Abstract
This study evaluated yellow, purple and orange passion fruit in pulp, peel, and seed for physicochemical characteristics, proximate composition, minerals, antioxidant capacity (DPPH and ABTS), phenolic compounds, carotenoids, flavonoids and anthocyanins. Yellow passion fruit presented higher concentrations of pectin (37.37 g/100 g) in peels; high cryptoxanthin, α-carotene, β-carotene, provitamin A, quercetin, and kaempferol in pulps and higher values of ash and total dietary fiber in seeds. The purple fruit was highlighted by a great value of anthocyanins (103.68 mg/100 g) in peels and seeds and the orange fruit reported higher levels of ash, carotenoids (mainly β-carotene with 21,274 μg/100 g), kaempferol in peels, higher contents of total soluble solids, lycopene (4405 μg/100 g), lutein, zeaxanthin, total carotenoids in pulps and phenolics in general. This research revealed that the pulp of passion fruit and his residues have a significant content of bioactive compounds, differing in type according the species analyzed.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3190-2) contains supplementary material, which is available to authorized users.
Keywords: Pectin, Total dietary fiber, Lycopene, Quercetin, Anthocyanin
Introduction
Passion fruit is a popular name given to several species of the genus Passiflora that belongs to Passifloraceae family, which there are more than 500 species distributed in regions of tropical and subtropical climate of the world. The variety Passiflora edulis Sims fo. flavicarpa, known as sour or passion fruit, is the most produced and marketed, and represents 95% of its fruit farm. Its cultivation is primarily focusing on the juice and pulp industry, especially due to its higher acidity and pulp yield. However, the species Passiflora edulis Sims fo. edulis and Passiflora caerulea, known as purple and orange passion fruit, respectively, have the sweetest flavor, so are best consumed as juice or as fresh pulp (Zeraik and Yariwake 2010). These fruits are of interest not only due to the pulps but also due to its peels and seeds which contain high levels of bioactive compounds. Furthermore, regarding species Passiflora caerulea, no research has been reported about bioactive compounds content.
The ideal condition for the development of passion fruit occurs in places where the tropical climate prevails, and it is emphasizing that the water content in the soil is one of the factors that most influences the flowering of passion fruit crop. The regions that this fruit is widely using are American and European countries, due to the favorable weather. The best variety known and more used is Passiflora edulis, not only because of its pulp but also due of the infusions made with the leaves (Silva et al. 2013).
The common consumption of fruit and vegetable in the diet can protect our organism of Manu chronic diseases such as cancer, neurological diseases, cardiovascular diseases, obesity, inflammations and infections (Volp et al. 2009). However, passion fruit appears to be an excellent source of nutrients as carbohydrates, vitamins, and minerals that are essential nutrients for life. The fruit has a high content in nutraceuticals, as phenolic acids, where anthocyanins and flavonoids are the majoritarian compounds of this group; carotenoids and β-carotene appear to be the principal component, with consequently increased provitamin A activity. These nutraceutical compounds have biological activities in the health, protective effect against degenerative and chronic diseases and act as mutagenesis and carcinogenesis inhibitors. Also, these compounds have been associated with antiviral, antiallergic, antiplatelet and anti-inflammatory activities (Morais et al. 2016; Castañeda-Ovando et al. 2009; Tanwar and Modgil 2012; González-Gallego et al. 2014).
A major problem of passion fruit juice in the manufacturing industry is the amounts of waste generated come from discarded that are the peels and seeds. These residues are excellent sources of nutrients, bioactive compounds and studies about your potential can even indicate a future application as food ingredients in the formulation of new products, encouraging the reutilization of food and offering a nutritious alternative diet at low cost.
Based on this, the aim of this study was to evaluate the physicochemical characteristics, color parameters, pectin, proximate composition, minerals, the content of bioactive compounds and antioxidant capacity in pulp, peel, and the seed of yellow, purple and orange passion fruit to future use of parts as functional ingredients.
Materials and methods
Sample preparation
The yellow passion fruit (Passiflora edulis Sims fo. flavicarpa) samples were obtained CEASA (Central Supply of Rio Grande do Sul), Caxias do Sul, RS, Brazil. The purple (Passiflora edulis Sims fo. edulis) and orange passion fruit (Passiflora caerulea) samples were collected from farmers in the Criuva District (Caxias do Sul), the Rio Grande do Sul, Brazil. The fruits were harvested when fully ripe, with their skin color (yellow, purple or orange). After the crop, the fruits were transported immediately to the laboratory where they were cleaned in running water. Then the fruits were cut in half and made a separation of the pulp, peel (albedo and flavedo) and seed, the parts were packed in plastic bags and stored in a freezer at − 18 °C until analysis.
Physicochemical and chemical analysis
Total soluble solids (TSS) were determined in pulps with a Brix refractometer. The pH was measured using a pH meter (Quimis, model Q-400A). The yield was calculated by the percentage ratio (%) of the weight of the fruit with and without seed. The titratable acidity was determined by titration method using standardized 0.1 M NaOH solution, and the results analyzing in g/100 g of citric acid following the Analytical Standards Instituto Adolfo Lutz (2008). All of these analyses were made in triplicate.
Yield
The yield calculation of the pulp was calculated by weight of whole fruit (one by one) and weight of the fruit without peel and seed (pulp). The result was expressed as percentage (%). The average number of passion fruit analyzed was around 30 units.
Color parameters
The color was analyzed using a portable colorimeter (Konica Minolta Model CR 400, Singapore) by the Commission Internationale de l’Eclairage (CIELAB system) by determining the values of L* (lightness), a* (component red-green) and b* (yellow-blue component). All of these analyses were made in triplicate.
Proximate composition
All analyses were performed according to AOAC (2000). Protein content was determined by the Kjeldahl method with a conversion factor of 5.75. Lipids were obtained by cold extraction and ash was determined in a muffle furnace at 550 °C. Total dietary fiber was determined by the enzymatic–gravimetric method, moisture by gravimetry. Carbohydrates were estimated by the difference of 100 per cent of the sum between proteins, lipids, water and ash. The results were expressed as % and the data presented is the average of triplicate analysis.
Determination of pectin
The pectin of peels was extracted using a method of Canteri-Schemin et al. (2005) and Seixas et al. (2014) with some modifications. The extraction process was carried out in beakers (600 mL), followed by heating in a microwave oven (Electrolux, ME21S 800 W). Each 2 g of passion fruit peel flour was added to 50 mL of distilled water in a beaker. Then, an addition of a tartaric acidic solution (10%), was added to maintain a final pH of 2 for the solutions. The beaker was put in a microwave heating for 3 min. The solution (still warm), was vacuum filtered on a filter paper and the filtrate (containing the soluble pectin) was cooled to 4 °C.
To isolate the soluble pectin from the filtrate, the solution was slowly added under magnetic stirring to two volumes of absolute ethyl alcohol. This mixture was stirred for 10 min, after which it was allowed to rest for 30 min to facilitate the flotation of the pectin. The pectin was separated by vacuum filtration on a filter paper. The extracted pectin in a gel form was immersed in absolute ethyl alcohol for about 12 h and then was partially dehydrated by immersion in acetone. The drying pectin was put in an air-circulated oven at 50 °C until constant weight (approximately 8 h). The results were expressed as g of pectin/100 g dry peels (triplicate).
Determination of minerals
All the samples (pulp, peel, and seed) of yellow, purple and orange passion fruit were lyophilized (Liotop, L101, Brazil) before mineral analysis. The analysis of minerals in the passion fruit samples were made in Plant Soil Laboratory Faculty of Agronomy, Federal University of Rio Grande do Sul (UFRGS), according to the methodology of atomic emission spectrometry with inductively coupled plasma source (ICP–OES) described by Tedesco and Gianello (2004) This method is compiling in Table 1S (supplementary material). The results were expressed as % and the data presented is the average of duplicate analysis.
Determination of carotenoid profile and provitamin A content
The profile of carotenoids was determined according to Mercadante and Rodriguez-Amaya (1998). The extraction of pigments was with acetone and the saponification in a KOH solution (10% in methanol) overnight. The extract was rotary evaporated (Fisatom, Model 801) (T < 25 °C) and stored in a freezer (− 18 °C) for quantification by high-performance liquid chromatography (HPLC).
For HPLC (High Performance Liquid Chromatography) analysis, the samples stored in a freezer were diluted with methyl tert-butyl ether (MTBE-JT Baker, CAS. Number 1634-04-4, purity 99.96%), sonicated (Unique, Model USC 1400) for 1 min and filtered (Millex LCR 0.45 μm, 13 mm) for injection into the HPLC (Agilent 1100 Series, Santa Clara, CA, USA), a UV–visible detector and with a quaternary system.
The column used was a C30 polymeric reverse phase (250 × 4.6 mm ID, 3 µm, YMC, model CT99SO3-2546WT). The mobile phase gradient (water:methanol:MTBE) (JT Baker, CAS Number 04.04.1634, 99.96% purity) commenced at 5:90:5, reaching 0:95:5 at 12 min, 0:89:11 at 25 min, 0:75:25 at 40 min, and finally 00:50:50 at 60 min. The temperature of column was 33 °C and a flow rate of 1 mL/min (Spectra were obtained at a fixed wavelength of 450 nm for carotenoids).
Compounds were identified by comparing the sample retention time’s with the retention times obtained for controls. For quantification, a standard curve was constructed for carotenoids over the following ranges: lutein 1–65 µg/mL (> 95%, Sigma-Aldrich); zeaxanthin 1–40 µg/mL (> 95%, Sigma-Aldrich); cryptoxanthin 4–100 µg/mL (> 97%, Sigma-Aldrich); α-carotene 2–25 µg/mL (> 95%, Sigma-Aldrich); β-carotene 5–50 µg/mL (> 97%, Sigma-Aldrich) and lycopene (≥ 85% Sigma-Aldrich).
The limits of detection (LOD) and quantification (LOQ) were calculated by injecting 10 times the blank of the sample at very low level were used for measurement of LOD and LOQ which were determined as follows: LOD = mean value + 3 standard deviation (SD) LOQ = mean value + 10 SD where, mean value is zero (Ertas et al. 2007). Lutein: 6.9 × 10−3 and 1.15 × 10−2 µg/g; zeaxanthin: 9.56 × 10−2 and 1.59 × 10−2 µg/g; cryptoxanthin: 2.11 × 10−2 and 3.51 × 10−2 µg/g; α-carotene: 1.97 × 10−2 and 3.28 × 10−2 µg/g; β-carotene: 6.53 × 10−2 and 10.89 × 10−2 µg/g and lycopene were 7 × 10−3 and 33 × 10−3 μg/g.
Provitamin A activity was calculated by the bioconversion factor following Institute of Medicine (2001), yielding a value of 12 mg of β-carotene with 1 mg of Retinol Activity Equivalent (RAE). The results were expressed as % and the data presented is the average of triplicate analysis.
Determination of phenolic compounds
Samples (5 g) were homogenized by exhaustive extraction with 20 mL of ethanol and centrifuged at 15 °C (Cientec, CTR—5000R, Brazil) at 5000 × g for 20 min. Then, 20 µL of supernatant was added to 1.58 mL of water and 100 µL of Folin-Ciocalteu (0.4 mol/L). After reaction (3 min), 300 µL of Na2CO3 was added, and the mixture kept at room temperature for 2 h. The absorbance was then read at 765 nm on a UV–visible spectrophotometer (Shimadzu, UV-1700 PharmaSpec, Japan). The ethanol was used as the blank and gallic acid was used for calibration of the standard curve (0–0.50 mg/L). The results were expressed as mg of gallic acid equivalent (GAE) per 100 g of dry sample (triplicates) (Swain and Hillis 1959).
Determination of quercetin and kaempferol
Quercetin and kaempferol contents were analyzed according to Zeraik and Yariwake (2010) with modifications. The sample (10 g) were homogenised in an Ultra-Turrax (T25, IKA, China) with 30 mL of methanol at room temperature. The extracts were centrifuged at 15,000 × g, 4 °C for 20 min and after in rotary evaporator giving 2 mL of extract. The resulting aqueous solution was filtered through a 0.45 μm Millex-HV PVDF membrane (Millipore, New Bedford, MA, USA) before HPLC analysis. The samples were prepared and analyzed in triplicate and the results were expressed as %.
The HPLC-/DAD analyses were carried out on a Waters Alliance 2695 (Milford, MA, USA) liquid chromatograph connected to a model 2996 (DAD) diode array detector and controlled by Waters Empower software. The separation was performed using a C18 polymer column (250 mm × 4.6 mm id, 5 µm Vydac, 218TP). The samples were injected automatically (10.0 μL). A flow rate of 0.8 mL/min was applied, using a linear gradient of 0.2% formic acid in water (solvent A) and 0.2% formic acid in acetonitrile (solvent B). The gradients were: 0–10 min, 15% B in 85% A and 10–30 min, 20% B in 80% A. The chromatogram was monitored at 330 nm, and UV spectra of individual peaks were recorded in the range of 200–400 nm.
The contents of quercetin and kaempferol were determined by comparison with an external standard, injecting a new standard daily at 30 mg/mL for Quercetin (> 98%, Sigma-Aldrich) and 4 mg/mL for kaempferol (> 99%, Sigma-Aldrich).
Determination of anthocyanins
The anthocyanins were analyzed according to Zanatta et al. (2005), so 5 g of sample were homogenized in an Ultra-Turrax (T25, IKA, China) with acidified methanol (HCl 1%) and then quantified by HPLC (Agilent 1100 Series, Santa Clara, CA, USA) equipped with a quaternary pump system solvent and a UV–visible detector was used with a C18Shim-PakCLC-ODScolumn(5 μm, 250 × 4.6 mm).
The mobile phase was 5% aqueous formic acid/methanol 85:15 (v/v) to 20:80 over 25 min and this isocratic ratio was maintained for 15 min. The mobile phase flow were 0.8 mL/min, the injection volume was 5 μL, and 29 °C was the temperature of column. The chromatograms were processed at a fixed wavelength of 520 nm.
The standards were from Sigma-Aldrich (USA): cyanidin-3-glucoside (CAS 7084-24-4, ≥ 95.0%), cyanidin-3,5-glucoside (CAS 2611-67-8, ≥ 90.0%), delphinidin-3-β-glucoside (CAS 6906-38-3, ≥ 97%), pelargonidin-3-glucoside (CAS 17334-58-6, ≥ 90%), aglycone delphinidin (CAS 528-53-0, > 95%), aglycone cyaniding (CAS 528-58-5, ≥ 95%), malvidin-3,5-diglucoside (CAS 643-84-5, ≥ 95%) and aglycone pelargonidin (CAS 17334-58-6, ≥ 90%). The quantification and identification of compounds were performed by comparing peak areas and retention times with their respective standards under the same chromatographic conditions. The results were expressed as % and the data presented is the average of triplicate analysis.
Determination of antioxidant capacity
The methodology used to determine antioxidant capacity was based on the sequestration of DPPH (2,2-diphenyl-1-picryl-hydrazyl) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radicals according to Brand-Williams et al. (1995). Samples (5 g) were placed in 20 mL of ethanol and then centrifuged at 15 °C at 5000 × g (Cientec, CTR—5000R, Brazil) for 20 min. The liquid was diluted in three concentrations (10, 30, and 100%). For the DPPH assay, aliquots of each concentration were treated with 2 mL of DPPH (0.06 mM). The absorbance was read at 517 nm (Shimadzu, UV-1700 PharmaSpec, Japan). The results were presented as IC (Effective Concentration of 50% radical inhibition) 50 (mg/100 mL).
For the ABTS assay, aliquots of each concentration were treated with 2 mL of ABTS (7 mM). The absorbance was read at 734 nm (Shimadzu, UV-1700 PharmaSpec, Japan). The results were presented as IC 50 (mg/100 mL).
Statistical analysis
Data were analyzed by ANOVA and Tukey’s mean comparison test with a significance level of 5%, followed by a principal component analysis (PCA) using the software Statistica 12.0 (Statsoft Inc, São Paulo, Brasil). Pearson’s correlation was to the results of phenolic compounds and antioxidant capacity using Statistica 12.0 (Statsoft, São Paulo, Brazil).
Results and discussion
Physicochemical analysis
The results of the physicochemical analysis in pulps of different species of passion fruit were: 9.10% of TSS (total soluble solids), 9.06% of titratable acidity (citric acid) and a pH of 2.66 in yellow passion fruit pulp. For purple passion fruit the results were 11.60% of TSS, 2.83% of titratable acidity and a pH of 2.72 and for orange passion fruit pulp, we found 12.30% of TSS, 2.21% of titratable acidity and a pH of 3.97. So, the pulp of orange passion fruit is sweeter because it showed the higher content of total soluble solids (TSS), pH and consequently, lower content of acidity. The pulp of yellow passion fruit showed higher acidity and lower pH and TSS and then it is more acid. Some factors such as soil type, fertilization, climate, irrigation and genetic traits of the cultivar can explain the variations between the physicochemical parameters.
Kishore et al. (2011) evaluated the physicochemical attributes as TSS and titrable acidity in purple passion fruit pulp (Passiflora edulis Sims) who the values were 15.30 and 3.80%, respectively. These results were higher when compared to purple passion fruit pulp of this research. In another study realized by Souza et al. (2012), who analysed the pH, titratable acidity and total soluble solids in sweet passion fruit pulp (Passiflora alata Dryand), the results were 3.31, 2.00, and 13.33%, respectively. These values were more similar to specie Passiflora caeurea analysed by us that was the passion fruit sweeter. Janzantti et al. (2012) did a research with yellow passion fruit pulp (P. edulis Sims f. flavicarpa Deg.) and the higher values were 4.32% for titratable acidity, 14.71% soluble solids and 3.53 for pH, different values found by us when compared to yellow passion fruit. López-Vargas et al. (2013) evaluated the pH in pulp and seed of yellow passion fruit (Passiflora edulis var. flavicarpa) and the results were 3.75. They found higher values of pH in relation to the same variety (yellow passion fruit) of our research. Pongener et al. (2013) found the highest total soluble solids of 16.2° Brix in purple passion fruit pulp (Passiflora edulis Sims) and titratable acidity of 2.34 g citric acid/100 mL extract. These values were different both for total soluble solids and titratable acidity when compared to purple passion fruit pulp analysed in this study.
The yield of different species of passion fruit was evaluated, and the results are shown in Table 1. As expected, pulp and peel of yellow passion fruit presented higher yield when compared to purple and orange passion fruit, but for seed, orange passion fruit showed higher yield. Thus, it appears that the residues that represent most of the fruit should be characterized to indicate the possible applications and uses. For example, replacing wheat flour in some formulations such as pasta, breads, cakes, biscuits, which will have a higher content of fibers and bioactive compounds.
Table 1.
Yield color parameters and pectin in different species of passion fruit (mean and standard deviation)
| Yellow | Purple | Orange | |
|---|---|---|---|
| Yield (%) | |||
| Pulp | 27.71 ± 1.00a | 25.23 ± 1.02c | 26.54 ± 0.98b |
| Peel | 64.05 ± 2.03a | 62.11 ± 1.90b | 57.28 ± 2.05c |
| Seed | 8.24 ± 0.05c | 12.66 ± 0.12b | 16.18 ± 0.08a |
| Color L* | |||
| Pulp | 58.05 ± 0.64b | 77.65 ± 0.57a | 26.90 ± 0.17c |
| Peel | 43.16 ± 1.47a | 21.58 ± 2.15c | 36.94 ± 0.45b |
| Seed | 33.86 ± 0.80a | 28.16 ± 0.78b | 26.96 ± 0.49b |
| Color a* | |||
| Pulp | 8.44 ± 0.01b | 0.29 ± 0.08c | 10.78 ± 0.11a |
| Peel | 2.29 ± 0.25b | 2.20 ± 0.22b | 13.23 ± 0.49a |
| Seed | 1.91 ± 0.09b | 0.30 ± 0.08c | 8.25 ± 0.24a |
| Color b* | |||
| Pulp | 42.83 ± 1.28a | 17.18 ± 1.08b | 6.08 ± 0.07c |
| Peel | 31.33 ± 0.53b | 3.83 ± 1.75c | 35.78 ± 1.64a |
| Seed | 19.36 ± 0.45a | 5.90 ± 0.21c | 8.89 ± 0.55b |
| Pectin | |||
| Peel | 37.67 ± 0.97a | 32.85 ± 1.20b | 21.55 ± 0.55c |
a,b,cDifferent superscript letters in the same row indicate statistically significant difference (p < 0.05)
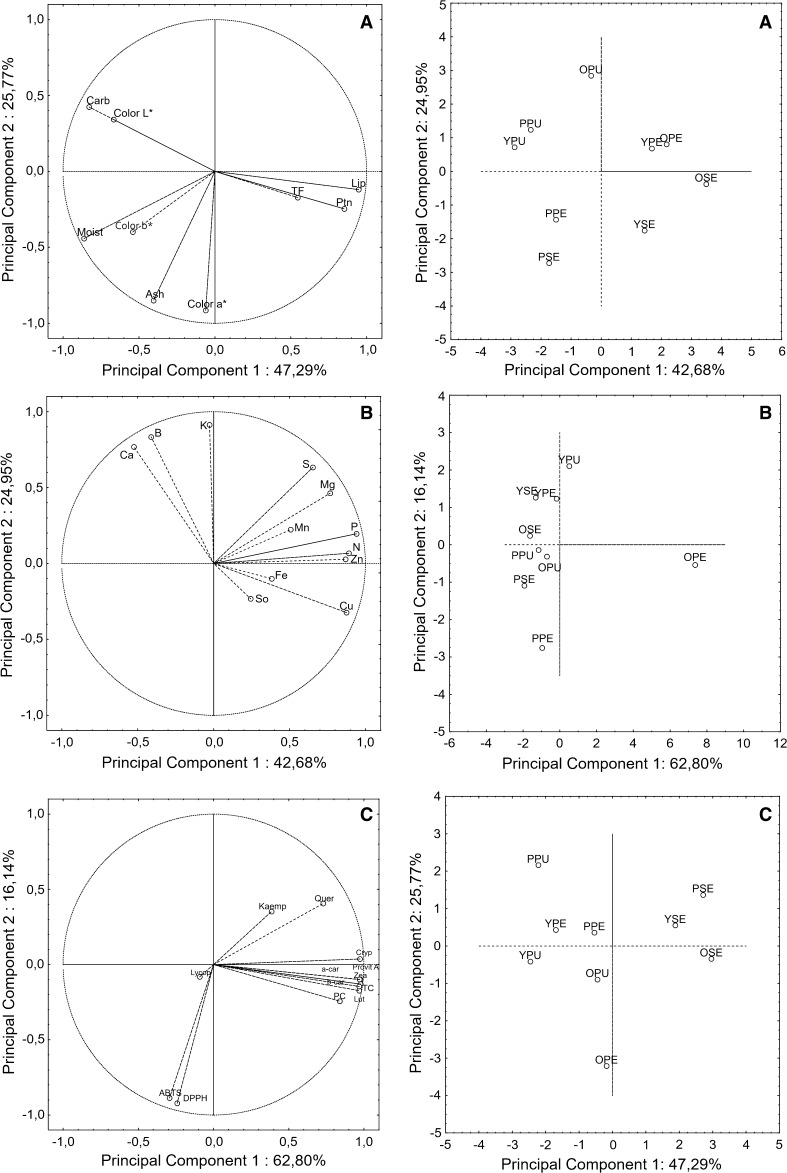
PCA (Principal Component Analysis) is a statistical technique used to reduce the dimensionality of a data set containing a large number of inter-related variables. The analysis is performed to maintain the maximum variance present in the data. This reduction produces a new reduced and uncorrelated set of variables, called principal components. These components are then chosen to ensure that the former retain the greater part of the variance present in the original variables.
The three passion fruits were evaluated for the color parameters, proximate composition, minerals, carotenoids, flavonoids, phenolic compounds, antioxidant capacity and the results are showed in Fig. 1.
Fig. 1.
Principal component analysis of yellow, purple and orange passion fruit in pulp, peel, and seed: proximate composition and color parameters (a); minerals (b); carotenoids, phenolic compounds and flavonoids (c). Legend: YPU: yellow passion fruit pulp; YPE: yellow passion fruit peel; YSE: yellow passion fruit seed; PPU: purple passion fruit pulp; PPE: purple passion fruit peel; PSE: purple passion fruit seed; OPU: orange passion fruit OPE: orange passion fruit peel; OSE: orange passion fruit seed; Moist: moisture; Ptn: protein; Lip: lipids; Carb: carbohydrates; TF: total fibre; N: nitrogen; P: phosphorus; K: potassium; Ca: calcium; Mg: magnesium; S: sulphur; Cu: copper; Zn: zinc; Fe: iron; Mn: manganese; B: boron; So: sodium; Lut: lutein; Zea: Zeaxanthin; Cryp: Cryptoxanthin; a-car: α-carotene; b-car: β-carotene; Lycop: lycopene; Provit A: provitamin A; TC: total carotenoids; PC: phenolic compounds; Quer: quercetin; Kaemp: kaempferol
By analyzing the components, the variance of the data was accounted for the significant contributions of 47.29% for the first principal component representing variable ash, color b and color L and 25.77% for the second principal components representing other variables (Fig. 1a).
Color
The passion fruit was evaluated within the context of color parameters (Fig. 1a). It was observed that for color L*, the pulp of purple passion fruit showed higher brightness. For color a* that indicates the intensity of colors red-green, orange passion fruit showed higher levels for pulp, peel, and seed when compared to yellow and purple passion fruit. For color b*, which indicates the intensity of colors yellow-blue, both for pulp and seed, yellow passion fruit presented more intensity, and for the peel, orange passion fruit showed more intensity when compared to other species of passion fruit. The differences in the color parameters can be explained mainly the species, soil and harvesting period are different.
Proximate composition
By principal component analysis (Fig. 1a) can be seen that the orange passion fruit stands out for its red coloration in the peel, high ash content present in the pulp and peel and the high content of proteins and lipids in the seeds. The yellow passion fruit, in turn, showed a high content of carbohydrates in the peel, probably due to the high content of pectin (37.37 g/100 g) in this species. The total fiber content in the pulp was greater in this species compared with others. The purple passion fruit highlights to have a high content of carbohydrates in the pulp compared with orange and yellow passion fruit (Table 2).
Table 2.
Proximate composition of different species of passion fruit (g/100 g of dry weight—except for moisture) with mean and standard deviation
| Yellow | Purple | Orange | |
|---|---|---|---|
| Moisture | |||
| Pulp | 90.06 ± 0.00a | 83.44 ± 0.99b | 88.18 ± 0.87a |
| Peel | 87.14 ± 3.29b | 87.02 ± 1.22b | 94.25 ± 0.26a |
| Seed | 57.09 ± 2.36a | 45.91 ± 0.97b | 58.46 ± 0.38a |
| Proteins | |||
| Pulp | 8.57 ± 0.10b | 6.53 ± 0.23c | 9.90 ± 0.35a |
| Peel | 3.40 ± 0.06c | 6.47 ± 0.04b | 11.60 ± 0.44a |
| Seed | 13.07 ± 0.12b | 13.23 ± 0.48b | 15.84 ± 0.15a |
| Lipids | |||
| Pulp | 1.11 ± 0.04b | 1.09 ± 0.04b | 2.92 ± 0.09a |
| Peel | 4.20 ± 0.03c | 4.89 ± 0.07b | 10.25 ± 0.12a |
| Seed | 12.31 ± 0.78c | 14.94 ± 0.41b | 19.64 ± 0.30a |
| Ash | |||
| Pulp | 6.94 ± 0.01a | 2.95 ± 0.14b | 7.31 ± 0.22a |
| Peel | 6.62 ± 0.24c | 7.93 ± 0. 05b | 13.29 ± 0.41a |
| Seed | 3.56 ± 0.05a | 1.85 ± 0.06c | 3.23 ± 0.18b |
| Carbohydrates | |||
| Pulp | 83.37 ± 0.00b | 89.42 ± 0.00a | 79.87 ± 0.00c |
| Peel | 85.78 ± 0.00a | 80.71 ± 0.00b | 64.86 ± 0.00c |
| Seed | 71.07 ± 0.00a | 69.98 ± 0.00a | 61.38 ± 0.00b |
| Total fibre | |||
| Pulp | 7.15 ± 0.07a | 1.40 ± 0.18c | 2.17 ± 0.11b |
| Peel | 61.16 ± 1.02a | 61.68 ± 1.31a | 62.14 ± 2.62a |
| Seed | 65.60 ± 0.52a | 55.06 ± 0.35b | 51.47 ± 0.60c |
a,b,c Different superscript letters in the same row indicate statistically significant difference (p < 0.05)
Souza et al. (2012) analyzed the proximate composition in sweet passion fruit pulp (Passiflora alata Dryand), and the results were (dry weight, except for moisture): 84.12% of moisture, 8.50% proteins, 0.63% lipids, 82.17% carbohydrates, 4.40% dietary fiber and 4.28% ash. The results of proteins and carbohydrates were similar with yellow passion fruit analyzed in this study, but for moisture, lipids, dietary fiber, and ash we found higher values.
Thereby, the peels and seeds of yellow, purple and orange passion fruit showed to be richer in total dietary fiber, proteins (seeds) and lipids, which should have an unsaturated origin.
Pectin
As it can be seen in Table 1, the peel of yellow passion fruit showed higher levels of pectin when compared to the purple and orange passion fruit (the latter presented the lowest content).
Liew et al. (2014) evaluated the production of pectin yellow passion fruit peel (Passiflora edulis f. flavicarpa) in extraction with citric acid. The authors found 14.60% yield of pectin from passion fruit peel. Kulkarni and Vijayanand (2010) also conducted a study to evaluate the pectin content of passion fruit peel (Passiflora edulis f. flavicarpa L.) yellow variety. The passion fruit peels were dehydrated for pectin extraction experiments. The conditions for the extraction of pectin from the passion fruit peel promoted a yield of 14.80 g/100 g of the dried peel. In another research carried out by Seixas et al. (2014), pectin extraction was investigated from the passion fruit peel (Passiflora edulis f. flavicarpa). The highest yield was found to be 18.20%. Different species of passion fruit of this study showed better results compared to the yield of pectin compared to all kinds of passion fruit analyzed.
Minerals
The minerals content of passion fruit is shown in Fig. 1B. By analyzing the components, the variance of the data was accounted for the significant contributions of 42.66% for the first and 24.95% for the second principal components. It can be observed that pulps of three species have high calcium, potassium, and boron. The yellow and orange passion fruit peels showed high zinc, manganese, copper, phosphorus, and sulfur. As for the purple passion fruit peel, these minerals had low content. Regarding seeds, orange passion fruit presented a considerable amount of copper followed by yellow passion fruit (Table 3).
Table 3.
Mineral composition of different species of passion fruit (mg/100 g of dry weight) with mean and standard deviation
| Yellow | Purple | Orange | |
|---|---|---|---|
| Zinc | |||
| Pulp | 5.20 ± 0.10b | 2.10 ± 0.05c | 6.50 ± 0.12a |
| Peel | 1.00 ± 0.02b | 0.90 ± 0.01c | 5.80 ± 0.05a |
| Seed | 4.10 ± 0.09c | 4.60 ± 0.05b | 8.90 ± 0.04a |
| Iron | |||
| Pulp | 5.50 ± 0.03a | 2.90 ± 0.02c | 3.20 ± 0.01b |
| Peel | 3.20 ± 0.04c | 4.60 ± 0.03a | 3.90 ± 0.02b |
| Seed | 5.20 ± 0.02a | 4.30 ± 0.03c | 4.50 ± 0.03b |
| Boron | |||
| Pulp | 0.70 ± 0.02a | 0.20 ± 0.01b | 0.70 ± 0.02a |
| Peel | 1.30 ± 0.03c | 1.40 ± 0.03b | 1.60 ± 0.04a |
| Seed | 0.40 ± 0.01b | 0.50 ± 0.02a | 0.50 ± 0.02a |
| Manganese | |||
| Pulp | 1.20 ± 0.05a | 0.40 ± 0.01b | 1.20 ± 0.01a |
| Peel | 0.50 ± 0.0c | 0.70 ± 0.02b | 7.30 ± 0.02a |
| Seed | 2.20 ± 0.05c | 2.30 ± 0.03b | 8.90 ± 0.01a |
| Copper | |||
| Pulp | 0.60 ± 0.02b | 0.20 ± 0.01c | 1.00 ± 0.02a |
| Peel | 0.10 ± 0.01c | 0.20 ± 0.01b | 0.30 ± 0.01a |
| Seed | 0.90 ± 0.02b | 0.70 ± 0.02c | 1.30 ± 0.03a |
| Phosphorus | |||
| Pulp | 380 ± 1.98b | 150 ± 1.70c | 390 ± 2.76a |
| Peel | 140 ± 1.30b | 70.00 ± 1.12c | 240 ± 1.71a |
| Seed | 310 ± 2.05b | 63.00 ± 1.19c | 390 ± 2.90a |
| Sulfur | |||
| Pulp | 170 ± 2.00b | 90.00 ± 0.85c | 330 ± 3.92a |
| Peel | 70.00 ± 0.40c | 160 ± 1.35b | 280 ± 2.80a |
| Seed | 150 ± 1.23b | 32 ± 0.10c | 230 ± 2.09a |
| Sodium | |||
| Pulp | 1.40 ± 0.02c | 5.30 ± 0.04b | 9.40 ± 0.20a |
| Peel | 2.20 ± 0.02c | 7.30 ± 0.12b | 11.50 ± 0.15a |
| Seed | 3.46 ± 0.07c | 4.80 ± 0.03a | 4.40 ± 0.05b |
| Magnesium | |||
| Pulp | 200 ± 1.23b | 120 ± 0.95c | 220 ± 1.30a |
| Peel | 120 ± 0.90c | 130 ± 0.97b | 140 ± 1.34a |
| Seed | 150 ± 1.10c | 290 ± 1.80a | 200 ± 1.22b |
| Nitrogen | |||
| Pulp | 2400 ± 20.0a | 1100 ± 8.0c | 1700 ± 12.0b |
| Peel | 620 ± 9.00c | 920 ± 7.50b | 940 ± 7.00a |
| Seed | 1800 ± 15.0b | 380 ± 1.50c | 2200 ± 18.5a |
| Potassium | |||
| Pulp | 3800 ± 25.5a | 1600 ± 16.0c | 2900 ± 18.5b |
| Peel | 2600 ± 15.7c | 2800 ± 16.3b | 4000 ± 32.0a |
| Seed | 760 ± 6.40b | 112 ± 3.00c | 1000 ± 7.80a |
| Calcium | |||
| Pulp | 50.00 ± 0.40a | 20.00 ± 0.12c | 30.00 ± 0.10b |
| Peel | 250 ± 1.98b | 310 ± 1.69a | 30.00 ± 0.11c |
| Seed | 30.00 ± 0.35b | 6.00 ± 0.02c | 330 ± 1.18a |
a,b,c Different superscript letters in the same row indicate statistically significant difference (p < 0.05)
Gondim et al. (2005) determined the mineral concentration in yellow passion fruit peels (Passiflora edulis). They found 44.51 mg of calcium, 0.89 mg of iron, 43.77 mg sodium, 27.82 magnesium, 0.32 mg zinc, 0.04 mg copper and 178.40 mg potassium in 100 g of fresh sample. These values were lower (except for sodium) when compared to the yellow passion fruit peel analyzed in this research due to they did not present the values in dry basis.
Souza et al. (2012) evaluated the mineral content (phosphorus, potassium, calcium, magnesium and iron) in passion fruit pulp (Passiflora alata Dryand). The authors found values of 34.95 mg for phosphorus, 375.42 mg potassium, 4.76 mg calcium, 19.82 mg magnesium and 1.06 mg of iron in 100 g of fresh pulp. They found significative amounts of calcium and potassium in pulp compared to our pulps studied.
As example, in adults above 19 years old, a portion of 100 g of orange passion fruit pulp presents 81 and 59% of recommended daily intake of zinc in women and men, respectively; 70 and 55% for magnesium in women and men; 111% for copper; 55% for phosphorus and 33% for calcium. In orange passion fruit peel (100 g), potassium presents 85% of recommended daily intake and manganese 405 and 317% in women and men. The pulp of yellow passion fruit (100 g) gives 30 and 69% of iron recommended daily intake for women and men; the concentrations of boron and sodium of all parts in passion fruit (portion of 100 g) is according to tolerable upper intake levels from 1 year old; sulphur and nitrogen not appears in recommended daily intake and tolerable upper intake levels.
Thus, it can be seen that from the nutritional matrix, we can ingest the recommended daily amount of minerals through the passion fruit, noting that the pulps have higher concentrations of minerals than peels.
Carotenoid profile and provitamin A content
The content of carotenoids and provitamin A are shown in Fig. 1c. By analyzing the components, the variance of the data was accounted for the significant contributions of 62.80% for the first and 16.14% for the second principal components. For peel, orange passion fruit stands out to all carotenoids evaluated and provitamin A about other species of passion fruit, where the majoritarian carotenoid was β-carotene because his peel color is orange. This species also presented in the pulp the highest content of lycopene (Table 4).
Table 4.
Analysis of carotenoids in different species of passion fruit (μg/100 g dry weight; mean and standard deviation)
| Pulp | Peel | |||||
|---|---|---|---|---|---|---|
| Yellow | Purple | Orange | Yellow | Purple | Orange | |
| Lutein | 44.28 ± 2.33b | 10.68 ± 0.11c | 105.36 ± 3.24a | 504.97 ± 24.77b | 366.88 ± 17.89b | 2881 ± 148.7a |
| Zeaxanthin | 65.51 ± 0.86b | 7.49 ± 0.05c | 91.22 ± 1.89a | 65.61 ± 0.22b | 48.70 ± 2.85b | 323.98 ± 11.11a |
| Cryptoxanthin | 254.38 ± 3.32a | 30.85 ± 0.07b | nd | 75.31 ± 0.05b | 74.56 ± 0.12b | 617.23 ± 37.71a |
| α-carotene | 86.43 ± 4.59a | 67.65 ± 2.16b | nd | nd | 37.19 ± 1.29b | 420.07 ± 15.02a |
| β-carotene | 1334 ± 78.8a | 171.88 ± 2.12c | 744.60 ± 15.47b | 272.52 ± 11.77b | 716.32 ± 30.65b | 21,274 ± 676a |
| Lycopene | nd | nd | 4405 ± 135.1a | nd | nd | nd |
| Provitamin A* | 111.16 ± 6.57a | 14.32 ± 0.18c | 62.05 ± 1.29b | 22.71 ± 0.98b | 59.69 ± 2.55b | 1773 ± 56.4a |
| Total carotenoids | 1785 ± 81.5b | 288.56 ± 0.03c | 5346 ± 145.4a | 918.41 ± 36.81b | 1244 ± 52.5b | 25,516 ± 561.9a |
a,b,cDifferent superscript letters in the same row indicate statistically significant difference (p < 0.05)
nd not detected
*Expressed as μg RAE (Retinol Activity Equivalent)
Souza et al. (2012) analyzed the content of β-carotene and lycopene in sweet passion fruit pulp (Passiflora alata Dryand), and they found 8249 and 5478 μg/100 g dry weight, confirming that these results were higher than our study for both β-carotene and lycopene. Pongener et al. (2013) evaluated the total carotenoids in purple passion fruit, and the higher value was 1467 μg/100 mL, being higher when compared to purple passion fruit analyzed in this research, but lower values compared to yellow and orange pulps.
Silva et al. (2014) evaluated the content of β-carotene and lycopene in pulp and peel of yellow passion fruit (Passiflora edulis Sims). They found 1362.07 and 57.93 μg β-carotene/100 g (dry basis), respectively and lycopene was not detected both in pulp and peel. The values of β-carotene were similar to our research for pulp (1333.97 μg/100 g dry basis), but for the peel, we detected more content of this compound (272.52 μg/100 g dry basis).
However, Pertuzatti et al. (2015) analyzed the carotenoids profile in yellow passion fruit (Passiflora edulis) and found: for lutein + zeaxanthin 1 μg/100 g, β-cryptoxanthin 24,990 μg/100 g, lycopene 28 μg/100 g and total carotenoids 25,100 μg/100 g. The values of β-cryptoxanthin and total carotenoids were much higher than our study, but for lutein + zeaxanthin, we found higher concentrations in all varieties and for lycopene, the variety ‘orange’ were higher too.
In another research realized by Septembre-Malaterre et al. (2016) where the content of β-carotene in passion fruit pulp (Passiflora edulis) was investigated, the values found were 3829.20 μg β-carotene equivalent/100 g. These results were higher when compared to all varieties analyzed in this study.
As an example (Institute of Medicine 2002), pulp of yellow passion fruit (100 g) presents 15 and 11% of DRI (dietary reference intakes) of vitamin A in women and men (> 14 years old), respectively. A portion of 100 g of orange passion fruit peel could contribute with 233 and 181% for women and men (> 14 years old), respectively according to recommended a daily intake of vitamin A.
Phenolic compounds
The presence of phenolic compounds in passion fruit makes this fruit an excellent candidate to evaluate several effects in vivo. As can be seen in Fig. 1c, the pulps and peels revealed the higher content of phenolic compounds, where the orange passion fruit stands out due to the higher concentrations. Therefore, the peels are residues that can be used and applied in formulations that would enrich the food due to the presence of these compounds (Table 5).
Table 5.
Analysis of phenolic compounds (mg/100 g dry weight), flavonoids (mg/100 g dry weight) and anthocyanins (μg/100 g dry weight) in different species of passion fruit with mean and standard deviation
| Pulp | Peel | Seed | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yellow | Purple | Orange | Yellow | Purple | Orange | Yellow | Purple | Orange | |
| Phenolic compounds | 1297.31 ± 13.43b | 788.93 ± 3.99c | 1559.15 ± 5.33a | 1061.87 ± 25.00c | 1570.80 ± 26.76b | 2584.91 ± 96.67a | 346.69 ± 6.58b | 325.69 ± 1.18c | 429.33 ± 0.19a |
| Quercetin | 506.45 ± 23.79a | 229.79 ± 10.99b | 16.28 ± 0.19c | 760.21 ± 32.07a | nd | 800.13 ± 24.18a | nd | nd | 120.41 ± 2.82 |
| Kaempferol | 199.66 ± 1.10a | 12.35 ± 0.08b | nd | nd | 74.70 ± 1.44b | 229.27 ± 8.90a | 375.32 ± 13.50 | nd | Nd |
| Cyanin | nd | nd | nd | nd | 1477.47 ± 20.85 | nd | nd | nd | Nd |
| Delphinidin-3,5-Glu | nd | nd | nd | nd | 8679.60 ± 341.32 | nd | nd | nd | Nd |
| Cyanidin-3-Glu | nd | nd | nd | nd | 2852.92 ± 177.93 | nd | nd | nd | Nd |
| Pelargonidin-3-Glu | nd | nd | 183.95 ± 6.52 | nd | 1551.94 ± 239.03 | nd | nd | nd | Nd |
| Aglycone Delphinidin | nd | nd | nd | nd | 90,998.72 ± 5218.53 | nd | nd | nd | Nd |
| Aglycone Cyanidin | nd | nd | nd | nd | 1237.73 ± 37.68 | nd | nd | nd | 159.18 ± 5.92 |
| Malvidin 3,5-Di | nd | nd | nd | nd | nd | nd | 4598.70 ± 119.73b | 8232.41 ± 6.54a | Nd |
| Aglycone Pelargonidin | nd | nd | nd | nd | nd | nd | nd | nd | 134.18 ± 0.84 |
| Total anthocyanins | nd | nd | 183.95 ± 6.52 | nd | 103,686.48 ± 542.11 | nd | 4598.70 ± 119.73 | 8232.41 ± 6.54a | 293.36 ± 6.75 |
| DPPH* | 0.20 ± 0.03a | 3.32 ± 0.02c | 2.41 ± 0.01b | 1.69 ± 0.03a | 6.98 ± 0.20c | 2.45 ± 0.03b | 1.18 ± 0.03a | 6.30 ± 0.08c | 2.68 ± 0.03b |
| ABTS* | 0.82 ± 0.03a | 4.59 ± 0.01c | 3.72 ± 0.05b | 2.22 ± 0.01a | 9.37 ± 0.05c | 2.95 ± 0.02b | 3.84 ± 0.08a | 4.76 ± 0.03b | 3.87 ± 0.00a |
a,b,cDifferent superscript letters in the same row and to the same part indicate statistically significant difference (p < 0.05)
nd not detected
*IC50 (g/100 mL): Effective Concentration of 50% radical inhibition
Silva et al. (2014) measured the total phenolic content in yellow passion fruit (Passiflora edulis Sims). They found 765.09 mg gallic acid equivalent/100 g (dry basis) in pulp and 451.06 mg gallic acid equivalent/100 g in peel (dry basis), similar values to our purple pulp. Already Septembre-Malaterre et al. (2016), found 286.6 mg gallic acid equivalent/100 g in passion fruit pulp (Passiflora edulis), different values about our research.
In summary, genotype, geographic effect, crop year, maturation and storage conditions are some characteristics that can influence the content of phenolic compounds, anthocyanins, flavonoids, carotenoids and other bioactive compounds in all fruit (Souza et al. 2008; Cardeñosa et al. 2016).
Flavonoids
Flavonoids were analyzed in all parts of different species of passion fruit, but the anthocyanins were analyzed only in seeds of all species of passion fruit, peel of purple passion fruit (because his color is purple) and pulp of orange passion fruit (because his color is red). In the other parts, we did not analyze because contains a negligible amount of these compounds.
The pulp of yellow passion fruit presented to be richer in quercetin and kaempferol that other species; for the peel, orange passion fruit showed more retention of kaempferol and for seed, only the species of yellow passion fruit detected this component.
Zeraik and Yariwake (2010) evaluated the total flavonoids, expressed as rutin and isoorientin in yellow passion fruit pulp (Passiflora edulis Sims f. flavicarpa Degener) and found 158.03 mg/L and 16.22 mg/L, respectively, suggesting that P. edulis fruits may be comparable with other flavonoid food sources such as orange juice or sugarcane juice.
Li et al. (2011) analyzed the flavonoid composition of Passiflora edulis ‘edulis’ and Passiflora edulis ‘flavicarpa’ more known as ‘purple’ and ‘yellow.’ The chromatograms revealed that the six major flavonoids obtained from Passiflora edulis ‘flavicarpa’ had not been detected in Passiflora edulis ‘edulis’ which suggested that the two population are originally disparate and that the fruit color is closely correlated with some other variabilities of the species.
Silva et al. (2014) measured the content of yellow flavonoids in pulp and peel of yellow passion fruit (Passiflora edulis Sims). They found 60.37 and 43.08 mg/100 g (dry basis), respectively, different values when compared to our study, because they analyzed by spectrophotometry and us by high-performance liquid chromatography.
In another research realized by Septembre-Malaterre et al. (2016) in which the content of total flavonoid content in passion fruit pulp (Passiflora edulis) was investigated, the values found were: 70.10 mg quercetin equivalent/100 g. Our study found different values to yellow (506.45 mg/100 g), purple (229.79 mg/100 g) and orange (16.28 mg/100 g).
Consequently, these fruit residues and pulp of passion fruit analyzed in this study can be regarded as a natural source of flavonoids and the part (pulp, peel, seed) where the color will indicate the concentration and type of flavonoid present in this matrix.
Anthocyanins
A large variety of anthocyanins were found in each part of different species passion fruit. As expected, the peel of purple passion fruit reveals a lot of anthocyanins, among them: cyanin, delphinidin-3,5-glucoside, cyanidin-3-glucoside, pelargonidin-3-glucoside, aglycone delphinidin (the majoritarian anthocyanin) and aglycone cyanidin. They were also found pelargonidin-3-glucoside in pulp of orange passion fruit (his color is red), aglycone cyanidin and aglycone delphinidin in seed of orange passion fruit and malvidin 3,5-diglucoside in seeds of yellow and purple passion fruit, which are the same species (Passiflora edulis), the purple showed higher content of this anthocyanin when compared to yellow passion fruit (Table 5).
As no data were found in the literature about the content of anthocyanins in purple passion fruit peel, we compared with fruits that have purple color in the peel. In a study realized by Todaro et al. (2009), delphinidin-3-rutinoside was extracted and identified as the major anthocyanin in eggplant peel (Solanum melongena var. esculentumpeel). Leite-Legatti et al. (2012) analyzed the content of anthocyanins in jaboticaba peel (Myrciaria jaboticaba). They found delphinidin 3-glucoside and cyanidin 3-glucoside (634.75 and 1963.57 mg/100 g, respectively) as anthocyanins. Cyanidin-3-O-glucoside was the dominant anthocyanin with 75.6% of the total anthocyanins.
Our results indicate promising perspectives for the exploitation of pulps of passion fruit species and their residues with significant levels of bioactive substances mainly peels of purple passion fruit which is rich in several anthocyanins.
Antioxidant capacity
The DPPH radical has purple color and ABTS the green color. With the addition of the radical fruit extract, the DPPH and ABTS are reduced, presenting yellow color, with consequent disappearance of absorption. From the results, it determines the percentage of antioxidant activity and scavenging of free radicals.
In the antioxidant capacity, pulp, peel and seed of yellow passion fruit has a greater power to scavenge the free radicals DPPH and ABTS (Table 5), which means that this fruit has a higher antioxidant capacity when compared to purple and orange passion fruit, except for seeds of yellow and orange passion fruit in ABTS analysis that no significative difference was detected.
Souza et al. (2012) evaluated the antioxidant capacity of different fruit, and they concluded that the smallest antioxidant capacity was observed in the jenipapo pulps, followed by sweet passion fruit, soursop, murici and marolo.
López-Vargas et al. (2013) also evaluated the antioxidant capacity DPPH in pulp and seed and albedo of yellow passion fruit (Passiflora edulis Sims fo. flavicarpa). They concluded that the albedo showed a higher ability to inhibit DPPH radical than the pulp and seed samples. Our study showed different results because we had higher antioxidant capacity in pulps compared with the peels, mainly due to the presence of high concentrations of β-carotene, lycopene, and quercetin in pulps.
Septembre-Malaterre et al. (2016) concluded that the highest antioxidant capacity was found in passion fruit (Passiflora edulis) pulp (64% of DPPH reduced) when compared to other fruits including mango, pineapple, banana, and litchi exerted lower free radical-scavenging activities (45–58%). In agreement with data of DPPH assays, passion fruit exercised the highest free radical scavenging capacity too through oxygen radical absorbance capacity (ORAC) (14.08 μM Trolox equivalent).
The antioxidant properties of yellow, purple and orange passion fruit are not affected by phenolic compounds by the Pearson’s Correlation analysis. So, the antioxidant capacity measured in passion fruit samples is certainly related to the content of carotenoids and anthocyanins of these samples, mainly in the content of lycopene (as can be seen in the Fig. 1c).
Conclusion
This research showed that pulp of passion fruit and its residues as peels and seeds have a significant content of bioactive compounds, differing in type according to the species examined, as example, peel of purple passion fruit has more anthocyanins; pulp of orange passion fruit present a good source of lycopene and his peels a large content of β-carotene and phenolic compounds; the pulp of yellow passion fruit present more content of quercetin and antioxidant capacity ABTS and DPPH. These residues can be added to formulations that can enrich the foods and have therapeutic effects to the human health and too reduce the waste promoting a positive environmental and economic impact.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors are grateful to the Brazilian Research Agency (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES) for their financial support.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3190-2) contains supplementary material, which is available to authorized users.
References
- AOAC International. Official methods of analysis (15th edn.) (2000) Association of Official Analytical Chemists, Washington, DC
- Brand-Williams W, Cuvelier ME, Berset C. 0 Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Canteri-Schemin MH, Fertonani HCR, Waszczynskyj N, Wosiacki G. Extraction of pectin from Apple pomace. Braz Arch Biol Technol. 2005;48:259–266. doi: 10.1590/S1516-89132005000200013. [DOI] [Google Scholar]
- Cardeñosa V, Girones-Vilaplana A, Muriel JL, Moreno DA, Moreno-Rojas JM. Influence of genotype, cultivation system and irrigation regime on antioxidant capacity and selected phenolics of blueberries (Vaccinium corymbosum L.) Food Chem. 2016;202:276–283. doi: 10.1016/j.foodchem.2016.01.118. [DOI] [PubMed] [Google Scholar]
- Castañeda-Ovando A, Pacheco-Hernández ML, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA. Review: chemical studies of anthocyanins. Food Chem. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- Ertas E, Õzer H, Alasalvar C. A rapid HPLC method for determination of Sudan dyes and Para Red in red chilli pepper. Food Chem. 2007;105:756–760. doi: 10.1016/j.foodchem.2007.01.010. [DOI] [Google Scholar]
- Gondim JAM, Moura MFV, Dantas AS, Medeiros RLS, Santos KM. Composição centesimal e de minerais em cascas de frutas. Food Sci Technol (Campinas) 2005;25(4):825–827. doi: 10.1590/S0101-20612005000400032. [DOI] [Google Scholar]
- González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Anti-inflammatory and immunomodulatory properties of dietary flavonoids. Polyphen Hum Health Dis. 2014;1:435–452. doi: 10.1016/B978-0-12-398456-2.00032-3. [DOI] [Google Scholar]
- Institute of Medicine . Food and nutrition board: dietary reference intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington: National Academy Press; 2001. pp. 65–126. [PubMed] [Google Scholar]
- Institute of Medicine . Dietary reference intakes for minerals and vitamin A. Washington: National Academy Press; 2002. [Google Scholar]
- Instituto Adolfo Lutz . Métodos físicos e químicos para análise de alimentos. São Paulo: Normas analíticas do Instituto Adolfo Lutz; 2008. [Google Scholar]
- Janzantti NS, Macoris MS, Garruti DS, Monteiro M. Influence of the cultivation system in the aroma of the volatile compounds and total antioxidant activity of passion fruit. LWT-Food Sci Technol. 2012;46:511–518. doi: 10.1016/j.lwt.2011.11.016. [DOI] [Google Scholar]
- Kishore K, Pathak KA, Shukla R, Bharali R. Effect of storage temperature on physico-chemical and sensory attributes of purple passion fruit (Passiflora edulis Sims) J Food Sci Technol. 2011;48:484–488. doi: 10.1007/s13197-010-0189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SG, Vijayanand P. Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.) LWT-Food Sci Technol. 2010;43:1026–1031. doi: 10.1016/j.lwt.2009.11.006. [DOI] [Google Scholar]
- Leite-Legatti AV, Batista AG, Dragano NRV, Marques AC, Malta LG, Riccio MF, Eberlin MN, Machado ART, Carvalho-Silva LB, Ruiz ALTG, Carvalho JE, Pastore GM, Júnior MRM. Jaboticaba peel: antioxidant compounds, antiproliferative and antimutagenic activities. Food Res Int. 2012;49:596–603. doi: 10.1016/j.foodres.2012.07.044. [DOI] [Google Scholar]
- Li H, Zhoua P, Yanga Q, Shena Y, Denga J, Li L, Zhaod D. Comparative studies on anxiolytic activities and flavonoid compositions of Passiflora edulis ‘edulis’ and Passiflora edulis ‘flavicarpa’. J Ethnopharmacol. 2011;133:1085–1090. doi: 10.1016/j.jep.2010.11.039. [DOI] [PubMed] [Google Scholar]
- Liew SQ, Chin NL, Yusof A. Extraction and characterization of pectin from passion fruit peels. Agric Agric Sci Procedia. 2014;2:231–236. doi: 10.1016/j.aaspro.2014.11.033. [DOI] [Google Scholar]
- López-Vargas JH, Fernández-López J, Pérez-Álvarez JA, Viuda-Martos M. Chemical, physico-chemical, technological, antibacterial and antioxidant properties of dietary fibre powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Food Res Int. 2013;51:756–763. doi: 10.1016/j.foodres.2013.01.055. [DOI] [Google Scholar]
- Mercadante AZ, Rodriguez-Amaya DB. Effects of ripening, cultivar differences, and processing on the carotenoid composition of mango. J Agric Food Chem. 1998;46:128–130. doi: 10.1021/jf9702860. [DOI] [PubMed] [Google Scholar]
- Morais CA, Rosso VV, Estadella D, Pisani LP. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J Nutr Biochem. 2016;33:1–7. doi: 10.1016/j.jnutbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Pertuzatti PB, Sganzerla M, Jacques AC, Barcia MT, Zambiazi RC. Carotenoids, tocopherols and ascorbic acid content in yellow passion fruit (Passiflora edulis) grown under different cultivation systems. LWT-Food Sci Technol. 2015;64:259–263. doi: 10.1016/j.lwt.2015.05.031. [DOI] [Google Scholar]
- Pongener A, Sagar V, Pal RK, Asrey R, Sharma RR, Singh SK. Physiological and quality changes during postharvest ripening of purple passion fruit (Passiflora edulis Sims) J Fruits. 2013;69:19–30. doi: 10.1051/fruits/2013097. [DOI] [Google Scholar]
- Seixas FL, Fukuda DL, Turbiani FRB, Garcia PS, Petkowicz CLO, Jagadevan S, Gimenes ML. Extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) by microwave-induced heating. Food Hydrocoll. 2014;38:186–192. doi: 10.1016/j.foodhyd.2013.12.001. [DOI] [Google Scholar]
- Septembre-Malaterre A, Stanislas G, Douraguia E, Gonthier M. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem. 2016;212:225–233. doi: 10.1016/j.foodchem.2016.05.147. [DOI] [PubMed] [Google Scholar]
- Silva JK, Cazarin CBB, Colomeu TC, Batista G, Meletti LMM, Paschoal JAR, Júnior SB, Furlan MF, Reyes FGR, Augusto F, Júnior MRM, Zollner RL. Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves In vitro and in vivo study. Food Res Int. 2013;53:882–890. doi: 10.1016/j.foodres.2012.12.043. [DOI] [Google Scholar]
- Silva LMR, Figueiredo EAT, Ricardo NMPS, Vieira IGP, Figueiredo RW, Brasil IM, Gomes CL. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014;143:398–404. doi: 10.1016/j.foodchem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Souza LM, Ferreira KS, Chaves JBP, Teixeira SL. L-ascorbic acid, b-carotene and lycopene content in papaya fruits (carica papaya) with or without physiological skin freckles. Sci Agric. 2008;65:246–250. doi: 10.1590/S0103-90162008000300004. [DOI] [Google Scholar]
- Souza VR, Pereira PAP, Queiroz F, Borges SV, Carneiro JDS. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012;134:381–386. doi: 10.1016/j.foodchem.2012.02.191. [DOI] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Tanwar B, Modgil R. Flavonoids: dietary occurrence and health benefits. Rev Artic Spatula DD. 2012;2:59–68. doi: 10.5455/spatula.20120328100506. [DOI] [Google Scholar]
- Tedesco MJ, Gianello C. Metodologia de análises de solo, plantas, adubos orgânicos e resíduos. Porto Alegre: Gênesis; 2004. pp. 61–66. [Google Scholar]
- Todaro A, Cimino F, Rapisarda P, Catalano AE, Barbagallo RN, Spagna G. Recovery of anthocyanins from eggplant peel. Food Chem. 2009;114:434–439. doi: 10.1016/j.foodchem.2008.09.102. [DOI] [Google Scholar]
- Volp ACP, Renhe IRT, Stringueta PC. Pigmentos naturais bioativos. Aliment Nutr. 2009;20:157–166. [Google Scholar]
- Zanatta CF, Cuevas E, Bobbio FO, Winterhalter P, Mercadante AZ. Determination of anthocyanins from camu–camu (Myrciaria dubia) by HPLC-PDA, HPLC-MS and NMR. J Agric Food Chem. 2005;53:9531–9535. doi: 10.1021/jf051357v. [DOI] [PubMed] [Google Scholar]
- Zeraik ML, Yariwake JH. Quantification of isoorientin and total flavonoids in Passiflora edulis fruit pulp by HPLC-UV/DAD. 2010. Microchem J. 2010;96:86–91. doi: 10.1016/j.microc.2010.02.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.