Abstract
Numerous bacterial species utilize quorum sensing molecules acyl-homoserine-lactones (AHLs) to communicate, however, crosstalk often complicates the dynamics and behaviors of mixed populations. In this study, we developed a luxR mutant of wild type Shewanella baltica SA03 (WT SA03), and aimed to investigate the role of S. baltica LuxR (AHLs receptor) involved in the spoilage of refrigerated shrimp (Litopenaeus vannamei) by inoculating WT SA03 and luxR mutant of S. baltica SA03 (ΔluxR SA03), respectively. The results indicated the maximum growth rate of total viable bacteria in shrimp inoculated with ΔluxR SA03 was 73.34% lower than that of WT SA03. The lag time of total bacteria in shrimp treated with ΔluxR SA03 were 87.6 h, significantly longer than that of WT SA03. Meanwhile, the total volatile basic nitrogen concentrations of shrimp treated with WT SA03 were significantly higher than that of ΔluxR SA03 after 2 days of storage, which were in agreement with the decrease of the content of AHLs of the shrimp. The results indicated S. baltica might utilize AHLs produced by other bacteria and accelerate the shrimp spoilage process through LuxR receptor system.
Keywords: Acyl-homoserine-lactones, Quorum sensing, Eavesdropping, Spoilage, Shrimp
Introduction
Quorum sensing (QS) is a cell to cell communication mechanism through which bacteria employ signal molecules to regulate their group behaviors responding to their local environment changes (Chhabra et al. 2005). Acyl-homoserine-lactones (AHLs) are the most studied signal molecule in gram-negative bacteria (Yang et al. 2016; Li et al. 2018). Generally, AHL-QS circuits contain a LuxR and LuxI homolog. LuxI is responsible for the production of AHLs, and LuxR can response to the AHLs to coordinate the bacteria behavior. However, LuxR solos have been shown to respond to exogenous AHLs produced by neighboring cells as well endogenously produced AHLs and some LuxR proteins have evolved from the ability to binding AHLs and respond to other molecules/signals (Patel et al. 2013). In order to compete bacteria and modulate the interaction with its competitor, some bacteria do exist eavesdropping on quorum sensing autoinducers (Case et al. 2008), such as Escherichia coli and Salmonella Enteritidis (Kim et al. 2014; Almeida et al. 2016).
The recent studies showed that Shewanella baltica, the specific spoilage organism in shrimp and fish, were capable of producing AI-2 and cyclic dipeptides as the signal molecules (Zhu et al. 2015, 2016; Gu et al. 2013). Though S. baltica possesses AHLs receptor LuxR, AHLs signal molecule was seldom detected in S. baltica. QS signal molecular and responses can be cooperative in vitro, but it remains unclear whether QS is cooperative in nature. Little is known about the relationship between S. baltica and other bacteria produced AHLs in the spoilage of shrimp, and S. baltica may do poorly in artificial petri dishes conditions because of the importance of metabolism networks in spoiled shrimp. Here, we constructed a luxR mutant of S. baltica, and the purpose of the study was to elucidate: (1) Can luxR mutant of S. baltica use exogenous AHLs to keep the quantity advantage in the microbiota development? (2) Whether the LuxR receptor system of S. baltica play an important role in the spoilage of shrimp or not.
Materials and methods
Materials and bacterial strains
Shrimp (Litopenaeus vannamei) with average length of 13.59 ± 0.52 cm and weight of 13.83 ± 0.32 g was purchased from a local market. The shrimp was transported in oxygenated package to laboratory within 1 h and was approved by the local ethical committee, Ocean university of China. Plate count agar (PCA) and iron agar (IA) mediums were purchased from Haibo Biotechnology Co., Ltd. (Qingdao, China). The synthetic N-hexanoyl-l-homoserine lactone (C6-HSL) and DNA kits were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals used in this study were of analytical grade and are commercially available.
The Shewanella baltica SA03 was isolated from spoiled refrigerated Litopenaeus vannamei using agar plate method and kept in our laboratory (Shewanella baltica SA03has identified using 16 s rDNA method, data not shown). S. baltica SA03 was routinely grown at 30 °C in Luria–Bertani (LB) broth under aerobic condition. E. coli WM3064 was grown at 37 °C in LB medium under aerobic condition. Appropriate concentration of antibiotics was added in E. coli WM3064.
Construction of the luxR mutant of S. baltica SA03
The S. baltica SA03 luxR mutant was constructed using the att-based fusion PCR method as described by Jin et al. (2013). Briefly, the target flanking gene was amplified with the primer containing attB and the specific sequence, then followed by a second round of PCR. The fusion PCR fragments were introduced into plasmid pHG1.0 by site-specific recombination using BP Clonase (Invitrogen, USA). The verified recombinant plasmid was transformed into and maintained in E. coil WM3064 and then transferred into S. baltica SA03 by conjugation with the ratio of the donor to the recipient being 2:1. Gentamycin-sensitive and sucrose-resistant colonies were screened by PCR for deletion of the target gene. Mutants were verified by sequencing the mutated regions. The primer used in this study was as follows: LF: TCACGGACTTGATAGACAA; LR: GTACTGGGATGCTGGTTT; SF: AATTGAGCCTGACACAGAC; SR: GTAGTTACGGTATCTTGAATGG.
Bacterial growth assay
The wild-type strain WT SA03 and ΔluxR SA03 mutant were incubated in AB medium in the presence of C6-HSL (10 μM) with shaking (biochemical incubator, 200 rpm) for 24 h at 30 °C, respectively. The control is added equivalent dimethyl sulfoxide. Bacterial population was estimated by the measure of OD600 using a spectrophotometer in 4 h intervals.
Shrimp inoculation and spoilage assays
Live shrimp were painless killed using − 20 °C sterile ice. The peeled and sterilized samples were randomly assigned into three groups (each group 9.0 ± 0.2 kg) and each subjected to processing in triplicate. The shrimp samples was combined inoculated with 1.0 × 104 colony-forming units (cfu)/mL initial microbiota (H2S bacteria (black colony) together with medium were removed using the small needle, then rinse the culture plate and suspension) of shrimp and 1.0 × 104 cfu/mL wild type S. baltica SA03 (WT SA03), or 1.0 × 104 cfu/mL initial microbiota of shrimp and 1.0 × 104 cfu/mL luxR mutant of S. baltica SA03 (ΔluxR SA03). The sterilized sample without inoculation was appointed as the control. Treated shrimp samples were air packaged and stored at 4 ± 1 °C inside the refrigerator for 6 days. Average 600 g samples were taken from each group for analysis at each sampling time. The experiments were performed in triplicates.
Total aerobic plate counts (TPC) were enumerated using nutrient agar, and incubated at 37 °C for 24 h. The results were expressed as the colony-forming units (cfu) per g of the sample. H2S-producing bacteria were determined in iron agar as described by Gu et al. (2013). The growth dynamics of total viable bacteria and H2S-producing bacteria were determined using the modified Gompertz model, which uses a static equation to describe bacterial growth N [cfu/g] as a function of time t [h] (Zwietering et al. 1990).
| 1 |
N0 is the initial population level of the bacteria at time t = 0 h (cfu/g); Nmax is the maximum microbial cell density (cfu/g); Lag is the lag time (h); and μmax is the maximum growth rate.
Total volatile basic nitrogen (TVB-N) of the shrimp was measured using steam distillation with a Kjeldahl distillation apparatus and titration (Kaewprachu et al. 2017). The distillate was collected in boric acid (0.3% w/v) and titrated with 0.05 mol/L standard hydrochloric acid using phenolphthalein as an indicator.
Extraction and detection of AHLs
Shrimp meat samples (100 g) were aseptically removed and subsequently homogenized with 200 mL ethyl acetate containing 0.1% (v/v) formic acid for 3 min in a stomacher and then centrifugation at 8000 rpm for 10 min at 4 °C. Ethyl acetate fractions were evaporated to dryness at 30 °C. The residues were re-dissolved in dimethyl sulfoxide (about 1.0 mL) to obtain the AHLs extracts. The AHLs was measured using the bioreporter strains A. tumefaciens A136 according to the method of Kawaguchi et al. (2008). A 200 μL mixture containing AHLs extracts, A. tumefaciens A136 cell-free lysate and 20 mM KH2PO4 buffer (pH 7.0) (v:v:v = 1:1:2) added into a 96-well plate, and then X-gal (500 μg/mL) was added to each solution and mixed. The plate was incubated at 30 °C for 3 h, and the absorbance at 635 nm was measured. The AHLs activity was expressed as OD635 value.
Statistical analysis
Analysis of variance (ANOVA) was performed and the mean comparisons were done by Duncan’s multiple range tests. The microbial growth dynamics were fitted using the modified Gompertz model by Sigmaplot 10.0 (Jandel Scientific, San Francisco, CA). All experiments were performed in triplicates (n = 3).
Results and discussion
Construction of ΔluxR SA03 mutant
In order to confirm the function of LuxR as a AHLs receptor, a knockout mutant of luxR was constructed. The strategy and results of ΔluxR SA03 mutant construction was shown in Fig. 1. ΔluxR SA03 was confirmed by PCR amplification and sequencing of luxR gene from chromosomal DNA of S. baltica. In the WT SA03, the intergenic region of luxR consists of 700 bp, whereas, in ΔluxR SA03, it consists of 200 base pairs, meaning that 500 bp up steam were lost during the recombination process. The results indicated that the luxR gene in S. baltica SA03 was knocked out successfully.
Fig. 1.
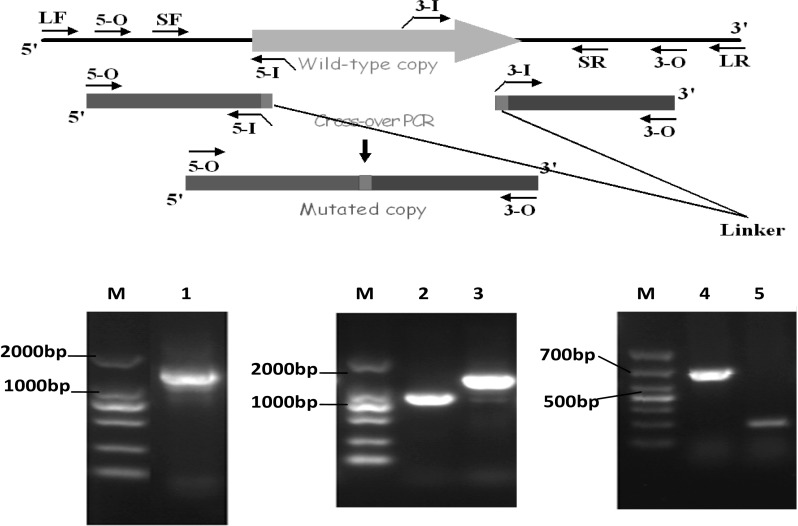
Construction and agarose gel result of Shewanella baltica SA03 luxR mutant. M: D2000 marker, 1. Fusion fragments of the target gene. 2. Gene products of primer LF/SR. 3. Gene products of primer SF/LR. 4. luxR gene fragment of wild type Shewanella baltica SA03. 5. luxR gene fragment of mutant luxR Shewanella baltica SA03
Growth curve of WT SA03 and ΔluxR SA03
The growth curves of WT SA03 and ΔluxR SA03 were similar (Fig. 2). However, exogenous signal molecule C6-HSL significantly improved the growth of S. baltica SA03, and showed no effect on ΔluxR SA03. It is widely believed that QS can regulate bacteria growth, and microbial growth and metabolism is the main major cause resulting in food spoilage or deterioration (Gram and Dalgaard 2002). Blana et al. (2017) found the growth of Salmonella may be affected by the presence of quorum sensing signaling compounds. Zhang et al. (2016) also found exogenous N-butanoyl-l-homoserine lactone (C4-HSL) and C6-HSL promoted the growth of Shewanella sp. Our results showed WT SA03 and ΔluxR SA03 have different response to exogenous C6-HSL, indicating LuxR system was involved in S. baltica QS system.
Fig. 2.
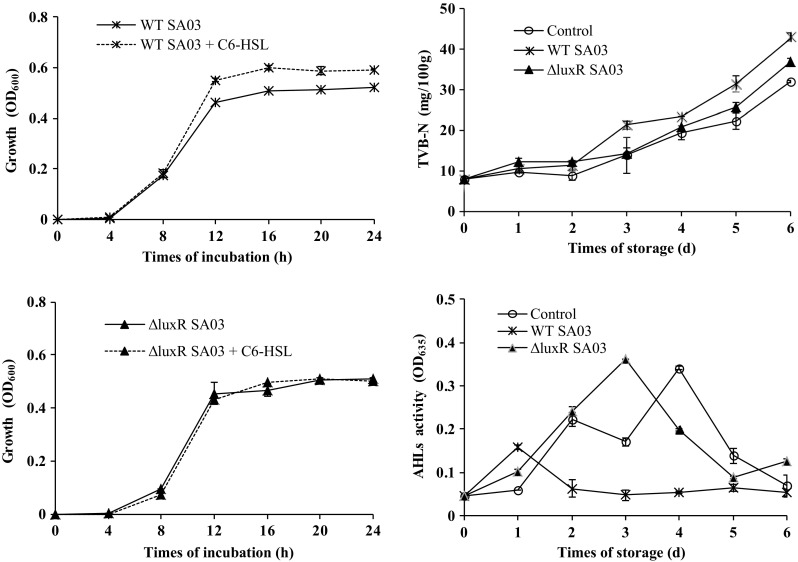
The growth changes of wild type Shewanella baltica SA03 (WT SA03) and luxR mutant Shewanella baltica SA03 (ΔluxR SA03) during inculation, and the total volatile basic nitrogen (TVB-N), acyl-homoserine-lactones (AHLs) activity changes of shrimp inoculated with WT SA03 and ΔluxR SA03 during cold storage
AHLs receptor LuxR of S. baltica and shrimp spoilage
In our previous study we have found Shewanella spp. was the only H2S-producing bacteria in spoiled shrimp, and exogenous C6-HSL can affect the microbiota change in refrigerated shrimp (Zhu et al. 2015). In this study, the effects of AHLs receptor LuxR on the microbiota changes in refrigerated shrimp were analyzed. The results showed total viable bacteria of ΔluxR SA03 treated samples exhibited a longer lag time ca. 87.6 h, and the maximum growth rate was significantly lower than that of WT SA03 (P < 0.05) (Table 1). Microorganisms are the major cause of spoilage of most seafood products, and extending lag phase durations of microorganism may extend the shelf-life of seafood products (Remya et al. 2017). Thus, we suggested that the LuxR-type QS may accelerate the development of microbiota in refrigerated shrimp.
Table 1.
Total viable bacteria growth changes in shrimp inoculation with WT SA03 or ΔluxR SA03
| Time of storage (days) | Total viable bacteria (× 105 CFU/g) | ||
|---|---|---|---|
| Control | WT SA03 | ΔluxR SA03 | |
| 0 | 0.1 ± 0.0a | 0.5 ± 0.1c | 0.4 ± 0.2b |
| 1 | 0.1 ± 0.0a | 1.1 ± 0.1c | 0.1 ± 0.0a |
| 2 | 0.1 ± 0.0a | 9.5 ± 1.1c | 0.3 ± 0.0b |
| 3 | 0.1 ± 0.0a | 5.3 ± 0.7c | 0.8 ± 0.1b |
| 4 | 0.2 ± 0.0a | 13.3 ± 0.7c | 2.2 ± 0.2b |
| 5 | 1.3 ± 0.1a | 42.0 ± 0.7c | 3.7 ± 0.7b |
| 6 | 2.1 ± 0.2a | 165.0 ± 0.2c | 81.0 ± 7.1b |
| μmax/h | 0.043 ± 0.006a | 0.236 ± 0.001b | 0.063 ± 0.006c |
| Lag (h) | 124.3 ± 0.6c | 37.2 ± 0.7a | 87.6 ± 4.3b |
| R2 | 0.9936 | 0.9838 | 0.9810 |
R2 is the coefficient of determination. Control: no inoculation
μmax is the maximum growth rate (per hour); Lag is the lag time (h)
Different letters in a row indicate significant differences (P < 0.05)
Level of TVB-N production is important quality parameter for the assessment of shrimp spoilage. As shown in Fig. 2, the TVB-N values of all treatments increased with storage time. However, the TVB-N concentrations of the samples treated with WT SA03 were significantly higher than those of ΔluxR SA03 and control after 2 days of storage (P < 0.05). These trends were in agreement with the changes of total viable bacteria. Meanwhile, the AHLs contents changes of three treatments were very different. The peak of AHLs content of WT SA03 treated samples appeared at the first day, and the ΔluxR SA03 and control appeared at the third and fourth day, respectively. The AHLs of WT SA03 treated samples kept lower level after 2 days of storage. We deduce WT SA03 might utilize AHLs produced by other bacteria through LuxR receptor system and avoid the accumulation of AHLs during storage, which accelerate the increase of total viable bacteria and the spoilage process of shrimp. However, the AHLs activity of control samples showed an obvious decrease at the storage day 3, which may be attributed to the errors of limited samples, or the utilization of AHLs as autoinducers, or the degradation of AHLs by quorum quenching enzymes (Chen et al. 2013).
Bacteria are now known to be highly social organisms. Their behaviors range from cooperatively forming complex multispecies communities to fiercely competing for resources (Michie et al. 2016). Since AHLs-based QS regulate the expression hydrolytic enzymes and other metabolic process, we hypothesized that AHL molecules may promote the competitiveness of S. baltica in refrigerated shrimp. If QS-regulated spoilage factors are cooperative, the benefits of signal response should be shared between both producers and nonproducers within the host (Zhou et al. 2014). We found WT SA03 tended to reach higher population densities, and ΔluxR SA03 did not benefit from the presence of AHLs. We inferred that WT SA03 gain a growth advantage in spoiled shrimp by avoiding the metabolic costs of QS-regulated traits (Zhou et al. 2014; Srimani and You 2014), and this reason may be related to the lower TVB-N level of ΔluxR SA03 treated samples during cold storage.
Conclusion
In this study, the luxR mutant of S. baltica ΔluxR SA03 was successfully developed. The strain of ΔluxR SA03 lost its sensitivity to AHLs in vitro and in vivo. S. baltica do not produce AHLs, however, S. baltica might utilize environmental AHLs produced by other bacteria through LuxR receptor system and accelerate the shrimp spoilage process, and ΔluxR SA03 did not benefit from the presence of AHLs. Our results suggest AHLs receptor LuxR of S. baltica was involved in the development of microbiota and spoilage of refrigerated shrimp, and the reason may be related to the AHLs eavesdropping of S. baltica through LuxR system.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 31671919). We would like to thank Professor Mclean (Texas State University, USA) for providing reporter strain of Agrobacterium tumefaciens A136 and Professor Gao (Zhejiang University, China) for providing Escherichia Coli WM3064 and plasmid pHG 1.0.
Compliance with ethical standards
Conflict of interest
All authors have no conflicts of interest.
Footnotes
Jinxin Jie, Honglei Yu, and Yunyan Han have contributed equally to the work and should be considered joint first authors.
Contributor Information
Zunying Liu, Phone: +86-532-82032400, Email: liuzunying@ouc.edu.cn.
Mingyong Zeng, Email: Mingyz@ouc.edu.cn.
References
- Almeida FA, Pinto UM, Vanetti MCD. Novel insights from molecular docking of SdiA from Salmonella Enteritidis and Escherichia coli with quorum sensing and quorum quenching molecules. Microb Pathog. 2016;99:178–190. doi: 10.1016/j.micpath.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Blana V, Georgomanou A, Giaouris E. Assessing biofilm formation by Salmonella enterica serovar Typhimurium on abiotic substrata in the presence of quorum sensing signals produced by Hafnia alvei. Food Control. 2017;80:83–91. doi: 10.1016/j.foodcont.2017.04.037. [DOI] [Google Scholar]
- Case RJ, Labbate M, Kjelleberg S. AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. ISME J. 2008;2:345–349. doi: 10.1038/ismej.2008.13. [DOI] [PubMed] [Google Scholar]
- Chen F, Gao Y, Chen X, Yu Z, Li X. Quorum quenching enzymes and their application in degrading signal molecules to block quorum sensing-dependent infection. Int J Mol Sci. 2013;9:17477–17500. doi: 10.3390/ijms140917477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra SR, Philipp B, Eberl L, Givskov M, Williams P, Cámara M. Extracellular communication in bacteria. In: Schulz S, editor. The chemistry of pheromones and other semiochemicals II. Berlin: Springer; 2005. pp. 279–315. [Google Scholar]
- Gram L, Dalgaard P. Fish spoilage bacteria—problems and solutions. Curr Opin Biotechnol. 2002;13:262–266. doi: 10.1016/S0958-1669(02)00309-9. [DOI] [PubMed] [Google Scholar]
- Gu Q, Fu L, Wang Y, Lin J. Identification and characterization of extracellular cyclic dipeptides as quorum-sensing signal molecules from Shewanella baltica, the specific spoilage organism of Pseudosciaena crocea during 4 °C storage. J Agric Food Chem. 2013;61:11645–11652. doi: 10.1021/jf403918x. [DOI] [PubMed] [Google Scholar]
- Jin M, Jiang Y, Sun L, Yin J, Fu H, Wu G, Gao H. Unique organizational and functional features of the cytochrome c maturation system in Shewanella oneidensis. PLoS ONE. 2013;8:e75610. doi: 10.1371/journal.pone.0075610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewprachu P, Osako K, Benjakul S, Suthiluk P, Rawdkuen S. Shelf life extension for Bluefin tuna slices (Thunnus thynnus) wrapped with myofibrillar protein film incorporated with catechin-Kradon extract. Food Control. 2017;79:333–343. doi: 10.1016/j.foodcont.2017.04.014. [DOI] [Google Scholar]
- Kawaguchi T, Chen YP, Norman RS, Decho AW. Rapid screening of quorum-sensing signal N-acyl homoserine lactones by an in vitro cell-free assay. Appl Environ Microb. 2008;74:3667–3671. doi: 10.1128/AEM.02869-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Duong T, Wu C, Choi J, Lan N, Kang SW, et al. Structural insights into the molecular mechanism of Escherichia coli SdiA, a quorum-sensing receptor. Acta Cryst. 2014;D70:694–707. doi: 10.1107/S1399004713032355. [DOI] [PubMed] [Google Scholar]
- Li SZ, Xu R, Ahmar M, Goux-Henry C, Queneau Y, Soulère L. Influence of the D/L configuration of N-acyl-homoserine lactones (AHLs) and analogues on their Lux-R dependent quorum sensing activity. Bioorg Chem. 2018;77:215–222. doi: 10.1016/j.bioorg.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Michie KL, Cornforth DM, Whiteley M. Bacterial tweets and podcasts signaling, eavesdropping, microbialfightclub. Mol Biochem Parasitol. 2016;208:41–48. doi: 10.1016/j.molbiopara.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HK, Suárez-Moreno ZR, Degrassi G, Subramoni S, Gonzalez JF, Venturi V. Bacterial LuxR solos have evolved to respond to different molecules including signals from plants. Front Plant Sci. 2013;4:447. doi: 10.3389/fpls.2013.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remya S, Mohan CO, Venkateshwarlu G, Sivaraman GK, Ravishankar CN. Combined effect of O2 scavenger and antimicrobial film on shelf life of fresh cobia (Rachycentron canadum) fish steaks stored at 2 °C. Food Control. 2017;71:71–78. doi: 10.1016/j.foodcont.2016.05.038. [DOI] [Google Scholar]
- Srimani JK, You L. Emergent dynamics from quorum eavesdropping. Chem Biol. 2014;21:1601–1602. doi: 10.1016/j.chembiol.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Yang C, Fang S, Chen D, Wang J, Liu F, Xia C. The possible role of bacterial signal molecules N-acyl homoserine lactones in the formation of diatom-biofilm (Cylindrotheca sp.) Mar Pollut Bull. 2016;107:118–124. doi: 10.1016/j.marpolbul.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhu S, Wu H, Jatt AN, Pan Y, Zeng M. Quorum sensing involved in the spoilage process of the skin and flesh of vacuum-packaged farmed turbot (Scophthalmus maximus) stored at 4 & #xB0;C. J Food Sci. 2016;81:M2776–M2784. doi: 10.1111/1750-3841.13510. [DOI] [PubMed] [Google Scholar]
- Zhou L, Slamti L, Nielsen-LeRoux C, Lereclus D, Raymond B. The social biology of quorum sensing in a naturalistic host pathogen system. Curr Biol. 2014;24:2417–2422. doi: 10.1016/j.cub.2014.08.049. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wu H, Zeng M, Liu Z, Wang Y. The involvement of bacterial quorum sensing in the spoilage of refrigerated Litopenaeus vannamei. Int J Food Microbiol. 2015;192:26–33. doi: 10.1016/j.ijfoodmicro.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhao A, Feng L, Gao H. Quorum sensing signals affect spoilage of refrigerated large yellow croaker (Pseudosciaena crocea) by Shewanella baltica. Int J Food Microbiol. 2016;217:146–155. doi: 10.1016/j.ijfoodmicro.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Zwietering MH, Jongenourger I, Rombouts FM, Van’t Riet K. Modeling of the bacterial growth curve. Appl Environ Microb. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


