Abstract
In this paper, the effects of erythorbic acid (EA) treatment with different concentrations on the quality of Grifola frondosa fruiting bodies stored at 4 °C for 27 days were studied by determining the changes in moisture content, weight loss, browning, electrolyte leakage, malondialdehyde (MDA), and nutritional compounds. The activities of polyphenoloxidase (PPO), cellulase and other antioxidant enzymes including superoxide dismutase (SOD), catalase, and peroxidase (POD) were also measured. Results showed that 0.1% EA-treated G. frondosa fruiting body maintained lower weight loss (< 6.0%, w/w), electrolyte leakage (< 45.8%), MDA (< 4.17 µmol kg−1), and higher moisture content (> 90.7%, w/w). Lower activities of PPO (< 72.64 × 103 U kg−1) and cellulase (< 189.86 × 103 U kg−1) in 0.1% EA-treated samples were observed compared with the other treatments. As a stereoisomer of ascorbic acid (AA), EA also could enhance SOD and POD activities of G. frondosa fruiting bodies. Our findings were the first time to evaluate the effect of EA on maintaining quality in G. frondosa fruiting bodies, and proved that low concentrations of EA (especially 0.1% EA, w/v) treatments were beneficial to preserve G. frondosa fruiting body with even higher efficiency than AA treatment. This study paved a foundation for the enhancement of quality retention of G. frondosa fruiting bodies.
Keywords: Grifola frondosa, Erythorbic acid, Quality retention, Physico-chemical properties, Antioxidant enzymes
Introduction
Fresh and preserved mushrooms are valuable sources of many important nutrients and bioactive components and consumed in many countries as a delicacy, particularly for their specific flavor, aroma, and taste (Pavel 2013). Grifola frondosa is a precious mushroom and has a long history of edibility and widely accepted medicinal use in China, Japan, and other Asian countries (Fan et al. 2011). G. frondosa is commercially cultivated on beech sawdust, cotton seed composts, or other wood-containing wastes to harvest its highly nutritious fruiting body used as flavor food (Xing et al. 2006).
In general, fresh mushrooms deteriorate and senesce rapidly at ambient temperature due to the high levels of respiration rate, metabolic activity and water content, making them to have the undesirable changes of appearance and general quality and lose their marketability (Aguirre et al. 2008). To improve the physiological quality and prolong the shelf life of mushroom, many methods including lower temperature, controlled atmosphere storage, chemical, or physical treatments were taken into account. Sreenivas et al. (2012) investigated pretreatments of mushroom by chemicals such as calcium chloride (CaCl2), potassium metabisulfite (KMS), ethylenediaminetetraacetic (sodium EDTA), and citric acid to improve shelf life of ash gourd fresh cuts from 2 to 30 days. In general, combinations of chemical and physical treatments showed the attractive preserving effect and increasing interest. Gamma irradiation and modified atmosphere packaging were proved to extend the storage life of shiitake mushroom up to 20 days (Jiang et al. 2010a). However, few reports are involved to investigate the postharvest changes or protect/prolong the shelf life of G. frondosa.
Erythorbic acid (EA) (synonyms: d-isoascorbic acid, d-araboascorbic acid) is a stereoisomer of ascorbic acid (vitamin C). It is a novel food preservative and antioxidant with excellent safe performance for inhibiting the decrease of color, preventing the food oxidation, aroma and flavors, and blocking the production of the carcinogen ammonium nitrite during food manufacturing process (Lanigan 1999). It had been classified and generally recognized as safe (GRAS) additives by US Food and Drug Administration (FDA) and used in processed foods in accordance with Good Manufacturing Practice (GMP) (FAO/WHO Food Standards. https://www.codexalimentarius.net/gsfaonline/index.htmL; REHWOLDT. 1986). However, to our knowledge, there are no reports related to the use of EA for extending the storage life of mushrooms. Hence, the present study aims to evaluate the effects of different doses of EA combined with cold storage (4 °C) on visible changes related to postharvest deterioration as well as on selected enzymes for extending the shelf life of the commercial G. frondosa fruiting bodies.
Materials and methods
Mushrooms and chemical pretreatment
Grifola frondosa fruiting bodies were purchased in Yizheng Agriculture Technology Firm (Changzhou, Jiangsu, China). EA with purity of over 99% was provided from Parchn Sodium Isovitamin C Co., Ltd (Dexing, Jiangxi, China). The mushrooms were selected with uniform size and maturity, and randomly divided into five groups as control, positive control [0.5% ascorbic acid (AA)], 0.1% EA, 0.5% EA, and 1% EA (w/v), respectively. After treated with EA or AA solution for 30 s, G. frondosa fruiting bodies were placed at ambient temperature for 20 min, and then packaged in polyethylene bags and stored at 4 ± 1 °C for 27 days. Samples were taken every 3 days to analyze the parameters including moisture content, weight loss, browning, electrolyte leakage, malondialdehyde (MDA), nutritional compounds and the activities of polyphenoloxidase (PPO), cellulase, superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD).
The crude extract of G. frondosa fruiting bodies was prepared by homogenizing with 1:2 (w/v) of 50 mM sodium phosphate buffer (pH 7.8) and collecting the supernatant fraction by centrifugation at 6793 g for 20 min.
Moisture content, weight loss, and texture analysis
The moisture content was determined by oven drying method (Nielsen 2010). Weight loss was determined before and after storage (Ping et al. 2017a). Weight loss (%) = (m0 − m1) × m0−1 × 100%, where m0 was the initial weight and m1 was the weight during storage.
The texture of G. frondosa cap was determined using a TA.XT2i texture analyzer (Stable Micro Systems, Godalming, UK), with a 2 mm diameter cylindrical probe. Samples were penetrated 5 mm in depth with 2.0 mm s−1 of probe speed. From the force versus time curves, firmness was defined as the maximum force. Measurements were determined on 10 replicates for each sample.
MDA content and electrolyte leakage analysis
MDA content was measured according to the method reported previously by Xing et al. (2008) with modifications. 2 mL of crude extract of G. frondosa fruiting bodies was mixed with 2 mL of 0.6% 2-thiobarbituric acid (TBA), boiled for 15 min, and centrifuged at 2504 g for 10 min. The absorbance of supernatant using trichloroacetic acid (TCA) solution as blank was measured at 532, 600, and 645 nm. The MDA content was calculated through the formula, MDA (µmol kg−1) = [6.45 × (D532 − D600) − 0.56 × D450] V × W−1, where V was the supernatant volume and W was the fresh weight.
Electrolyte leakage was measured according to the method reported by Autio and Bramlage (1986) with modifications. The tissue discs of G. frondosa fruiting bodies with 5 mm thick and 5 mm diameter were prepared using a cork borer, and suspended in 40 mL of deionized water. The electrical conductivity was measured immediately (P0) and again after 10 min (P1). After boiled for 15 min and cooled, a final conductivity (P2) was measured. The relative electrolyte leakage rate was calculated as follows, relative electrolyte leakage rate (%) = (P1 − P0) × (P2 − P0)−1 × 100%.
Total soluble protein and sugar contents analysis
The concentrations of total soluble protein and sugar contents in the fresh G. frondosa were determined according to the Bradford (Kruger 1994; Liu et al. 2018) and dinitrosalicylic acid (DNS) methods (Miller 1959; Ping et al. 2017b), respectively.
Browning degree and PPO analysis
Browning degree was performed by a UV spectrophotometer (UV-1801, Beifen-Ruili Analytical Instrument, Beijing, China). The absorbance of G. frondosa crude extract was measured at 410 nm using deionized water as blank. Browning degree was expressed as Α410 × 10.
Spectrophotometric measurement of PPO activity was followed the method reported by Soliva et al. (2000). Briefly, 1 mL of 0.1 M catechol was used as substrate, 3.9 mL of sodium acetate buffer (pH 4.8, 50 mM) and 0.8 mL of the enzyme solution were added. The absorbance of crude enzyme solution was measured at 420 nm and recorded the increase in absorbance at 420 nm for 3 min. One unit (U) of PPO activity was defined as the amount of enzyme which increased the absorbance by 0.001 per min under the assay condition. The specific activity of PPO was expressed as U kg−1 fresh weight.
Cellulase activity analysis
Cellulase activity was assayed using DNS method to estimate reducing sugars released from CMC solubilized in 50 mmol acetate buffer 1% CMC (pH 4.8). 1 mL of crude extract was added to 2 mL of 1% CMC and incubated at 50 °C for 30 min. Reaction was stopped by the addition of 3 mL of DNS reagent and the reaction mixture was boiled for 5 min. The absorbance of the reaction using inactivated cellulase as blank was measured at 540 nm. One unit (U) of cellulase was defined as the amount of enzyme which liberated reducing sugars equivalent to 1 mg of glucose per h. The cellulase activity was expressed as follows, cellulase activity (U kg−1 fresh weight) = 5.56 × B × VT × (M × 0.5 × V1)−1, where B was the amount of C6H12O6 found by standard curve line (mg), VT was the supernatant volume (mL), M was the fresh weight (kg), V1 was the measurement volume (mL), and 0.5 was the reaction time (h).
Antioxidase (SOD, CAT, and POD) analysis
SOD, CAT, and POD were determined by antioxidase kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of 1 mL reaction system per kg protein. One unit of CAT was defined as the amount of enzyme that decomposes 1 µmol of H2O2 min−1 per kg tissues protein. One unit of POD was defined as the amount of enzyme that catalyzed 1 µg of substrate min−1 per kg tissues protein.
Statistical analysis
All experiments were performed in triplicate in this study unless otherwise noted (Liu et al. 2014; Ping et al. 2017c, d). Analysis of variance was carried out using the SAS program version 8.1 (SAS Institute Inc., Cary, NC, USA). The results were expressed as means ± standard deviations (Liu et al. 2017a, b). Statistical comparisons were made by one-way analysis of variance, followed by Duncan’s multiple-comparison test. Least significant differences represented as a, b, c, d and e were computed at P < 0.05. Figures presented in this study were drawn using the origin software version 8.0 (OriginLab Corp., Northampton, MA, USA).
Results and discussion
Effect of EA pretreatment on appearance of G. frondosa fruiting bodies
Figure 1 presents the appearance of treated or untreated G. frondosa fruiting bodies during 27-day storage at 4 °C. The treatment of G. frondosa fruiting bodies with different EA concentrations, especially at 0.1% (w/v) EA had prolonged the storage life from 3 to 12 days keeping G. frondosa fruiting bodies with stable color without spoilage, while brown rot was observed for untreated G. frondosa fruiting bodies (CK) at day 9, and the brown rot increased with the elongation of storage time led to rancidity of G. frondosa fruiting bodies. At the end of storage, all samples spoilage completely.
Fig. 1.
Appearance of G. frondosa fruiting bodies treated by dipping with EA and AA after 0, 3, 9, 12, 15, and 27 days of storage
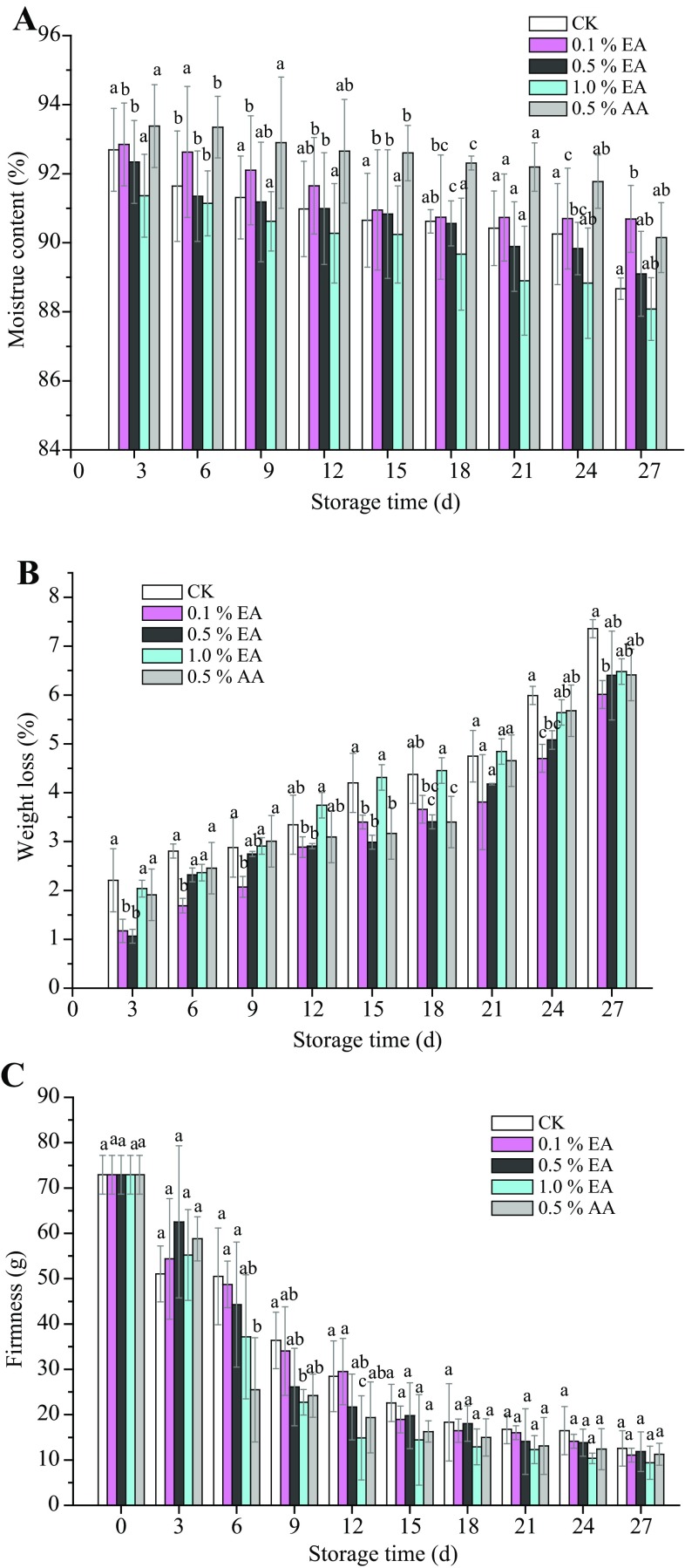
Effect of EA pretreatment on moisture content, weight, and texture
Owing to transpiration and respiration, moisture of all samples gradually decreased during storage (Fig. 2a). EA concentration of 0.1% (w/v) resulted in the minimum moisture loss of 2.2%. With the increase of EA concentration to 1.0% (w/v), moisture loss increased to 3.3%, which was comparable to that of AA treatment (0.5%, w/v), and lower than that of control samples (4.0%). Dehydration was the main factor involved in the deterioration of mushroom quality during postharvest storage (Khan et al. 2014). From Fig. 2b, weight loss of all treated groups reached to the highest level of 6.0–7.4% after 27-day storage. In general, 4–6% weight loss of fresh fruits and vegetables lose their practicability. At day 15, weight loss of control samples or 1% EA (w/v) treatment was 4.2 and 4.3%, respectively. In addition, loss commercial value with deteriorated appearance after 15-day storage. 0.1% EA, 0.5% EA, or 0.5% AA (w/v) treatments delayed the deterioration to over 21 days with weight loss 3.8, 4.2, and 4.7%, respectively. Specifically, 0.1% EA (w/v) treatment had the lowest weight loss (6.0%), which suggested that combination of EA or AA, polyethylene bags package and cold storage could reduce the weight loss of G. frondosa fruiting bodies. In some cases, package is also a key factor for mushroom freshness and weight loss. For example, unpackaged shitake mushrooms showed a weight loss of 72% after 6 days of storage, while application of packaging could prevent the rapid loss of water (Antmann et al. 2008). The firmness/texture change of mushrooms is one of the key issues during their deterioration. Figure 2c shows that the firmness of all samples decreased gradually from 72.93 to 12.90–18.32 g at the 18-day storage and kept at a stable level of about 12 ± 3 g within the following 9-day storage time. In general, the untreated G. frondosa fruiting bodies had the higher firmness (about 1.10 times) than those in EA or AA-treated samples. Among these treated samples, 0.1% EA (w/v) treatment showed a slight firmness reduction from 72.93 to 14.15 g during 24-day storage, indicating that 0.1% EA (w/v) could delay shriveling and avoid excessive softening of G. frondosa. Similar results were also found in shiitake mushrooms (Lentinula edodes) with slow firmness reduction from 3 to 2 N by modified atmosphere packaging (Jiang et al. 2010b).
Fig. 2.
Changes in moisture (a), weight (b), and firmness (c) of G. frondosa fruiting bodies treated in EA and AA. All mushrooms were packaged in polyethylene bags and stored at 4 ± 1 °C for 27 days. Each data point was the mean of three samples. Vertical bars represent standard deviation of means
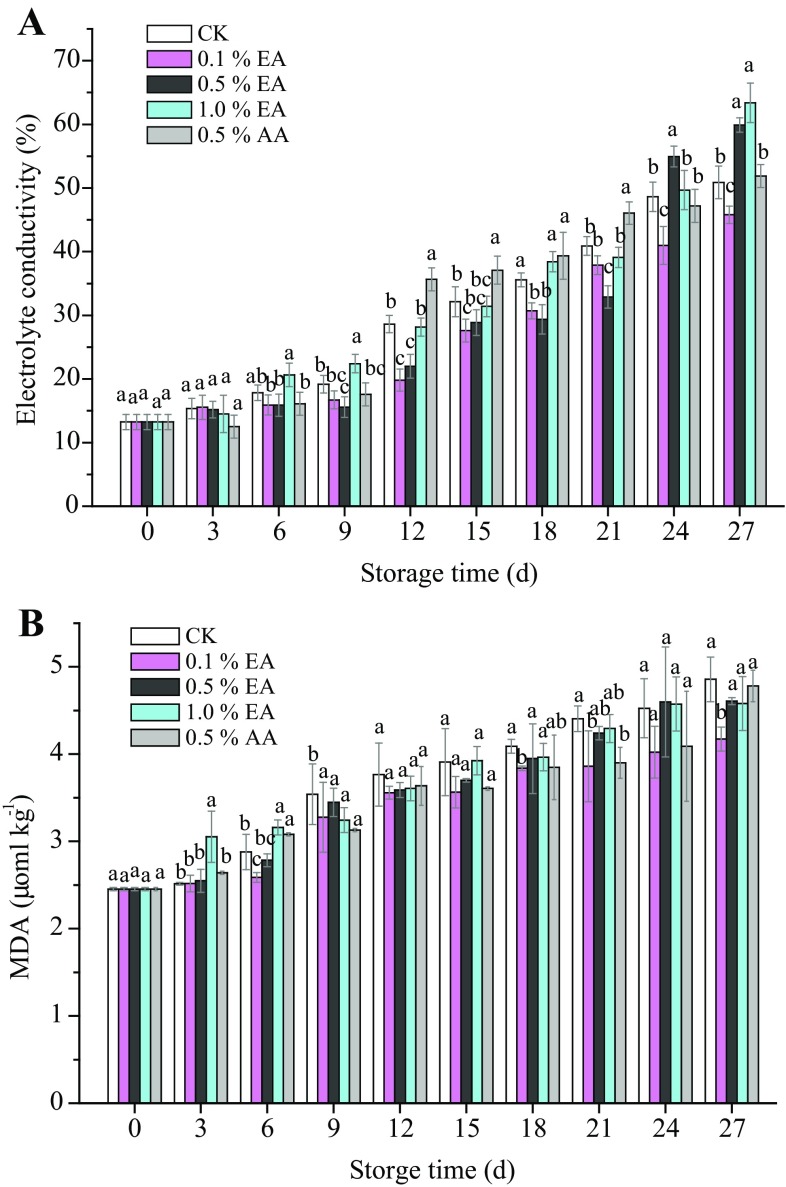
Effect of EA pretreatment on electrolyte leakage and MDA
Electrolyte leakage of plant tissues had been used as an indicator for tissue and membrane integrity in many studies (Liu et al. 2010). As shown in Fig. 3a, the electrolyte leakage changes of all G. frondosa samples were observed during 27-day storage. 0.1% EA (w/v) treatment contributed to the lowest electrolyte leakage of 45.8%. However, increase of EA concentration to 1% (w/v) resulted in the increased damage of G. frondosa cell membrane with the electrolyte leakage of over 50%.
Fig. 3.
Changes in electrolyte conductivity (a), MDA (b) of G. frondosa fruiting bodies treated in EA and AA. All mushrooms were packaged in polyethylene bags and stored at 4 ± 1 °C for 27 days. Each data point was the mean of three samples. Vertical bars represent standard deviation of means
MDA is regarded as an index of oxidative peroxidation, causing a reduction in membrane integrity, which can increase membrane leakage and mushrooms deterioration. The MDA content had the similar profiles to electrolyte leakage during the 27 days of storage (Fig. 3b). During storage, EA (w/v) or AA (w/v) treatment delayed the increase of MDA content from 2.45 µmol kg−1 to less 4.86 µmol kg−1. After 27-day storage, MDA contents of G. frondosa treated with 0.1, 0.5, 1% EA or 0.5% AA (w/v) increased to 4.17, 4.61, 4.58, and 4.78 µmol kg−1, respectively. Hence, it could be concluded that 0.1% of EA could effectively inhibit the electrolyte leakage and MDA production.
Effect of EA pretreatment on nutritional compounds
Total soluble sugars in mushrooms are closed to the carbohydrate metabolism and temporary energy storage and generally regarded as an indicator of postharvest deterioration of mushroom products (Jiang et al. 2013). The results shown in Fig. 4 indicated that the soluble sugar and protein concentrations in G. frondosa fruiting bodies reduced rapidly from 14944.04 mg kg−1 to 7059.04–7801.21 mg kg−1 (Fig. 4a), and 7431.82 mg kg−1 to 4154.92–4743.69 mg kg−1 (Fig. 4b), respectively, in the initial 6-day storage, and kept a steady decrease trend in the following period. In general, the initial storage period holds a high concentration of O2 and temperature which led to fast respiration. Jahangir et al. (2011) found the similar phenomena with the consumption of total soluble sugar and protein with 16-day storage in Agaricus bisporus fruiting bodies.
Fig. 4.
Changes in total soluble sugar (a) and soluble protein (b) of G. frondosa fruiting bodies treated in EA and AA. All mushrooms were packaged in polyethylene bags and stored at 4 ± 1 °C for 27 days. Each data point was the mean of three samples. Vertical bars represent standard deviation of means
Among these experiments, 0.5% of EA (w/v) treatment significantly inhibited the consumption of total soluble sugar (reduction ratios of 11924.75 mg kg−1) and soluble protein (reduction ratios of 4596.87 mg kg−1) in G. frondosa. EA and AA acted as antioxidants which might attribute to its antioxidant activity eliminating the produced active oxygen.
Effect of EA pretreatment on browning degree and PPO
Figure 5a shows the changes of PPO activity in G. frondosa fruiting bodies within 27-day postharvest period. The PPO activity in untreated G. frondosa (control) increased to a maximum level of 88.21 × 103 U kg−1 at day 9 storage, while that of 1% EA (w/v) treatment reached to the highest activity of 98.45 × 103 U kg−1 within 6 days. The lowest enzyme activity was detected in mushrooms treated in 0.1% EA (w/v) (P < 0.05). Lagnika et al. (2013) also found that A. bisporus fruiting bodies had the increased PPO activity from 4371 ± 2.64 to 6763 ± 6.02 U kg−1 at the initial 3-day storage, and then decreased to 3000 ± 21.82 U kg−1 in the following 9 days.
Fig. 5.
Changes in PPO (a), browning (b) of G. frondosa treated in EA and AA. All mushrooms were packaged in polyethylene bags and stored at 4 ± 1 °C for 27 days. Each data point was the mean of three samples. Vertical bars represent standard deviation of means
PPO can promote enzymatic browning by oxidizing mono- and di-phenols to o-quinones with the polymerization to produce brown pigments. The results in Fig. 5b indicated that 0.1% EA and 0.5% AA (w/v) treatments lowered the browning which correlating with results of determined PPO activity (P < 0.05). It proved that PPO played an important role of mushrooms browning. Cacace et al. (2002) also revealed that 5% (w/v) EA and 1% (w/v) citric acid treatment delayed L values (lightness) changes from 93.2 to 99.9, the growth of total aerobic microbial populations, and sensory alteration of fresh-cut potato storage at 6 °C.
Effect of EA pretreatment on cellulase activity
Cellulose played an important role during the development of plant cell walls. Cellulase promoted cellulose degradation leading to softing and decay of fruits and vegetables. The change of cellulase activity of all groups is shown in Fig. 6. 0.1% EA (w/v) treatments seemed to inhibit significantly cellulase activity in G. frondosa fruiting bodies from 189.86 × 103 to 10.12 × 103 U kg−1, followed by 0.5% EA and 0.5% AA (w/v) treatments, with the cellulase activities changes from 183.58 × 103 to 20.50 × 103 U kg−1 and from 194.19 × 103 to 31.17 × 103 U kg−1, respectively.
Fig. 6.
Changes in cellulase activity of G. frondosa fruiting bodies treated in EA and AA. All mushrooms were packaged in polyethylene bags and stored at 4 ± 1 °C for 27 days. Each data point was the mean of three samples. Vertical bars represent standard deviation of means
Effect of EA pretreatment on antioxidases
In mushrooms, the antioxidant enzymes including SOD, CAT, and POD respond to physiological changes as direct antioxidants scavenging O2− and H2O2. Combination of ascorbic acid with high O2 modified atmosphere packaging could alleviate browning and delay quality loss of fresh-cut eggplants (Li et al. 2014) and inhibit the PPO activity and improve radical scavenging activity of the DPPH and ABTS in A. bisporus (Hyejin and Gunhee 2014). In the present study, SOD activity in all groups rapidly increased at the beginning of 6 days, and decreased gradually in the following storage period (Table 1). EA and AA treatments maintained the higher SOD activities than control samples within the initial 9 days. Among these groups, 0.1% EA (w/v) treatments gave the highest SOD activity of (298.40 ± 2.97) × 106 U kg−1 protein on the 6th day. CAT activity in each experimental group kept increase during the 27-day storage. However, storage stage also affected the CAT activities among groups. For example, 0.1% EA (w/v)-treated G. frondosa had a higher CAT activity at the initial 15-day storage, and then lowered within the following days than those of control sample. POD activity in EA treatment kept the higher level than those in untreated or AA-treated group. Among the groups, 0.1% EA (w/v) gave the highest POD activities of 37.76, 48.33, or 35.89 × 106 U kg−1 protein at 3rd, 24th, and 27th storage day, respectively. In this study, EA as a stereoisomer of AA could enhance SOD, CAT, and POD activity of G. frondosa fruiting bodies and the efficacy was better than that of AA, especially 0.1% EA (w/v) treatment.
Table 1.
Changes in SOD, CAT, and POD activity of G. frondosa fruiting bodies treated in EA and AA, respectively
| Days at 4 °C | CK | 0.1% EA | 0.5% EA | 1% EA | 0.5% AA |
|---|---|---|---|---|---|
| T-SOD (106 U kg−1 protein) | |||||
| 3 | 187.44 ± 49.46a | 219.73 ± 39.76a | 219.47 ± 72.51a | 198.49 ± 58.00a | 208.43 ± 2.98a |
| 6 | 241.79 ± 53.07b | 298.40 ± 2.97a | 274.51 ± 0.16ab | 232.11 ± 4.40b | 265.53 ± 0.53ab |
| 9 | 169.10 ± 9.71d | 255.28 ± 7.27a | 220.69 ± 9.04b | 198.39 ± 7.54c | 215.51 ± 4.57b |
| 15 | 152.96 ± 33.24a | 141.67 ± 18.24a | 168.59 ± 32.89a | 132.09 ± 8.65a | 148.12 ± 2.08a |
| 18 | 129.69 ± 9.71ab | 138.20 ± 24.38a | 125.34 ± 1.90ab | 120.26 ± 4.40ab | 113.36 ± 3.75b |
| 21 | 85.89 ± 4.71b | 128.63 ± 2.50a | 95.95 ± 2.01b | 91.66 ± 23.53b | 80.56 ± 14.93b |
| 24 | 76.28 ± 3.22b | 76.29 ± 1.29b | 77.87 ± 1.09b | 86.42 ± 3.34a | 83.18 ± 8.71ab |
| 27 | 65.85 ± 4.41ab | 74.17 ± 6.42a | 89.31 ± 35.99a | 56.90 ± 5.01ab | 37.19 ± 1.78b |
| CAT (106 U kg−1 protein) | |||||
| 3 | 1190.42 ± 54.41b | 1295.04 ± 55.01a | 1331.47 ± 2.92a | 1223.26 ± 19.95b | 1205.88 ± 12.17b |
| 6 | 1166.34 ± 4.91c | 1410.48 ± 17.52a | 1310.92 ± 27.70b | 1278.23 ± 63.37b | 1302.80 ± 29.58b |
| 9 | 1220.25 ± 9.65a | 1243.97 ± 40.72a | 1234.26 ± 22.20a | 1234.26 ± 24.80a | 1252.34 ± 12.17a |
| 15 | 1296.31 ± 17.52b | 1456.37 ± 23.55a | 1329.10 ± 30.78b | 1314.91 ± 19.95b | 1223.30 ± 13.71c |
| 18 | 1239.31 ± 25.61ab | 1213.14 ± 60.36b | 1245.25 ± 38.74ab | 1279.30 ± 14.94ab | 1311.80 ± 35.82a |
| 21 | 1864.14 ± 28.35a | 1802.33 ± 23.66ab | 1566.76 ± 59.12c | 1451.39 ± 53.14d | 1753.42 ± 42.26b |
| 24 | 2516.36 ± 49.93a | 2144.52 ± 10.90b | 1764.64 ± 26.34c | 1684.12 ± 26.96d | 1775.36 ± 28.58c |
| 27 | 2829.21 ± 28.43a | 2857.74 ± 27.13a | 2560.44 ± 23.45c | 2061.56 ± 15.26d | 2734.91 ± 30.21b |
| POD (106 U kg−1 protein) | |||||
| 3 | 32.03 ± 0.28a | 37.76 ± 1.65a | 32.50 ± 11.59a | 30.97 ± 6.58a | 25.20 ± 5.41a |
| 6 | 15.73 ± 0.44a | 24.30 ± 2.33a | 24.52 ± 8.27a | 21.06 ± 1.98a | 19.89 ± 5.57a |
| 9 | 16.79 ± 1.59b | 24.20 ± 5.14a | 25.16 ± 5.45a | 23.23 ± 1.82ab | 18.47 ± 2.01ab |
| 15 | 16.34 ± 3.52a | 18.90 ± 5.57a | 20.96 ± 1.15a | 16.86 ± 1.38a | 17.60 ± 3.42a |
| 18 | 34.81 ± 2.95b | 34.24 ± 1.65b | 33.59 ± 0.92b | 33.02 ± 6.86b | 43.93 ± 1.77a |
| 21 | 42.45 ± 5.10a | 45.14 ± 3.03a | 45.66 ± 4.38a | 39.38 ± 0.81a | 32.13 ± 0.90b |
| 24 | 31.60 ± 1.17c | 48.33 ± 3.70a | 46.88 ± 2.42a | 39.37 ± 2.34b | 24.90 ± 1.07d |
| 27 | 24.32 ± 3.22c | 35.89 ± 1.64a | 28.81 ± 1.33b | 27.56 ± 1.76bc | 8.17 ± 1.80d |
All mushrooms were packaged in polyethylene bags and stored at 4 ± 1 °C for 27 days
Each data point was the mean of three replicate samples. a, b, c and d represent the significant difference for p < 0.05.
Conclusions
Effects of EA treatment on maintaining quality in G. frondosa fruiting bodies during the postharvest storage were investigated. The results suggested that EA and AA treatments controlled the increase of weight loss and MDA by inhibiting cellulase activity and improving antioxidase activities when compared with control treatment. Higher moisture content, lower electrolyte leakage, and PPO activity were observed in 0.1% EA (w/v) treatment compared with other treatments. 0.5% EA (w/v) treatment had higher level of total soluble protein and soluble sugar content followed by 0.1% EA treatment. In summary, treatment in low EA concentrations (especially 0.1% EA, w/v) was a simple, economical, and effective method for maintaining quality and extending postharvest life of G. frondosa fruiting bodies at relatively low temperature. This study laid a foundation for the enhancement of quality retention of Grifola frondosa fruiting bodies.
Acknowledgements
The authors gratefully acknowledge the financial supports from the National Natural Science Foundation of China (31771961), the earmarked fund for Shanghai Modern Edible Fungi-industry Technology Research System (2017, No. 9), China Postdoctoral Science special Foundation (2013T60648), 2012 Excellent Key Young Teachers Project of Jiangsu University, Science & Technology Platform Construction Program of Jiangxi Province.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Lifeng Ping and Fengmei Chen contributed equally to this work.
Contributor Information
Lifeng Ping, Phone: +86-571-85070528, Email: lfping2015@zust.edu.cn.
Fengjie Cui, Phone: +86-511-88780201, Email: fengjiecui@163.com.
References
- Aguirre L, Jesus MF, Catherine BR, Helen G. Modeling browning and brown spotting of mushrooms (Agaricus bisporus) stored in controlled environmental conditions using image analysis. J Food Eng. 2008;91:280–286. doi: 10.1016/j.jfoodeng.2008.09.004. [DOI] [Google Scholar]
- Antmann G, Ares G, Lema P, Lareo C. Influence of modified atmosphere packaging on sensory quality of shiitake mushrooms. Postharvest Biol Technol. 2008;49:164–170. doi: 10.1016/j.postharvbio.2008.01.020. [DOI] [Google Scholar]
- Autio WR, Bramlage WJ. Chilling sensitivity of tomato fruit in relation to ripening and senescence. J Am Soc Hortic Sci. 1986;111:201–204. [Google Scholar]
- Cacace JE, Delaquis PJ, Mazza G. Effect of chemical inhibitors and storage temperature on the quality of fresh-cut potatoes. J Food Qual. 2002;25:181–195. doi: 10.1111/j.1745-4557.2002.tb01018.x. [DOI] [Google Scholar]
- Fan YN, Wu XY, Zhang M. Physical characteristics and antioxidant effect of polysaccharides extracted by boiling water and enzymolysis from Grifola frondosa. Int J Biol Macromol. 2011;48:798–803. doi: 10.1016/j.ijbiomac.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Hyejin P, Gunhee K. Effect of application of rice bran extract on quality of Agaricus Bisporus during storage. Korean J Hortic Sci Technol. 2014;32:834–844. doi: 10.7235/hort.2014.13185. [DOI] [Google Scholar]
- Jahangir MM, Jiang TJ, Jiang ZH, Amjad M, Ying TJ. Methyl jasmonate enhances postharvest physicochemical and microbial quality of button mushroom (Agaricus bisporus) J Food Agric Environ. 2011;9:91–95. [Google Scholar]
- Jiang TJ, Luo SS, Chen QP. Effect of integrated application of gamma irradiation and modified atmosphere packaging on physicochemical and microbiological properties of shiitake mushroom (Lentinus edodes) Food Chem. 2010;122:761–767. doi: 10.1016/j.foodchem.2010.03.050. [DOI] [Google Scholar]
- Jiang T, Wang Q, Xu S, Jahangir MM, Ying T. Structure and composition changes in the cell wall in relation to texture of shitake mushrooms (Lentinula edodes) stored in modified atmosphere packaging. J Soc Chem Ind. 2010;90:742–749. doi: 10.1002/jsfa.3876. [DOI] [PubMed] [Google Scholar]
- Jiang T, Feng L, Zheng X, Li JR. Physicochemical responses and microbial characteristics of shiitake mushroom (Lentinus edodes) to gum arabic coating enriched with natamycin during storage. Food Chem. 2013;138:1992–1997. doi: 10.1016/j.foodchem.2012.11.043. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Aisikaer G, Khan RU. Effects of composite chemical pretreatment on maintaining quality in button mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biol Technol. 2014;95:36–41. doi: 10.1016/j.postharvbio.2014.04.001. [DOI] [Google Scholar]
- Kruger NJ. The Bradford method for protein quantitation. In: Walker JM, editor. Basic protein and peptide protocols. Methods in molecular biology. New York: Humana Press; 1994. [Google Scholar]
- Lagnika C, Zhang M, Mothibe KJ. Effects of ultrasound and high pressure argon on physico-chemical properties of white mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biol Technol. 2013;82:87–94. doi: 10.1016/j.postharvbio.2013.03.006. [DOI] [Google Scholar]
- Lanigan RS. Final report on the safety assessment of ascorbyl palmitate, ascorbyl dipalmitate, ascorbyl stearate, erythorbic acid, and sodium erythorbate. Int J Toxicol. 1999;18:1–26. [Google Scholar]
- Li X, Jiang Y, Li W, Tang Y, Yun J. Effects of ascorbic acid and high oxygen modified atmosphere packaging during storage of fresh-cut eggplants. Food Sci Technol. 2014;20:99–108. doi: 10.1177/1082013212472351. [DOI] [PubMed] [Google Scholar]
- Liu ZL, Wang XY, Zhu JY, Wang J. Effect of high oxygen modified atmosphere on post-harvest physiology and sensorial qualities of mushroom. Int J Food Sci Technol. 2010;45:1097–1103. doi: 10.1111/j.1365-2621.2010.02199.x. [DOI] [Google Scholar]
- Liu ZQ, Zhang XH, Xue YP, Xu M, Zheng YG. Improvement of alcaligenes faecalis nitrilase by gene site saturation mutagenesis and its application in stereospecific biosynthesis of (R)-()-mandelic acid. J Agric Food Chem. 2014;62:4685–4694. doi: 10.1021/jf405683f. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Dong SC, Yin HH, Xue YP, Tang XL, Zhang XJ, He JY, Zheng YG. Enzymatic synthesis of an ezetimibe intermediate using carbonyl reductase coupled with glucose dehydrogenase in an aqueous-organic solvent system. Bioresour Technol. 2017;229:26–32. doi: 10.1016/j.biortech.2016.12.098. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Wu L, Zhang XJ, Xue YP, Zheng YG. Directed evolution of carbonyl reductase from Rhodosporidium toruloides and its application in stereoselective synthesis of tert-Butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate. J Agric Food Chem. 2017;65:3721–3729. doi: 10.1021/acs.jafc.7b00866. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Wu L, Zheng L, Wang WZ, Zhang XJ, Jin LQ, Zheng YG. Biosynthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate by carbonyl reductase from Rhodosporidium toruloides in mono and biphasic media. Bioresour Technol. 2018;249:161–167. doi: 10.1016/j.biortech.2017.09.204. [DOI] [PubMed] [Google Scholar]
- Miller G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Nielsen SS. Determination of moisture content. In: Nielsen SS, editor. Food analysis laboratory manual (Food Science Texts Series) Boston: Springer; 2010. [Google Scholar]
- Pavel K. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J Sci Food Agric. 2013;93:209–218. doi: 10.1002/jsfa.5960. [DOI] [PubMed] [Google Scholar]
- Ping LF, Chen XY, Yuan XL, Zhang M, Chai YJ, Shan SD. Application and comparison in biosynthesis and biodegradation by Fusarium solani and Aspergillus fumigatus cutinases. Int J Biol Macromol. 2017;104:1238–1245. doi: 10.1016/j.ijbiomac.2017.06.118. [DOI] [PubMed] [Google Scholar]
- Ping L, Wang M, Yuan X, Cui F, Huang D, Sun W, Zou B, Huo S, Wang H. Production and characterization of a novel acidophilic and thermostable xylanase from Thermoascus aurantiacu. Int J Biol Macromol. 2017;109:1270–1279. doi: 10.1016/j.ijbiomac.2017.11.130. [DOI] [PubMed] [Google Scholar]
- Ping LF, Guo Q, Chen XY, Yuan XL, Zhang CR, Zhao H. Biodegradation of pyrene and benzo[a]pyrene in the liquid matrix and soil by a newly identified Raoultella planticola strain. 3 Biotech. 2017;7:56. doi: 10.1007/s13205-017-0704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping LF, Zhang CR, Cui H, Yuan XL, Cui JT, Shan SD. Characterization and application of a newly isolated pyrene-degrading bacterium, Pseudomonas monteilii. 3 Biotech. 2017;7:309. doi: 10.1007/s13205-017-0945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliva RC, Elez P, Sebastia MM, Martin O. Evaluation of browning effect on avocado purée preserved by combined methods. Innov Food Sci Emerg. 2000;1:261–268. doi: 10.1016/S1466-8564(00)00033-3. [DOI] [Google Scholar]
- Sreenivas KM, Singhal RS, Lele SS. Chemical pretreatments and partial dehydration of ash gourd (Benincasa hispida) pieces for preservation of its quality attributes. LWT Food Sci Technol. 2012;44:2281–2284. doi: 10.1016/j.lwt.2011.06.009. [DOI] [Google Scholar]
- Xing ZT, Cheng JH, Tan Q, Pan YJ. Effect of nutritional parameters on laccase production by the culinary and medicinal mushroom, Grifola frondosa. World J Microbiol Biotechnol. 2006;22:1215–1221. doi: 10.1007/s11274-006-9163-z. [DOI] [Google Scholar]
- Xing ZT, Wang YS, Feng ZY, Tan Q. Effect of different packaging films on postharvest quality and selected enzyme activities of Hypsizygus marmoreus mushrooms. J Agric Food Chem. 2008;56:11838–11844. doi: 10.1021/jf8024387. [DOI] [PubMed] [Google Scholar]