145 These inflammasome-containing exosomes may fuse with peripheral immune cells to activate inflammatory and immune response.72, 73 Therefore, immunotherapy targeting inflammasome-containing exosomes might be a promising strategy for the treatment of CNS injury.146
Lipid-lowering drugs for immunomodulation
The lipid-lowering drugs such as statins have extensive immunomodulatory and anti-inflammatory properties. Among statins, atorvastatin exhibits neuroprotective effect in many preclinical studies on both TBI147, 148, 149, 150, 151, 152, 153, 154 and SCI.155, 156, 157, 158 Although many mechanisms may contribute to atorvastatin's neuroprotective effects, the microglia/macrophage polarization may play a more predominant role.159 Acute atorvastatin administration in mice with TBI effectively reduced inflammatory responses by suppressing microglia/macrophage activation and immune cell invasion.159 Several clinical trials with atorvastatin showed very promising therapeutic effects for TBI147 and SCI,160 but some clinical studies did not identify any improvement in functional recovery.148, 160 The discrepancies may result from different modeling, treatment patterns and sample sizes. Further clinical trials with larger cohort sizes and longer multi-center evaluation periods are needed.
Inflammasomes as therapeutic targets for CNS injury
The priming and activation of inflammasomes are the major components of the innate immune response and sterile inflammation after CNS injury.41, 77, 144 Thus, immunotherapies targeting the inflammasome response might be a promising anti-inflammatory approach for CNS injury (TBI and SCI). Although the complexities and mechanisms underlying the inflammasome response after CNS injury remain under extensive investigation,77, 144 several proof-of-concept studies hold promising in contacting the inflammasome assembly and activation.161
Early studies showed that treatment with anti-ASC neutralizing antibody immediately after fluid-percussion brain injury in rats significantly improves the histopathology and functional recovery.162 Such treatment reduced caspase-1 activation, IL-1β production and XIAP cleavage.162 The CSF from TBI patients can activate neuronal AIM2 inflammasome and ASC oligomerization.163, 164 Blocking pyroptosis using caspase-1 inhibitors (Ac-YVAD-cmk, VX-765) or pannexin-1 inhibitors (Probenecid and Brilliant Blue FCF) prevents inflammasome-mediated inflammation and improves CNS injury.161, 163 Omega-3 fatty acids attenuate neuroinflammation and improve neurological outcome via inhibiting the NLRP3 inflammasome activation.165 Propofol, a lipid-soluble intravenous anesthetic, has been shown to protect against TBI via inhibiting ROS-dependent activation of the NLRP3 inflammasome.166 The angiotensin II receptor antagonist Telmisartan reduces traumatic cerebral edema by inhibiting the NLRP3 inflammasome-mediated accumulation of IL-1β and IL-18.167 Melatonin treatment attenuates the early brain injury after subarachnoid hemorrhage by inhibiting NLRP3 inflammasome-associated pyroptosis.168 Treatment with estrogen or stromal cell-derived factor-1 alpha (SDF-1α) after SCI exhibits neuroprotective function via inhibiting local inflammasome activation.169, 170 Resveratrol attenuates the inflammatory response and ameliorates TBI by reducing ROS production and inhibiting NLRP3 activation via SIRT1.171 The treatment with hyperbaric oxygen (HBO) alleviates the inflammatory response in experimental TBI via modulating microglial NLRP3 inflammasome signaling and reducing IL-1β/IL-18 accumulation.172, 173 NLRP3 inhibitors such as BAY 11-7082 (via NFκB) or A438079 (via P2X7) have been shown to inhibit the inflammatory response and improve functional recovery after TBI.75, 174 Mangiferin has been extensively used as an anti-inflammatory drug and its neuroprotective effect after CNS injury is also attributed to NLRP3 inhibition.175 Several other treatments for neuroprotective effects against SCI have been shown to target inflammasomes, such as heme oxygenase-1146, Rho kinase inhibitor fasudil176, the citus flavonoid glycoside rutin177 and quercetin178, the natural triterpenoid compound asiatic acid179 and the dopamine receptor agonist A-68930.180
However, some studies using Nlrp1 (−/−) and Asc(−/−) mice demonstrated a non-essential role of the NLRP1 inflammasome after TBI.181 This may result from the developmental compensation of this specific inflammasome knockout. Since IL-1β is the major end-point product of the inflammasome activation, the neuroprotective effect of the therapeutic treatment targeting IL-1β with blockers such as IL-1ra and IL-1β neutralizing antibodies after TBI182, 183 and SCI184 also support the conclusion that inflammasome therapy holds highly promising for the treatment of CNS injury. Given the majority of preclinical studies targeting inflammasomes showed positive therapeutic benefits against TBI and SCI, clinical trials may be immediately needed for CNS injury.185
HDAC inhibitor for SCI and TBI
The histone deacetylases (HDAC) remove acetyl groups on a histone, allowing for tighter DNA wrapping around the histones, thus suppressing gene transcription. HDAC inhibitors have been widely developed and utilized to activate gene transcription. After TBI, treatments with various HDAC inhibitors such as Valproate, sodium butyrate, ITF2357, trichostatin-A, Scriptaid, and 4-dimethylamino-N-[5-(2-mercaptoacetylamino) pentyl]benzamide (DMA-PB) significantly mitigate neuroinflammation, improve motor functional recovery and promote learning/memory in several animal models.186, 187, 188, 189, 190 These HDAC inhibitors preferentially upregulate the transcriptional expression of many neuroprotective genes involved in cell survival, proliferation, and differentiation.191 Both grey matter and white matter tracts are significantly preserved by HDAC inhibition after TBI.186, 192 Similarly, several HDAC inhibitors such as Valproate and RGFP966 have been shown to have neuroprotective effect against SCI by suppressing inflammatory response, promoting neurogenesis and stimulating axonal regeneration.193, 194, 195, 196 The expression of HDAC3 is upregulated in the innate immune cells (microglia/macrophages) at the injury site after SCI.196 For both TBI and SCI, the inhibition of microglia/macrophage activation is the major mechanism underlying the neuroprotective benefits from HDAC inhibition therapy.186, 194, 196 These exciting preclinical findings provide a promising future therapy using HDAC inhibitors targeting microglia for the treatment of CNS injury.193
Nanoparticles or drugs targeting monocytes
Monocyte-derived macrophages contribute primarily to the initial inflammatory damage after CNS injury.197 Selective blockage of monocyte infiltration during early stage of CNS injury may prevent detrimental effects of the early innate immune response while preserving the beneficial effects of the residential microglia/macrophages.198 The highly negatively charged, synthetic, 500 nm-diameter immune-modifying nanoparticles have been used to sequester monocytes in the spleen where they undergo caspase-3-mediated apoptosis.199 Thus, intravenous administration of the biodegradable nanoparticles after SCI safely and selectively limits monocyte infiltration into the injury site and significantly improves functional recovery after both moderate and severe SCI.198 These immune-modifying nanoparticles may offer a promising treatment for CNS injury because of the multifaceted advantages and continuous improvement of the nanomedicine.200
Laquinimod is an immunomodulatory oral drug in the clinical practice for the treatment of multiple sclerosis201, 202 and other autoimmune diseases.203 Its immunomodulation results mainly from the inhibition of the monocytes infiltration into CNS in neurodegenerative diseases.204 A recent study shows that laquinimod treatment reduces lesion volume and axonal damage and restored neurogenesis after TBI.205 Laquinimod might be a potential immunotherapy for CNS injury.
A new bioengineered protein comprised of the human leukocyte antigen (HLA)-DRα1 domain linked covalently to mouse MOG-35-55 peptide (DRα1-MOG-35-55) has been shown to modulate monocyte response206, 207 and improve histological and clinical outcomes after TBI.208
Immunotherapy at the adaptive immune response after CNS injury
Emerging neuroprotective therapeutics targeting B cells
Intravenous immunoglobulin G (IVIG)
IVIG contains polyclonal IgG and has been extensively used as a first-line therapy with a good pharmacological safety profile in the clinical patients with immunodeficiency and autoimmune diseases such as Guillain-Barre syndrome, chronic inflammatory demyelinating polyneuropathy and Kawasaki disease.209, 210 IVIG has been also examined for neuroprotective effects in ischemia-type insults.210, 211, 212 In animal studies, IVIG administration acutely after SCI213 or TBI214 significantly improves neural functional recovery. IVIG not only suppresses the excessive immune responses via inhibiting inflammatory cytokine production, immune cell invasion/activation and complement activation in the CNS, but also reverses the concomitant immunosuppression against invading pathogens after CNS injury.17, 213, 215, 216, 217 Given the long-term clinical use of IVIG for the treatment of autoimmune and immunodeficiency conditions and the promising efficacy for CNS injury in animal studies,211, 213, 214 IVIG remains a potential candidate for clinical trials in SCI and TBI.218, 219
Monoclonal antibodies after CNS injury
Robust B-cell response occurs after SCI by generating pathogenic antibodies.117, 118 B cell deficiency in RAG knockout mice improves functional recovery after SCI.220 Antibody-mediated depletion of B cells through the glycoengineered anti-muCD20 antibody (18B12) in a mouse model significantly inhibits the NFκB-dependent production of pro-inflammatory mediators and improves functional recovery after SCI.221 The therapeutic CD20 antibodies such as rituximab or obinutuzumab may provide a new immunotherapy for the treatment of CNS injury.
Targeting T cells (Peripheral and CNS)
Targeting T cell trafficking and infiltration is neuroprotective in CNS injury.222 The chemokine CXCL10 is a potent recruiter for T cells and has been implicated in the pathogenesis of CNS injury.223, 224, 225 CXCL10 antagonist226 or neutralizing antibodies227, 228 have been shown to attenuate T cell infiltration, suppresses neuronal death, increase axonal regeneration and improve functional recovery after SCI.
Fingolimod is an orally-effective immunosuppressant targeting sphingosine 1-phosphate receptor S1P1, and clinically used for the treatment of relapsing-remitting multiple sclerosis.229, 230 It induces S1P1 internalization and sequesters lymphocytes in the lymph nodes, reducing the circulating population of lymphocytes and their trafficking into tissues.231, 232 In addition to multiple sclerosis and other autoimmune diseases, fingolimod therapeutic benefits have been reported in many other neurodegenerative diseases and CNS injury. For SCI, systemic treatment with fingolimod blocks neuroinflammation and improves motor function and bladder function.233, 234 Local administration of fingolimod reduces reactive gliosis, prevents neuronal death and improves motor functional recovery after SCI.235 A 3-day consecutive fingolimod treatment starting at 1 h after TBI significantly reduces as many as 20 kinds of cytokines/chemokines and the infiltrated T and NK cells, but increases the percentage of regulatory T cells, and the concentration of anti-inflammatory IL-10.236 Fingolimod attenuates the general microglia activation, BBB damage and axonal injury, and improves neurological functions after TBI. Given the extensive clinical application and widely reported therapeutic efficacy, fingolimod or other immunosuppressor targeting T cell trafficking or extravasation may be a promising therapy for the treatment of CNS injury.237
However, the adaptive immune responses may also serve as a protective autoimmunity to help neurorepair after CNS injury.238, 239 In the protective autoimmunity, the adaptive immune cells (particularly T cells) recognize self-constituents and potentiate an autoreactive response.240, 241 Immunization with a synthetic peptide A91 derived from the myelin basic protein shows strong neuroprotective efficacy after SCI in preclinical studies.240, 242 However, the safety, dosage and schedule of this peptide need to be addressed before translating into clinical therapy.
Challenging questions and future directions
Both the innate and adaptive immune responses play key roles in the secondary injury progression of both TBI and SCI. Several immunomodulatory strategies are likely to see translation to patients within the next few decades. However, several challenging questions could be addressed or investigated for future studies. The patrolling immune cells, particularly T cells and monocytes at the border between CNS and immune system play important roles in both physiological and pathological conditions (Fig. 1). As of now, many unknown functions remain to be determined. For example, what factors or molecular mechanisms determine the specificity and diversity of meningeal immune cells? Additionally, how do these meningeal immune cells invade the CNS parenchyma during pathological states? How does the crosstalk between the resident and invading immune cells contribute to the initial inflammatory damages and late neurorepair process? Various subtypes of innate and adaptive immune cells in the CNS are identified. Their characteristics and functions and the mechanisms underlying the subtype mutual conversion remain to be better delineated. Most importantly, what is the timing/window and transit process for the beneficial or detrimental role of innate and adaptive immune responses after CNS injury? Novel immunotherapeutics are needed to guide the maladaptive immune responses to the favorable wound-healing responses after CNS injury. The peripheral immune system exhibits immunosuppression after CNS injury, but whether T cell exhaustion occurs within CNS and affects neurodegeneration or neurorepair during the chronic injury remains largely unknown.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Taylor C.A., Bell J.M., Breiding M.J. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon D.W., McGeachy M.J., Bayır H. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majdan M., Plancikova D., Brazinova A. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health. 2016;1:e76–e83. doi: 10.1016/S2468-2667(16)30017-2. [DOI] [PubMed] [Google Scholar]
- 4.Cheng P., Yin P., Ning P. Trends in traumatic brain injury mortality in China, 2006-2013: a population-based longitudinal study. PLoS Med. 2017;14:e1002332. doi: 10.1371/journal.pmed.1002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh A., Tetreault L., Kalsi-Ryan S. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinal cord injury (SCI) 2016 facts and figures at a glance. J Spinal Cord Med. 2016;39:493–494. doi: 10.1080/10790268.2016.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frontera J.E., Mollett P. Aging with spinal cord injury: an update. Phys Med Rehabil Clin N Am. 2017;28:821–828. doi: 10.1016/j.pmr.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy K., Laskowitz D.T. Cellular and molecular mechanisms of secondary neuronal injury following traumatic brain injury. In: Laskowitz D., Grant G., editors. Translational Research in Traumatic Brain Injury. CRC Press/Taylor and Francis Group; Boca Raton (FL): 2016. [Chapter 5] [PubMed] [Google Scholar]
- 9.Dorsett C.R., McGuire J.L., Niedzielko T.L. Traumatic brain injury induces alterations in cortical glutamate uptake without a reduction in glutamate Transporter-1 protein expression. J Neurotrauma. 2017;34:220–234. doi: 10.1089/neu.2015.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill R.L., Singh I.N., Wang J.A. Time courses of post-injury mitochondrial oxidative damage and respiratory dysfunction and neuronal cytoskeletal degradation in a rat model of focal traumatic brain injury. Neurochem Int. 2017;111:45–56. doi: 10.1016/j.neuint.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterfield D.A., Reed T.T. Lipid peroxidation and tyrosine nitration in traumatic brain injury: insights into secondary injury from redox proteomics. Proteom Clin Appl. 2016;10:1191–1204. doi: 10.1002/prca.201600003. [DOI] [PubMed] [Google Scholar]
- 12.Tymianski M., Charlton M.P., Carlen P.L. Secondary Ca2+ overload indicates early neuronal injury which precedes staining with viability indicators. Brain Res. 1993;607:319–323. doi: 10.1016/0006-8993(93)91523-u. [DOI] [PubMed] [Google Scholar]
- 13.Murugan M., Santhakumar V., Kannurpatti S.S. Facilitating mitochondrial calcium uptake improves activation-induced cerebral blood flow and behavior after mTBI. Front Syst Neurosci. 2016;10:19. doi: 10.3389/fnsys.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipinski M.M., Wu J., Faden A.I. Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal. 2015;23:565–577. doi: 10.1089/ars.2015.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banjara M., Ghosh C. Sterile neuroinflammation and strategies for therapeutic intervention. Int J inflam. 2017;2017:8385961. doi: 10.1155/2017/8385961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahuja C.S., Nori S., Tetreault L. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 17.Ulndreaj A., Badner A., Fehlings M.G. Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000Res. 2017;6:1907. doi: 10.12688/f1000research.11633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negi N., Das B.K. CNS: not an immunoprivilaged site anymore but a virtual secondary lymphoid organ. Int Rev Immunol. 2017;37:57–68. doi: 10.1080/08830185.2017.1357719. [DOI] [PubMed] [Google Scholar]
- 19.Engelhardt B., Vajkoczy P., Weller R.O. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 20.Louveau A., Harris T.H., Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Q., Ineichen B.V., Detmar M. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. 2017;8:1434. doi: 10.1038/s41467-017-01484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Absinta M., Ha S.K., Nair G. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6:e29738. doi: 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louveau A., Smirnov I., Keyes T.J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aspelund A., Antila S., Proulx S.T. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laman J.D., Weller R.O. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol. 2013;8:840–856. doi: 10.1007/s11481-013-9470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochmeister S., Zeitelhofer M., Bauer J. After injection into the striatum, in vitro-differentiated microglia- and bone marrow-derived dendritic cells can leave the central nervous system via the blood stream. Am J Pathol. 2008;173:1669–1681. doi: 10.2353/ajpath.2008.080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiefenhövel F., Immig K., Prodinger C. Indications for cellular migration from the central nervous system to its draining lymph nodes in CD11c-GFP+ bone-marrow chimeras following EAE. Exp Brain Res. 2017;235:2151–2166. doi: 10.1007/s00221-017-4956-x. [DOI] [PubMed] [Google Scholar]
- 28.Hatterer E., Davoust N., Didier-Bazes M. How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood. 2006;107:806–812. doi: 10.1182/blood-2005-01-0154. [DOI] [PubMed] [Google Scholar]
- 29.Louveau A., Plog B.A., Antila S. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127:3210–3219. doi: 10.1172/JCI90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saghazadeh A., Rezaei N. The role of timing in the treatment of spinal cord injury. Biomed Pharmacother. 2017;92:128–139. doi: 10.1016/j.biopha.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 31.Khalid S.I., Ampie L., Kelly R. Immune modulation in the treatment of amyotrophic lateral sclerosis: a review of clinical trials. Front Neurol. 2017;8:486. doi: 10.3389/fneur.2017.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer B., Masliah E. Immunotherapy for Alzheimer's disease: past, present and future. Front Aging Neurosci. 2014;6:114. doi: 10.3389/fnagi.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y.T., Lu X.M., Chen K.T. Immunotherapy strategies for spinal cord injury. Curr Pharm Biotechnol. 2015;16:492–505. doi: 10.2174/138920101606150407112646. [DOI] [PubMed] [Google Scholar]
- 34.Nizamutdinov D., Shapiro L.A. Overview of traumatic brain injury: an immunological context. Brain Sci. 2017;7:E11. doi: 10.3390/brainsci7010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jassam Y.N., Izzy S., Whalen M. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–1265. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kigerl K.A., Lai W., Wallace L.M. High mobility group box-1 (HMGB1) is increased in injured mouse spinal cord and can elicit neurotoxic inflammation. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.11.018. S0889–S1591(17)30519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun M., Vaibhav K., Saad N.M. White matter damage after traumatic brain injury: a role for damage associated molecular patterns. Biochim Biophys Acta. 2017;1863(10 Pt B):2614–2626. doi: 10.1016/j.bbadis.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadani S.P., Walsh J.T., Smirnov I. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wicher G., Wallenquist U., Lei Y. Interleukin-33 promotes recruitment of microglia/macrophages in response to traumatic brain injury. J Neurotrauma. 2017;34:3173–3182. doi: 10.1089/neu.2016.4900. [DOI] [PubMed] [Google Scholar]
- 40.Du L.X., Wang Y.Q., Hua G.Q. IL-33/ST2 pathway as a rational therapeutic target for CNS diseases. Neuroscience. 2018;369:222–230. doi: 10.1016/j.neuroscience.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 41.Fann D.Y., Lim Y.A., Cheng Y.L. Evidence that NF-kappaB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke. Mol Neurobiol. 2018;55:1082–1096. doi: 10.1007/s12035-017-0394-9. [DOI] [PubMed] [Google Scholar]
- 42.Su W.J., Zhang Y., Chen Y. NLRP3 gene knockout blocks NF-kappaB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav Brain Res. 2017;322(Pt A):1–8. doi: 10.1016/j.bbr.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Kong X., Yuan Z., Cheng J. The function of NOD-like receptors in central nervous system diseases. J Neurosci Res. 2017;95:1565–1573. doi: 10.1002/jnr.24004. [DOI] [PubMed] [Google Scholar]
- 44.Chen K.B., Uchida K., Nakajima H. High-mobility group box-1 and its receptors contribute to proinflammatory response in the acute phase of spinal cord injury in rats. Spine (Phila Pa 1976) 2011;36:2122–2129. doi: 10.1097/BRS.0b013e318203941c. [DOI] [PubMed] [Google Scholar]
- 45.Rao Z., Zhang N., Xu N. 1,25-Dihydroxyvitamin D inhibits LPS-induced high-mobility group box 1 (HMGB1) secretion via targeting the NF-E2-related factor 2-Hemeoxygenase-1-HMGB1 pathway in macrophages. Front Immunol. 2017;8:1308. doi: 10.3389/fimmu.2017.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X., Wu S., Chen C. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-kappaB pathway following experimental traumatic brain injury. J Neuroinflammation. 2017;14:143. doi: 10.1186/s12974-017-0917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makinde H.M., Cuda C.M., Just T.B. Nonclassical monocytes mediate secondary injury, neurocognitive outcome, and neutrophil infiltration after traumatic brain injury. J Immunol. 2017;199:3583–3591. doi: 10.4049/jimmunol.1700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merkel S.F., Andrews A.M., Lutton E.M. Dexamethasone attenuates the Enhanced rewarding effects of cocaine following experimental traumatic brain injury. Cell Transplant. 2017;26:1178–1192. doi: 10.1177/0963689717714341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziegler-Heitbrock L. Blood monocytes and their subsets: established features and open questions. Front Immunol. 2015;6:423. doi: 10.3389/fimmu.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greter M., Lelios I., Croxford A.L. Microglia versus myeloid cell nomenclature during brain inflammation. Front Immunol. 2015;6:249. doi: 10.3389/fimmu.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Bonilla L., Faraco G., Moore J. Spatio-temporal profile, phenotypic diversity, and fate of recruited monocytes into the post-ischemic brain. J Neuroinflammation. 2016;13:285. doi: 10.1186/s12974-016-0750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auffray C., Fogg D., Garfa M. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 53.Yona S., Kim K.W., Wolf Y. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gyoneva S., Kim D., Katsumoto A. Ccr2 deletion dissociates cavity size and tau pathology after mild traumatic brain injury. J Neuroinflammation. 2015;12:228. doi: 10.1186/s12974-015-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsieh C.L., Niemi E.C., Wang S.H. CCR2 deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J Neurotrauma. 2014;31:1677–1688. doi: 10.1089/neu.2013.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morganti J.M., Jopson T.D., Liu S. CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J Neurosci. 2015;35:748–760. doi: 10.1523/JNEUROSCI.2405-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donnelly D.J., Longbrake E.E., Shawler T.M. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J Neurosci. 2011;31:9910–9922. doi: 10.1523/JNEUROSCI.2114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michaud J.P., Pimentel-Coelho P.M., Tremblay Y. The impact of Ly6Clow monocytes after cerebral hypoxia-ischemia in adult mice. J Cereb Blood Flow Metab. 2014;34:e1–e9. doi: 10.1038/jcbfm.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellavance M.A., Gosselin D., Yong V.W. Patrolling monocytes play a critical role in CX3CR1-mediated neuroprotection during excitotoxicity. Brain Struct Funct. 2015;220:1759–1776. doi: 10.1007/s00429-014-0759-z. [DOI] [PubMed] [Google Scholar]
- 60.Lee J., Costantini T.W., D'Mello R. Altering leukocyte recruitment following traumatic brain injury with ghrelin therapy. J Trauma Acute Care Surg. 2014;77:709–715. doi: 10.1097/TA.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 61.Yokota K., Saito T., Kobayakawa K. The feasibility of in vivo imaging of infiltrating blood cells for predicting the functional prognosis after spinal cord injury. Sci Rep. 2016;6:25673. doi: 10.1038/srep25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aubé B., Lévesque S.A., Paré A. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol. 2014;193:2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- 63.Semple B.D., Trivedi A., Gimlin K. Neutrophil elastase mediates acute pathogenesis and is a determinant of long-term behavioral recovery after traumatic injury to the immature brain. Neurobiol Dis. 2015;74:263–280. doi: 10.1016/j.nbd.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gucluler G., Adiguzel E., Gungor B. Impaired toll like receptor-7 and 9 induced immune activation in chronic spinal cord injured patients contributes to immune dysfunction. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171003. e0171003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin X., Yamashita T. Microglia in central nervous system repair after injury. J Biochem. 2016;159:491–496. doi: 10.1093/jb/mvw009. [DOI] [PubMed] [Google Scholar]
- 66.Dropp J.J. Mast cells in the human brain. Acta Anat (Basel) 1979;105:505–513. doi: 10.1159/000145157. [DOI] [PubMed] [Google Scholar]
- 67.Moretti R., Chhor V., Bettati D. Contribution of mast cells to injury mechanisms in a mouse model of pediatric traumatic brain injury. J Neurosci Res. 2016;94:1546–1560. doi: 10.1002/jnr.23911. [DOI] [PubMed] [Google Scholar]
- 68.Khalil M., Ronda J., Weintraub M. Brain mast cell relationship to neurovasculature during development. Brain Res. 2007;1171:18–29. doi: 10.1016/j.brainres.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X., Dong H., Li N. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J Neuroinflammation. 2016;13:127. doi: 10.1186/s12974-016-0592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong H., Zhang X., Wang Y. Suppression of brain mast cells Degranulation inhibits microglial activation and central nervous system inflammation. Mol Neurobiol. 2017;54:997–1007. doi: 10.1007/s12035-016-9720-x. [DOI] [PubMed] [Google Scholar]
- 71.Nyúl-Tóth A., Kozma M., Nagyöszi P. Expression of pattern recognition receptors and activation of the non-canonical inflammasome pathway in brain pericytes. Brain Behav Immun. 2017;64:220–231. doi: 10.1016/j.bbi.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 72.Mortezaee K., Khanlarkhani N., Beyer C. Inflammasome: its role in traumatic brain and spinal cord injury. J Cell Physiol. 2017 doi: 10.1002/jcp.26287. [DOI] [PubMed] [Google Scholar]
- 73.Wallisch J.S., Simon D.W., Bayir H. Cerebrospinal fluid NLRP3 is increased after severe traumatic brain injury in infants and children. Neurocrit Care. 2017;27:44–50. doi: 10.1007/s12028-017-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mamik M.K., Power C. Inflammasomes in neurological diseases: emerging pathogenic and therapeutic concepts. Brain. 2017;140:2273–2285. doi: 10.1093/brain/awx133. [DOI] [PubMed] [Google Scholar]
- 75.Jiang W., Li M., He F. Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J Neuroinflammation. 2017;14:207. doi: 10.1186/s12974-017-0980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fleshner M., Frank M., Maier S.F. Danger signals and inflammasomes: stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology. 2017;42:36–45. doi: 10.1038/npp.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Rivero Vaccari J.P., Dietrich W.D., Keane R.W. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab. 2014;34:369–375. doi: 10.1038/jcbfm.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anwar M.A., Al Shehabi T.S., Eid A.H. Inflammogenesis of secondary spinal cord injury. Front Cell neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gehrmann J. Microglia: a sensor to threats in the nervous system? Res Virol. 1996;147:79–88. doi: 10.1016/0923-2516(96)80220-2. [DOI] [PubMed] [Google Scholar]
- 80.Siddiqui T.A., Lively S., Schlichter L.C. Complex molecular and functional outcomes of single versus sequential cytokine stimulation of rat microglia. J Neuroinflammation. 2016;13:66. doi: 10.1186/s12974-016-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cherry J.D., Olschowka J.A., O'Banion M.K. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gensel J.C., Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 83.Walker D.G., Lue L.F. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther. 2015;7:56. doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Miranda A.S., Zhang C.J., Katsumoto A. Hippocampal adult neurogenesis: does the immune system matter? J Neurol Sci. 2017;372:482–495. doi: 10.1016/j.jns.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 85.York E.M., Bernier L.P., MacVicar B.A. Microglial modulation of neuronal activity in the healthy brain. Dev Neurobiol. 2017 doi: 10.1002/dneu.22571. [DOI] [PubMed] [Google Scholar]
- 86.Hu X., Leak R.K., Shi Y. Microglial and macrophage polarization─new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawabori M., Yenari M.A. The role of the microglia in acute CNS injury. Metab Brain Dis. 2015;30:381–392. doi: 10.1007/s11011-014-9531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loane D.J., Kumar A. Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp Neurol. 2016;275(Pt 3):316–327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sochocka M., Diniz B.S., Leszek J. Inflammatory response in the CNS: friend or foe? Mol Neurobiol. 2017;54:8071–8089. doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marinelli C., Di Liddo R., Facci L. Ligand engagement of Toll-like receptors regulates their expression in cortical microglia and astrocytes. J Neuroinflammation. 2015;12:244. doi: 10.1186/s12974-015-0458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan H., Zhang K., Shan L. Reactive astrocytes undergo M1 microglia/macrohpages-induced necroptosis in spinal cord injury. Mol Neurodegener. 2016;11:14. doi: 10.1186/s13024-016-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haan N., Zhu B., Wang J. Crosstalk between macrophages and astrocytes affects proliferation, reactive phenotype and inflammatory response, suggesting a role during reactive gliosis following spinal cord injury. J Neuroinflammation. 2015;12:109. doi: 10.1186/s12974-015-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmed A., Patil A.A., Agrawal D.K. Immunobiology of spinal cord injuries and potential therapeutic approaches. Mol Cell Biochem. 2017 doi: 10.1007/s11010-017-3184-9. [DOI] [PubMed] [Google Scholar]
- 94.Jiang H., Wang Y., Liang X. Toll-like receptor 4 knockdown attenuates brain damage and neuroinflammation after traumatic brain injury via inhibiting neuronal autophagy and astrocyte activation. Cell Mol Neurobiol. 2017 doi: 10.1007/s10571-017-0570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robinson C., Apgar C., Shapiro L.A. Astrocyte hypertrophy contributes to aberrant neurogenesis after traumatic brain injury. Neural Plast. 2016;2016:1347987. doi: 10.1155/2016/1347987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kopitar-Jerala N. Innate immune response in brain, NF-kappa B signaling and cystatins. Front mol neurosci. 2015;8:73. doi: 10.3389/fnmol.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brambilla R., Bracchi-Ricard V., Hu W.H. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brambilla R., Hurtado A., Persaud T. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem. 2009;110:765–778. doi: 10.1111/j.1471-4159.2009.06190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dvoriantchikova G., Barakat D., Brambilla R. Inactivation of astroglial NF-kappa B promotes survival of retinal neurons following ischemic injury. Eur J Neurosci. 2009;30:175–185. doi: 10.1111/j.1460-9568.2009.06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bracchi-Ricard V., Lambertsen K.L., Ricard J. Inhibition of astroglial NF-kappaB enhances oligodendrogenesis following spinal cord injury. J Neuroinflammation. 2013;10:92. doi: 10.1186/1742-2094-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okada S., Hara M., Kobayakawa K. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res. 2018;126:39–43. doi: 10.1016/j.neures.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 102.Rauvala H., Paveliev M., Kuja-Panula J. Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans. Neural Regen Res. 2017;12:687–691. doi: 10.4103/1673-5374.206630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Engelhardt B., Ransohoff R.M. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 104.Schlager C., Korner H., Krueger M. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530:349–353. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 105.Kivisäkk P., Mahad D.J., Callahan M.K. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neumann J., Riek-Burchardt M., Herz J. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129:259–277. doi: 10.1007/s00401-014-1355-2. [DOI] [PubMed] [Google Scholar]
- 107.Yednock T.A., Cannon C., Fritz L.C. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 108.Fleming J.C., Bao F., Chen Y. Timing and duration of anti-alpha4beta1 integrin treatment after spinal cord injury: effect on therapeutic efficacy. J Neurosurg Spine. 2009;11:575–587. doi: 10.3171/2009.6.SPINE08915. [DOI] [PubMed] [Google Scholar]
- 109.Bao F., Omana V., Brown A. The systemic inflammatory response after spinal cord injury in the rat is decreased by alpha4beta1 integrin blockade. J Neurotrauma. 2012;29:1626–1637. doi: 10.1089/neu.2011.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Itano A.A., Jenkins M.K. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 111.Kumamoto Y., Mattei L.M., Sellers S. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc Natl Acad Sci U S A. 2011;108:8749–8754. doi: 10.1073/pnas.1100567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirahara K., Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol. 2016;28:163–171. doi: 10.1093/intimm/dxw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jandl C., Loetsch C., King C. Cytokine Expression by T Follicular helper cells. Methods mol Biol. 2017;1623:95–103. doi: 10.1007/978-1-4939-7095-7_8. [DOI] [PubMed] [Google Scholar]
- 114.Filiano A.J., Gadani S.P., Kipnis J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci. 2017;18:375–384. doi: 10.1038/nrn.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Radjavi A., Smirnov I., Kipnis J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav Immun. 2014;35:58–63. doi: 10.1016/j.bbi.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Derecki N.C., Cardani A.N., Yang C.H. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ankeny D.P., Guan Z., Popovich P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ankeny D.P., Lucin K.M., Sanders V.M. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- 119.Cabrera-Aldana E.E., Ruelas F., Aranda C. Methylprednisolone administration following spinal cord injury reduces aquaporin 4 expression and Exacerbates edema. Mediators Inflamm. 2017;2017:4792932. doi: 10.1155/2017/4792932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sunshine J.E., Dagal A., Burns S.P. Methylprednisolone therapy in acute traumatic spinal cord injury: analysis of a regional spinal cord model systems Database. Anesth Analg. 2017;124:1200–1205. doi: 10.1213/ANE.0000000000001906. [DOI] [PubMed] [Google Scholar]
- 121.Shultz R.B., Zhong Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res. 2017;12:702–713. doi: 10.4103/1673-5374.206633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simon D.W., Aneja R.K., Alexander H. Minocycline attenuates high mobility group box 1 translocation, microglial activation, and thalamic neurodegeneration after traumatic brain injury in postnatal day 17 rats. J Neurotrauma. 2018;35:130–138. doi: 10.1089/neu.2017.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hanlon L.A., Raghupathi R., Huh J.W. Differential effects of minocycline on microglial activation and neurodegeneration following closed head injury in the neonate rat. Exp Neurol. 2017;290:1–14. doi: 10.1016/j.expneurol.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chin T.Y., Kiat S.S., Faizul H.G. The effects of minocycline on spinal root avulsion injury in rat model. Malays J Med Sci. 2017;24:31–39. doi: 10.21315/mjms2017.24.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanchez Mejia R.O., Ona V.O., Li M. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–1399. doi: 10.1097/00006123-200106000-00051. discussion 1399–1401. [DOI] [PubMed] [Google Scholar]
- 126.Papa S., Caron I., Erba E. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials. 2016;75:13–24. doi: 10.1016/j.biomaterials.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 127.Bin S., Zhou N., Pan J. Nano-Carrier mediated co-delivery of methyl prednisolone and minocycline for improved post-traumatic spinal cord injury conditions in rats. Drug Dev Ind Pharm. 2017;43:1033–1041. doi: 10.1080/03639045.2017.1291669. [DOI] [PubMed] [Google Scholar]
- 128.Sangobowale M., Grin'kina N.M., Whitney K. Minocycline plus N-acetylcysteine reduce behavioral deficits and improve Histology with a clinically useful time window. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5348. [DOI] [PubMed] [Google Scholar]
- 129.Casha S., Zygun D., McGowan M.D. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135(Pt 4):1224–1236. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- 130.Thibault-Halman G., Rivers C.S., Bailey C.S. Predicting recruitment feasibility for acute spinal cord injury clinical trials in Canada using national registry data. J Neurotrauma. 2017;34:599–606. doi: 10.1089/neu.2016.4568. [DOI] [PubMed] [Google Scholar]
- 131.Swinehart I.T., Badylak S.F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev Dyn. 2016;245:351–360. doi: 10.1002/dvdy.24379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu Y., Wang J., Shi Y. Implantation of brain-derived extracellular matrix enhances neurological recovery after traumatic brain injury. Cell Transplant. 2017;26:1224–1234. doi: 10.1177/0963689717714090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang S., Ge X., Yu J. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018;32:512–528. doi: 10.1096/fj.201700673R. [DOI] [PubMed] [Google Scholar]
- 134.Li Y., Yang Y.Y., Ren J.L. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res Ther. 2017;8:198. doi: 10.1186/s13287-017-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haggerty A.E., Marlow M.M., Oudega M. Extracellular matrix components as therapeutics for spinal cord injury. Neurosci Lett. 2017;652:50–55. doi: 10.1016/j.neulet.2016.09.053. [DOI] [PubMed] [Google Scholar]
- 136.Yang Y., Ye Y., Su X. Mscs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci. 2017;11:55. doi: 10.3389/fncel.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xiong Y., Mahmood A., Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res. 2017;12:19–22. doi: 10.4103/1673-5374.198966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Y., Chopp M., Zhang Z.G. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. doi: 10.1016/j.neuint.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Manek R., Moghieb A., Yang Z. Protein biomarkers and neuroproteomics characterization of microvesicles/exosomes from human cerebrospinal fluid following traumatic brain injury. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moyron R.B., Gonda A., Selleck M.J. Differential protein expression in exosomal samples taken from trauma patients. Proteomics Clin Appl. 2017;11(9–10) doi: 10.1002/prca.201700061. [DOI] [PubMed] [Google Scholar]
- 141.Kong F.L., Wang X.P., Li Y.N. The role of exosomes derived from cerebrospinal fluid of spinal cord injury in neuron proliferation in vitro. Artif Cells Nanomed Biotechnol. 2018;46:200–205. doi: 10.1080/21691401.2017.1304408. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y., Chopp M., Meng Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lankford K.L., Arroyo E.J., Nazimek K. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190358. e0190358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Rivero Vaccari J.P., Brand F., 3rd, Adamczak S. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136(suppl 1):39–48. doi: 10.1111/jnc.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Adamczak S., Dale G., de Rivero Vaccari J.P. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117:1119–1125. doi: 10.3171/2012.9.JNS12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin W.P., Xiong G.P., Lin Q. Heme oxygenase-1 promotes neuron survival through down-regulation of neuronal NLRP1 expression after spinal cord injury. J Neuroinflammation. 2016;13:52. doi: 10.1186/s12974-016-0521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Farzanegan G.R., Derakhshan N., Khalili H. Effects of atorvastatin on brain contusion volume and functional outcome of patients with moderate and severe traumatic brain injury; a randomized double-blind placebo-controlled clinical trial. J Clin Neurosci. 2017;44:143–147. doi: 10.1016/j.jocn.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 148.Robertson C.S., McCarthy J.J., Miller E.R. Phase II clinical trial of atorvastatin in mild traumatic brain injury. J Neurotrauma. 2017 doi: 10.1089/neu.2016.4717. [DOI] [PubMed] [Google Scholar]
- 149.Turkoglu O.F., Eroglu H., Okutan O. Atorvastatin efficiency after traumatic brain injury in rats. Surg Neurol. 2009;72:146–152. doi: 10.1016/j.surneu.2008.07.004. discussion 152. [DOI] [PubMed] [Google Scholar]
- 150.Lu D., Qu C., Goussev A. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang H., Lynch J.R., Song P. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206:59–69. doi: 10.1016/j.expneurol.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 152.Qu C., Lu D., Goussev A. Effect of atorvastatin on spatial memory, neuronal survival, and vascular density in female rats after traumatic brain injury. J Neurosurg. 2005;103:695–701. doi: 10.3171/jns.2005.103.4.0695. [DOI] [PubMed] [Google Scholar]
- 153.Lu D., Mahmood A., Goussev A. Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J Neurosurg. 2004;101:813–821. doi: 10.3171/jns.2004.101.5.0813. [DOI] [PubMed] [Google Scholar]
- 154.Peng W., Yang J., Yang B. Impact of statins on cognitive deficits in adult male rodents after traumatic brain injury: a systematic review. Biomed Res Int. 2014;2014:261409. doi: 10.1155/2014/261409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nacar O.A., Eroglu H., Cetinalp N.E. Systemic administration of atorvastatin improves locomotor functions and hyperacute-acute response after experimental spinal cord injury: an ultrastructural and biochemical analysis. Turk Neurosurg. 2014;24:337–343. doi: 10.5137/1019-5149.JTN.8131-13.1. [DOI] [PubMed] [Google Scholar]
- 156.Kardes O., Civi S., Tufan K. Effects of atorvastatin on experimental spinal cord ischemia-reperfusion injury in rabbits. Turk Neurosurg. 2017;27:594–602. doi: 10.5137/1019-5149.JTN.16627-15.2. [DOI] [PubMed] [Google Scholar]
- 157.Gao S., Zhang Z.M., Shen Z.L. Atorvastatin activates autophagy and promotes neurological function recovery after spinal cord injury. Neural Regen Res. 2016;11:977–982. doi: 10.4103/1673-5374.184498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pathak N.N., Balaganur V., Lingaraju M.C. Antihyperalgesic and anti-inflammatory effects of atorvastatin in chronic constriction injury-induced neuropathic pain in rats. Inflammation. 2013;36:1468–1478. doi: 10.1007/s10753-013-9688-x. [DOI] [PubMed] [Google Scholar]
- 159.Xu X., Gao W., Cheng S. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation. 2017;14:167. doi: 10.1186/s12974-017-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aghazadeh J., Samadi Motlagh P., Salehpour F. Effects of atorvastatin in patients with acute spinal cord injury. Asian Spine J. 2017;11:903–907. doi: 10.4184/asj.2017.11.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.García-Fernández A., García-Laínez G., Ferrándiz M.L. Targeting inflammasome by the inhibition of caspase-1 activity using capped mesoporous silica nanoparticles. J Control Release. 2017;248:60–70. doi: 10.1016/j.jconrel.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 162.de Rivero Vaccari J.P., Lotocki G., Alonso O.F. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Adamczak S.E., de Rivero Vaccari J.P., Dale G. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab. 2014;34:621–629. doi: 10.1038/jcbfm.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee H., Shin E.A., Lee J.H. Caspase inhibitors: a review of recently patented compounds (2013-2015) Expert Opin Ther Pat. 2018;28:47–59. doi: 10.1080/13543776.2017.1378426. [DOI] [PubMed] [Google Scholar]
- 165.Lin C., Chao H., Li Z. Omega-3 fatty acids regulate NLRP3 inflammasome activation and prevent behavior deficits after traumatic brain injury. Exp Neurol. 2017;290:115–122. doi: 10.1016/j.expneurol.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 166.Ma J., Xiao W., Wang J. Propofol inhibits NLRP3 inflammasome and attenuates blast-induced traumatic brain injury in rats. Inflammation. 2016;39:2094–2103. doi: 10.1007/s10753-016-0446-8. [DOI] [PubMed] [Google Scholar]
- 167.Wei X., Hu C.C., Zhang Y.L. Telmisartan reduced cerebral edema by inhibiting NLRP3 inflammasome in mice with cold brain injury. J Huazhong Univ Sci Technolog Med Sci. 2016;36:576–583. doi: 10.1007/s11596-016-1628-1. [DOI] [PubMed] [Google Scholar]
- 168.Cao S., Shrestha S., Li J. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci Rep. 2017;7:2417. doi: 10.1038/s41598-017-02679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zendedel A., Mönnink F., Hassanzadeh G. Estrogen attenuates local inflammasome expression and activation after spinal cord injury. Mol Neurobiol. 2018;55:1364–1375. doi: 10.1007/s12035-017-0400-2. [DOI] [PubMed] [Google Scholar]
- 170.Zendedel A., Johann S., Mehrabi S. Activation and regulation of NLRP3 inflammasome by intrathecal application of SDF-1a in a spinal cord injury model. Mol Neurobiol. 2016;53:3063–3075. doi: 10.1007/s12035-015-9203-5. [DOI] [PubMed] [Google Scholar]
- 171.Zou P., Liu X., Li G. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol Med Rep. 2018;17:3212–3217. doi: 10.3892/mmr.2017.8241. [DOI] [PubMed] [Google Scholar]
- 172.Qian H., Li Q., Shi W. Hyperbaric oxygen alleviates the activation of NLRP3inflammasomes in traumatic brain injury. Mol Med Rep. 2017;16:3922–3928. doi: 10.3892/mmr.2017.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Baratz-Goldstein R., Toussia-Cohen S., Elpaz A. Immediate and delayed hyperbaric oxygen therapy as a neuroprotective treatment for traumatic brain injury in mice. Mol Cell Neurosci. 2017;83:74–82. doi: 10.1016/j.mcn.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 174.Irrera N., Pizzino G., Calò M. Lack of the Nlrp3 inflammasome improves mice recovery following traumatic brain injury. Front Pharmacol. 2017;8:459. doi: 10.3389/fphar.2017.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fan K., Ma J., Xiao W. Mangiferin attenuates blast-induced traumatic brain injury via inhibiting NLRP3 inflammasome. Chem Biol Interact. 2017;271:15–23. doi: 10.1016/j.cbi.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 176.Impellizzeri D., Mazzon E., Paterniti I. Effect of fasudil, a selective inhibitor of Rho kinase activity, in the secondary injury associated with the experimental model of spinal cord trauma. J Pharmacol Exp Ther. 2012;343:21–33. doi: 10.1124/jpet.111.191239. [DOI] [PubMed] [Google Scholar]
- 177.Wu J., Maoqiang L., Fan H. Rutin attenuates neuroinflammation in spinal cord injury rats. J Surg Res. 2016;203:331–337. doi: 10.1016/j.jss.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 178.Jiang W., Huang Y., Han N. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord. 2016;54:592–596. doi: 10.1038/sc.2015.227. [DOI] [PubMed] [Google Scholar]
- 179.Jiang W., Li M., He F. Neuroprotective effect of asiatic acid against spinal cord injury in rats. Life Sci. 2016;157:45–51. doi: 10.1016/j.lfs.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 180.Jiang W., Li M., He F. Dopamine D1 receptor agonist A-68930 inhibits NLRP3 inflammasome activation and protects rats from spinal cord injury-induced acute lung injury. Spinal Cord. 2016;54:951–956. doi: 10.1038/sc.2016.52. [DOI] [PubMed] [Google Scholar]
- 181.Brickler T., Gresham K., Meza A. Nonessential role for the NLRP1 inflammasome complex in a murine model of traumatic brain injury. Mediators Inflamm. 2016;2016:6373506. doi: 10.1155/2016/6373506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Helmy A., Guilfoyle M.R., Carpenter K.L. Recombinant human interleukin-1 receptor antagonist promotes M1 microglia biased cytokines and chemokines following human traumatic brain injury. J Cereb Blood Flow Metab. 2016;36:1434–1448. doi: 10.1177/0271678X15620204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sun M., Brady R.D., Wright D.K. Treatment with an interleukin-1 receptor antagonist mitigates neuroinflammation and brain damage after polytrauma. Brain Behav Immun. 2017;66:359–371. doi: 10.1016/j.bbi.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 184.Zong S., Zeng G., Wei B. Beneficial effect of interleukin-1 receptor antagonist protein on spinal cord injury recovery in the rat. Inflammation. 2012;35:520–526. doi: 10.1007/s10753-011-9341-5. [DOI] [PubMed] [Google Scholar]
- 185.Amin J., Boche D., Rakic S. What do we know about the inflammasome in humans? Brain Pathol. 2017;27:192–204. doi: 10.1111/bpa.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang G, Shi Y, Jiang X, et al HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci U S A. 2015;112:2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yu F., Wang Z., Tanaka M. Posttrauma cotreatment with lithium and valproate: reduction of lesion volume, attenuation of blood-brain barrier disruption, and improvement in motor coordination in mice with traumatic brain injury. J Neurosurg. 2013;119(3):766–773. doi: 10.3171/2013.6.JNS13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dash P.K., Orsi S.A., Zhang M. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One. 2010;5:e11383. doi: 10.1371/journal.pone.0011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shein N.A., Grigoriadis N., Alexandrovich A.G. Histone deacetylase inhibitor ITF2357 is neuroprotective, improves functional recovery, and induces glial apoptosis following experimental traumatic brain injury. FASEB J. 2009;23:4266–4275. doi: 10.1096/fj.09-134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang B., West E.J., Van K.C. HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res. 2008;1226:181–191. doi: 10.1016/j.brainres.2008.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Georgoff P.E., Nikolian V.C., Higgins G. Valproic Acid Induces Pro-Survival Transcriptomic Changes in Swine Subjected to Traumatic Injury and Hemorrhagic Shock. J Trauma Acute Care Surg. 2017 doi: 10.1097/TA.0000000000001763. [DOI] [PubMed] [Google Scholar]
- 192.Kumar S., Patel R., Moore S. Estrogen receptor beta ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiol Dis. 2013;56:131–144. doi: 10.1016/j.nbd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chu T., Zhou H., Lu L. Valproic acid-mediated neuroprotection and neurogenesis after spinal cord injury: from mechanism to clinical potential. Regen Med. 2015;10:193–209. doi: 10.2217/rme.14.86. [DOI] [PubMed] [Google Scholar]
- 194.Lu W.H., Wang C.Y., Chen P.S. Valproic acid attenuates microgliosis in injured spinal cord and purinergic P2X4 receptor expression in activated microglia. J Neurosci Res. 2013;91:694–705. doi: 10.1002/jnr.23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lv L., Sun Y., Han X. Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition. Brain Res. 2011;1396:60–68. doi: 10.1016/j.brainres.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 196.Kuboyama T., Wahane S., Huang Y. HDAC3 inhibition ameliorates spinal cord injury by immunomodulation. Sci Rep. 2017;7:8641. doi: 10.1038/s41598-017-08535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Evans T.A., Barkauskas D.S., Myers J.T. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol. 2014;254:109–120. doi: 10.1016/j.expneurol.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jeong S.J., Cooper J.G., Ifergan I. Intravenous immune-modifying nanoparticles as a therapy for spinal cord injury in mice. Neurobiol Dis. 2017;108:73–82. doi: 10.1016/j.nbd.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Getts D.R., Terry R.L., Getts M.T. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014;6:1–14. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bharadwaj V.N., Nguyen D.T., Kodibagkar V.D. Nanoparticle-Based Therapeutics for Brain Injury. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201700668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ziemssen T., Tumani H., Sehr T. Safety and in vivo immune assessment of escalating doses of oral laquinimod in patients with RRMS. J Neuroinflammation. 2017;14:172. doi: 10.1186/s12974-017-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thöne J., Linker R.A. Laquinimod in the treatment of multiple sclerosis: a review of the data so far. Drug Des Devel Ther. 2016;10:1111–1118. doi: 10.2147/DDDT.S55308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Narain S., Furie R. Update on clinical trials in systemic lupus erythematosus. Curr Opin Rheumatol. 2016;28:477–487. doi: 10.1097/BOR.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 204.Mishra M.K., Wang J., Keough M.B. Laquinimod reduces neuroaxonal injury through inhibiting microglial activation. Ann Clin Transl Neurol. 2014;1:409–422. doi: 10.1002/acn3.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Katsumoto A., Miranda A.S., Butovsky O. Laquinimod attenuates inflammation by modulating macrophage functions in traumatic brain injury mouse model. J Neuroinflammation. 2018;15:26. doi: 10.1186/s12974-018-1075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Benedek G., Meza-Romero R., Jordan K. HLA-DRalphα1-mMOG-35-55 treatment of experimental autoimmune encephalomyelitis reduces CNS inflammation, enhances M2 macrophage frequency, and promotes neuroprotection. J Neuroinflammation. 2015;12:123. doi: 10.1186/s12974-015-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang J., Ye Q., Xu J. DRalphα1-MOG-35-55 reduces permanent ischemic brain injury. Transl Stroke Res. 2017;8:284–293. doi: 10.1007/s12975-016-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang L., Liu Z., Ren H. DRalphα1-MOG-35-55 treatment reduces lesion volumes and improves neurological deficits after traumatic brain injury. Metab Brain Dis. 2017;32(5):1395–1402. doi: 10.1007/s11011-017-9991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gogou M., Papadopoulou-Alataki E., Spilioti M. Clinical applications of intravenous immunoglobulins in child neurology. Curr Pharm Biotechnol. 2017;18:628–637. doi: 10.2174/1389201018666170915123700. [DOI] [PubMed] [Google Scholar]
- 210.Thom V., Arumugam T.V., Magnus T. Therapeutic potential of intravenous immunoglobulin in acute brain injury. Front Immunol. 2017;8:875. doi: 10.3389/fimmu.2017.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen X., Arumugam T.V., Cheng Y.L. Combination therapy with low-Dose IVIG and a C1-esterase inhibitor ameliorates brain damage and functional deficits in experimental ischemic stroke. Neuromolecular Med. 2018 doi: 10.1007/s12017-017-8474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Akyol G.Y., Manaenko A., Akyol O. IVIG activates FcgammaRIIB-SHIP1-PIP3 Pathway to stabilize mast cells and suppress inflammation after ICH in mice. Sci Rep. 2017;7:15583. doi: 10.1038/s41598-017-15455-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brennan F.H., Kurniawan N.D., Vukovic J. IVIg attenuates complement and improves spinal cord injury outcomes in mice. Ann Clin Transl Neurol. 2016;3:495–511. doi: 10.1002/acn3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jeong S., Lei B., Wang H. Intravenous immunoglobulin G improves neurobehavioral and histological outcomes after traumatic brain injury in mice. J Neuroimmunol. 2014;276(1–2):112–118. doi: 10.1016/j.jneuroim.2014.08.626. [DOI] [PubMed] [Google Scholar]
- 215.Fehlings M.G., Nguyen D.H. Immunoglobulin G: a potential treatment to attenuate neuroinflammation following spinal cord injury. J Clin Immunol. 2010;30(Suppl. 1):S109–S112. doi: 10.1007/s10875-010-9404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tzekou A., Fehlings M.G. Treatment of spinal cord injury with intravenous immunoglobulin G: preliminary evidence and future perspectives. J Clin Immunol. 2014;34(Suppl. 1):S132–S138. doi: 10.1007/s10875-014-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ulndreaj A., Chio J.C., Ahuja C.S. Modulating the immune response in spinal cord injury. Expert Rev Neurother. 2016;16:1127–1129. doi: 10.1080/14737175.2016.1207532. [DOI] [PubMed] [Google Scholar]
- 218.Relkin N.R., Thomas R.G., Rissman R.A. A phase 3 trial of IV immunoglobulin for Alzheimer disease. Neurology. 2017;88:1768–1775. doi: 10.1212/WNL.0000000000003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kasai T., Kondo M., Ishii R. Abeta levels in the jugular vein and high molecular weight Abeta oligomer levels in CSF can be used as biomarkers to indicate the anti-amyloid effect of IVIg for Alzheimer's disease. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174630. e0174630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wu B., Matic D., Djogo N. Improved regeneration after spinal cord injury in mice lacking functional T- and B-lymphocytes. Exp Neurol. 2012;237:274–285. doi: 10.1016/j.expneurol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 221.Casili G., Impellizzeri D., Cordaro M. B-cell depletion with CD20 antibodies as new approach in the treatment of inflammatory and immunological Events associated with spinal cord injury. Neurotherapeutics. 2016;13:880–894. doi: 10.1007/s13311-016-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Clausen F., Lorant T., Lewén A. T lymphocyte trafficking: a novel target for neuroprotection in traumatic brain injury. J Neurotrauma. 2007;24:1295–1307. doi: 10.1089/neu.2006.0258. [DOI] [PubMed] [Google Scholar]
- 223.Jones T.B., Hart R.P., Popovich P.G. Molecular control of physiological and pathological T-cell recruitment after mouse spinal cord injury. J Neurosci. 2005;25:6576–6583. doi: 10.1523/JNEUROSCI.0305-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Israelsson C., Bengtsson H., Lobell A. Appearance of Cxcl10-expressing cell clusters is common for traumatic brain injury and neurodegenerative disorders. Eur J Neurosci. 2010;31:852–863. doi: 10.1111/j.1460-9568.2010.07105.x. [DOI] [PubMed] [Google Scholar]
- 225.Israelsson C., Bengtsson H., Kylberg A. Distinct cellular patterns of upregulated chemokine expression supporting a prominent inflammatory role in traumatic brain injury. J Neurotrauma. 2008;25:959–974. doi: 10.1089/neu.2008.0562. [DOI] [PubMed] [Google Scholar]
- 226.Ghirnikar R.S., Lee Y.L., Eng L.F. Chemokine antagonist infusion promotes axonal sparing after spinal cord contusion injury in rat. J Neurosci Res. 2001;64:582–589. doi: 10.1002/jnr.1110. [DOI] [PubMed] [Google Scholar]
- 227.Glaser J., Gonzalez R., Sadr E. Neutralization of the chemokine CXCL10 reduces apoptosis and increases axon sprouting after spinal cord injury. J Neurosci Res. 2006;84:724–734. doi: 10.1002/jnr.20982. [DOI] [PubMed] [Google Scholar]
- 228.Gonzalez R., Hickey M.J., Espinosa J.M. Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regen Med. 2007;2:771–783. doi: 10.2217/17460751.2.5.771. [DOI] [PubMed] [Google Scholar]
- 229.Juif P.E., Kraehenbuehl S., Dingemanse J. Clinical pharmacology, efficacy, and safety aspects of sphingosine-1-phosphate receptor modulators. Expert Opin Drug Metab Toxicol. 2016;12:879–895. doi: 10.1080/17425255.2016.1196188. [DOI] [PubMed] [Google Scholar]
- 230.Jeffery D.R., Rammohan K.W., Hawker K. Fingolimod: a review of its mode of action in the context of its efficacy and safety profile in relapsing forms of multiple sclerosis. Expert Rev Neurother. 2016;16:31–44. doi: 10.1586/14737175.2016.1123094. [DOI] [PubMed] [Google Scholar]
- 231.Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 232.Healy LM, Antel JP Sphingosine-1-Phosphate Receptors in the Central Nervous and Immune Systems. Curr Drug Targets. 2016;17:1841–1850. doi: 10.2174/1389450116666151001112710. [DOI] [PubMed] [Google Scholar]
- 233.Norimatsu Y., Ohmori T., Kimura A. FTY720 improves functional recovery after spinal cord injury by primarily nonimmunomodulatory mechanisms. Am J Pathol. 2012;180:1625–1635. doi: 10.1016/j.ajpath.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 234.Lee K.D., Chow W.N., Sato-Bigbee C. FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J Neurotrauma. 2009;26:2335–2344. doi: 10.1089/neu.2008.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang J., Wang J., Lu P. Local delivery of FTY720 in PCL membrane improves SCI functional recovery by reducing reactive astrogliosis. Biomaterials. 2015;62:76–87. doi: 10.1016/j.biomaterials.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 236.Gao C., Qian Y., Huang J. A three-day consecutive fingolimod administration improves neurological functions and modulates multiple immune responses of CCI mice. Mol Neurobiol. 2017;54:8348–8360. doi: 10.1007/s12035-016-0318-0. [DOI] [PubMed] [Google Scholar]
- 237.Huwiler A., Zangemeister-Wittke U. The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: recent findings and new perspectives. Pharmacol Ther. 2017 doi: 10.1016/j.pharmthera.2017.11.001. S0163–S7258(17)30285-1. [DOI] [PubMed] [Google Scholar]
- 238.Rodríguez-Barrera R., Flores-Romero A., Fernández-Presas A.M. Immunization with neural derived peptides plus scar removal induces a permissive microenvironment, and improves locomotor recovery after chronic spinal cord injury. BMC Neurosci. 2017;18:7. doi: 10.1186/s12868-016-0331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Coder B., Wang W., Wang L. Friend or foe: the dichotomous impact of T cells on neuro-de/re-generation during aging. Oncotarget. 2017;8:7116–7137. doi: 10.18632/oncotarget.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Martiñón S., Garcia-Vences E., Toscano-Tejeida D. Long-term production of BDNF and NT-3 induced by A91-immunization after spinal cord injury. BMC Neurosci. 2016;17:42. doi: 10.1186/s12868-016-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Schwartz M., Kipnis J. Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol Med. 2001;7:252–258. doi: 10.1016/s1471-4914(01)01993-1. [DOI] [PubMed] [Google Scholar]
- 242.García E., Silva-García R., Mestre H. Immunization with A91 peptide or copolymer-1 reduces the production of nitric oxide and inducible nitric oxide synthase gene expression after spinal cord injury. J Neurosci Res. 2012;90:656–663. doi: 10.1002/jnr.22771. [DOI] [PubMed] [Google Scholar]
- 243.Shechter R., Schwartz M. CNS sterile injury: just another wound healing? Trends Mol Med. 2013;19:135–143. doi: 10.1016/j.molmed.2012.11.007. [DOI] [PubMed] [Google Scholar]