Abstract
Development of oat-based fermented beverages started in Europe in the past 30 years with the rise of the functional foods market. It is based on the increasing consumer demand for health drinks and value added foods and on the scientific insights on the nutrition composition of oats. The main health effects of oats are attributed to their high β-glucan content, which is proved to lower blood cholesterol and the intestinal absorption of glucose thus preventing diseases like cardiovascular injury, dyslipidemia, hypertension, inflammatory state and diabetes type 2. Another important role of β- glucan is its prebiotic function in the gastrointestinal tract, supporting the growth of beneficial microbial groups. The slowly digestible fraction of oat starch has a functional role as it moderates the glycaemic response. Oats are also a valuable source of highquality proteins, unsaturated lipids and antioxidants. In addition, oats are appropriate for people suffering from celiac disease because they do not contain gluten. Oat grain processing involves several steps, including thermal processes aiming to prevent oat products from rapid enzymatic deterioration and ensure storage stability. Oat drinks are formulated through processing oat with a liquid ingredient. Further, this matrix is inoculated with lactic acid bacteria to produce a fermented beverage. In some, drinks, probiotic lactic acid bacteria were used to increase product functional value. Thus, the ancient concept of cereal-based fermented foods is implemented into development of new functional oat-based fermented beverages and several products are already marketed in Europe as healthy, fast and convenient supplementary foods.
Keywords: Fermented oat beverages, Functional oat beverages, Oat composition, Functional foods
Introduction
Functional foods are considered to promote health-boosting effects in addition to their nutritional value, which are attributed to the content of biologically active components in adequate amounts (Staka et al. 2015; Chua et al. 2013). Fermented oat beverages are referred to as functional because of the synbiotic effect of probiotic starter cultures and the prebiotic fiber β–glucan (Nyanzi and Jooste 2012; Iserliyska et al. 2015; Chua et al. 2013; Russo et al. 2017). It is important to note that the manufacturing processes and food matrices have a significant effect on the functional performance of the probiotic strains (Sánchez et al. 2012). Nowadays the market for functional food and beverages has evolved considerably, as people become more and more concerned for their health and lifestyle quality (Shah et al. 2016; Salmerón et al. 2015). Oat grain has a high functional potential due to its composition. Oats are known to be an excellent source of dietary fiber, antioxidants and a well balanced protein fraction (Shah et al. 2016; Londono et al. 2013; Staka et al. 2015). They provide more protein, fiber, calcium, iron, zinc and essential amino acids than other whole grain crops (Sangwan et al. 2014; Shah et al. 2016; Tosh and Chu 2015). Oats are known to be unique among cereals as they are therapeutically active against diabetes, dyslipidemia, hypertension, inflammatory state and vascular injury (Londono et al. 2013; Sangwan et al. 2014; Shah et al. 2016; Staka et al. 2015; Vasudha and Mishra 2013). Oats health effects have been primarily attributed to the highly viscous β-glucan fraction, which is proved to lower blood cholesterol and the intestinal absorption of glucose (Hu et al. 2014; Iserliyska et al. 2015; Lampi et al. 2015; Tosh and Chu 2015). Oat drinks are formulated through processing oat with milk or other liquid ingredient, and they are marketed as healthy, fast and convenient supplementary food. The rising consumer preferences to health drink and value added products, the changing lifestyle and increasing urbanization are factors expected to expand significantly the global oat drink market over the next 10 years (http://www.futuremarketinsights.com/reports/oat-drinks-market). The aim of the present review is to highlight the valuable aspects of oat composition which make it a preferred raw material for the development of new functional fermented beverages. The review also explores the specific steps in oat processing and the various approaches applied to enhance the health effects of these products.
Composition of oats
People have used different oat species since ancient times as high calorie feed and wholesome food. Oats are grown worldwide and consumed in many countries as part of their daily diet. Contemporary research reveals the health promoting properties and high nutritional value of oats are due to the availability of soluble fibre, essential amino acids, unsaturated fatty acids (oleic, linoleic and linolenic acid), vitamins (A, B12, D, E), minerals (calcium, phosphorous and iron) and phytochemicals (avenanthramides) (Arendt and Zannini 2013; Clemens and van Klinken 2014; Murphy 2011; Regand et al. 2011; Russo et al. 2017; Tosh and Chu 2015). Consuming oats is also beneficial for people suffering from celiac disease (CD) because they do not contain gluten (Decker et al. 2014; Gangopadhyay et al. 2015; Londono et al. 2013).
There are about seventy species of oats around the world, grown mostly in cool, moist and moderate climates (Clemens and van Klinken 2014). The most commonly cultivated are Avena sativa L. (common oat), Avena nuda L. (naked oat) and Avena byzantina (red oat). Avena sativa L. has hulls that surround the kernel and may be white, grey, yellow or black. Avena byzantina is generally grown in the southern half of the United States and in the Near and Middle East. Avena nuda L. (Hull-less Oat, Large Naked Oat) is grown only to a very limited extent (Hu et al. 2014).
Starch is the main source of available carbohydrates in oat products (Regand et al. 2011; Tosh and Chu 2015). It is located in the endosperm in the form of starch granules embedded in the network of protein and other non protein components (Rasane et al. 2015; Shah et al. 2016). It represents 82% (dry basis) of the oat endosperm (Kedia et al. 2008). Oat starch is characterised by small size granules with a well developed granule surface and oval or irregular shape (Rasane et al. 2015; Shah et al. 2016). There are lipids bound to starch residing inside the native starch granules either in the amylose helix or in the gap between amylose and amylopectin (Angioloni and Collar 2011). The starch granules are surrounded by cell walls which should be broken by adequate processing to make starch accessible by the digestive enzymes of the fermenting microorganisms or the human digestive enzymes. Starch digestibility depends on the type of starch molecules, which can be rapidly digestible (RDS) or slowly digestible (SDS). The sum of RDS and SDS gives the total digestible starch (DS). Resistant starch is the fraction that passes undigested through the digestive system and acts as functional fiber (Rasane et al. 2015; Robert et al. 2016). SDS is the most important functional fraction of oat starch as it moderates the glycaemic response (Regand et al. 2011). Glycaemic index (GI) is defined as the blood glucose raising potential of carbohydrate foods (Tosh and Chu 2015). The slower the glucose levels rise in the blood, the smaller the GI and safer the insulin response is (Regand et al. 2011).
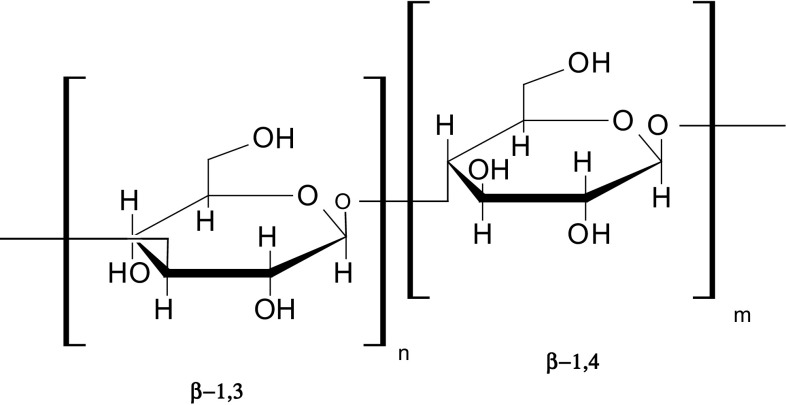
The carbohydrate fraction of oats that possesses highly functional properties is β-glucan. It is a linear polysaccharide, with D-glucopyranosyl residues arranged as mixtures of consequent β-(1 → 4)-linked glucose units in blocks that are separated by single β-(1 → 3) linkages (Fig. 1). The β-glucan content in oat ranges from 1.8 to 8.1% (dm) (Flander et al. 2007; Flander 2012). Oat β-glucan has been extensively investigated and proven to have physiological effects on lowering cholesterol and postprandial blood glucose levels (Brindzová et al. 2008; Iserliyska et al. 2015; Staka et al. 2015). β-glucan is one of the few compounds that is approved for its cholesterol lowering properties by both EFSA and FDA. FDA has accepted a health claim stating that a daily intake of 3 g of soluble oat β-glucan can lower the risk of coronary heart disease (Rasane et al. 2015), while in the European Union, the amount required for a cholesterol-lowering health claim is 1.0 g β-glucan content per portion of a product (Flander 2012). The benefits of oat β-glucan are due to the viscosity formed in the human gastrointestinal tract (Staka et al. 2015). β-glucan increases luminal viscosity and results in slower absorption of carbohydrates (Regand et al. 2011; Staka et al. 2015). Viscosity depends on solubility, molecular weight and the branched structure of β-glucan. It passes through the stomach and small intestines undigested by the human digestive enzymes and acts as a prebiotic in the gastrointestinal tract (Staka et al. 2015). Prebiotics are defined as “selectively fermented ingredients that allow specific changes, both in the composition and/or activity in the gastrointestinal microbiota that confers benefits upon host well-being and health” (Gibson et al. 2004; Robert et al. 2016). There are two related health claims, the first stating that β-glucan lowers the blood cholesterol when taken at least 3 g/day and the other states that β-glucan reduces the post-prandial glycaemic response if people consume at least 4 g of β-glucan from barley or oats per meal for each 30 g of available carbohydrates in the meal (Kariluoto et al. 2014). The interaction between oat β-glucan and starch has the potential to influence starch digestibility and consequently affects its bioavailability.
Fig. 1.
Molecule structure of β-glucan
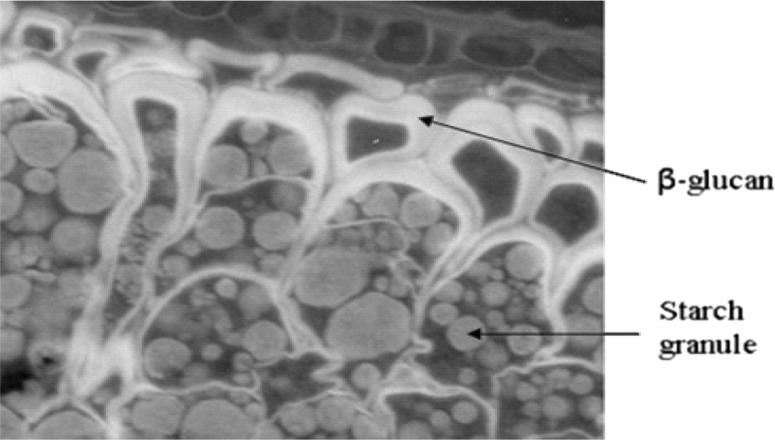
Oat β-glucan, together with other non-starch polysaccharides can be seen in the walls of the endosperm cells which enclose starch, matrix protein and lipid reserves of the grain (Fig. 2). Extraction of β-glucan usually requires inactivation of endogenous enzymes (β-glucanases and amylases), as leaving these enzymes active may transform the β-glucan to a lower molecular weight product. Molecular weight of β-glucan is an important factor as it determines its viscosity, which defines the cholesterol-lowering capacity of β-glucan (Niniosa et al. 2011; Gangopadhyay et al. 2015).
Fig. 2.
Electron microscopy picture of the oat grain (www.oatsandhealth.org)
Oat protein has a high nutritional value and low cost. Protein content in oats (11–15% of the grain) can be divided into four fractions. Albumins (water soluble, 1–12% of the total protein) are the enzyme-containing fraction. Globulins (salt water soluble fraction) in oat enosperm represent about 80% of the total protein (Rasane et al. 2015). The globulin fraction in oats is the major storage protein fraction unlike other cereal grains (Londono et al. 2013). Prolamins (soluble in alcohol) in oats are called avenins and vary between 10 and 15% of the total protein content in contrast to wheat, in which prolamins represent 80% of the total seed protein content (Rasane et al. 2015; Londono et al. 2013). Glutelins (soluble in acid or bases) vary between 5 and 66% depending on oat variety (Rasane et al. 2015). Protein found in oats is known to be nutritionally superior to that of wheat due to higher lysine content which is the main limiting amino acid in cereals (Kedia et al. 2009). Based on the recognised value of oat proteins, some current research efforts are specifically focused on the production of oat protein concentrates and their application as food ingredients in different food matrices (http://www.oatpro.eu/).
Avenins are regarded as safe for CD patients. With the increasing demand for gluten-free products, oat is considered as a good alternative to diversify the diet of CD patients, also contributing with healthy compounds such as essential amino acids, unsaturated fatty acids, beta-glucans, polyphenols and vitamins. However, a guaranteed gluten-free oat production chain is an essential requirement for CD-safe oat production (Londono et al. 2013).
Oats contain relatively high amounts of lipids compared to other cereal grains (Schnitzenbaumer and Arendt 2013; Zhou et al. 1999). Oat lipid content varies from 5 to 9% in different cultivars. Oat lipids are commonly found within the endosperm (Rasane et al. 2015) and can exist in the oat grain as lipid bodies dispersed like emulsion droplets, each small droplet isolated by phospholipid—protein surrounding layer (Decker et al. 2014). Lipids that are bound to starch are less than those found in any other cereal grains (Angioloni and Collar 2011). The fat fraction of oat grain consists of triglycerides, phospholipids, glycolipids, unsaturated fatty acids, and sterols. The excellent fatty acid composition has a significant impact on the nutritional quality of oats (Zhou et al. 1999). It comprises high level of unsaturated fatty acids like oleic acid (18:1) and linoleic acid (18:2) (Brindzová et al. 2008; Rasane et al. 2015; Lehtinen and Lasskso 2004; Zhou et al. 1999).
High lipid content of oats, however, may cause various processing problems such as poor flavour and excessive browning of toasted products. Oat also contains considerable amount of lipases, which are capable of acting under low moisture condition and, if not controlled, cause rancidity of oat products. A year of storage at room temperature doesn’t affect substantialy the lipid content of intact oat kernels. However, the disintegrated oat products and oat flours are faster affected by lipid oxidation and have shorter shelf-life (Keying et al. 2009; Decker et al. 2014; Lehtinen and Lasskso 2004, Rasane et al. 2015).
Antioxidants in oats such as tocopherols, tocotrienols, L-ascorbic acid, thiols, phenolic amino acids and other phenolic compounds act as natural protectors of the grain (Keying et al. 2009). They prevent free radical damage to lipids, proteins, DNA, RNA, and cellular organelles (Hitayezua et al. 2015). The main antioxidants in oats are polyphenolic compounds and vitamin E.
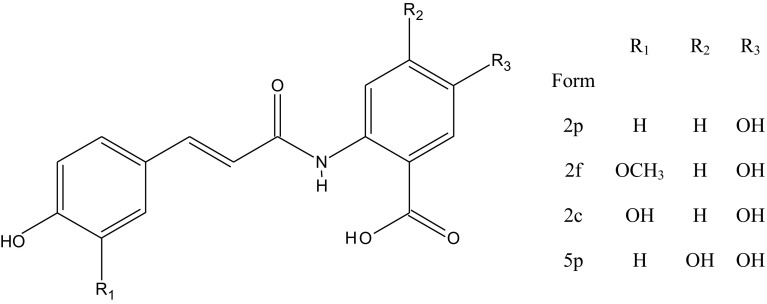
Polyphenolic compounds in oats account for 5.7% and include phenolic acids, flavonoids and a unique group of N-cinnamoylanthranilic acid derivatives referred to as avenanthramides (AVs) (Fig. 3) (Hitayezua et al. 2015; Shah et al. 2016; Gangopadhyay et al. 2015, Peterson 2001; Rasane et al. 2015; Schnitzenbaumer and Arendt 2013). Phenolic acids in oats are mainly vanillic, caffeic, p-coumaric, ferulic, p-cinnamic acids. Avenanthramides are low-molecular weight, soluble phenolic compounds which are unique for oat grain and are not found in other cereals. There are more AVs in oats but so far the structure of only five has been completely elucidated. The most abundant AVs are N-[3′, 4′-Dihydroxy-(E)-cinnamoyl]-5-hydroxyanthranilic acid (AV2c), N-[4′-Hydroxy-3′-methoxy-(E)-cinnamoyl]-5-hydroxyanthranilic acid (AV2f), and N-[4′-Hydroxy-(E)-cinnamoyl]-5-hydroxyanthranilic acid (AV2p). Avenanthramides possess strong antioxidant activity. They have been demonstrated to reduce oxidation of low density lipoprotein cholesterol (LDL cholesterol) in synergy with vitamin C in animals and humans (Hitayezua et al. 2015).
Fig. 3.
Structure of avenanthramides
The other group of oat bioactives that have the ability to scavange free radicals are tocols. Tocols (tocopherols and tocotrienols), also known as vitamin E, are natural antioxidants in grains, which are able to donate phenolic hydrogen atoms to free radicals thus stopping destructive chain reactions of oxidation. They exist as lipid-soluble compounds and are unevenly distributed within the grain: almost all tocopherols are found in the germ, while most of the tocotrienols are located within the endosperm, with α-tocotrienol as the predominant homologue (Peterson 2001). Different studies show total tocols in oats to range from 15 to 48 mg/kg (Peterson 2001; Rasane et al. 2015).
Besides vitamin E, oats are a good source of other vitamins such as Thiamin, Riboflavin, Niacin, Panthotenic acid, vitamin B6 and Pholate (Sangwan et al. 2014; Kedia et al. 2008).
Minerals in the oat grain are unevenly distributed. Bran is the fraction with the highest concentration of minerals. It contains high levels of potassium, sodium, magnesium and calcium. The aleurone layer is rich in phosphorous (Kedia et al. 2008).
Oats processing
One of the challenges of oat processing is associated with its high lipase and lipoxygenase activity (Lehtinen and Lasskso 2004). Inactivation of the lipases and lipoxygenases is achieved by heat treatment of the oat kernels (Tosh and Chu 2015; Lehtinen and Lasskso 2004). The thermal processes are applied to prevent oat products from rapid enzymatic deterioration and ensure storage stability. Heat treatment alter not only the lipase activity of oats, but also other physicochemical and sensory characteristics such as the antioxidant activity, β-glucan and free fatty acids composition. It modifies the overall nutritional composition, the profile of the flavour active molecules, and the overall sensory profile (Runyon et al. 2015; Decker et al. 2014; Lehtinen and Lasskso 2004).
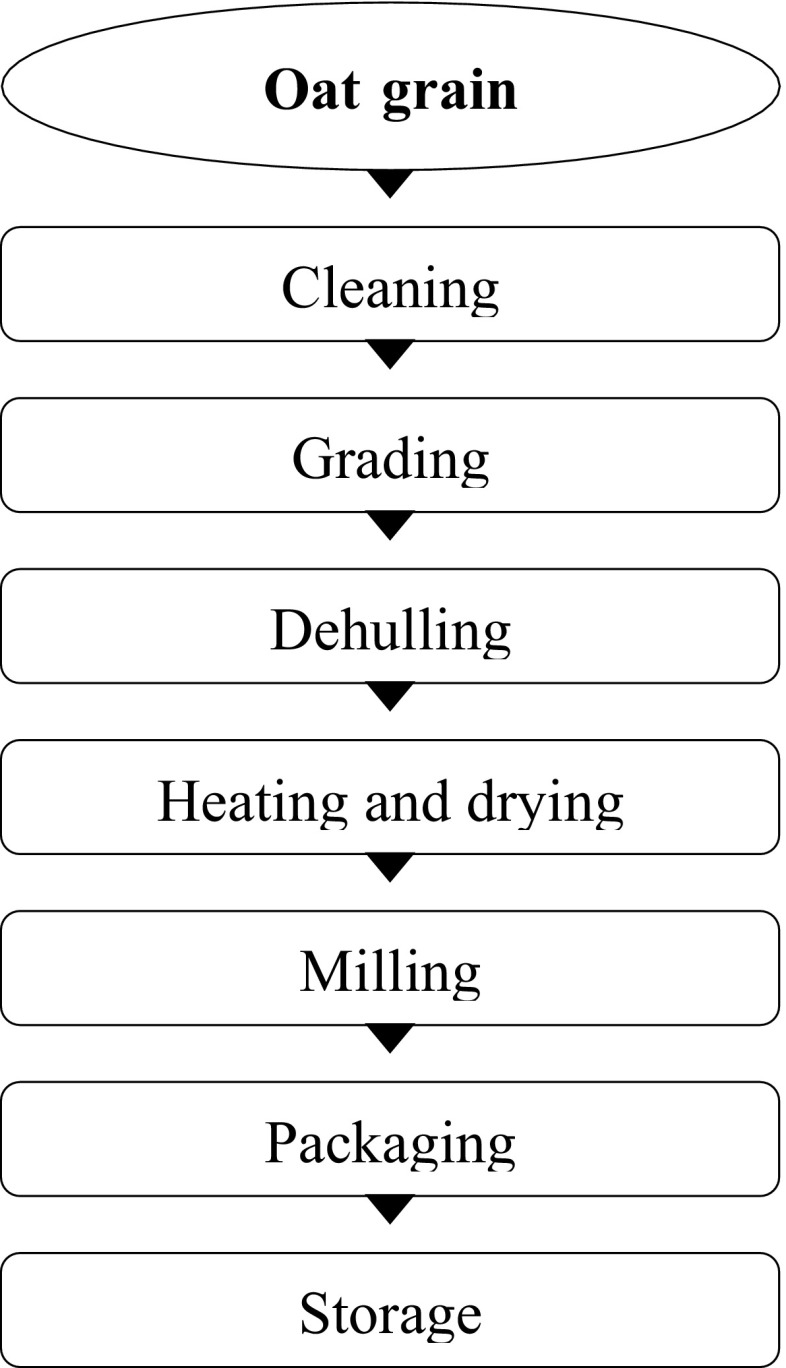
The grain of most oat varieties consists of a groat or kernel and a hull (or husk) in covered oats. Hulls comprise 28–32% of the oat grain. Naked oats lack the hull and are therefore preferred for processing (Zhou et al. 1999). Oat groats consist of three different layers: bran, endosperm and germ (Decker et al. 2014; Kedia et al. 2009). The bran layer of oats is not as structurally distinct as, for example, the bran of wheat, and the oat grain is softer therefore it needs different milling processes than those used for milling wheat (Zhou et al. 1999). In order to obtain an easily digestible and an easy-to-prepare product, oat kernels are subjected to multiple step processing. Milling requires some preliminary steps such as cleaning, grading and dehulling (Fig. 4).
Fig. 4.
Flow diagram of oat flour production
Cleaning includes series of separations to remove foreign components present in the grain. Grading is applied to divide the grain into different sized fractions in order to ensure effective milling. Dehulling separates the digestible part of the kernel—the groat from the hulls. Hulls can be used as high fiber ruminant feed, for biomass power plants or for other purposes (Decker et al. 2014). Further, oat groats are subjected to heat treatment in order to inactivate the lipid degrading enzymes causing poor sensory quality and shorter shelf life of the products. Heating processes employed are kiln-drying, extrusion or steam stabilizing (Murphy 2011; Lampi et al. 2015; Lehtinen and Lasskso 2004). Depending on the time of heat treatment, lipases may be either reversibly inhibited or permanently deactivated. The treatment should be efficient enough to inactivate the lipolytic enzymes but mild enough to prevent degradation of the naturally occurring valuable bioactive molecules e.g. antioxidants and vitamins (Lampi et al. 2015).
Milling processes are employed for deriving wholegrain oat flours. After the bran layer is removed from the endosperm during milling, the individual cell walls are broken down and the oat lipids come into contact with the highly reactive lipase enzyme. The decomposition of lipids into free fatty acids (FFA) is the reason for instability of the milled oat products. The FFA get subsequently changed by lipoxygenase enzymes and a chain of oxidative reaction is initiated. This process causes hydrolytic rancidity as well as off-flavours and bitterness (Lehtinen and Lasskso 2004). The most used indicator for occurring oxidative reactions is the presence of hexanal in the food matrix. The extent of lipid oxidation is characterized by various parameters such as amount of remaining intact unsaturated fatty acids, presence of fatty acids hydroperoxides and presence of secondary oxidation products (Decker et al. 2014; Keying et al. 2009; Lehtinen and Lasskso 2004; Lampi et al. 2015).
Fermented oat beverages
Fermentation is one of the oldest processes used for food preservation and fermented foods and beverages are of great importance for people in the developing world (Agati et al. 1998; Blandino et al. 2003; Nyanzi and Jooste 2012; Tchekessi et al. 2014). Fermentation is traditionally used to obtain products from various cereals around the world. There are numerous benefits related to food fermentaion such as saving energy for matrix processing, and desirable biochemical changes which result in enhanced nutritional value, improved sensory properties, food safety and shelf-life (Agati et al. 1998; Blandino et al. 2003; Guyot 2012; Nionelli et al. 2014; Tchekessi et al. 2014). There is a wide variety of traditional cereal-based beverages around the world (Mäkinen et al. 2015; Nyanzi and Jooste 2012). Such products are mostly consumed in Africa and are prepared from maize, millet and/or sorghum by spontaneous fermentation from mixed microbial cultures involving lactic acid bacteria and yeasts. Some examples for African fermented beverages are: Ben-saalga (Burkina Faso), Kanun-zaki (Nigeria), Koko (Ghana), Mageu (Southern Africa and Arabian Gulf countries), Munkoyo (Zamibia and Congo), Thobwa (Malawi and Tanzania), Uji (Uganda, Kenya and Tanzania), Bushera (Uganda) and many others. They are used by the general population and as food for infants (Agati et al. 1998; Nionelli et al. 2014; Mäkinen et al. 2015; Nyanzi and Jooste 2012; Tchekessi et al. 2014; Vasudha and Mishra 2013). Boza is a traditional non-alcoholic beverage consumed in the Balkan region (Bulgaria, Romania, Albania and Turkey) which is prepared from wheat, rye, millet or other cereals mixed with water and sugar, (Mäkinen et al. 2015; Nyanzi and Jooste 2012; Vasudha and Mishra 2013). Kvass is consumed in Eastern Europe and is made from rye or barley malt flour and stale rye bread (Nyanzi and Jooste 2012). Mexican Pozol is obtained by soaking maize in lime water. Dough balls (nixtamal) are wrapped in banana leaves, fermented for 0.5–4 days and dissolved in water prior consumption (Nyanzi and Jooste 2012; Vasudha and Mishra 2013). The microorganisms involved in cereal fermentations mainly belong to the genera Lactobacillus (Lb. plantarum, Lb. fermentum, Lb. casei, Lb. reuteri, Lb. rhamnosus, Lb. Acidophilus, Lb. brevis and others); Pediococcus (P. acidilactici, P. pentosaceus, P. pentisaceus etc.), Bifidobacterium; Candida; Debaryomyces; Endomycopsis; Hansenula; Pichia; Saccharomyces and Trichosporon (Guyot 2012; Nionelli et al. 2014; Nyanzi and Jooste 2012; Russo et al. 2016; Vasudha and Mishra 2013).
Development of oat-based fermented beverages started in Europe in the past 30 years with the rise of the functional foods market. Based on the increased knowledge on oats high nutritional value and consumers’ demand for healthy foods, several oat-based probiotic beverages were introduced in Europe. Proviva is produced by Skane Dairy, Sweden, and is fermented by Lactobacillus plantarum 299v (Nyanzi and Jooste 2012; Vasudha and Mishra 2013). Yosa is a product from Finland, fermented by probiotic strains Lactobacillis acidophilus LA5 and Bifidobacterium lactis Bb12 (Nyanzi and Jooste 2012). Grainsfield Wholegrain Liquid is an effervescent liquid made from organic malted oats, maize, rice, alfalfa seed, pearl barley, linseed, rye grain, wheat and millet and fermented by Lb. acidophilus, Lb. delbreukii, Saccharomyces boulardii and Saccharomyces cerevisiae species (Tchekessi et al. 2014). In Sweden, a non-dairy fermented product named Adavena M40 was formulated based on oat, which was comparable to yoghurt regarding consumer acceptability (Martensson et al. 2000, 2001). An oat-banana fermented beverage with a β-glucan additive was studied by Goncerzewicz et al. (2016). Another new wholegrain oat beverage named Biovessina is fermented by probiotic LAB (Lb. plantarum) and is produced in several varieties—with sweeteners or sugar and with natural flavourings (Angelov et al. 2005, 2006; Gotcheva et al. 2002; Iserliyska et al. 2015).
Probiotic lactic acid bacteria are incorporated in the newly developed fermented oat beverages in order to enhance the functionl properties of the products. Probiotics and their health benefits are discovered in 1907 by Metchnioff and have been prospected ever since (Vijaya Kumar et al. 2015). According to the most widely accepted definition, probiotics are live microorganisms which when administered in adequate amounts confer a health benefit on the host (Chua et al. 2013; Sánchez et al. 2012). The quantity per serving should be minimum 106–107 CFU (Nyanzi and Jooste 2012; Salmerón et al. 2015; Vasudha and Mishra 2013; Vijaya Kumar et al. 2015). The first criterium for selection of such strains is their safety for food applications. The strains should be with Generally Recognized as Safe (GRAS)—status or Qualified Presumption of Safety (QPS)—status, which was implemented by EFSA in 2007 (EFSA 2007). Further, the strains should meet a number of selection criteria such as resistance to acid and bile, production of antimicrobial compounds, attachment to the human epithelial cells and colonization in the human intestine, antibitoc resistance and other properties (Gotcheva et al. 2002). To be successfully applied in oat products, the probiotic starters should endure the physico-chemical conditions of the matrix during processing and storage and should remain viable for a long period to ensure an adequate product shelf life. They also should have low production cost (Prado et al. 2008; Sánchez et al. 2012; Zhao et al. 2015).
One of the important characteristics of probiotic strains for application in fermented oat beverages is their ability to produce bacteriocins during fermentation. Bacteriocins are bioactive proteinaceous antimicrobial substances produced by some strains of Lactobacillus, Lactococcus, Pediococcus, some yeasts and filamentous fungi (Kormin et al. 2001; Nyanzi and Jooste 2012). Bacteriocin-producing microorganisms have the ability to prevent the growth of pathogens like Staphilococcus aureus, Bacillus cereus, Listeria monocytogenes and Clostridium botulinum (Kormin et al. 2001; Nyanzi and Jooste 2012). They act as “biopreservatives” replacing the use of some chemical additives. All bacteriocins are active in a nanomolar range and have no toxicity, and besides being excellent biopreservatives of the fermented foods, they make the intestinal environment less comfortable for pathogenic microbes (Kormin et al. 2001; Parada et al. 2007; Vijaya Kumar et al. 2015). An example for food application is Nisin, a bacteriocin produced by Lactococcus lactis which is applied for preservation of dairy products, fish and meat products to replace nitrate additives (Kormin et al. 2001; Parada et al. 2007).
Most existing probiotics have been isolated from the gut microbiota of humans (Salmerón et al. 2015; Sánchez et al. 2012); Kedia et al. (2009) used mixed culture of human fecal bacteria to ferment oat bran and wholegrain oat flours in order to prove the prebiotic effect of the oat products. Salmerón et al. (2015) used human derived Lactobacillus acidophilus, Lactobacillus plantarum and Lactobacillus reuteri in fermented cereal (oats, barley and malt) beverages and evaluated cell viability, the physicochemical composition and acceptance of the obtained products (Salmerón et al. 2015). Other strains were isolated from traditional fermented foods, and especially those from cereal-based products were considered with good potential for oat fermentation (Gotcheva et al. 2002; Angelov et al. 2005).
Probiotic foods comprise between 60 and 70% of the total functional food market (Salmerón et al. 2015). Probiotics are primarily used in dairy products therefore these products have the biggest share of the probiotic food market (Mäkinen et al. 2015; Vasudha and Mishra 2013; Sánchez et al. 2012). Nevertheless, some drawbacks of the dairy probiotic products have been pointed out: lactose intolerance, which is a problem for many people worldwide; allergy to β-caseine in cow’s milk which leads to inflammation; the high content of saturated lipids and cholesterol in dairy products and the high cost of milk (Mäkinen et al. 2015; Nyanzi and Jooste 2012; Singhal et al. 2017; Vasudha and Mishra 2013; Vijaya Kumar et al. 2015). Probiotic cereal beverages are a good alternative but their potential has not been revealed completely yet in the Western world mainly because of objectionable to the mainstream palate sensory characteristics (Mäkinen et al. 2015). Up to date, non-dairy fermented beverages are a growing segment of the functional food market (Russo et al. 2016; Sánchez et al. 2012). As an answer to the demand for milk substitutes, research efforts are directed towards designing optimal synbiotic drinks using wholegrain oat flours or brans and probiotic LAB and yeasts (Kedia et al. 2008; Lindström et al. 2015; Leroy and De Vuyst 2014; Martins et al. 2013; Rasane et al. 2015; Sangwan et al. 2014; Staka et al. 2015). Fermented oat beverages have the potential to prevent certain disorders and increasing number of consumers choose plant-based milk substitutes for medical reason or as a dietary choice (Mäkinen et al. 2015; Martins et al. 2013; Salmerón et al. 2015; Tchekessi et al. 2014). Chronic diseases that can be prevented by fermented cereal-based probiotic beverages are obesity, cardiovascular disease, type 2 diabetes and some cancers (Table 1) (Kedia et al. 2008; Mäkinen et al. 2015; Rasane et al. 2015; Salmerón et al. 2015; Staka et al. 2015; Vijaya Kumar et al. 2015).
Table 1.
Disorders that can be prevented by fermented oat beverages
| Disorder to prevent | Oats instead of milk | Probiotic microorganisms | References |
|---|---|---|---|
| Lactose intolerance | • | Mäkinen et al. (2015), Chua et al. (2013), Salmerón et al. (2015), Vasudha and Mishra (2013) and Vijaya Kumar et al. (2015) | |
| Allergies to milk protein | • | Mäkinen et al. (2015), Salmerón et al. (2015), Vasudha and Mishra (2013) and Vijaya Kumar et al. (2015) | |
| Irritable bowel syndrome | • | • | Kedia et al. (2008) and Staka et al. (2015) |
| Inflammatory bowel disease | • | Salmerón et al. (2015), Staka et al. (2015) and Vasudha and Mishra (2013) | |
| Diarrhea | • | Salmerón et al. (2015) and Vasudha and Mishra (2013) | |
| Constipation | • | Chua et al. (2013) and Vasudha and Mishra (2013) | |
| High cholesterol | • | • | Chua et al. (2013), Clemens and van Klinken (2014), Rasane et al. (2015), Salmerón et al. (2015), Vijaya Kumar et al. (2015) and Vasudha and Mishra (2013) |
| Low immunity | • | Chua et al. (2013) | |
| Salmerón et al. (2015) and Vasudha and Mishra (2013) | |||
| Colorectal cancer | • | Chua et al. (2013), Kedia et al. (2008), Rasane et al. (2015), Salmerón et al. (2015) and Vasudha and Mishra (2013) | |
| Respiratory tract infections | • | Vasudha and Mishra (2013) | |
| Helicobacter pylori infection | • | Salmerón et al. (2015) and Vasudha and Mishra 2013 | |
| Flatulence | • | Vijaya Kumar et al. (2015) |
Russo et al. (2016) obtained a fermented oat-based drink mixing oat flour with water. They heated the mixture at 95 °C in a water bath for 10 min with shaking, cooled the mixure to 37 °C and added a starter culture of Lactobacillus plantarum strains. After 16 h fermentation at 37 °C, strain Lb. plantarum B2 showed an increase of the riboflavin content, which increased more during product storage. Exopolysaccharide-producing strain Lb. plantarum Lp90 was proved to positively affect viscosity of the beverages and improve the probiotic potential of the strain. Exopolysaccharides (EPS) play a role in bacterial adaptation to harsh environmental conditions. Such compounds are produced by some Lactobacillus and Bifidobacterium strains and act as a colonization factor in the host displacing pathogens on the surface of intestinal epithelium. EPS can modify the composition and the metabolic activity of the microbiota and, along with surface associated proteins in the cell-wall of a probiotic strain, are factors responsible for protection, adhesion and colonization onto the gut mucosa of the host (Sánchez et al. 2012).
Folate is also produced by LAB during fermentation. Kariluoto et al. used different microorganisms to explore biofortification with folate in oat and barley matrices. Folate deficiency causes megaloblastic anaemia and neural tube defects (NTD) in foetus and suboptimal intake of folate leads to risk of cardiovascular disease, stroke and certain cancers. Thus fortification of cereal food with folate is important for public health. The tested microorganisms included a number of yeast and bacterial strains, including lactic acid bacteria. The yeast strains Saccharomyces cerevisiae ALKO743, Saccharomyces cerevisiae CBS7764 and Candida milleri ABM4949 showed the greatest folate producing potential which was even higher when sugar was added for yeasts to metabolise (Kariluoto et al. 2014).
During mixed fermentations yeasts supply LAB with simple sugars, B vitamins and enzymes. Lipases and esterases contribute to the flavour of fermented beverages which makes them more appealing, while phytase lowers phytic acid—an antinutrient that can seriously affect protein and mineral digestibility (Nyanzi and Jooste 2012).
Gupta et al. (2010) prepared a fermented oat drink by mixing oat flour, sugar and water, heating the mixture to 95 °C, cooling and inoculation with a starter culture of Lactobacillus plantarum. The study showed that oat and sugar concentrations were the factors with greatest influence on Lb. plantarum growth. Nionelli et al. (2014) obtained a beverage made of oat flakes milled into flour and fermented with Lactobacillus plantarum, Lactobacillus casei and Lactobacillus paracasei strains. Different drinks were prepared, some fermented by LAB alone while in others—addition of enzymes were added as well (with xylanase or α-amylase activity). All beverages were incubated at 30 °C for different periods After fermentation the beverages were pasteurized at 63 °C for 30 min. The parameters of the beverages assayed during 30 days of storage were pH, titrable acidity, water holding capacity, viscosity, total dry matter, color, organic acids, ethanol, free amino acids, antioxidant activities, dietary fiber concentration, in vitro starch hydrolysis and sensory analyses. The processing of oat flakes through fermentation with selected LAB was found to improve physical, nutritional, functional and most of all sensory properties. Fermentation increased the polyphenols availability and the antioxidant activity and decreased the hydrolysis index in vitro.
Fermentation with lactic acid bacteria improves the nutritional value and sensory properties by enriching the media with metabolites, and at the same time enhances microbial shelf-life of food by lowering the pH value (Agati et al. 1998; Mäkinen et al. 2015; Russo et al. 2017; Salmerón et al. 2015). Carbohydrates fermentation by amylolytc strains facilitates starchy food digestion (Agati et al. 1998). The microorganisms involved in the fermentation produce enzymes and other bioactive compounds such as short chain fatty acids, vitamins, folate, amino acids, bacteriocins and exopolysaccarides (EPS). Fermentation is proved to enhance content of phenolics, avenanthramides and flavonoids of oats and elevates the protective role of antioxidants against DNA damage in oats. Also, fermentation makes antioxidants and microelements more accessible, decreases the levels of antinutrients such as phytates and trypsin inhibitors, and reduces hexanal levels that are responsible for undesirable beany off flavour (Blandino et al. 2003; Kariluoto et al. 2014; Mäkinen et al. 2015). EPS produced by LAB are considered promising molecules in the functional food area as prebiotic fermentable substrates are able to modulate the intestinal microbiota (Nionelli et al. 2014; Russo et al. 2016). Fermentation results in improved texture, taste and aroma of the oat beverages (Blandino et al. 2003).
Acceptance and future perspectives for functional oat beverages
Oat probiotic beverages are healthy but not satisfactory enough in terms of shelf-life and organoleptic acceptance when compared to dairy ones. Therefore there is a need for further research in this area (Vijaya Kumar et al. 2015). Oat synbiotic beverages need to be balanced by taste, composition and price. Lower protein content may be compensated by adding nut milks (hazelnut, almond, coconut, peanuts) and legumes (soy) while fortification with minerals and adding fruit extracts or concentrates (blueberries, aronia, forest fruits etc.) can contribute to raising antioxidant activity, nutritional and sensory profile (Leroy and De Vuyst 2014; Mäkinen et al. 2015). Shelf-stability issues could be solved by keeping strict refrigeration conditions at temperatures below 4 °C to preserve for a longer storage period sufficient probiotic cell numbers that provide functionality.
The overall design of the product including viable probiotic cells, a sufficient β-glucan content, a good nutrient profile of the product, enough clinical evidence, a rational manufacturing process as well as choosing a proper marketing strategy including target population groups play role in successful commersialisation of functional oat beverages (Vijaya Kumar et al. 2015). Target groups can be vegans, vegetarians, training or dieting people as well as people with allergies having demands for non-diary or gluten-free products (Russo et al. 2017). Increasing awareness among the consumers towards healthy lifestyle results in a shifting preference to health drink products, which will boost the oat beverage market. Other marketing factors like hypermarket/supermarket segment growth and continuous product launch in developing regions like Asia Pacific, Middle East and others are expected to support rising oat drink sales in the next 10 years. Western Europe is expected to represent the major market in terms of value for oat drinks, while North America is expected to represent a favorable market for oat drinks in terms of consumption (http://www.futuremarketinsights.com/reports/oat-drinks-market).
References
- Agati V, Guyot JP, Morlon-Guyot J, Talamond P, Hounhouigan DJ. Isolation and characterization of new amylolytic strains of Lactobacillus fermentum from fermented maize doughs (mawa and ogi) from Benin. J Appl Microbiol. 1998;85:512–520. doi: 10.1046/j.1365-2672.1998.853527.x. [DOI] [Google Scholar]
- Angelov A, Gotcheva V, Hristozova T, Gargova S. Application of pure and mixed probiotic lactic acid bacteria and yeast cultures for oat fermentation. J Sci Food Agric. 2005;85:2134–2141. doi: 10.1002/jsfa.2223. [DOI] [Google Scholar]
- Angelov A, Gotcheva V, Kuncheva R, Hristozova T. Development of a new oat-based probiotic drink. Int J Food Microbiol. 2006;112:75–80. doi: 10.1016/j.ijfoodmicro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Angioloni A, Collar C. Significance of lipid binding on the functional and nutritional profiles of single and multigrain matrices. Eur Food Res Technol. 2011;233:141–150. doi: 10.1007/s00217-011-1503-z. [DOI] [Google Scholar]
- Arendt EK, Zannini E. Cereal grains for the food and beverage industries. Sawston: Woodhead Publishing; 2013. [Google Scholar]
- Blandino A, Al-Aseeri ME, Pandiella SS, Cantero D, Webb C. Cereal-based fermented foods and beverages. Food Res Int. 2003;36:527–543. doi: 10.1016/S0963-9969(03)00009-7. [DOI] [Google Scholar]
- Brindzová L, Čertík M, Rapta P, Zalibera M, Mikulajová A, Takácsová M. Antioxidant activity, β-glucan and lipid contents of oat varieties. Czech J Food Sci. 2008;26:163–173. doi: 10.17221/2564-CJFS. [DOI] [Google Scholar]
- Chua Y-F, Wiseb ML, Gulvadya AA, Changc T, Kendrad DF, Van Klinkena B-W, Shia Y, O’Sheaa M. In vitro antioxidant capacity and anti-inflammatory activity of seven common oats. Food Chem. 2013;139:426–431. doi: 10.1016/j.foodchem.2013.01.104. [DOI] [PubMed] [Google Scholar]
- Clemens R, Van Klinken BJ-W. Oats, more than just a whole grain: an introduction. Br J Nutr. 2014;112:S1–S3. doi: 10.1017/S0007114514002712. [DOI] [PubMed] [Google Scholar]
- Decker EA, Rose DJ, Stewart D. Processing of oats and the impact of processing operations on nutrition and health benefits. Br J Nutr. 2014;112:S58–S64. doi: 10.1017/S000711451400227X. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA J. 2007;587:1–16. [Google Scholar]
- Flander L (2012) Bioprocessing to improve oat bread quality (Doctoral thesis), VTT Science 8
- Flander L, Salmenkallio-Marttila M, Suortti T, Autio K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT- Food Sci Technol. 2007;40:860–870. doi: 10.1016/j.lwt.2006.05.004. [DOI] [Google Scholar]
- Gangopadhyay N, Hossain MB, Rai DK, Brunton NP. A review of extraction and analysis of bioactives in oat and barley and scope for use of novel food processing technologies. Molecules. 2015;20:10884–10909. doi: 10.3390/molecules200610884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Goncerzewicz A, Misiewicz A, Owczarek L, Jasińska U, Skąpska S. The effect of a newly developed oat-banana fermented beverage with a beta-glucan additive on ldhL gene expression in Streptococcus thermophilus TKM3 KKP 2030p. Curr Microbiol. 2016;73:773–780. doi: 10.1007/s00284-016-1126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotcheva V, Hristozova E, Hristozova T, Guo M, Roshkova Z, Angelov A. Assessment of potential probiotic properties of lactic acid bacteria and yeast strains. Food Biotechnol. 2002;16:211–225. doi: 10.1081/FBT-120016668. [DOI] [Google Scholar]
- Gupta S, Cox S, Abu-Ghannam N. Process optimisation for the development of a functional beverage based on lactic acid fermentation of oats. Biochem Eng J. 2010;52:199–204. doi: 10.1016/j.bej.2010.08.008. [DOI] [Google Scholar]
- Guyot J-P. Cereal-based fermented foods in developing countries: ancient foods for modern research. Int J Food Sci Technol. 2012;47:1109–1114. doi: 10.1111/j.1365-2621.2012.02969.x. [DOI] [Google Scholar]
- Hitayezua R, Baakdaha MM, Kinninb J, Hendersonc K, Tsopmoa A. Antioxidant activity, avenanthramide and phenolic acid contents of oat milling fractions. J Cer Sci. 2015;63:35–40. doi: 10.1016/j.jcs.2015.02.005. [DOI] [Google Scholar]
- https://www.futuremarketinsights.com/reports/oat-drinks-market. Accessed July 2017
- http://www.oatsandhealth.org. Accessed July 2017
- http://www.oatpro.eu (ERA-NET project OATPRO—engineering of oat proteins: consumer driven sustainable food development process). Accessed July 2017
- Hu X-Z, Zheng J-M, Li X, Xu C, Zhao Q. Chemical composition and sensory characteristics of oat flakes: a comparative study of naked oat flakes from China and hulled oat flakes from western countries. J Cer Sci. 2014;60:297–301. doi: 10.1016/j.jcs.2014.05.015. [DOI] [Google Scholar]
- Iserliyska D, Aleksandrov S, Angacheva E, Iliev A, Angelov A (2015) Acceptability of a nutritious flavoured oat based beverage “Biovessina”. In: Conference proceedings of “food, technology health”, pp 54–59
- Kariluoto S, Edelmann M, Nyström L, Sontag-Strohm T, Salovaara H, Kivelä R, Herranen M, Korhola M, Piironen V. In situ enrichment of folate by microorganisms in beta-glucan rich oat and barley matrices. Int J Food Microbiol. 2014;176:38–48. doi: 10.1016/j.ijfoodmicro.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Kedia G, Vazquez J, Pandiella SS. Enzymatic digestion and in vitro fermentation of oat fraction by human lactobacillus strains. Enzyme Microb Technol. 2008;43:355–361. doi: 10.1016/j.enzmictec.2008.03.015. [DOI] [Google Scholar]
- Kedia G, Vazquez JA, Charalampopoulos D, Pandiella SS. In vitro fermentation of oat bran obtained by debranning with a mixed culture of human fecal bacteria. Curr Microbiol. 2009;58:338–342. doi: 10.1007/s00284-008-9335-1. [DOI] [PubMed] [Google Scholar]
- Keying Q, Changzhong R, Zaigui L. An investigation on pretreatments for inactivation of lipase in naked oat kernels using microwave heating. J Food Eng. 2009;95:280–284. doi: 10.1016/j.jfoodeng.2009.05.002. [DOI] [Google Scholar]
- Kormin S, Rusul G, Radu S, Ling FH. Bacteriocin-producing lactic acid bacteria isolated from traditional fermented food. Malays J Med Sci. 2001;8:63–68. [PMC free article] [PubMed] [Google Scholar]
- Lampi A-M, Damerau A, Li J, Moisio T, Partanen R, Forssell P, Piironen V. Changes in lipids and volatile compounds of oat flours and extrudates during processing and storage. J Cereal Sci. 2015;62:102–109. doi: 10.1016/j.jcs.2014.12.011. [DOI] [Google Scholar]
- Lehtinen P, Lasskso S. Role of lipid reactions in quality of oat products. Agric Food Sci. 2004;13:88–99. doi: 10.2137/1239099041838085. [DOI] [Google Scholar]
- Leroy F, De Vuyst L. Fermented food in the context of a healthy diet: how to produce novel functional foods? Curr Opin Clin Nutr Metab Care. 2014;17:574–581. doi: 10.1097/MCO.0000000000000108. [DOI] [PubMed] [Google Scholar]
- Lindström C, Voinot A, Forslund A, Holst O, Rascón A, Öste R, Östman E. An oat bran-based beverage reduces postprandial glycaemia equivalent to yoghurt in healthy overweight subjects. Int J Food Sci Nutr. 2015;66:700–705. doi: 10.3109/09637486.2015.1035233. [DOI] [PubMed] [Google Scholar]
- Londono DM, van’t Westende WPC, Goryunova S, Salentijn EMJ, Van den Broeck HC, Van der Meer IM, Visser RGF, Gilissen LJWJ, Smulders MJM. Avenin diversity analysis of the genus Avena (oat). Relevance for people with celiac disease. J Cer Sci. 2013;58:170–177. doi: 10.1016/j.jcs.2013.03.017. [DOI] [Google Scholar]
- Mäkinen OE, Wanhalinna V, Zannini E, Arendt EK. Foods for special dietary needs: non-dairy plant based milk substitutes and fermented dairy type products. Crit Rev Food Sci Nutr. 2015 doi: 10.1080/10408398.2012.761950. [DOI] [PubMed] [Google Scholar]
- Martensson O, Oeste R, Holst O. Lactic acid bacteria in an oat-based non-dairy milk substitute: fermentation characteristics and EPS formation. Food Sci Technol/LWT. 2000;33:525–530. [Google Scholar]
- Martensson O, Andersson C, Andersson K, Oste R, Holst O. Formulation of an oat based fermented product and its comparison with yoghurt. J Sci Food Agric. 2001;81:1314–1321. doi: 10.1002/jsfa.947. [DOI] [Google Scholar]
- Martins EMF, Ramos AM, Vanzela ESL, Stringheta PC, de Oliveira Pinto CL, Martins JM. Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Res Int. 2013;51:764–770. doi: 10.1016/j.foodres.2013.01.047. [DOI] [Google Scholar]
- Murphy DL. Oats: Cultivation. Uses and Health Effects: Nova Science Publishers, Hauppauge, New York; 2011. [Google Scholar]
- Niniosa A-I, Sibakovb J, Mandalaa I, Fasseasa K, Poutanen K, Nordlund E, Lehtinen P (2011) Enzymatic depolymerisation of oat β-glucan. In: Conference on proceedings of 11th international congress engineering food (ICEF11)
- Nionelli L, Coda R, José AC, Poutanen K, Gobbetti M, Carlo GR. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int J Food Microbiol. 2014;185:17–26. doi: 10.1016/j.ijfoodmicro.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Nyanzi R, Jooste PJ (2012) Cereal-based functional foods. In: Rigobelo EC (ed) Probiotics InTech, pp 161–196 10.5772/50120
- Parada JL, Caron CR, Medeiros PAB, Soccol CR. Bacteriocins from lactic acid bacteria: purification, properties and use as biopreservatives. Braz Arch Biol Technol. 2007;50:521–542. doi: 10.1590/S1516-89132007000300018. [DOI] [Google Scholar]
- Peterson DM. Oat antioxidants. J Cereal Sci. 2001;33:115–129. doi: 10.1006/jcrs.2000.0349. [DOI] [Google Scholar]
- Prado FC, Parada JL, Pandey A, Soccol CR. Trends in non-dairy probiotic beverages. Food Res Int. 2008;41:111–123. doi: 10.1016/j.foodres.2007.10.010. [DOI] [Google Scholar]
- Rasane P, Jha A, Sabikhi L, Kumar A, Unnikrishnan VS. Nutritional advantages of oats and opportunities for its processing as value added foods. J Food Sci Technol. 2015;52:662–675. doi: 10.1007/s13197-013-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regand A, Chowdhury Z, Tosh SM, Wolever TMS, Wood P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011;129:297–304. doi: 10.1016/j.foodchem.2011.04.053. [DOI] [PubMed] [Google Scholar]
- Robert W, Krumbeck JA, Bindels LB, Cani PD, Fahey GJ, Goh YJ, Hamaker B, Martens EC, Mills DA, Rastal RA, Vaughan E, Sanders ME. Prebiotics: why definitions matter. Curr Opin Biotechnol. 2016;37:1–7. doi: 10.1016/j.copbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon JR, Sunilkumar BA, Nilsson L, Rascon A, Bergenståhl B. The effect of heat treatment on the soluble protein content of oats. J Cer Sci. 2015;65:119–124. doi: 10.1016/j.jcs.2015.06.008. [DOI] [Google Scholar]
- Russo P, de Chiara MLV, Capozzi V, Arena MP, Amodio ML, Rascón A, Dueñas MT, López P, Spano G. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT-Food Sci Technol. 2016;68:288–294. doi: 10.1016/j.lwt.2015.12.040. [DOI] [Google Scholar]
- Russo P, Arena MP, Fiocco D, Capozzi V, Drider D, Spano G. Lactobacillus plantarum with broad antifungal activity: a promising approach to increase safety and shelf-life of cereal-based products. Int J Food Microbiol. 2017;247:48–54. doi: 10.1016/j.ijfoodmicro.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Salmerón I, Thomas K, Pandiella SS. Effect of potentially probiotic lactic acid bacteria on the physicochemical composition and acceptance of fermented cereal beverages. J Funct Foods. 2015;15:106–115. doi: 10.1016/j.jff.2015.03.012. [DOI] [Google Scholar]
- Sánchez B, Ruiz L, Gueimonde M, Ruas-Madiedo P, Margolles A. Toward improving technological and functional properties of probiotics in foods. Trends Food Sci Technol. 2012;26:56–63. doi: 10.1016/j.tifs.2012.02.002. [DOI] [Google Scholar]
- Sangwan S, Singh R, Tomar SK. Nutritional and functional properties of oats: an update. J Innov Biol. 2014;1:003. [Google Scholar]
- Schnitzenbaumer B, Arendt EK. A comparative study of oat (Avena sativa) cultivars as brewing adjuncts. Eur Food Res Technol. 2013;236:1015–1025. doi: 10.1007/s00217-013-1965-2. [DOI] [Google Scholar]
- Shah A, Masoodi FA, Gani A, Ashwar BA. Newly released oat varieties of himalayan region- techno-functional, rheological, and nutraceutical properties of flour. LWT-Food Sci Technol. 2016;70:111–118. doi: 10.1016/j.lwt.2016.02.033. [DOI] [Google Scholar]
- Singhal S, Baker RD, Baker SS. A comparison of the nutritional value of cow’s milk and nondairy beverages. J Pediatr Gastroenterol Nutr. 2017;64:799–805. doi: 10.1097/MPG.0000000000001380. [DOI] [PubMed] [Google Scholar]
- Staka A, Bodnieks E, Puķītis A. Impact of oat-based products on human gastrointestinal tract. Proc Latv Acad Sci. 2015;69:145–151. [Google Scholar]
- Tchekessi CKC, Bokossa IY, Azokpota P, Agbangla C, Daube G, Scippo ML, Korsak N, Gotcheva V, Blagoeva G, Angelov A. Isolation and quantification of lactic acid bacteria from traditional fermented products in Benin. Int J Curr Microbiol App Sci. 2014;3:1–8. [Google Scholar]
- Tosh SM, Chu YF. Systematic review of the effect of processing of whole-grain oat cereals on glycaemic response. Br J Nutr. 2015;114:1256–1262. doi: 10.1017/S0007114515002895. [DOI] [PubMed] [Google Scholar]
- Vasudha S, Mishra HN. Non-dairy probiotic beverages. Int Food Res J. 2013;20:7–15. [Google Scholar]
- Vijaya Kumar B, Vijayendra SVN, Reddy OVS. Trends in dairy and non-dairy probiotic products—a review. J Food Sci Technol. 2015;52:6112–6124. doi: 10.1007/s13197-015-1795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao FL, Zhong K, Tong LT, Liu LY, Zhou XR, Zhou SM. Selection of lactic acid bacterial strains for a fermented oat beverage. Mod Food Sci Technol. 2015;31:263–270. [Google Scholar]
- Zhou M, Robards K, Glennie-Holmes M, Helliwell S. Oat Lipids. J Amer Oil Chem Soc. 1999;76:159–169. doi: 10.1007/s11746-999-0213-1. [DOI] [Google Scholar]