Abstract
Adequate intravenous fluid therapy is essential in renal transplant recipients to ensure a good allograft perfusion. Central venous pressure (CVP) has been considered the cornerstone to guide the fluid therapy for decades; it was the only available simple tool worldwide. However, the revolutionary advances in assessing the dynamic preload variables together with the availability of new equipment to precisely measure the effect of intravenous fluids on the cardiac output had created a question mark on the future role of CVP. Despite the critical role of fluid therapy in the field of transplantation. There are only a few clinical studies that compared the CVP guided fluid therapy with the other modern techniques and their relation to the outcome in renal transplantation. Our work sheds some light on the available published data in renal transplantation, together with data from other disciplines evaluating the utility of central venous pressure measurement. Although lager well-designed studies are still required to consolidate the role of new techniques in the field of renal transplantation, we can confidently declare that the new techniques have the advantages of providing more accurate haemodynamic assessment, which results in a better patient outcome.
Keywords: Fluid monitoring, Central venous pressure, Renal transplantation
Core tip: We suggest that central venous pressure (CVP) measurement should be abandoned in renal transplantation since it may be misleading. We recommend using intra-operative and post-operative cardiac output monitoring devices for guiding fluid therapy in renal transplant recipients. Although lager well-designed studies are still required to consolidate the role of new techniques in comparison to CVP monitoring in the field of renal transplantation. We Suggest that the new methods have the advantage of providing a more accurate haemodynamic assessment in renal transplant cases.
INTRODUCTION
Central venous pressure (CVP) measurement have been in use for more than half a century to assess intravascular fluid status of renal transplant recipients and, thereby, be used as a guide for intravenous fluid therapy in renal transplantation. With the current advances in the diagnostic tools, the value of CVP is a point of debate. Several studies proved that CVP measurements are neither correlated to cardiac output nor have a precise correlation with intravascular volume status, therefore it’s value in fluid management of renal transplant recipient is at the best speculative. On the other hand, the traditionalists continue to believe that CVP values are of sufficiently good enough as a benchmark in determining resuscitation goals for a given patient.
It is well recognised that optimum fluid resuscitation is essential to maximise the outcomes in critically ill patients. However, only a few studies have reliably endeavoured to assess the role of CVP in comparison to other modern techniques in the field of renal transplantation. We aim to answer this question in regards to clinical application of CVP and objectively review from the point of view of its benefits and inherent limitations.
HISTORICAL USE OF CVP
The clinical correlation between CVP and the intravascular fluid volume were established more than 50 years ago[1]. Theoretical basis of CVP is to measure the pressure in the superior vena cava (SVC) or right atrium pressure, which reflects the right ventricle preload[2]. Indeed, several textbooks have dogmatically stated that CVP provides a clinically relevant and reliable information in regards to circulatory and volume status of patients[3].
Marik et al[3] published a systematic review article that evaluated the relationship between CVP and the fluid status of the patients and concluded that CVP is an unreliable indicator of the fluid status and should not be used as a guide to fluid management. Furthermore, Marik et al[4] as per updated meta-analysis for evaluation of CVP reliability in clinical practice, reiterated abandoning the use of CVP as a guide in fluid management.
Cecconi et al[5] pointed that commonly used preload measurements such as CVP or end diastolic volume, when used in isolation, cannot be used reliably as a guide to fluid resuscitation. They rather recommend using more than one hemodynamic variable for patient evaluation and management. Nonetheless, the study validated the role of CVP in certain situations as severe congestive heart failure or hypovolemia, where the use of CVP is valuable in guiding fluid management[5].
CVP IN THE CURRENT PRACTICE
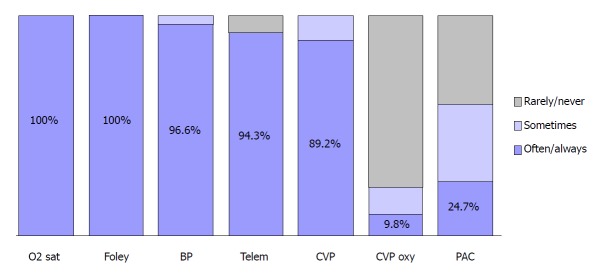
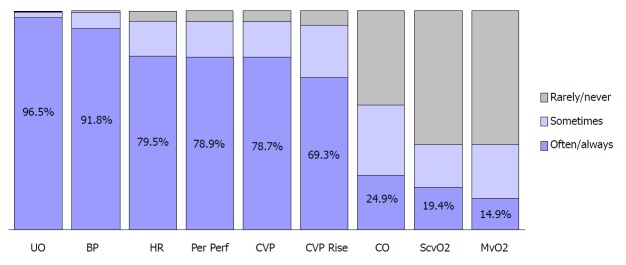
CVP measurement continues to be a pedestal in day to day clinical practice. A survey studying the resuscitation practices of Canadian physicians have shown that 89.2% of them would use CVP as a monitoring parameter in septic shock as shown in Figure 1[6]. Additionally, CVP-determined endpoints were considered the end-point of volume resuscitation in the early phases of septic shock by 78.7% of the Canadian clinicians as illustrated in Figure 2[6].
Figure 1.
Monitoring parameters used by intensive care unit physicians[6]. BP: Intra-arterial blood pressure; CVP: Central venous pressure; CVP oxy: Continuous monitoring of central venous oxygen saturation; Foley: Foley catheter; O2 sat: Oxygen saturation; PAC: Pulmonary artery catheter; Telem: Telemetry.
Figure 2.
Volume resuscitation end-points[6]. BP: Blood pressure; CO: Cardiac output; CVP: Central venous pressure; CVP rise: Sustained rise in central venous pressure; HR: Heart rate; MvO2: Mixed venous oxygen saturation; Per Perf: Peripheral perfusion; ScvO2: Central venous oxygen saturation; UO: Urine output.
Bignami et al[7] addressed the current clinical practice in hemodynamic monitoring after cardiac surgery in Italy. They analysed data collected from 71 centres using a 33-item questionnaire from. For monitoring intravascular volume status, CVP was used most frequently (26.7%), followed by arterial BP (19.7%) and echocardiography (5.6%)[7]. Sondergaard et al[8] reported that CVP, though not a direct measure of preload, can be used to assess volume status, heart performance and systemic vascular resistance.
DRAWBACKS AND LIMITATIONS OF CVP IN RELATION TO RENAL TRANSPLANTATION
Recent medical advances in understanding haemodynamic of the vascular system together with the availability of new technology have changed the scope of diagnostic approaches. We strongly feel that CVP is not the right tool in assessing the fluid balance and guide fluid therapy in renal transplantation. CVP reading is affected by several physical and anatomical factors as illustrated in Table 1[9].
Table 1.
Factors affecting the measured central venous pressure reading[9]
| Central venous blood volume | Venous return/cardiac output Total blood volume Regional vascular tone |
| Compliance of central compartment | Vascular tone Right ventricular compliance: Myocardial disease Pericardial disease Tamponade |
| Tricuspid valve disease | Stenosis Regurgitation |
| Cardiac rhythm | Junctional rhythm Atrial fibrillation Atrio-ventricular dissociation |
| Reference level of transducer | Positioning of patient |
| Intrathoracic pressure | Respiration Intermittent positive pressure ventilation Positive end-expiratory pressure Tension pneumothorax |
During kidney transplant operation, the recipient is exposed to many intraoperative factors which may alter the CVP reading, hence, can be misleading in decision making. These factors can be summarised in the following points: (1) During the operation, the position of the patient is not always in flat supine position. The surgeon may be tilting the table in a different direction, commonly head down while elevating the left or the right side to improve the access to the iliac vessels. The effect of posture changes on CVP reading was documented since a long time[10]; (2) transplant surgery always entails the use of abdominal retractors. These retractors must have a pressure effect on the viscera and subsequently affect the venous return. Moreover, the tension created by the retractors will resist movement of the diaphragm and will eventually affect the intrathoracic pressure. These mechanical factors again will give a false CVP reading[11]; (3) there is positive pressure ventilation (PPV) during the transplant operation will affect the CVP reading as mentioned in Table 1[9]. There is no convincing evidence demonstrating to how much the CVP is affected by PPV; (4) the target intra-operative CVP remains elusive. While aggressive hydration ensures good allograft perfusion. On the other hand, overhydration carries the risk of pulmonary congestion, pulmonary oedema, and prolonged intubation especially in patients with pre-existing cardiac conditions[12]; (5) CKD patients on dialysis fluctuate between the volume overload state and the dry state during the post-dialysis period, which makes it difficult to declare which CVP reading should be considered as a normal reading. Additionally, the effect of ageing, long-standing hypertension and the use of various medications affecting the peripheral vascular resistance (alpha blockers, beta blockers and calcium channel blockers) would be further confounding parameters[9]; and (6) we should not forget that placement of central venous catheters and other devices may result in central vein stenosis. Central vein stenosis can jeopardise the future of arteriovenous fistula and arteriovenous graft in the ipsilateral extremity when the renal graft fails, and the patient returns to dialysis[13-15].
POSSIBLE ALTERNATIVES FOR FLUID STATUS MONITORING
The introduction of commercially available equipment for assessing dynamic preload variables [e.g., stroke volume variation (SVV)] considered a revolutionary advance in peri-operative fluid management. Srivastava et al[16] evaluated the use of intraoperative transesophageal Doppler (TED) to estimate the corrected flow time and variation in stroke volume values. TED was used to guide intraoperative fluid management in 110 living donor renal transplant recipients, and the outcome was compared with the historical records of 104 control recipients who received CVP guided fluid management over the previous year. They concluded that TED was associated with a similar rate of immediate graft function. Moreover, it was associated with a significantly less amount of intra-operative intravenous (IV) fluids, and reduced incidence of postoperative fluid overload[16].
Similarly, Kumar et al[17] studied the use of SVV (obtained from minimally invasive cardiac output monitor) to guide the perioperative fluid therapy in major abdominal surgery. The study documented a significantly lower amount of IV fluids used with the new technique, not only that but also there was a significantly shorter ICU stay, and a non-significant shorter hospital stay[17]. These non-invasive tools were used successfully as a part of enhanced recovery programs in kidney transplantation to improve patient outcomes and speed up patient’s recovery after surgery[18].
Furthermore, several other non-invasive techniques are utilised for cardiac output assessment and IV fluid guidance like lithium dilution technology (e.g., LiDCOplusTM machine) and arterial pulse wave analysis (e.g., FloTrac/VigileoTM)[19,20]. However, each one of these novel, non-invasive techniques has its own limitations. Clinicians should be aware of the underlying principles and limitations of each technique to choose the best modality for each clinical scenario individually[19,20]. Advantages and limitations of some of the currently available non-invasive approaches are summarised in Table 2[19,20].
Table 2.
Advantages and limitations of some commercially available (minimally invasive) cardiac output monitoring[19,20]
| Modality | Examples | Advantages | Limitations |
| Pulse wave analysis | LiDCOrapid™ and FloTrac/Vigileo™ | Requires only arterial line; Beat-by-beat CO monitoring (this may help to evaluate response to IV fluids). - Validated by clinical studies in different medical and surgical conditions | Presence of arterial line with optimum waveform signal is a prerequisite; Accuracy may be reduced by sever arrhythmia; Needs frequent recalibration during periods of hemodynamic Instability |
| Lithium dilution | LiDCOplus™ | Simple technique (can use peripheral arterial line); Continuous CO monitoring | Arterial line required; Accuracy affected by some neuromuscular blocking drugs; Lithium chloride is contraindicated in patients undergoing treatment with lithium salts |
| Electrical bioimpedance | BioZ® | Completely non-invasive | Numerous mathematical assumptions; Limited validity in patients with dysrhythmias |
| Partial CO2 rebreathing | NICO™ | Easy to set up | Requires intubation and mechanical ventilation with minimal gas exchange abnormalities and fixed ventilator settings; Accuracy decreased with haemodynamic instability |
| Pulsed dye densitometry | DDG-330® | Non-invasive | Intermittent assessment; Accuracy may be affected by vasoconstriction, movement of the sensor and interstitial oedema |
CO: Cardiac output; OR: Operating room.
The reliability of these new techniques to guide fluid therapy in surgical cases has been investigated in several clinical trials. The conclusion of these trials is summarized in Table 3.
Table 3.
Dynamic evaluation of fluid status in comparison to conventional approach
| Author | Patients No. | Study group | Conclusion |
| Berkenstadt et al[21], 2001 | 15 | Patients undergoing brain surgery | SVV could predict fluid responsiveness to even a small volume loading of 100 mL of 6% hydroxyethyl starch given for two minutes; There was no correlation between the changes in SV and the values of the CVP and heart rate before or after loading |
| Rex et al[22], 2004 | 14 | Coronary artery bypass grafting (CABG) patients | The dynamic index SVV allowed real-time monitoring of left ventricular preload. Moreover, it allowed assessing the haemodynamic effect of a fluid challenge; Other preload variables (i.e., PAOP, CVP, LVEDAI and ITBI) failed to predict fluid responsiveness |
| Preisman et al[23], 2005 | 18 | Coronary artery bypass grafting (CABG) patients | Functional haemodynamic indices were superior to static indicators of cardiac preload in predicting fluid responsiveness; Use of CVP for the evaluation of intravascular volume status, have been found to lack any predictive value |
| Hofer et al[24], 2005 | 40 | CABG patients | Stroke volume index was significantly correlated with SVV (P < 0.001) and PPV (P < 0.001) only; While CVP failed to have a significant correlation (P = 0.235) |
| Wiesenack et al[25], 2005 | 20 | CABG patients | Stroke volume index correlated significantly with SVV and PPV derived from pulse contour analysis (P < 0.05) but not with CVP or pulmonary artery wedge pressure |
| Cannesson et al[26], 2006 | 18 | CABG patients | Left ventricular stroke area measured by transoesophageal echocardiographic automated border detection is not only sensitive to changes in preload but also, can quantify the effects of volume expansion on cardiac output; The difference in CVP reading did not reach statistical significance in the study groups |
| Lee et al[27], 2007 | 20 | Neurosurgical patients | Corrected flow time by oesophageal Doppler and PPV are better than CVP and LVEDAI in predicting fluid responsiveness |
| Cannesson et al[28], 2007 | 25 | CABG patients | ΔPOP can predict response to volume expansion as well as quantify the effects of volume expansion on hemodynamic parameters during cardiac surgery; There was no statistically significant relation between CVP and increase in cardiac index after volume expansion |
| Belloni et al[29], 2008 | 19 | CABG patients | Their results confirm the ability of SVV (P = 0.0005) and PPV (P = 0.001) to predict fluid responsiveness in ventilated patients during cardiac surgery No significant differences were found in mean LVEDA and CVP before and after fluid administration |
| Biais et al[30], 2008 | 35 | Postoperative period of liver transplantation | SVV and PPV measurement by arterial waveform analysis can be used to predict the effects of volume expansion in mechanically ventilated patients after liver transplantation; The failure of CVP and PAOP to predict fluid responsiveness agrees with increasing evidence that static preload indicators are not suitable for functional haemodynamic monitoring |
| Hofer et al[31], 2008 | 40 | CABG patients | Conventional static preload parameters failed to reflect the fluid status or to predict fluid responsiveness. CVP is therefore unsuitable for predicting ventricular response to fluid loading; SVV measured by the FloTrac™/Vigileo™ and the PiCCOplus™ systems exhibited similar performances regarding predicting fluid responsiveness |
| de Waal et al[32], 2009 | 18 | CABG patients | SVV of > 8% can predict fluid responsiveness with 100% sensitivity and 78% specificity, while PPV ≥ 10% can identify fluid-responders with 64% sensitivity and 100% specificity; CVP readings were not better in predicting fluid responsiveness than random chance |
| Cannesson et al[33], 2009 | 25 | CABG patients | SVV of 10% helped in discrimination of responders to volume expansion with an 82% sensitivity and 88% specificity; SVV may be a potential alternative to DeltaPP which is an accurate predictor of fluid responsiveness in ventilated patients; SVV was significantly a better predictor of fluid responsiveness than CVP and PCWP in this study |
| Zimmermann et al[34], 2010 | 20 | Elective major abdominal surgery | Both SVV and PVI are valid indicators of fluid responsiveness in ventilated patients during major abdominal surgery; CVP did not adequately reflect circulating blood volume and failed to predict fluid responsiveness in this study |
| Desgranges et al[35], 2011 | 28 | CABG patients | PVI can predict fluid responsiveness during general anaesthesia whatever the site of measurement in the operating room (the finger, the ear, and the forehead); PCWP and CVP showed no significant difference between responders and non-responders |
| Shin et al[36], 2011 | 33 | Elective living donor liver transplantation | Femoral SVV > 8% can predict responders to fluid loading with a specificity of 80% and a sensitivity of 89%; CVP and PAOP did not correlate with the changes in the cardiac index that occurred with a fluid challenge |
| Broch et al[37], 2011 | 81 | CABG patients | SVV (P = 0.002) and PPV (P < 0.0001) were found to be reliable indicators for fluid responsiveness unlike CVP (P = 0.13) that failed to predict it; PVI ability to predict fluid responsiveness is limited in the presence of low perfusion indices |
| Cannesson et al[38], 2011 | 413 | Multicentre study of different abdominal and cardiac surgeries | PPV [AUC 0.89 (0.86; 0.92)] is superior to CVP [AUC 0.57 (0.54; 0.59)] in prediction of fluid responsiveness (P < 0.001) |
| Yazigi et al[39], 2012 | 60 | CABG patients older than 70 yr | PPV is a reliable predictor of fluid responsiveness while CVP and PAOP were not better than a random chance in predicting the response to fluid; PPV reliability was not affected by the decreased arterial compliance and increased arterial stiffness related to aging |
| Bogović et al[40], 2017 | 24 | Major (abdominal or trauma) surgery | The study stressed on the inability of CVP to provide a valid evaluation of the preload; SVV and PPV monitored by LiDCO™ were better alternatives for preload assessment |
AUC: Area under the receiver operator characteristic curve; CVP: Central venous pressure; DeltaPP: Respiratory variations in arterial pulse pressure; ITBI: Intrathoracic blood volume index; LVEDA: Left ventricular end-diastolic area; LVEDAI: Left ventricular end-diastolic area index; PAOP: Pulmonary artery occlusion pressure; PCWP: Pulmonary capillary wedge pressure; PPV: Pulse pressure variation; PVI: Pleth variability index; SV: Stroke volume; SVV: Stroke volume variation; ΔPOP: Respiratory variations in the pulse oximetry plethysmographic waveform amplitude.
CONCLUSION
Although CVP measurement continues to be popular, yet it is not ideal for guiding and monitoring of fluid management in renal transplantation. It is noteworthy that there may be large variations in intravascular volume status and the patients have limited range of intravascular volume that can be called euvolemia (because of co-morbidities, vascular complications, drugs and the effects of disease on the autonomic nervous system). Therefore, the volume that is infused in a patient whose fluid balance status is doubtful is going to be imprecise if CVP is to be relied upon to appreciate their baseline value. Pulmonary oedema could be the first sign of fluid overload. Other variables such as the patient position, the use of abdominal retractors, and the positive pressure ventilation make any CVP reading meaningless. As clearly evident from the data presented in Tables 1-3, we suggest that CVP measurement be abandoned in renal transplantation since it may be misleading. Alternative to CVP, we recommend using intra-operative and post-operative cardiac output monitoring devices for guiding fluid therapy in renal transplant recipients. Understanding their limitations helps to provide more robust monitoring of fluid therapy. Giving that these novel tools are only used in the ITU/HDU and operating theatre settings, management of these patients on the ward relies mainly on regular vital signs monitoring including daily body weight rather than being misled by erroneous CVP reading.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other co-authors contributed their efforts in this manuscript.
Manuscript source: Unsolicited manuscript
Peer-review started: January 1, 2018
First decision: January 31, 2018
Article in press: April 1, 2018
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Markic D, Pandey CK, Salvadori M, Tarantino G S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
Contributor Information
Ahmed Aref, Department of Nephrology, Sur hospital, Sur 411, Sultanate of Oman; Faculty of Health and Science, Institute of Learning and Teaching, University of Liverpool, Liverpool L69 3GB, United Kingdom.
Tariq Zayan, Department of Nephrology, Sur hospital, Sur 411, Sultanate of Oman; Faculty of Health and Science, Institute of Learning and Teaching, University of Liverpool, Liverpool L69 3GB, United Kingdom.
Ajay Sharma, Faculty of Health and Science, Institute of Learning and Teaching, University of Liverpool, Liverpool L69 3GB, United Kingdom; Department of Transplantation Surgery, Royal Liverpool University Hospital, Liverpool L7 8XP, United Kingdom.
Ahmed Halawa, Faculty of Health and Science, Institute of Learning and Teaching, University of Liverpool, Liverpool L69 3GB, United Kingdom; Department of Transplantation Surgery, Sheffield Teaching Hospitals, Sheffield S5 7AU, United Kingdom. ahmed.halawa@sth.nhs.uk.
References
- 1.GAUER OH, HENRY JP, SIEKER HO. Changes in central venous pressure after moderate hemorrhage and transfusion in man. Circ Res. 1956;4:79–84. doi: 10.1161/01.res.4.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Zochios V, Ansari B, Jones N. Is central venous pressure a reliable indicator of fluid responsiveness in the critically ill? Br J Hosp Med (Lond) 2014;75:598. doi: 10.12968/hmed.2014.75.10.598. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 4.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 5.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntyre LA, Hébert PC, Fergusson D, Cook DJ, Aziz A; Canadian Critical Care Trials Group. A survey of Canadian intensivists' resuscitation practices in early septic shock. Crit Care. 2007;11:R74. doi: 10.1186/cc5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bignami E, Belletti A, Moliterni P, Frati E, Guarnieri M, Tritapepe L. Clinical practice in perioperative monitoring in adult cardiac surgery: is there a standard of care? Results from an national survey. J Clin Monit Comput. 2016;30:347–365. doi: 10.1007/s10877-015-9725-4. [DOI] [PubMed] [Google Scholar]
- 8.Sondergaard S, Parkin G, Aneman A. Central venous pressure: soon an outcome-associated matter. Curr Opin Anaesthesiol. 2016;29:179–185. doi: 10.1097/ACO.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 9.Smith T, Grounds RM, Rhodes A. Update in Intensive Care and Emergency Medicine (Book 42). Central Venous Pressure: Uses and Limitations. Springer. 2005:99–110. [Google Scholar]
- 10.Amoroso P, Greenwood RN. Posture and central venous pressure measurement in circulatory volume depletion. Lancet. 1989;2:258–260. doi: 10.1016/s0140-6736(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 11.Pilat J, Dabrowski W, Biernacka J, Bicki J, Rudzki S. Changes in intra-abdominal, iliac venous and central venous pressures in patients undergoing abdominal surgery due to large tumors of the colon--a pilot study. Acta Clin Croat. 2010;49:381–388. [PubMed] [Google Scholar]
- 12.De Gasperi A, Narcisi S, Mazza E, Bettinelli L, Pavani M, Perrone L, Grugni C, Corti A. Perioperative fluid management in kidney transplantation: is volume overload still mandatory for graft function? Transplant Proc. 2006;38:807–809. doi: 10.1016/j.transproceed.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal AK. Central vein stenosis. Am J Kidney Dis. 2013;61:1001–1015. doi: 10.1053/j.ajkd.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal AK, Patel BM, Haddad NJ. Central vein stenosis: a nephrologist's perspective. Semin Dial. 2007;20:53–62. doi: 10.1111/j.1525-139X.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 15.Geerts W. Central venous catheter-related thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014:306–311. doi: 10.1182/asheducation-2014.1.306. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava D, Sahu S, Chandra A, Tiwari T, Kumar S, Singh PK. Effect of intraoperative transesophageal Doppler-guided fluid therapy versus central venous pressure-guided fluid therapy on renal allograft outcome in patients undergoing living donor renal transplant surgery: a comparative study. J Anesth. 2015;29:842–849. doi: 10.1007/s00540-015-2046-4. [DOI] [PubMed] [Google Scholar]
- 17.Kumar L, Rajan S, Baalachandran R. Outcomes associated with stroke volume variation versus central venous pressure guided fluid replacements during major abdominal surgery. J Anaesthesiol Clin Pharmacol. 2016;32:182–186. doi: 10.4103/0970-9185.182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halawa A, Rowe S, Roberts F, Nathan C, Hassan A, Kumar A, Suvakov B, Edwards B, Gray C. A Better Journey for Patients, a Better Deal for the NHS: The Successful Implementation of an Enhanced Recovery Program After Renal Transplant Surgery. Exp Clin Transplant. 2018;16:127–132. doi: 10.6002/ect.2016.0304. [DOI] [PubMed] [Google Scholar]
- 19.Alhashemi JA, Cecconi M, Hofer CK. Cardiac output monitoring: an integrative perspective. Crit Care. 2011;15:214. doi: 10.1186/cc9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108:887–897. doi: 10.1213/ane.0b013e31818ffd99. [DOI] [PubMed] [Google Scholar]
- 21.Berkenstadt H, Margalit N, Hadani M, Friedman Z, Segal E, Villa Y, Perel A. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth Analg. 2001;92:984–989. doi: 10.1097/00000539-200104000-00034. [DOI] [PubMed] [Google Scholar]
- 22.Rex S, Brose S, Metzelder S, Hüneke R, Schälte G, Autschbach R, Rossaint R, Buhre W. Prediction of fluid responsiveness in patients during cardiac surgery. Br J Anaesth. 2004;93:782–788. doi: 10.1093/bja/aeh280. [DOI] [PubMed] [Google Scholar]
- 23.Preisman S, Kogan S, Berkenstadt H, Perel A. Predicting fluid responsiveness in patients undergoing cardiac surgery: functional haemodynamic parameters including the Respiratory Systolic Variation Test and static preload indicators. Br J Anaesth. 2005;95:746–755. doi: 10.1093/bja/aei262. [DOI] [PubMed] [Google Scholar]
- 24.Hofer CK, Müller SM, Furrer L, Klaghofer R, Genoni M, Zollinger A. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest. 2005;128:848–854. doi: 10.1378/chest.128.2.848. [DOI] [PubMed] [Google Scholar]
- 25.Wiesenack C, Fiegl C, Keyser A, Prasser C, Keyl C. Assessment of fluid responsiveness in mechanically ventilated cardiac surgical patients. Eur J Anaesthesiol. 2005;22:658–665. doi: 10.1017/s0265021505001092. [DOI] [PubMed] [Google Scholar]
- 26.Cannesson M, Slieker J, Desebbe O, Farhat F, Bastien O, Lehot JJ. Prediction of fluid responsiveness using respiratory variations in left ventricular stroke area by transoesophageal echocardiographic automated border detection in mechanically ventilated patients. Crit Care. 2006;10:R171. doi: 10.1186/cc5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Kim JT, Yoon SZ, Lim YJ, Jeon Y, Bahk JH, Kim CS. Evaluation of corrected flow time in oesophageal Doppler as a predictor of fluid responsiveness. Br J Anaesth. 2007;99:343–348. doi: 10.1093/bja/aem179. [DOI] [PubMed] [Google Scholar]
- 28.Cannesson M, Attof Y, Rosamel P, Desebbe O, Joseph P, Metton O, Bastien O, Lehot JJ. Respiratory variations in pulse oximetry plethysmographic waveform amplitude to predict fluid responsiveness in the operating room. Anesthesiology. 2007;106:1105–1111. doi: 10.1097/01.anes.0000267593.72744.20. [DOI] [PubMed] [Google Scholar]
- 29.Belloni L, Pisano A, Natale A, Piccirillo MR, Piazza L, Ismeno G, De Martino G. Assessment of fluid-responsiveness parameters for off-pump coronary artery bypass surgery: a comparison among LiDCO, transesophageal echochardiography, and pulmonary artery catheter. J Cardiothorac Vasc Anesth. 2008;22:243–248. doi: 10.1053/j.jvca.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Biais M, Nouette-Gaulain K, Cottenceau V, Revel P, Sztark F. Uncalibrated pulse contour-derived stroke volume variation predicts fluid responsiveness in mechanically ventilated patients undergoing liver transplantation. Br J Anaesth. 2008;101:761–768. doi: 10.1093/bja/aen277. [DOI] [PubMed] [Google Scholar]
- 31.Hofer CK, Senn A, Weibel L, Zollinger A. Assessment of stroke volume variation for prediction of fluid responsiveness using the modified FloTrac and PiCCOplus system. Crit Care. 2008;12:R82. doi: 10.1186/cc6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Waal EE, Rex S, Kruitwagen CL, Kalkman CJ, Buhre WF. Dynamic preload indicators fail to predict fluid responsiveness in open-chest conditions. Crit Care Med. 2009;37:510–515. doi: 10.1097/CCM.0b013e3181958bf7. [DOI] [PubMed] [Google Scholar]
- 33.Cannesson M, Musard H, Desebbe O, Boucau C, Simon R, Hénaine R, Lehot JJ. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2009;108:513–517. doi: 10.1213/ane.0b013e318192a36b. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann M, Feibicke T, Keyl C, Prasser C, Moritz S, Graf BM, Wiesenack C. Accuracy of stroke volume variation compared with pleth variability index to predict fluid responsiveness in mechanically ventilated patients undergoing major surgery. Eur J Anaesthesiol. 2010;27:555–561. doi: 10.1097/EJA.0b013e328335fbd1. [DOI] [PubMed] [Google Scholar]
- 35.Desgranges FP, Desebbe O, Ghazouani A, Gilbert K, Keller G, Chiari P, Robin J, Bastien O, Lehot JJ, Cannesson M. Influence of the site of measurement on the ability of plethysmographic variability index to predict fluid responsiveness. Br J Anaesth. 2011;107:329–335. doi: 10.1093/bja/aer165. [DOI] [PubMed] [Google Scholar]
- 36.Shin YH, Ko JS, Gwak MS, Kim GS, Lee JH, Lee SK. Utility of uncalibrated femoral stroke volume variation as a predictor of fluid responsiveness during the anhepatic phase of liver transplantation. Liver Transpl. 2011;17:53–59. doi: 10.1002/lt.22186. [DOI] [PubMed] [Google Scholar]
- 37.Broch O, Bein B, Gruenewald M, Höcker J, Schöttler J, Meybohm P, Steinfath M, Renner J. Accuracy of the pleth variability index to predict fluid responsiveness depends on the perfusion index. Acta Anaesthesiol Scand. 2011;55:686–693. doi: 10.1111/j.1399-6576.2011.02435.x. [DOI] [PubMed] [Google Scholar]
- 38.Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B, Tavernier B. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a "gray zone" approach. Anesthesiology. 2011;115:231–241. doi: 10.1097/ALN.0b013e318225b80a. [DOI] [PubMed] [Google Scholar]
- 39.Yazigi A, Khoury E, Hlais S, Madi-Jebara S, Haddad F, Hayek G, Jabbour K. Pulse pressure variation predicts fluid responsiveness in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2012;26:387–390. doi: 10.1053/j.jvca.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Bogović TZ, Bulum A, Hrabač P, Perić M, Tonković D, Pavlović DB, Baronica R. CVP vs. dynamic hemodynamic parameters as preload indicators in hemodynamically unstable patients after major surgery. Signa Vitae. 2017;13:56–60. [Google Scholar]