Abstract
In the present investigation d-limonene oil (4-isopropenyl-1-methylcyclohexene) was encapsulated by ultra-sonication method using whey protein (WP)–maltodextrin (MD) conjugates as coating material and their characterization was done with respect to physico-chemical and antimicrobial properties. Antimicrobial activity of limonene oil (LO) nanoemulsion and bulk LO dissolved in dimethyl sulphoxide (DMSO) were assessed by agar well diffusion method. Stable formulation of d-limonene oil nanoemulsion [5.0% LO + 9.0% WP–MD (1:2 w/w) conjugate] had shown mean particle size, zeta potential and poly dispersity index of 116.60 ± 5.30 nm, − 19.64 ± 0.23 mV and 0.205 ± 0.02 respectively. LO nanoemulsion were stable to different food processing conditions like heat treatments, ionic strength (0.1–1.0 M) and pH (3.0–7.0). LO nanoemulsion was stable for 15 days at 25 °C and it had shown particle size of 332.20 ± 5.40 nm at 15th day. It was observed that minimum inhibitory concentration (MIC) of both LO nanoemulsion and bulk LO dissolved in DMSO were at 12.50 µl/ml against Bacillus cereus (ATCC 14459), Escherichia coli (ATCC 25922), Enterococcus faecalis (NCDC 115) and Salmonella typhi (NCDC 6017). Since d-limonene has been considered to be a safer alternative compared to synthetic antimicrobial food additives, the present investigation will be helpful in developing a more effective antimicrobial system for the production and preservation of foods.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3198-7) contains supplementary material, which is available to authorized users.
Keywords: Whey protein–maltodextrin conjugate, d-Limonene oil, Nanoemulsion, Antimicrobial activity, Essential oils
Introduction
Now a days, the foodborne illness is a serious problem threatening public health around the world. A total of 37 outbreaks involving 3485 persons who have been affected due to food poisoning have been reported in India (Rao Vemula et al. 2012). As per the aggregate analysis of the Integrated Disease Surveillance Programme (IDSP) data between 2011 and 2015, food-borne outbreaks, together with acute diarrhoeal disease, constitute nearly half of all reported outbreaks under IDSP for the period between 2011 and 2016. The essential oils (EOs), considered as natural antimicrobials and of plant origin have received increasing attention because of their efficacy against a broad spectrum of pathogens (Burt 2004) and to meet the consumer’s requirements in terms of food quality and safety. EOs are natural concentrated aromatic hydrophobic liquid products obtained from plants by steam distillation or hydro-distillation, expression or solvent extraction (Edris 2007). EOs and their extracts have been classified by the USFDA (Food and Drug Administration) as “Generally Recognized as Safe” (GRAS) status (CFR 2009). From thousands of years various EOs find their applications for wide variety of purposes including perfumes, cosmetics, medicines, foods and drinks. Some of the EOs exert an antimicrobial and antifungal activity that formed the basis of their applications as a natural antimicrobial additive in food manufacturing and preservation to extend the shelf life of food products (Cimanga et al. 2002). It is a best option to decrease the use of synthetic and semisynthetic antimicrobial compounds.
d-Limonene (4-isopropenyl-1-methylcyclohexene), a natural and functional monoterpene is the major component of all citrus EOs. Because of its pleasant citrus-like flavor, limonene is widely used in food, cosmetics and consumer products. It is listed in the code of federal regulation as GRAS for flavoring agent and food preservative. Limonene has been found to possess antifungal, antimicrobial, antioxidant, anticarcinogen, Chemo-preventive and antidiabetic properties. Therefore, its use as a food preservative has also been proposed.
However, the technological limitations limits the use of d-limonene in food system. Properties like low water solubility, high volatility, and strong odor of d-limonene limit their applications in foods and beverages. It’s hydrophobic nature limits the use in hydrophilic rich surfaces or solid–liquid interfaces (Soottitantawat et al. 2003). It undergoes oxidative degradation under normal storage condition which leads to loss of lemon like flavor (Li and Chiang 2012). Therefore, it is challenging phenomenon to use d-limonene in food system. Hence, there is a need to encapsulate lipophilic bioactive compounds. Probably, nanoemulsion lipid delivery system is the good alternative to encapsulate lipophilic compounds.
Nanoemulsions are oil-in-water or water in oil type emulsions in which the average globule size of the range 100–500 nm that are isotopically clear dispersion stabilized by interfacial layer of surfactant molecule (Lovelyn and Attama 2012). Smaller particle size and narrow particle size distribution of nanoemulsions leads to distinct physicochemical properties like optical transparency, bulk viscosity and physical stability and that contributes to superiority of nanoemulsions over conventional emulsions (Donsi et al. 2012). There is a need of emulsifiers to reduce surface tension and stabilize globular interfaces in emulsions. Milk proteins, particularly whey proteins, have been widely used as emulsifiers in foods (Lee and McClements 2010) due to their amphipathic nature (Shah et al. 2012). But, the functional properties of whey proteins can become poor under certain conditions due to aggregation or precipitation of protein particularly around at pH close to the isoelectric point of the proteins. It has been speculated that the chemical modification of proteins via conjugation with polysaccharides may improve emulsifying properties, especially at low pH as the isoelectric point and solubility will be altered and molecular integrity maintained (Song et al. 2002). Hence, there is a huge scope for protein-polysaccharide conjugate to replace the usage of protein alone.
In the present investigation, d-limonene oil nanoemulsion was prepared by Ultrasonication method using natural food grade emulsifier (whey protein–maltodextrin conjugates). Attempt was made to find the efficacy of prepared whey protein–maltodextrin conjugates to use as coating material for the preparation of nanoemulsion. The prepared d-limonene oil nanoemulsion was characterized with respect to physical and antimicrobial properties.
Materials and methods
Materials
Whey protein concentrate (WPC-70) was procured from Modern Dairy Pvt. Ltd. Karnal, Haryana. Maltodextrin (MD), Brain Heart Infusion Broth, Nutrient Agar and Agar was procured from Hi Media Laboratories Pvt. Ltd., Mumbai. d-limonene oil was purchased from Siva aromatics Pvt. Ltd., Delhi. All the microbial cultures are obtained from National Collection of Dairy Cultures (NCDC) and American Type Culture Collection (ATCC) of Dairy Microbiology, NDRI, Karnal.
Preparation of whey protein–maltodextrin conjugate
WPC (70) and MD were separately dissolved in distilled water at a mass ratio of 1:2 (WPC:MD). After hydration of 8 h, whey protein and maltodextrin solutions were mixed and pH was adjusted to 7.0 using 0.1 N HCl and 0.1 N NaOH. The samples were then freeze dried to remove water, and were ground to make powder. The powder was subjected for dry heat treatment at 90 °C for 2 h at 80% relative humidity (RH). The Maillard reaction can take place in solutions (Li et al. 2012) and powder (Akhtar and Dickinson 2003). In aqueous solutions, the glycation rate is low because water inhibits the initial Amadori reaction and subsequent reactions (Li and Chiang 2012). Hence, we have selected dry condition for the preparation of whey protein–maltodextrin conjugates. 80% RH was maintained by using preheated desiccator containing saturated solution of KCl.
Preparation of d-limonene oil nanoemulsion
Emulsions were prepared by using method described by Jafari et al. (2007) with slight modifications. The two stages involved were: (a) pre-emulsions were obtained by using high speed magnetic stirrer, and (b) these coarse emulsions were further emulsified using an ultrasonicator.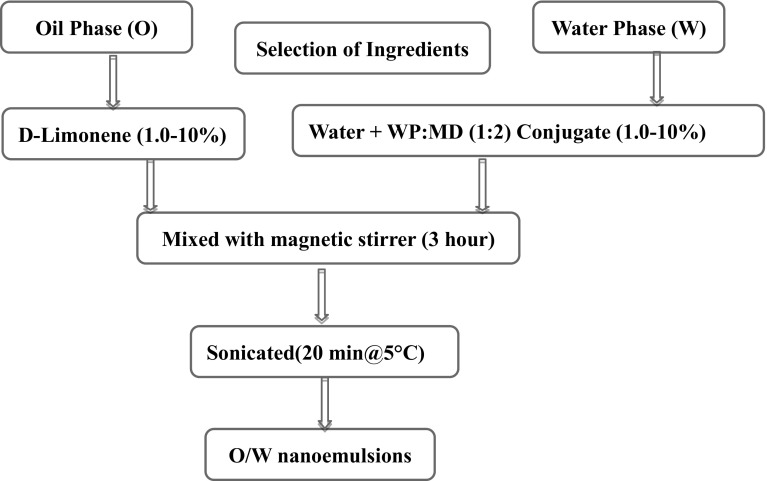
Emulsion stability was measured by the method of Dalev and Simeonova (1995) with slight modifications by two approaches:
Centrifugation of freshly prepared nanoemulsions were done at 1300×g for 30 min to observe their sedimentation or phase separation behavior.
The freshly prepared nanoemulsions were kept at 80°C for 30 min followed by an ice bath for 15 min and further the emulsions were centrifuged at 1300×g for 30 min to observe their sedimentation or phase separation behavior.
Physico-chemical characterization of d-limonene oil nanoemulsions
In the present study, the prepared nanoemulsions were studied for their physico-chemical characteristics particle size and zeta potential which are discussed below.
Particle size and zeta potential
The mean particle size (Z-average), zeta-potential and Poly Dispersity Index (PDI) of the nanoemulsions were measured by using Malvern Nanoparticle Analyzer. The experiments were carried out on the 50 times diluted (Distilled water was used for dilution purpose) freshly prepared nanoemulsions. Disposable four-side plain cuvettes were used under an operating temperature of 25 °C and humidity 85%. The lower and upper size limits for this instrument is 0.3 nm and 8.0 µm respectively. Droplet size measurements were carried out in triplicate for each emulsion.
Determination of effect of different processing conditions on the stability of d-limonene oil nanoemulsions
The effect of different processing conditions like heat treatment [pasteurization (63 °C for 30 min), forewarming (80 °C for 10 min), boiling (95 °C for 10 min) and autoclave (121 °C)], pH (3.0–7.0) and ionic strength (0.1–1.0 M NaCl) was studied which will be applicable for their commercial utilization. Freshly prepared nanoemulsions were used to serve the purpose. The particle size distribution and zeta potential were measured after exposing the nanoemulsions to each treatment.
Antimicrobial assay
The d-limonene oil nanoemulsions were examined for their antimicrobial efficacy against various food borne pathogens. The microbial cultures were inoculated in Brain Heart Infusion (BHI) broth before analysis. Experiments were carried out on all microbial strains incubated in an aerated incubator.
The antimicrobial activity of nanoemulsions was determined in the form of minimum inhibitory concentration (MIC). The MIC value was determined as the lowest concentration of the antimicrobial agent that inhibited the visible growth of the test microorganism with the help of agar well-assay method. MIC was assessed for microorganisms like Bacillus cereus (ATCC 14459), E. coli (ATCC 25922), Enterococcus faecalis (NCDC 115) and Salmonella typhi (NCDC 6017).
Minimum inhibitory concentration (MIC) by using agar well diffusion assay
Cultures were screened for their antimicrobial activity against food borne pathogens using agar well diffusion assay. To measure the antimicrobial activity, the nutrient agar was poured in petri plates to solidify and dried for 24 h in incubator at 37 °C. These plates were then overlaid with 7 ml of nutrient soft agar inoculated with 70 μl of 3.0–4.0 h grown cultures (~ 106 CFU/ml) of test microorganisms in BHI broth. The soft agar was allowed to solidify and wells were punched out of the agar with sterile well borer (5.0 mm Dia.). Then different concentrations of nanoemulsions such as 25–100 μl were added into the wells. Same concentration of d-limonene oil as in nanoemulsion was dissolved in dimethyl sulphoxide (DMSO) and 25–100 μl was added into the wells. This was taken as positive control to examine the antimicrobial efficacy of encapsulated (nanoemulsion) and unencapsulated (oil in DMSO) Oil. Sterile saline was taken as negative control to compare the antimicrobial activity of prepared nanoemulsions. The plates were then refrigerated at 4 °C for 10 min to facilitate the diffusion of nanoemulsions and were incubated at 37 °C for 24 h. The diameters of zone of inhibition extending laterally around the wells were measured and clear zones of 3.0 mm or more (excluding diameters of well) were considered as positive zone of inhibition. Each measurement was replicated two times.
Statistical analysis
All statistical analysis was performed using MS-EXCEL-2010 package. Results are presented as mean ± standard error (SE) of three replicates, and significance was tested by employing analysis of variance (ANOVA).
Results and discussion
Optimization and characterization of d-limonene oil nanoemulsion
Different concentration of both core (d-limonene oil) and coating materials [whey protein–maltodextrin (1:2 w/w) conjugates] were screened to fabricate stable nanoemulsion. The conditions for the preparation of nanoemulsions were optimized and stable nanoemulsions were obtained by sonification at 5 °C for 20 min. The most stable formulation of d-limonene oil nanoemulsion [5.0% LO + 9.0% WP–MD (1:2 w/w) conjugate] had shown mean particle size, Zeta potential and poly dispersity index of 116.60 ± 5.30 nm, − 19.64 ± 0.23 mV and 0.205 ± 0.02 respectively (Table 1). The pH of the optimized d-limonene oil nanoemulsion was 6.50 ± 0.15. The relatively lower value of PDI for nanoemulsion can be correlated with more stability on storage. The stability of nanoemulsion might be due to the improved emulsifying and stabilizing properties of whey proteins after glycation with maltodextrin. Akhtar and Dickinson (2007) showed that whey proteins are highly compatible with MD at the 1:2 (w/w) ratio in the formation of conjugates with excellent emulsifying properties. In emulsion system containing both proteins and polysaccharides, proteins form an adsorbed coherent viscoelastic layer at the oil–water interface, while polysaccharide confer stability through their thickening and gelation behavior in the aqueous phase. The improved stability of the conjugate stabilized emulsions in comparison to the whey protein stabilized emulsions is attributed to the conjugated protein molecule forming a bulky polymeric layer than the non-conjugated protein on the droplet surface, with the MD portion extruding outwards into the continuous phase providing better steric stabilization, thus preventing droplet aggregation and coalescence. Akhtar and Dickinson 2007 reported that the improved emulsifying and stabilizing properties of WPI after glycation with MD were previously reported for orange oil and triglyceride oils emulsions due to the adsorption of more hydrophobic protein moiety onto the oil–water interface and the hydrophilic oligosaccharide protruding in the water phase providing steric hindrance against aggregation. At a high concentration of surfactant (S/O = 1.5), d-limonene nanoemulsions could be obtained. Li and Chiang (2012) optimized the process for preparation of d-limonene in water nanoemulsion using ultrasonic emulsification using mixed surfactants of sorbitan etrioleate and polyoxyethylene (20) oleyl ether and they revealed that 10% d-limonene nanoemulsion formed at So ratio (d-limonene concentration to mixed surfactant concentration) 0.6–0.7 and applied power 18 W for 120 s had droplet size below 100 nm. Xue et al. (2013) investigated emulsifying properties of WPI–MD conjugates at pH 6.4. Up to 1.50% w/v of thymol can be emulsified by 7.0% w/v conjugates and 10 v/v propylene glycol (PG) without showing turbidity. Shah et al. (2012) dispersed the thymol in whey protein isolate-maltodextrin conjugate capsule using the emulsion–evaporation technique and they reported that usage of WPI conjugated with MD improves dispersibility, transparency and thermal stability of the emulsion compared to the usage of non-conjugated mixtures of WPI and MD.
Table 1.
Physico-chemical characterization of 5% d-limonene oil nanoemulsion stabilized by 9% whey protein–maltodextrin conjugate
| Particle size (nm) | 116.6 ± 5.3 |
| Zeta potential (mV) | − 19.64 ± 0.23 |
| Poly dispersity index | 0.205 ± 0.02 |
| pH | 6.5 ± 0.15 |
Values are mean ± standard error (n = 3)
Effect of processing conditions on the stability of d-limonene nanoemulsions
The stability of the prepared nanoemulsions to various processing conditions were analyzed by changing various parameters like pH (3.0–7.0); ionic strength (NaCl: 0.1–1.0 M); and heat treatments (pasteurization, forewarming, boiling and sterilization). The state of aggregation of protein in solution is dependent on pH, ionic strength and temperature. The physical and chemical stability of nanoemulsions greatly dependent on storage temperature, pH, and ionic strength.
Effect of pH on the particle size and zeta potential
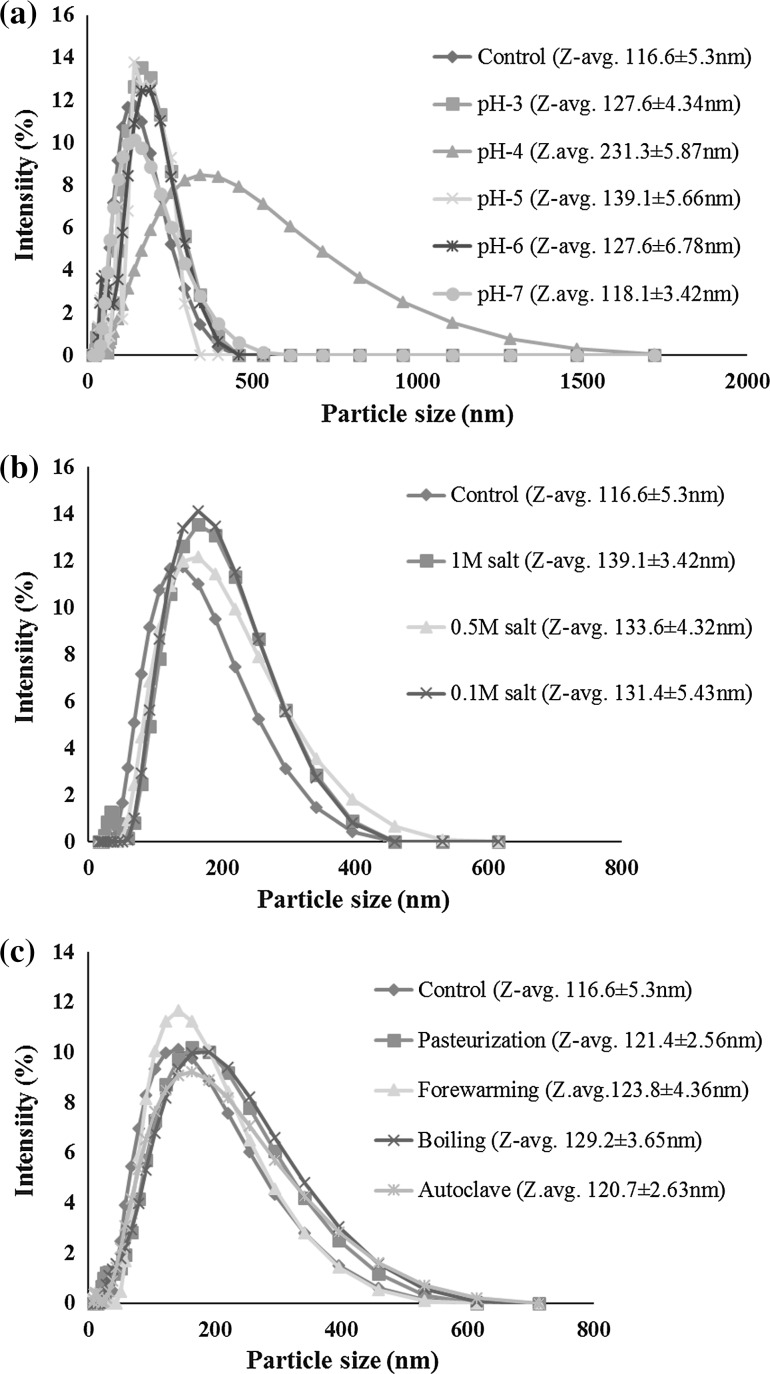
The particle size distribution of LO nanoemulsion prepared using whey protein–maltodextrin conjugate were determined as a function of pH as shown in the Fig. 1a. The mean particle size of nanoemulsion decreased with increase in pH from 3.0 to 7.0 with the exception at pH 4.0 (231.30 ± 5.87 nm). i.e., from 127.60 ± 4.34 nm (pH 3.0) to 118.10 ± 3.42 nm (pH 7.0). The mean particle size of control nanoemulsion was 116.60 ± 5.30 nm (pH 6.5). The increase in the particle size at pH 4.0 is mainly due to the effect of isoelectric point of WP–MD (1:2 w/w) conjugate. The magnitude of the zeta potential decreased and the isoelectric point was shifted towards lower pH after whey proteins glycated with maltodextrin. The prepared nanoemulsion was stable to the different pH range (3.0–7.0) tested. Similar results were shown by Akhtar and Dickinson (2007) that whey protein–maltodextrin conjugate can be used as an effective emulsifier for formulating food emulsions under acidic conditions. Shah et al. (2012) reported that thymol nanoemulsion prepared by using whey protein isolate-maltodextrin complex were stable between pH 3.0 and 7.0.
Fig. 1.
Effect of processing conditions on particle size and zeta potential of d-limonene oil nanoemulsion stabilized by 9% whey protein–maltodextrin (1:2 w/w) conjugate. a Effect of pH on particle size distribution of 5% d-limonene oil nanoemulsion stabilized by whey protein–maltodextrin (1:2 w/w) conjugate. b Effect of ionic strength on particle size distribution of 5% d-limonene oil nanoemulsion stabilized by whey protein–maltodextrin (1:2 w/w) conjugate. c Effect of heat treatments on particle size distribution of 5% d-limonene oil nanoemulsion stabilized by 9% whey protein–maltodextrin (1:2 w/w) conjugate
The magnitude of zeta potential of LO nanoemulsion prepared using whey protein–maltodextrin conjugate, changes with increase in pH from 3.0 (+ 5.98 ± 0.49 mV) to 7.0 (− 20.89 ± 0.26 mV) as shown in Table 2. The magnitude of zeta potential at pH 4.0 (+0.86 ± 0.39 mV) was towards slight positive side. But, at pH to 5.0 the magnitude of zeta potential increased towards the negative side (− 17.21 ± 0.78 mV). This indicates that isoelectric point of the whey protein shifts towards lower pH after glycated with maltodextrin as compared to whey protein alone which provides stability to the droplets at near isoelectric point of the proteins. The shift of isoelectric point towards lower values after glycation was shown previously (Liu and Zhong 2012). Conjugation of whey proteins alters the distribution of protein surface charge, leading to decrease of isoelectric point (Achouri et al. 2005). The decrease of isoelectric point is attributed to the decrease content of basic lysine on protein surface after glycation with MD, which is supported by the lower magnitude of zeta potential of glycated WPC than WPC at pH below isoelectric point.
Table 2.
Effect of processing conditions (pH, ionic strength and heat treatment) on zeta potential and poly dispersity index (PDI) of 5% d-limonene oil nanoemulsion stabilized by whey protein–maltodextrin (1:2 w/w) conjugate
| Treatments | Zeta potential (mV) | PDI |
|---|---|---|
| Control | − 19.64 ± 0.23 | 0.205 ± 0.02 |
| Pasteurization (63 °C/30 min) | − 17.29 ± 0.38 | 0.287 ± 0.01 |
| Forewarming (80 °C/10 min) | − 18.13 ± 0.59 | 0.273 ± 0.03 |
| Boiling (95 °C/10 min) | − 17.92 ± 0.43 | 0.297 ± 0.02 |
| Autoclave (121 °C/15 min) | − 17.60 ± 0.36 | 0.279 ± 0.01 |
| 0.1 M NaCl | − 16.19 ± 0.64 | 0.237 ± 0.05 |
| 0.5 M NaCl | − 14.29 ± 0.53 | 0.265 ± 0.02 |
| 1.0 M NaCl | − 13.02 ± 0.78 | 0.279 ± 0.04 |
| pH 3.0 | +5.98 ± 0.49 | 0.255 ± 0.01 |
| pH 4.0 | +0.86 ± 0.39 | 0.289 ± 0.04 |
| pH 5.0 | − 17.21 ± 0.78 | 0.255 ± 0.03 |
| pH 6.0 | − 19.12 ± 0.32 | 0.225 ± 0.03 |
| pH 7.0 | − 20.89 ± 0.26 | 0.237 ± 0.02 |
Values are mean ± standard error (n = 3)
Effect of ionic strength on the particle size distribution and zeta potential
The effect of ionic concentration on the particle size distribution of LO nanoemulsion was shown in the Fig. 1b. The prepared nanoemulsion was stable to tested ionic concentration (NaCl: 0.1–1.0 M). Particle size was relatively increased with increase in ionic concentration from control i.e. without addition of salt (116.60 ± 5.3 nm) to the 1.0 M NaCl added nanoemulsion (139.10 ± 3.42 nm). McClements and Rao (2011) reported similar results that protein stabilized corn oil in water nanoemulsions were found to be stable to droplet aggregation and creaming from 0 to 1000 mM concentration of NaCl. This may be due to the weaker attractive and repulsive interactions between the droplets. Shah et al. (2012) found that the insignificant difference between nanoemulsion samples containing 0–50 mM concentration of NaCl. Stability at higher concentration of NaCl indicates the dominance of steric repulsion, the strength of which is independent on the ionic environment for non ionizable maltodextrin, over electrostatic repulsion that is weakened by the addition of NaCl.
The effect of ionic concentration on the magnitude of zeta potential of LO nanoemulsion was shown in Table 2. It was found that as ionic concentration increases, the magnitude of zeta potential of the nanoemulsion decreases (i.e. towards zero). This might be due to the decrease in the electrostatic repulsion between the molecules. The magnitude of the zeta potential reduced from − 19.64 ± 0.23 mV (control nanoemulsion, i.e. without addition of salt) to − 13.02 ± 0.78 mV (nanoemulsion added with 1.0 M NaCl). This results commemorates the findings of Onsaard et al. (2013). i.e. The magnitude of zeta-potential of the WPC emulsions containing maltodextrin and carrageenan was negative at all ionic strengths, but the magnitude of zeta-potential decreased when the ionic strength increased from 0 to 500 mM.
Effect of heat treatments (pasteurization, forewarming, boiling and sterilization) on the particle size distribution and zeta potential
The effect of heat treatment on the particle size distribution of LO nanoemulsion was shown in the Fig. 1c. The prepared nanoemulsion was stable to different heat processing conditions like pasteurization (63 °C/30 min), forewarming (80 °C/10 min), boiling (100 °C/10 min) and sterilization (121 °C/15 min). This might be due to the improved emulsifying and stabilizing properties of whey proteins after glycation with maltodextrin were previously reported for orange, flavor, and triglyceride oils (Akhtar and Dickinson 2007) due to the adsorption of more hydrophobic protein moiety onto the oil–water interface and the hydrophilic oligosaccharide protruding in the water phase providing steric hindrance against aggregation. Likewise, the maltodextrin glycated to whey proteins effectively prevents the aggregation of whey proteins during heating at various pH and ionic conditions and improves heat stability (Liu and Zhong 2012).
The effect of heat treatment on the magnitude of zeta potential of LO nanoemulsion was shown in Table 2 and it was found that the different heat treatments did not cause any significant change in the magnitude of zeta potential of nanoemulsion. The improved thermal stability can be attributed to steric effects provided by the glycated MD. NaCN–MD mixture, crude conjugate and purified conjugate stabilized o/w emulsions were as thermally stable as NaCN stabilized emulsions on heating for up to 2.5 min at 140 °C, as minimal changes in the fat globule size distributions and mean fat globule size were observed on heating for up to this time. Extending the heating time to between 5.0 and 20 min progressively shifted the fat globule size distribution and mean fat globule size to larger distribution/sizes for all of the emulsions (O’Regan and Mulvihill 2010).
Effect of storage time on the particle size distribution of nanoemulsion
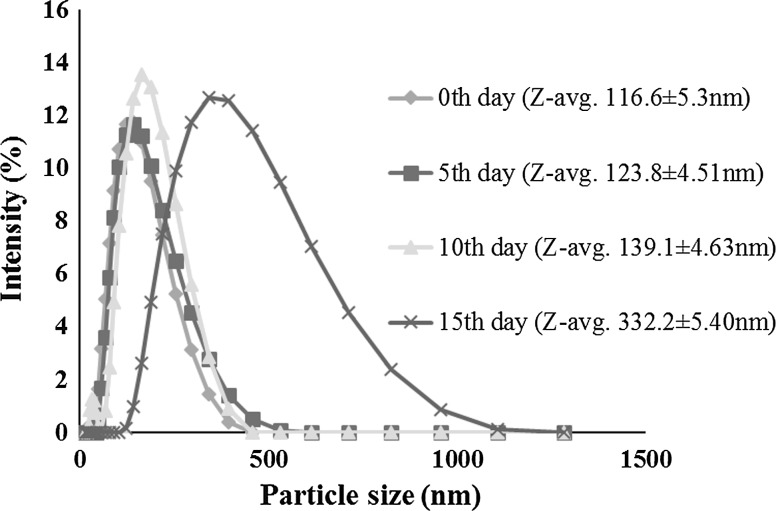
The storage study of LO nanoemulsion was done for 15 days. The effect of storage time on particle size distribution was shown in the Fig. 2. The nanoemulsion was found to be stable for studied storage period. It was found that there was a progressive increase in particle size of the nanoemulsion with increase in storage time from 0th day (116.60 ± 5.30 nm) to 15th day (332.20 ± 5.40 nm). The increase in the average size was may be due to the movement of the dispersed droplet pervaded the dispersing phase, then the chances of droplet collisions increased (Zahi et al. 2015). The mean particle size of the droplets in an emulsion/nanoemulsion increases over time due to diffusion of oil molecules from small to large droplets through the intervening fluid. Li and Lu (2016) reported that d-limonene nanoemulsion was seemed to be stable against flocculation and coalescence during storage at 25 °C as compared with 4 °C and 50 °C. NaCN–MD conjugate stabilized o/w emulsions showed improved stability in comparison to NaCN stabilized emulsions after storage for 20 days under accelerated shelf life testing conditions (O’Regan and Mulvihill 2010).
Fig. 2.
Effect of storage time on particle size distribution of 5% d-limonene oil nanoemulsion stabilized by 9% whey protein–maltodextrin (1:2 w/w) conjugate
Antimicrobial activity of 5% d-limonene oil nanoemulsion stabilized by 9% whey protein–maltodextrin (1:2 w/w) conjugate
The antimicrobial activity of 5% LO nanoemulsion and bulk LO (5%) dissolved in dimethyl sulphoxide (DMSO) was evaluated against different microorganisms like B. cereus (ATCC 14459), E. coli (ATCC 25922), E. faecalis (NCDC 115) and Salmonella typhi (NCDC 6017) by agar well diffusion method.
Minimum inhibitory concentration (MIC) of 5% d-limonene oil nanoemulsion stabilized by 9% whey protein–maltodextrin (1:2 w/w) conjugate and bulk d-limonene oil dissolved in DMSO against above mentioned organisms
Zone of inhibition of different concentration of5% LO nanoemulsion and bulk LO (5%) dissolved in dimethyl sulphoxide (DMSO) against different microorganisms like B. cereus (ATCC 14459), E. faecalis (NCDC 115), E. coli (ATCC 25922) and Salmonella typhi (NCDC 6017) were shown in the Fig. 3a–d. It was found that there was no significant difference in the antimicrobial activity of LO nanoemulsion and bulk LO dissolved in DMSO against the tested organisms. Both LO nanoemulsion (5.0% oil phase) and bulk LO dissolved in DMSO (5.0% oil phase) were exhibited MIC of 12.50 µl/ml (Fig. 3) against the above mentioned organisms. Zhang et al. (2014) reported that d-limonene nanoemulsions with nisin exhibit excellent antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and S. cerevisiae (yeast). Donsi et al. (2011) reported a reduction in E. coli population after being in contact during 24 h incubation with a d-limonene-loaded nanoemulsion stabilized with soy lecithin or sugar esters processed by high pressure homogenization. Wu et al. (2014) reported that there was no difference in inactivation of L. monocytogenes by thyme oil emulsion and thyme oil pre-dissolved in ethanol, while the emulsion was initially more effective than the pre-dissolved thyme oil when treating E. coli O157:H7 and S. enteritidis. Zahi et al. (2015) reported that there was an improvement in the antimicrobial activity of d-limonene after its incorporation into the organogel-based nanoemulsion as compared to free d-limonene, the improvement may be attributed due to the small droplet size of the d-limonene organogel-based nanoemulsion which may easily fuse with the bacterial cells and cause the death of the microorganisms due to the increase in its water solubility. However, the antimicrobial mechanism of EOs is not fully understood. It was proposed to involve membrane disruption by the lipophilic compounds (Tajkarimi et al. 2010). It has been demonstrated that the level of hydrophobicity of EOs affects the toxicity to bacteria (Goni et al. 2009). However, after being incorporated into oil-in-water (O/W) emulsions, the antimicrobial activity of EOs would be affected by the composition of the whole system.
Fig. 3.
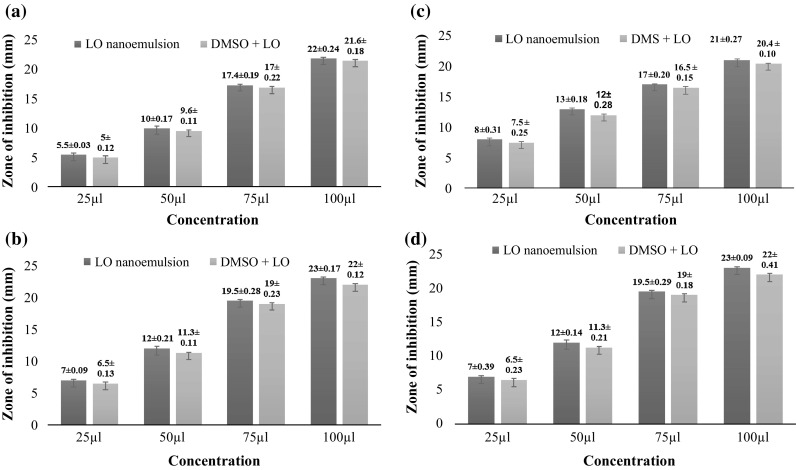
Antimicrobial activity of 5% d-limonene oil nanoemulsion and 5% bulk d-limonene oil dissolved in dimethyl sulphoxide against B. cereus (ATCC 14459), E. faecalis (NCDC 115), E. coli (ATCC 25922) and Salmonella typhi (NCDC 6017). Zone of inhibition of 5% d-limonene oil nanoemulsion and 5% bulk d-limonene oil dissolved in dimethyl sulphoxide against a B. cereus (ATCC 14459), b E. faecalis (NCDC 115), c E. coli (ATCC 25922) and d Salmonella typhi (NCDC 6017)
Conclusion
In the present study, d-limonene oil nanoemulsion were prepared by Ultrasonication method using whey protein–maltodextrin conjugate as coating material. The stable formulation of limonene oil nanoemulsion (5.0% oil + 9.0% conjugate) had a mean particle size of 116.6.0 ± 5.30 nm, zeta potential of − 19.64 ± 0.23 mV and poly dispersity index of 0.205 ± 0.02 and stable for 15 days at 25 °C with an increase in particle size to 332.20 ± 5.40 nm at 15th day. The stable formulation of limonene oil nanoemulsion (5.0% oil + 9.0% conjugate) was stable to different processing conditions like different pH (3.0–7.0), ionic concentrations (0.1–1.0 M) and different heat treatments like pasteurization (63 °C/30 min), forewarming (80 °C/10 min), boiling (95 °C/10 min) and sterilization (121 °C/15 min). The antimicrobial activity of stable formulation of limonene oil nanoemulsion (5.0% oil + 9.0% conjugate) was evaluated by agar well diffusion method. It has shown minimum inhibitory concentration (MIC) of 12.50 µl/ml against B. cereus (ATCC 14459), E. coli (ATCC 25922), E. faecalis (NCDC 115) and Salmonella typhi (NCDC 6017). Since d-limonene has been considered to be a safer alternative compared to synthetic antimicrobial food additives, the present investigation will be helpful in developing a more effective antimicrobial system for the production and preservation of foods. WP–MD conjugates can be used as a novel emulsifier to produce limonene nanoemulsions suitable for use as preservatives in food applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank to Director, ICAR-National Dairy Research Institute, Karnal, Haryana (India) for providing necessary facilities, and ICAR for providing funds under CRP on Nanotechnology platform.
Compliance with ethical standards
Conflict of interest
The authors declared no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3198-7) contains supplementary material, which is available to authorized users.
Contributor Information
K. S. Sonu, Email: sonuksgowda@gmail.com
Bimlesh Mann, Phone: +91-184-2259143, Phone: 2259152, Email: bimleshmann@gmail.com, Email: bmann@rediffmail.com, Email: headdairychemistry@gmail.com, http://www.ndri.res.in.
Rajan Sharma, Email: rajansharma21@gmail.com.
Rajesh Kumar, Email: rbajaj1375@gmail.com.
Richa Singh, Email: richasingh.ndri@gmail.com.
References
- Achouri A, Boye JI, Yaylayan VA, Yeboah FK. Functional properties of glycated soy 11S glycinin. J Food Sci. 2005;70:C269–C274. doi: 10.1111/j.1365-2621.2005.tb07172.x. [DOI] [Google Scholar]
- Akhtar M, Dickinson E. Emulsifying properties of whey protein–dextran conjugates at low pH and different salt concentrations. Colloids Surf B. 2003;31:125–132. doi: 10.1016/S0927-7765(03)00049-3. [DOI] [Google Scholar]
- Akhtar M, Dickinson E. Whey protein–maltodextrin conjugates as emulsifying agents: an alternative to gum Arabic. Food Hydrocoll. 2007;21:607–616. doi: 10.1016/j.foodhyd.2005.07.014. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- CFR (2009) Code of Federal Regulations. Title 21, Part 172.515. Food additives permitted for direct addition to food for human consumption: synthetic flavoring substances and adjuvants, pp 56–63
- Cimanga K, Kambu K, Tona L, Apers S, De Bruyne T, Hermans N, Totte J, Pieters L, Vlietinck AJ. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J Ethnopharmacol. 2002;79:213–220. doi: 10.1016/S0378-8741(01)00384-1. [DOI] [PubMed] [Google Scholar]
- Dalev PG, Simeonova LS. Emulsifying properties of protein–pectin complexes and their use in oil containing foodstuffs. J Sci Food Agric. 1995;68(2):203–206. doi: 10.1002/jsfa.2740680211. [DOI] [Google Scholar]
- Donsi F, Annunziata M, Vincensi M, Ferrari G. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J Biotechnol. 2011;159:342–350. doi: 10.1016/j.jbiotec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Donsi F, Annunziata M, Vincensi M, Ferrari G. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J Biotechnol. 2012;159:342–350. doi: 10.1016/j.jbiotec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- Goni P, Lopez P, Sanchez C, Gomez-Lus R, Becerril R, Nerin C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009;116:982–989. doi: 10.1016/j.foodchem.2009.03.058. [DOI] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J Food Eng. 2007;82:478–488. doi: 10.1016/j.jfoodeng.2007.03.007. [DOI] [Google Scholar]
- Lee SJ, McClements DJ. Fabrication of protein-stabilized nanoemulsions using a combined homogenization and amphiphilic solvent dissolution/evaporation approach. Food Hydrocoll. 2010;24(6–7):560–569. doi: 10.1016/j.foodhyd.2010.02.002. [DOI] [Google Scholar]
- Li PH, Chiang BH. Process optimization and stability of d-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason Sonochem. 2012;19(1):192–197. doi: 10.1016/j.ultsonch.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Li B, Jiang Y, Liu F, Chai Z, Li Y, Leng X. Synergistic effects of whey protein-polysaccharide complexes on the controlled release of lipid soluble and water soluble vitamins in W1/O/W2 double emulsion system. Int J Food Sci Technol. 2012;47(2):248–254. doi: 10.1111/j.1365-2621.2011.02832.x. [DOI] [Google Scholar]
- Li PH, Lu WC. Effect of storage condition on the physical stability of d-limonene nanoemulsion. Food Hydrocoll. 2016;53:218–224. doi: 10.1016/j.foodhyd.2015.01.031. [DOI] [Google Scholar]
- Liu G, Zhong Q. Glycation of whey protein to provide steric hindrance against thermal aggregation. J Agric Food Chem. 2012;60:9754–9762. doi: 10.1021/jf302883b. [DOI] [PubMed] [Google Scholar]
- Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2012;2:626–639. doi: 10.4236/jbnb.2011.225075. [DOI] [Google Scholar]
- McClements DJ, Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr. 2011;51:285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- O’Regan J, Mulvihill DM. Heat stability and freeze-thaw stability of oil-in-water emulsions stabilized by sodium caseinate–maltodextrin conjugates. Food Chem. 2010;119:182–190. doi: 10.1016/j.foodchem.2009.06.019. [DOI] [Google Scholar]
- Onsaard E, Putthanimon J, Singthong J, Thammarutwasik P. Influence of maltodextrin and environmental stresses on stability of whey protein concentrate/k-carrageenan stabilized sesame oil-in-water emulsions. Food Sci Technol Int. 2013 doi: 10.1177/1082013213498247. [DOI] [PubMed] [Google Scholar]
- Rao Vemula S, Naveen Kumar R, Polasa K. Foodborne diseases in India—a review. Br Food J. 2012;114(5):661–680. doi: 10.1108/00070701211229954. [DOI] [Google Scholar]
- Shah B, Ikeda S, Davidson PM, Zhong Q. Nanodispersing thymol in whey protein isolate–maltodextrin conjugate capsules produced using the emulsion–evaporation technique. J Food Eng. 2012;113:79–86. doi: 10.1016/j.jfoodeng.2012.05.019. [DOI] [Google Scholar]
- Song Y, Babiker E, Usui M, Saito A, Kato A. Emulsifying properties and bactericidal action of chitosan–lysozyme conjugates. Food Res Int. 2002;35:459–466. doi: 10.1016/S0963-9969(01)00144-2. [DOI] [Google Scholar]
- Soottitantawat A, Yoshii H, Furuta T, Ohkawara M, Linko P. Microencapsulation by spray drying: influence of emulsion size on the retention of volatile compounds. J Food Sci. 2003;68(7):2256–2262. doi: 10.1111/j.1365-2621.2003.tb05756.x. [DOI] [Google Scholar]
- Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- Wu JE, Lin J, Zhong Q. Physical and antimicrobial characteristics of thyme oil emulsified with soluble soybean polysaccharide. Food Hydrocoll. 2014;39:144–150. doi: 10.1016/j.foodhyd.2013.12.029. [DOI] [Google Scholar]
- Xue J, Davidson PM, Zhong Q. Thymol nanoemulsified by whey protein–maltodextrin conjugates: the enhanced emulsifying capacity and antilisterial properties in milk by propylene glycol. J Agric Food Chem. 2013;61:12720–12726. doi: 10.1021/jf4043437. [DOI] [PubMed] [Google Scholar]
- Zahi MR, Liang H, Yuan Q. Improving the antimicrobial activity of d-limonene using a novel organogel-based nanoemulsion. Food Control. 2015;50:554–559. doi: 10.1016/j.foodcont.2014.10.001. [DOI] [Google Scholar]
- Zhang Z, Vriesekoop F, Yuan Q, Liang H. Effects of nisin on the antimicrobial activity of d-limonene and its nanoemulsion. Food Chem. 2014;150:307–312. doi: 10.1016/j.foodchem.2013.10.160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.