Abstract
This study investigated the recovery of phytochemical antioxidants in Dacryodes rostrata fruit using different extraction solvents. The effects of solvent of varying polarities with sequential extraction method on the recovery of phenolics, flavonoids, carotenoids and anthocyanins from different parts of the fruit (seed, pulp and peel) were determined. Their antioxidant activities were further determined using DPPH radical, ferric reducing antioxidant power (FRAP), hydroxyl radical scavenging, superoxide anion radical scavenging and phosphomolybdenum method. Dacryodes Rostrata seed had the highest total phenolic content with 50% ethanol as the most efficient extraction solvent. The highest total flavonoid content was obtained in ethyl acetate extract of fruit pulp, whereas peel extracted with hexane and 50% ethanol was the highest in total carotenoid content and total anthocyanin content, respectively. The seed extracted with 50% ethanol exhibited the strongest DPPH radical scavenging activity. Iron chelating activity measured by FRAP assay was the best in seed extracts, particularly in those polar extracts derived from water and 50% ethanol. Antioxidant activities of 50% ethanol extract of D. rostrata seed was the highest when determined by FRAP and phosphomolydenum assays. However, the influence of extraction solvents is not distinctly shown by hydroxyl radical and superoxide anion radical scavenging activities. This is the first report on the effect of various extraction solvents on the recovery of phytochemicals in D. rostrata fruit parts and the seed of D. rostrata is a potential source of polar antioxidants.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3170-6) contains supplementary material, which is available to authorized users.
Keywords: Anthocyanin, Antioxidant, Carotenoid, Flavonoid, Kembayau, Polyphenol
Introduction
Dacryodes rostrata (DR) is a wildly grown higher plant in Malaysia with limited scientific information. The fruit of DR is better known as “kembayau”. It is one of the indigenous fruits found in Borneo Island. DR fruit is a single-seeded drupe and ovoid to oblong in shape, measuring height range of 3–4 cm and has an average weight of 12.0 ± 2.5 g. The weight of the fruit is mainly contributed by its seed (54.01–56.72%), followed by pulp and peel. The skin of unripe DR fruit is in pale yellow or to be white in colour and subsequently turns black when it ripens. Owing to its hard texture, DR fruit is normally consumed after blanching with hot water by the local people. It is also preserved using salts or black soya sauce and taken as an accompaniment of rice or porridge (Kong et al. 2011).
Besides exploring the nutritional composition of DR fruit, appraisal and recovery of its phytochemical antioxidants using different extraction solvents will enable the development of natural antioxidants and nutraceuticals for food, cosmetic and other application. It has been suggested by Nikousaleh and Prakash (2016) that varying solvents and extraction conditions have a prominent effect on the accuracy of quantification of antioxidants content. To date, aqueous ethanol has been used in the extraction of antioxidant from DR fruit (Kong et al. 2011). However, the influence of other solvents on the recovery of antioxidants in seed, pulp and peel of DR has not been identified (Tee et al. 2015).
Antioxidative compounds in natural sources have different chemical properties, polarity and stability, hence affect their solubility (Nikousaleh and Prakash 2016). Type of solvent employed in the extraction and recovery of antioxidative compounds from fruit was reported to be very influential due to the interaction between bioactive compounds and the solvents are depending on their chemical structures and polarities (Prasad et al. 2010). Therefore, the present study aimed to measure the abilities of different extraction solvents in recovering phytochemical antioxidants of the fruit parts. This study also comprehensively measured the antioxidant potential of all fruit parts.
Materials and methods
Analytical reagents and chemicals
Analytical grade ethanol, butanol, ethyl acetate and n-hexane and other chemicals were obtained from the general suppliers. Analytical standards including gallic acid, quercetin, β-carotene and butylated hydroxytoluene were purchased from Sigma-Aldrich (M) Sdn. Bhd. (Kuala Lumpur, Malaysia).
Sample preparation and extraction
About 10 kg of fresh DR fruit was obtained from Sarawak, Malaysia. Upon arrival, the fruit was washed with tap water before separated into three parts: peel, pulp and seed. All samples were cut into smaller pieces of about an average of 1.0 × 1.0 cm in size. The samples were then lyophilised and ground into powder using a form before stored at − 20 °C for further analyses.
Extraction and recovery of phytochemical antioxidants in the samples (10 g each of seed, pulp and peel) were performed according to the sequential extraction method using different extraction solvents including hexane, ethyl acetate, butanol, 50% ethanol and water. The extraction method was adapted from the method described by Prasad et al. (2013) with some modifications. Each solvent extraction was done using 250 mL of solvent (solvent to solid ratio of 25:1) at 30 °C and 300 rpm assisted by an orbital shaker for 90 min. Each extract was then filtered and the remaining residue was subjected to re-extraction. All extracts were pooled and concentrated to dryness using a rotary evaporator and water extract was dried under lyophilisation.
Total phenolic content
Total phenolic content (TPC) was determined according to a modified method described by Kong et al. (2011). Briefly, 50 μL of the previously diluted sample (80 μg/mL) or standard at different concentrations was added with 50 μL of 1 N Folin–Ciocalteu reagent and incubated for 2 min. A 900 μL of NaCl (2% w/v) was then added to the mixture. The solution was left at ambient temperature for 30 min. The absorbance was measured at 750 nm using a spectrophotometer. A standard calibration curve of gallic acid (0.01–1.00 mg/mL) was plotted to calculate the TPC and expressed as mg gallic acid equivalent (GAE)/100 g freeze-dried sample (FDS).
Total flavonoid content
Total flavonoid content (TFC) was determined based on a modified method of Liu et al. (2008). A 500 μL of previously diluted sample (80 μg/mL) was added with 30 μL of 5% NaNO2, followed by the addition of 30 μL of 10% AlCl3. After 6 min, 200 μL of 1 M NaOH was added. Then, 95% ethanol was used to top up the mixture to a final volume of 1 mL before the absorbance was measured at 510 nm. The calibration curve was plotted using quercetin as a standard (0.01–1.00 mg/mL) and all values were expressed as mg quercetin equivalent (QE)/100 g of FDS.
Total anthocyanin content
Total anthocyanin content (TAC) was determined using a pH differential method described by Giusti and Wrolstad (2005). A 125 μL of sample (80 μg/mL) was evenly mixed with 875 μL of 0.025 M KCl buffer (pH 1). The mixture was allowed to stand for 15 min and the absorbance was measured at 510 and 700 nm. Following the similar procedure, the sample was then added to 0.025 M sodium acetate buffer at pH 4.5 and the absorbance was read at 510 nm and 700 nm after 15 min. The total anthocyanin content was calculated as follows:
| 1 |
where A is the absorbance of the sample determined as follows:
| 2 |
MW = Molecular weight for cyanidin-3-glucoside which is 449.2 g/mol; DF = Dilution factor of the sample, ε = Molar absorptivity of cyanidin-3-glucoside (26,900 M/cm). The result was expressed as mg cyanidin-3-glucoside equivalent (CE)/100 g FDS.
Total carotenoid content
A spectrophotometric method for estimation of total carotenoid content in fruits was employed (Khoo et al. 2008). The diluted sample (80 μg/mL) was centrifuged for 10 min at 3000 rpm. The supernatant was deliberately collected for the absorbance reading. The absorbance value was taken at 450 nm. A standard calibration curve of β-carotene (0.01–0.05 mg/mL) was plotted to calculate the results. Data were expressed as mg β-carotene equivalent (BE)/100 g FDS.
DPPH scavenging activity
DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity was measured based on the modified method of Brand-Williams et al. (1995). An aliquot of 100 μL sample or butylated hydroxytoluene (BHT) at varying concentrations (20-100 μg/mL) was mixed with 900 μL of 0.2 mΜ DPPH solution prepared in methanol and was left to react for 30 min at room temperature. The absorbance reading was then taken at 515 nm using the spectrophotometer. Absorbance of a control, containing only 0.2 mΜ DPPH solution, was measured. The percentage (%) of DPPH radical scavenging activity was calculated. Data were presented as IC50 value for each sample. IC50 is the concentration of sample or standard (BHT) that inhibits 50% of the DPPH radicals in µg/mL.
Ferric reducing antioxidant power
Ferric reducing antioxidant power (FRAP) was done based on the modified method of Benzie and Strain (1996). Initially, the three reagents were prepared, namely 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM hydrochloric acid and 20 mM FeCl3. FRAP reagent was then prepared by mixing acetate buffer, TPTZ solution and FeCl3 solution at a ratio of 10:1:1 (v/v/v), respectively. Briefly, 50 μL of the sample or BHT at varying concentrations (20–100 μg/mL) was added with 950 μL of the FRAP reagent, followed by an incubation at 37 °C. Absorbance reading was taken at 593 nm after 30 min of incubation. Data were calculated using a calibration curve of FeSO4 at concentrations ranged 0.2-1.0 mM. The results were presented as mM Fe(II) equivalent.
Antioxidant activity by phosphomolybdenum method
Phosphomolybdenum method was performed according to the method described by Prasad et al. (2010). Phosphomolybdenum reagent was first prepared to consist 0.6 M sulphuric acid, 4 mM ammonium molybdate and 28 mM sodium phosphate. An aliquot of 0.1 mL of sample or BHT at varying concentrations (20–100 μg/mL) was then mixed with 1 mL of phosphomolybdenum reagent. The mixture was incubated in a water bath at 95 °C over a period of 90 min. The absorbance was taken at 695 nm. Antioxidant activity was expressed as absorbance value read at 695 nm in which a higher absorbance value represents a higher antioxidant activity.
Hydroxyl radical scavenging activity
Measurement of hydroxyl radical scavenging activity was done based on the method reported by Jan et al. (2013) with some modifications. A reactive mixture was first prepared by mixing 400 μL of 0.2 Μ phosphate buffer (pH 7.4), 50 μL of 50 mΜ 2-deoxyribose, 50 μL of 50 mΜ hydrogen peroxide, 50 μL of 3.2 mΜ iron chloride, 50 μL of 1 mΜ disodium EDTA and 50 μL of 1.8 mΜ ascorbate. Then, 50 μL of sample or BHT (20–100 μg/mL) was added to the reacting mixture and total volume of the mixture was adjusted to 800 μL with phosphate buffer. The mixture was thoroughly shaken and incubated at 50 °C for 20 min. The reaction was terminated with 250 μL of 10% trichloroacetic acid. The colour was developed by adding 150 μL of 5% 2-thiobarbituric acid (prepared in 1.25% sodium hydroxide). The mixture was heated in water bath at 95 °C for 15 min and then left to cool to room temperature before measuring the absorbance at 532 nm. The percentage (%) of hydroxyl radical scavenging activity was calculated. Data were expressed as IC50 value (µg/mL) of each sample.
Superoxide anion radical scavenging activity
Superoxide anion radical scavenging activity was determined based on the method described by Li et al. (2012) with slight modification. The sample or BHT (150 μL) at varying concentrations (20–100 μg/mL) was added to a reacting mixture consisted of 1310 μL of 0.05 M Tris–HCl (pH 7.4) and 1 mM EDTA. A 40 μL of 60 mM pyrogallol (prepared in 1 mM HCl) was then added to the mixture, followed by an immediate shaking at room temperature. The initial absorbance (time = 0 min) at 325 nm was immediately measured and another reading was taken after 5 min. The experiment was carried out at 37 °C. The percentage (%) of superoxide anion radical scavenging activity was calculated. Data were expressed as IC50 value (µg/mL) of each sample.
Statistical analysis
All experiments were conducted in triplicates and all values were expressed as mean ± standard deviation. SPSS Statistics version 22 was used to analyse all the data obtained. Significant differences were evaluated using analysis of variance (ANOVA), followed by Duncan’s new multiple range tests, where p value less than 0.05 was considered significantly different.
Results and discussion
Total phenolic content
The highest TPC was recovered from the seed of DR, ranged between 22.3 and 3016.5 mg GAE/100 g FDS (Table 1). This was followed by peel and pulp of the fruit which ranged from 20.5 to 1651.5 mg GAE/100 g FDS and 12.7 to 1135.5 mg GAE/100 g FDS, respectively. The trend of current finding was in agreement with a previous study done by Kong et al. (2011), where a similar trend on TPC of different fruit parts of DR was reported. Statistical results also showed that TPC significantly differed among different parts of DR extracted with the solvents used, except hexane and water.
Table 1.
Antioxidant components of Dacryodes rostrata fruit parts extracted using different solvents
| Antioxidant component | Solvents | Seed | Pulp | Peel |
|---|---|---|---|---|
| Total phenolic content (mg GAE/100 g FDS) | Hexane | 22.3 ± 3.1e,x | 22.3 ± 3.1d,x | 20.5 ± 3.1d,x |
| Ethyl acetate | 122.9 ± 2.9c,x | 12.7 ± 1.1f,y | 44.6 ± 4.4b,z | |
| Butanol | 1035.2 ± 10.5b,x | 60.2 ± 5.0b,y | 35.0 ± 2.2c,z | |
| Ethanol | 3016.5 ± 17.7a,x | 1135.5 ± 4.4a,y | 1651.5 ± 19.6a,z | |
| Water | 47.1 ± 1.1d,x | 30.9 ± 1.1c,y | 43.9 ± 1.9b,x | |
| Total flavonoid content (mg QE/100 g FDS) | Hexane | 35.4 ± 12.2c,x | 177.2 ± 12.2c,y | 148.8 ± 56.2c,y |
| Ethyl acetate | 5879.6 ± 45.5b,x | 9289.8 ± 60.7a,y | 3372.8 ± 52.6a,z | |
| Butanol | 860.9 ± 122.8a,x | 30.3 ± 13.1d,y | 25.3 ± 8.7d,y | |
| Ethanol | 8189.0 ± 241.2a,x | 387.4 ± 22.7b,y | 941.9 ± 80.3b,z | |
| Water | 17.7 ± 4.3d,x,y | 10.1 ± 4.3e,x | 30.3 ± 15.1d,y | |
| Total anthocyanin content (mg CE/100 g FDS) | Hexane | 2.18 ± 0.01b,x,y | 2.49 ± 0.01b,y | 1.87 ± 0.01b,x |
| Ethyl acetate | 1.11 ± 0.01c,x | 2.22 ± 0.01b,y | 1.56 ± 0.01b,z | |
| Butanol | 1.11 ± 0.01c,x | 2.00 ± 0.00c,y | 1.11 ± 0.01c,x | |
| Ethanol | 4.90 ± 0.01a,x | 4.68 ± 0.02a,x | 35.85 ± 0.03a,y | |
| Water | 2.34 ± 0.01b,x | 0.89 ± 0.01d,y | 1.56 ± 0.01b,z | |
| Total carotenoid content (mg BE/100 g FDS) | Hexane | 6.5 ± 0.2f,x | 27.3 ± 0.9d,y | 48.8 ± 2.7b,z |
| Ethyl acetate | 6.1 ± 0.6f,x | 4.6 ± 0.1 g,y | 12.9 ± 1.2f,z | |
| Butanol | 5.2 ± 0.1 g,x | 5.3 ± 0.1 g,x | 5.4 ± 0.0e,x | |
| Ethanol | 19.6 ± 1.1e,x | 16.2 ± 0.7e,y | 22.3 ± 1.0d,z | |
| Water | 2.1 ± 0.6 h,x | 1.5 ± 0.1 h,x | 3.5 ± 0.0e,y |
All data are presented as mean ± standard deviation (n = 3)
GAE gallic acid equivalent, QE quercetin equivalent, CE cyanidin-3-glucoside equivalent, BE β-carotene equivalent, FDS freeze dried sample
Different superscript lowercase letters (a–h) between different extraction solvents used to denote significant difference at p < 0.05. Different superscript lowercase letters (x,y,z) between different DR parts denote significant difference at p < 0.05. The ethanol used was of 50%
The current finding also clearly portray the substantial influence of different extraction solvents on the recovery of TPC. The extracts of fruit parts derived using 50% ethanol showed a maximum amount of TPC, ranged from 1135.5 to 3016.5 mg GAE/100 g FDS, whereas the extracts prepared from the least polar hexane was found to contain the lowest TPC (20.5–22.3 mg GAE/100 g FDS). In general, the solubility of phenolic compounds from DR increases with increasing solvent polarity. Those intermediate polar solvents such as 50% ethanol and butanol were found to be more potent in extracting phenolic compounds. The results were in agreement with previous studies that compared the effectiveness of different extraction solvents in the derivation of polyphenols from various samples. A study on Limnophila aromatica indicated that TPC obtained by 50% ethanol was about sixfold higher than its water extract (Do et al. 2014). Another study also revealed a higher TPC in 50% ethanol than hexane and ethyl acetate from roots of Armoracia rusticana (Tomsone and Kruma 2013). Our finding showed that TPC of hexane extract of DR peel was significantly lower than the ethyl acetate extract. The result is supported by Babbar et al. (2014) that total phenolics extracted from tomato peel using ethyl acetate were higher than the hexane extract.
Hydroxyl groups of phenolic compounds are more susceptible for hydrogen bonding with electronegative oxygen of organic alcohol such as ethanol, whereas hydroxyl group of ethanol also has a tendency to form hydrogen bonds with oxygen atom that found in the phenol molecules such as oleuropein, ferulic acid, syringic acid and synaptic acid, these may explain the current observation, where TPC is higher in ethanol compared to ethyl acetate and hexane (António et al. 2009). On the other hand, the results indicated that peel contained purple colour pigments exhibited a higher TPC than pulp, which may due to the presence of anthocyanins, such as pelargonidin and petunidin (Harzallah et al. 2016).
Total flavonoid content
Table 1 shows the TFC of different fruit parts of DR extracted with varying solvent systems. It can be observed that the ethyl acetate extract of DR pulp (9289.8 mg QE/100 g FDS) had the highest TFC at p < 0.05, followed by 50% ethanol extract of the seed (8189 mg QE/100 g FDS). TFC of the pulp extracts increased as follows: ethyl acetate > 50% ethanol > butanol > hexane > water. The results also showed that TPC of the ethyl acetate and ethanol extracts was not significantly differed among different DR parts. This finding is substantiated by literature in which ethyl acetate is selectively the best solvent for extracting polyphenols from grape seeds due to its capabilities of extracting proanthocyanidins (Spigno et al. 2007).
In this study, the ethyl acetate extracts of pulp and peel exhibited the highest TFC, whereas the ethanolic extract of DR seed displayed the highest value. Current findings implied that most of the flavonoids in DR fruit are less polar or semi-polar in nature. Hence, the main difference in the flavonoid content extracted from the solvents is attributed to the difference in polarity of these solvents, which affects the solubility of flavonoid compounds from the fruit parts (Prasad et al. 2010).
A previous study also reported that the recovery of flavonoids in ethanolic extract of Limnophila aromatica was fourfold higher than the polar water extract (Do et al. 2014). On top of that, a research conducted using Monotheca buxifolia fruit suggested that hexane is a poorer solvent for flavonoids recovery among the plant extracts compared to those more polar solvents such as butanol and ethyl acetate (Jan et al. 2013).
Total anthocyanin content
DR fruit has a purple colour peel, where 50% ethanol extract of the fruit peel contained the highest TAC of 35.85 mg CE/100 g FDS, which is almost seven-fold higher than the seed extract (4.9 mg CE/100 g FDS). As shown in Table 1, TAC in DR pulp extracted by the non-polar solvents was the highest. However, DR peel and seed with polar solvent such as 50% ethanol and water had significantly higher TAC than DR pulp at p < 0.05. The finding is consistent with a previous study which showed that aqueous ethanol was an effective solvent in the recovery of anthocyanins from grape (Cheng et al. 2012).
Similarly, the use of alcoholic solvents has been widely adopted in extraction and recovery of polyphenols from plant products due to their effectiveness in mass transfer as opposed to the use of a mono-component solvent (Yilmaz and Toledo 2006). Owing to the uneven distribution of polyphenolic compounds in different fruit parts, selection of the most fitting solvent system is significant to maximise the recovery of TAC.
Total carotenoid content
Based on the results shown in Table 1, TCC of the different parts of DR fruit is contradicted with the TPC. The results showed that DR peel extracted with all the solvent used contained the highest TCC which ranged between 3.5 and 48.8 mg BE/100 g FDS, followed by the pulp and seed (1.5–27.3 and 2.1–19.6 mg BE/100 g FDS, respectively). A previous study reported that the peel of Canarium odontophyllum fruit, a fruit which has a similar appearance as DR fruit, contained the greatest amount of carotenoids (Prasad et al. 2011). The high carotenoids content in the peel of DR could be due to the exposure of its outer part to the sunlight while growing, where carotenogenesis takes place. This process will cause the conversion of chlorophylls into carotenoids under photo-oxidative conditions (Prasad et al. 2011).
Among the extraction solvents used for recovery of carotenoid, a very interesting trend has been observed for TCC, whereby hexane as the least polar solvent yields the highest amount of TCC from the peel of DR fruit, which was 48.8 mg BE/100 g FDS. At the same time, 50% ethanol is the more polar organic solvent provided with the second highest amount of TCC which was 22.3 mg BE/100 g FDS. The results suggest that there are two different groups of carotenoids present in DR fruit, carotenes which are lipophilic such as α-carotene and β-carotene that were extracted using hexane; whereas the semi-polar xanthophylls, for example, lutein and zeaxanthin, may be attracted towards the ethanolic solution (Khoo et al. 2011).
Antioxidant assays based on electron transfer reaction
DPPH radical scavenging activity
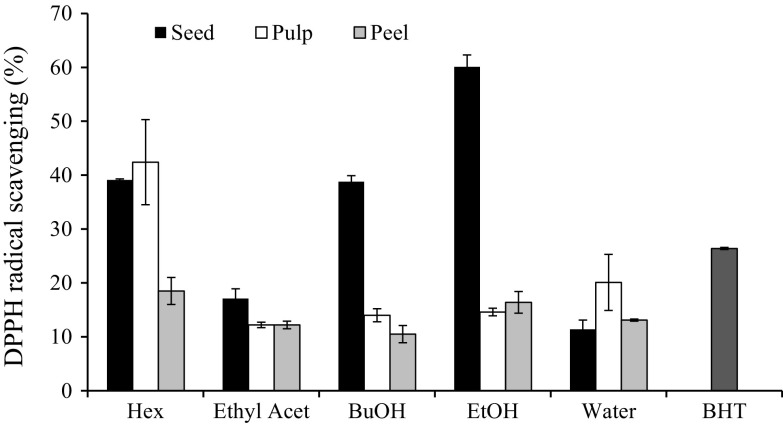
Based on our preliminary data, the scavenging ability of the extracts against DPPH radicals increased with the increasing concentration (Fig. S1). Despite a moderate scavenging ability was exhibited by most of the extracts, the maximum inhibition activities were found between 10 and 75%. This finding is in agreement with Prasad et al. (2011), where moderate inhibition activities have been reported for the seed, peel and pulp of C. odontophyllum. Among the three studied fruit parts, seed extracts had higher DPPH radical scavenging activity than the other samples with the highest was found in the ethanolic extract (Fig. 1). DR peel and pulp extracted with hexane at extract concentration of 80 μg/mL had the highest DPPH radical scavenging activity among the extracts of the other solvents used. Current findings are agreed by Kong et al. (2011) who reported an increasing trend of DPPH scavenging activity for the DR extracts, where seed extract exhibited the highest antioxidant activity.
Fig. 1.
DPPH radical scavenging activities of seed, pulp and peel extracted with different extraction solvents. The results were based on the extracts at the concentration of 80 µg/mL. Hex: hexane; Ethyl Acet: ethyl acetate; BuOH: buthanol; EtOH: 50% ethanol; BHT: butylated hydroxytoluene
Although all the extracts exhibited scavenging capacity against DPPH radical, the results varied from one another and were highly attributed to the influence of extraction solvents used. Based on the IC50 values obtained, ethanolic extract showed a very strong DPPH radical scavenging activity, where the ethanolic extract of seed had 50% inhibition ability at an IC50 concentration of 52.4 μg/mL. Meanwhile, IC50 values of all the other extracts were higher than 100 μg/mL due to these extracts required a higher concentration to achieve 50% scavenging activity. It is also noteworthy that the ethanolic extract of seed had a better antioxidant activity than positive control (BHT), where the IC50 value was lower than 100 μg/mL. The result indicated that DR seed is a potential source of natural antioxidants. Besides the amount and strength of antioxidant compound itself, the ability of the antioxidant in DR seed to transfer an electron to a free radical can also be affected by the environment where the reaction takes place, for example in different types of solvent.
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay is considered as the simplest colourimetric method. This assay is based on the principle that the radical scavenger or antioxidant will serve as a hydrogen donor for the DPPH· which reduce to DPPH2; hence, the reaction results in a change in colour intensity (Brand-Williams et al. 1995). Kinetics of hydrogen atom transfer activity for the phenolic compound, from phenol to DPPH, show that every solvent possesses unique hydrogen bond atom acceptor (βH2) and donator (αH2) (Nenadis and Tsimidou 2002). These acceptor and donator are required to form intermolecular hydrogen bonds with phenolic compound and to donate a hydrogen atom to the free radicals. The higher polarity and hydrogen bond donating ability of an antioxidant increase the susceptibility in transferring its hydrogen atom to DPPH radicals (Jabbari and Gharib 2012). Hence, 50% ethanol has greater αH2 than βH2 enables the phenolic compounds to exist in DR extract for easily donating hydrogen atoms, whereas water with greater βH2 lead to lower antioxidant activities (Oliferenko et al. 2004). However, many other factors can also affect the antioxidant activities. Therefore, the use of different antioxidant assays is important to elucidate the antioxidant potential of DR extracts.
Ferric reducing antioxidant power (FRAP)
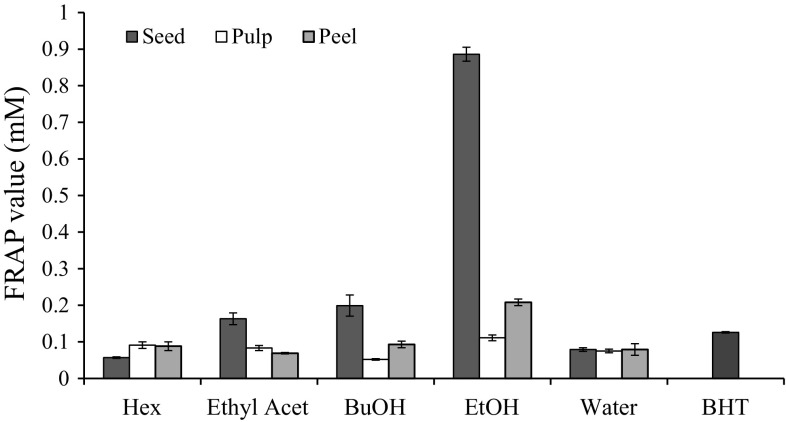
FRAP values of phytochemical antioxidants from different parts of DR fruit extracted using the extraction solvents were determined. The FRAP values increased with increasing extract concentration and reached a maximum value at a concentration of 80 μg/mL and decreased thereafter, except for 50% ethanol extract of seed and BHT (Fig. S2). Due to the high phenolic and flavonoid content in DR seed, FRAP value of the seed extracts was generally higher than the extract of the other parts which ranged from 0.023 to 0.886 mM Fe(II). However, hexane extract of seed had FRAP value lower than the FRAP values of hexane extracts of pulp and peel of DR. The result also showed that ethanolic extract had the highest FRAP value compared to other extracts.
As shown in Fig. 2, FRAP values of DR seed extracts exhibited a decreasing trend of 50% ethanol > butanol > ethyl acetate > water > hexane. Among the peel extracts derived from different solvents, the FRAP values were from 0.069 to 0.208 mM Fe(II). Similar to the seed, the ethanolic extract of peel had the highest ferric reducing power, followed by butanol, hexane, water and ethyl acetate extracts. FRAP values of the pulp extracted with different extraction solvents are decreasing as follows: 50% ethanol > hexane > ethyl acetate > water > butanol.
Fig. 2.
FRAP values of seed, pulp and peel extracted with different extraction solvents that expressed as mM FeSO4 equivalent. The results were based on the extracts at the concentration of 80 µg/mL. Hex: hexane; Ethyl Acet: ethyl acetate; BuOH: buthanol; EtOH: 50% ethanol; BHT: butylated hydroxytoluene
It is worth noting that ethanolic extracts of DR peel and seed surpassed the reducing capacity of synthetic antioxidant used in this assay. It might be due to the high amount of polyphenolic compounds found in both seed and peel of DR, which are responsible to exert the antioxidant activity via single electron transfer reaction (Pulido et al. 2000). Therefore, this study shows the potential of phytochemicals in DR fruit extracts as the possible natural substitutes for synthetic antioxidants.
FRAP assay determines the reducing ability of an antioxidant. The FRAP procedure was performed in an acidic environment, where ferric-tripyridyltriazine (Fe3+-TPTZ) complex is reduced to ferrous tripyridyltriazine (Fe2+-TPTZ) complex by an antioxidant, causing the formation of intense blue colour. This phenomenon is regarded as single electron transfer mechanism. Phytochemical antioxidants in DR fruit extracts act as reductants and have a robust electron-donating capacity which serves as a cursor for determining the potential antioxidant activity of the fruit extracts.
Phosphomolybdenum method
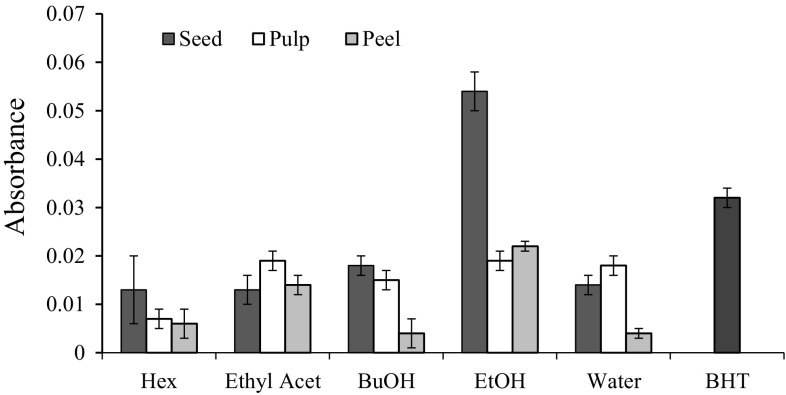
Antioxidant activity of the extracts of different parts of DR fruit was also evaluated using phosphomolybdenum method. Based on the screening tests, the absorbance changed of the extracts determined using the method increased with the increasing concentration (Fig. S3). As shown in Fig. 3, the peel extracts had absorbance changed between 0.001 and 0.036, where 50% ethanol > ethyl acetate > hexane > butanol > water. As for the pulp, the absorbance values ranged between 0.007 and 0.019. Conversely, the seed extracts had higher absorbance values of 0.013–0.054.
Fig. 3.
Values for the increased absorbance of seed, pulp and peel extracted with different extraction solvents determined using phosphomolydenum assay. The results were based on the extracts at the concentration of 80 µg/mL. Hex: hexane; Ethyl Acet: ethyl acetate; BuOH: buthanol; EtOH: 50% ethanol; BHT: butylated hydroxytoluene
This method was performed based on the reduction of Mo (IV) to Mo (V) by the antioxidants in DR fruit extracts and subsequently formed a green phosphate or Mo (V). A higher absorbance value indicating a higher antioxidant activity. In this study, the antioxidant activities of all the extracts are reacted in a concentration-dependent manner. The highest absorbance value was noted to be the ethanolic extract of seed which indicates its good antioxidant activity. The high antioxidant activity attributed to the high level of polyphenols especially flavonoids which act as the reductant. Even though ethyl acetate extract had a higher level of flavonoids, the antioxidant capacity of the extract is not as good as ethanol. The finding suggests that other polyphenolic compounds recovered in the ethanolic extract, which may synergistically act as the reductants of phosphomolybdate ion (Liu et al. 2008). Moreover, heat treatment during the extraction is able to significantly increase the antioxidant activity of clove sample accessed using phosphomolybdenum method (Nikousaleh and Prakash 2016).
Assays based on reactive oxygen species scavenging mechanism
Hydroxyl radical scavenging activity
In this study, the capability of antioxidants in all extracts recovered from DR fruit parts to scavenge hydroxyl radicals further justifies the antioxidant potential of DR fruit extracts. Overall, all the extracts performed equally well in scavenging ·OH but seed extracts are better than the pulp and peel extract. The ·OH scavenging activity of DR extracts increased in a concentration-dependent manner; however, some of the extracts reached a plateau stage at a concentration of 20 μg/mL. Moreover, hexane and water extracts of all fruit parts were less active in scavenging hydroxyl radical and require a higher extract concentration to obtain an IC50 value.
Based on the IC50 values calculated (Table 2), the values were comparable and not significantly different among ethyl acetate, butanol and 50% ethanol extracts of all fruit parts, which require the least and a similar range of concentration (37.1–40.6 μg/mL) to promote 50% inhibition of the hydroxyl radicals. IC50 values of these extracts were also comparable to BHT, where the IC50 value of BHT was 37.3 μg/mL. The higher amount of phytochemicals in these extracts may serve as important candidates in scavenging hydroxyl radicals. According to Hagerman et al. (1998), phenolic compounds with many aromatic rings and OH group tends to have a better hydroxyl radical scavenging activity.
Table 2.
Radical scavenging activities of Dacryodes rostrata fruit parts extracted using different solvents
| Samples | Solvent | IC50 (μg/mL) | |
|---|---|---|---|
| Hydroxyl radical scavenging | Superoxide anion radical scavenging | ||
| Seed | Hexane | > 100 | 55.7 ± 0.8a |
| Ethyl acetate | 40.6 ± 0.2a | 53.4 ± 1.5a | |
| Butanol | 38.2 ± 0.1a | > 100 | |
| Ethanol | 40.6 ± 0.3a | > 100 | |
| Water | > 100 | > 100 | |
| Pulp | Hexane | > 100 | > 100 |
| Ethyl acetate | 40.1 ± 0.1a | 57.7 ± 3.4a | |
| Butanol | 38.7 ± 0.1a | 56.4 ± 2.2a | |
| Ethanol | 40.1 ± 0.1a | 66.6 ± 5.0b | |
| Water | > 100 | > 100 | |
| Peel | Hexane | 68.8 ± 1.8b | > 100 |
| Ethyl acetate | 39.2 ± 0.1a | 87.5 ± 11.0c | |
| Butanol | 37.1 ± 0.1a | 88.6 ± 9.1c | |
| Ethanol | 37.8 ± 0.1a | 82.6 ± 9.0c | |
| Water | > 100 | > 100 | |
| BHT | Positive control | 37.3 ± 0.1a | 178.2 ± 40.9d |
All data are presented as mean ± standard deviation (n = 3)
BHT butylated hydroxytoluene
Different superscript lowercase letters (a–d) between different extraction solvents used to denote significant difference at p < 0.05
IC50 value of the sample is the concentration of sample needed to inhibit 50% of the activity. The ethanol used was of 50%
The assay of hydroxyl radical scavenging is an alternate method for identifying the antioxidant capacity of any antioxidants present in the fruit extracts, which based on the principle of deoxyribose degradation (Li 2013). Unlike electron transfer reaction assay, this antioxidant assay deals with the hydroxyl radicals that could also be produced in human cells. Hydroxyl radicals can cause DNA strand breakage which leads to undesirable biological effects like mutagenesis and cell death. This assay employs Fenton reagent to generate ·OH which oxidises the 2-deoxy-d-ribose molecule. The damage of cyclic furan ring of the deoxyribose results in the formation of malondialdehyde which reacts with 2-thiobarbituric acid upon heating to yield TBARS (thiobarbituric acid-reactive substance), a pink chromogen with an absorbance reading of 530 nm (Li 2013).
Superoxide radical anion scavenging activity
Superoxide radical anion scavenging activities of different parts of DR fruit extracts were determined based on different concentrations. The peel extracts showed O·−2 scavenging activity ranged between 14.6 and 68.6%. On the other hand, the extracts of DR pulp demonstrated O·−2scavenging activity ranged 10.1–83.5%, whereas seed extracts ranged between 13.5 and 59.4%. Overall, the peel and pulp extracts had better scavenging activities than the seed extract, in which butanol extracts of pulp (83.5%) and peel (68.6%) displayed the highest scavenging activity compared to the ethyl acetate extract of DR seed (59.4%). The O·−2 scavenging ability of the peel extracts in the studied solvents is decreasing as follows: butanol > ethyl acetate > 50% ethanol > water > hexane. A similar trend was also observed in the pulp extracts. In contrast, O·−2scavenging ability of the seed extracts in solvents is decreasing as follows: ethyl acetate > butanol > hexane > 50% ethanol > water.
IC50 values of all the extracts of DR fruit are tabulated in Table 2. The overall performance of all the extracts was relatively high and surpassed the radical scavenging capacity of BHT, in which a higher concentration of this synthetic antioxidant is required to cater 50% of inhibition activity. The less polar hexane and ethyl acetate extracts of DR seed, as well as the butanol and ethyl acetate extracts of DR pulp, had the lowest IC50 values compared with the other extracts. The low IC50 values attributed to flavonoids content in the pulp and could be the major contributor to the high scavenging activity. It is because flavonoids are regarded as potent radical scavengers. Carotenoids, the less polar compounds which contain many double bonds in their chemical structure, are also known to be the potent singlet oxygen quenchers (Khoo et al. 2011). Hence, the extracts derived from the non-polar solvent including hexane, possess moderate O·−2 scavenging activity even though these extracts had lower TPC, TFC and TAC.
Superoxide radical anion (O·−2) is another type of reactive oxygen species abundantly found in human body. The ability of DR extracts in scavenging this radical indicates their possibilities of inhibiting a similar type of radical in the body. The addition of pyrogallol into the mixture containing EDTA, Tris–HCl buffer and the extracts initiates production of O·−2 via auto-oxidation of pyrogallol (Li 2012). The formation of a yellow–brown solution indicates generation of O·−2 which can be measured at 325 nm. Antioxidants in the extracts that eliminate the O·−2 production with a lower absorbance value shows a higher percentage of inhibition activity toward the oxidation process (Li 2012).
Conclusion
The recovery of phytochemicals in DR fruit parts using different extraction solvents are varied. Among different fruit parts, DR peel had the highest amounts of total anthocyanins and total carotenoids, whereas DR seed had the highest total phenolic content. The use of polar to non-polar extraction solvents in extraction and recovery of phytochemicals implies a variation in antioxidative activities that assessed by electron transfer and hydrogen transfer reaction assays. Water extracts contained the more polar compounds which including some of the polar phenolic compounds such as phenolic acids, whereas non-polar hexane is able to extract the semi-polar to non-polar carotenoids.
Non-polar compounds such as carotenoids have the higher ability in scavenging superoxide anion radical. The semi-polar polar antioxidants including anthocyanins and some of the flavonoids are also found to be the most effective in scavenging hydroxyl radicals. Due to a range of phytochemicals was recovered from the solvents of different polarity, the application of DR fruit extracts as nutraceuticals should consider the extraction solvent used. Also, the extracts of DR fruit parts contain potent natural antioxidants and can be used to substitute the synthetic antioxidants in the food industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our gratitude to the technical supports from laboratory staffs of School of Engineering, Monash University Malaysia and the laboratory facilities provided.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3170-6) contains supplementary material, which is available to authorized users.
Contributor Information
Krishnamurthy Nagendra Prasad, Email: nagendra.prasad@monash.edu, Email: knag76@gmail.com.
Kin Weng Kong, Phone: +603-79674717, Email: kongkm@um.edu.my.
References
- António JQ, Mota FL, Pinho SP, Macedo EA. Solubilities of biologically active phenolic compounds: measurements and modeling. J Phys Chem B. 2009;113(11):3469–3476. doi: 10.1021/jp808683y. [DOI] [PubMed] [Google Scholar]
- Babbar N, Oberoi HS, Sandhu SK, Bhargav VK. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J Food Sci Technol. 2014;51(10):2568–2575. doi: 10.1007/s13197-012-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cheng VJ, Bekhit AEDA, McConnell M, Mros S, Zhao J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012;134(1):474–482. doi: 10.1016/j.foodchem.2012.02.103. [DOI] [Google Scholar]
- Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22(3):296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV–visible spectroscopy. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith D, Sporns P, editors. Handbook of food analytical chemistry. Hoboken: Wiley; 2005. pp. 19–31. [Google Scholar]
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46(5):1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Harzallah A, Bhouri AM, Amri Z, Soltana H, Hammami M. Phytochemical content and antioxidant activity of different fruit parts juices of three figs (Ficus carica L.) varieties grown in Tunisia. Ind Crops Prod. 2016;83:255–267. doi: 10.1016/j.indcrop.2015.12.043. [DOI] [Google Scholar]
- Jabbari M, Gharib F. Solvent dependence on antioxidant activity of some water-insoluble flavonoids and their cerium(IV) complexes. J Mol Liq. 2012;168:36–41. doi: 10.1016/j.molliq.2012.02.001. [DOI] [Google Scholar]
- Jan S, Khan MR, Rashid U, Bokhari J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public Health Res Perspect. 2013;4(5):246–254. doi: 10.1016/j.phrp.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo HE, Ismail A, Mohd-Esa N, Idris S. Carotenoid content of underutilized tropical fruits. Plant Foods Hum Nutr. 2008;63(4):170–175. doi: 10.1007/s11130-008-0090-z. [DOI] [PubMed] [Google Scholar]
- Khoo HE, Prasad KN, Kong KW, Jiang Y, Ismail A. Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules. 2011;16(2):1710–1738. doi: 10.3390/molecules16021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KW, Chew LY, Prasad KN, Lau CY, Ismail A, Sun J, Hosseinpoursarmadi B. Nutritional constituents and antioxidant properties of indigenous kembayau (Dacryodes rostrata (Blume) H. J. Lam) fruits. Food Res Int. 2011;44(7):2332–2338. doi: 10.1016/j.foodres.2010.10.039. [DOI] [Google Scholar]
- Li X. Improved pyrogallol autoxidation method: a reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J Agric Food Chem. 2012;60(25):6418–6424. doi: 10.1021/jf204970r. [DOI] [PubMed] [Google Scholar]
- Li X. Solvent effects and improvements in the deoxyribose degradation assay for hydroxyl radical-scavenging. Food Chem. 2013;141(3):2083–2088. doi: 10.1016/j.foodchem.2013.05.084. [DOI] [PubMed] [Google Scholar]
- Li X, Lin J, Han W, Mai W, Wang L, Li Q, Lin M, Bai M, Zhang L, Chen D. Antioxidant ability and mechanism of rhizoma Atractylodes macrocephala. Molecules. 2012;17(11):13457–13472. doi: 10.3390/molecules171113457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Qiu N, DingH Yao R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res Int. 2008;41(4):363–370. doi: 10.1016/j.foodres.2007.12.012. [DOI] [Google Scholar]
- Nenadis N, Tsimidou M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH·) tests. J Am Oil Chem Soc. 2002;79(12):1191–1195. doi: 10.1007/s11746-002-0626-z. [DOI] [Google Scholar]
- Nikousaleh A, Prakash J. Antioxidant components and properties of dry heat treated clove in different extraction solvents. J Food Sci Technol. 2016;53(4):1993–2000. doi: 10.1007/s13197-015-2113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliferenko AA, Oliferenko PV, Huddleston JG, Rogers RD, Palyulin VA, Zefirov NS, Katritzky AR. Theoretical scales of hydrogen bond acidity and basicity for application in QSAR/QSPR studies and drug design. Partitioning of aliphatic compounds. J Chem Inform Comput Sci. 2004;44(3):1042–1055. doi: 10.1021/ci0342932. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Chew LY, Khoo HE, Kong KW, Azlan A, Ismail A. Antioxidant capacities of peel, pulp, and seed fractions of Canarium odontophyllum Miq. fruit. J Biomed Biotechnol. 2010;2010:871379. doi: 10.1155/2010/871379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KN, Chew LY, Khoo HE, Yang B, Azlan A, Ismail A. Carotenoids and antioxidant capacities from Canarium odontophyllum Miq. fruit. Food Chem. 2011;124(4):1549–1555. doi: 10.1016/j.foodchem.2010.08.010. [DOI] [Google Scholar]
- Prasad N, Yang B, Kong KW, Khoo HE, Sun J, Azlan A, Ismail A Romli ZB (2013) Phytochemicals and antioxidant capacity from Nypa fruticans Wurmb. fruit. Evid Based Complement Alternat Med Article ID 154606, 9 pages [DOI] [PMC free article] [PubMed]
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48(8):3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Spigno G, Tramelli L, De Faveri DM. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng. 2007;81(1):200–208. doi: 10.1016/j.jfoodeng.2006.10.021. [DOI] [Google Scholar]
- Tee LH, Ramanan RN, Tey BT, Chan ES, Azrina A, Amin I, Yang B, Lau CY, Prasad KN. Phytochemicals and antioxidant capacities from Dacryodes rostrata fruits. Med Chem. 2015;5:023–027. [Google Scholar]
- Tomsone L, Kruma Z. Comparison of different solvents for isolation of phenolic compounds from horseradish (Armoracia rusticana L.) leaves. In: Treija S, Skujeniece S, editors. Research for rural development. Jelgava: Latvia University of Agriculture; 2013. pp. 104–110. [Google Scholar]
- Yilmaz Y, Toledo RT. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J Food Compos Anal. 2006;19(1):41–48. doi: 10.1016/j.jfca.2004.10.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.