Abstract
Apple contributes significantly to the livelihood and health of the people living in Himalayan regions. Among others, the Delicious group of apple is known for their health promoting and income generating attributes. However, the systematic investigation on morphological and phytochemical attributes of the apple growing in Indian Himalayan region is poorly known. An altitude-dependent variation in the fruit quality traits and phytochemical diversity was observed. The Royal Delicious was found rich in total tannin, flavonoids, flavonols, antioxidant activity [2,2-azinobis (3-ethylbenzothiazoline-6-sulphonic acid), 2,2-Diphenyl-1-picryhydrazyl, Ferric reducing antioxidant power], procyanidin B2, phloridzin and gallic acid; Red Delicious for total phenol and epicatechin and Golden Delicious for chlorogenic acid. The cultivar and altitude-dependent variation of phenolic profile in peel and pulp portion emphasizes genotype-specific biosynthesis of phenolic compounds in regulatory mechanisms. It is suggested that selection of suitable altitude and cultivars is required for harnessing the maximum phytochemical and antioxidant activity for benefits to human consumption.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3179-x) contains supplementary material, which is available to authorized users.
Keywords: Apple, High performance liquid chromatography, Phenolics, Western Himalaya
Introduction
Apple (Malus X domestica) is one of the world’s most ancient and widely cultivated temperate crops and its consumption is widespread all across the globe. The fruits are generally consumed fresh (about 71%) while about 20% are processed into different value-added products (Shalini and Gupta 2010). Most of the cultivars are processed into five main products viz., juice, canned sauce, canned slices, dried apples and frozen slices. Few apple cultivars are however processed into vinegar, jelly, apple butter, apple wine, and apple essence (Downing 2012; de Jesús et al. 2018).
Apple consumption has been considered as a major source of dietary antioxidants owing to the presence of polyphenolics and flavonoids, leading to many health benefits. For instance, epidemiological studies have shown the inverse correlation between consumption of apple fruits and many chronic diseases of human, specifically cardiovascular diseases, lung dysfunctions, and cancer (Kalinowska et al. 2014). In addition, the presence of polyphenolic compounds are also known to influence the quality of the fruit like shelf life, susceptibility to diseases (Khanizades et al. 2008), colour, flavour and taste, which add on to the price of apple fruits in the market. Thus, polyphenol compounds not only impart health benefits but also contribute to the appearance of apple (Kalinowska et al. 2014).
A number of reports are available on the different apple cultivars, which have shown the presence of five major polyphenolics namely hydroxycinnamic acids, flavan-3-ols/procyanidins, anthocyanins, flavonols, and dihydrochalcones (Boyer and Liu 2004). However, their concentration varies among the cultivars and fruit portion. For instance, procyanidin B2, catechin, epicatechin, phloridzin, and the quercetin are higher in apple peel than pulp. Chlorogenic acid is reported higher in the pulp than peel (Escarpa and Gonzalez 1998). In addition, the profile of phenolic phytochemicals and their concentration is reported to be influenced by changes in the growth period, growing season, and geographical locations. Similarly, type of cultivars, nutrient conditions, and location within the tree is also reported to influence the polyphenolic content (Khanizadeh et al. 2008).
Delicious group apple such as Golden Delicious, Red Delicious, and Royal Delicious are known for their higher economic and nutritional value. This group is popular among consumers for their taste and appearance. The behavior pattern of all these cultivars, by and large, remains similar and, therefore, they are handled in one group. The fruits of this group have high soluble solids, low acidity, and a very firm texture. The juice of these fruits is sweet, aromatic and bland. In addition to fruit juice, apple pomace is reported for dietary fibre, pectins and natural antioxidants (Bhushan et al. 2008). Studies have found that Delicious apples are good for dehydrofrozen slices, fair for canned or frozen slices and poor for the sauce (Downing 2012).
Among different cultivars of apple, Red Delicious, Royal Delicious, and Golden Delicious are widely cultivated in Western Himalaya, mainly in the states of Jammu and Kashmir, Himachal Pradesh and Uttarakhand (Indian Horticulture Database 2014). Cultivation and post-harvest processing of these cultivars is a source of income and livelihood for a large number of the people in the region. However, the expansion of apple cultivation to marginal areas and abnormal climatic conditions is considered to have an adverse effect on quantity and quality of apple production in Western Himalaya (Jindal and Mankotia 2004). Since all these cultivars are introduced in the Himalayan region and have now been naturalized, systematic assessment of their morphological and phenolic profile is needed, in view of changing climate and apple expansion in the Himalayas (Jindal and Mankotia 2004; de Jesús et al. 2018). The present investigation is an attempt to study the variation in the morphological and phytochemical profile of different fruit portions along with an altitude gradient in most widely grown Delicious group of apple cultivars growing in Western Himalaya, India.
Material and method
Site selection and fruit collection
An extensive field survey was conducted during the year 2014–2016 for the selection of apple growing sites, ranging between 1771 and 2780 m amsl in Western Himalaya. Apple fruits (30 No.) were randomly collected from 10 different trees of selected cultivars i.e., Golden Delicious, Red Delicious, Royal Delicious (on the basis of relevance to commercial cultivation and livelihood of the farmers) from different major apple growing regions at different altitudes in Western Himalaya [Naugaoun (1771 m amsl), Shitlakhet (2000 m amsl), Chaubatiya (2040 m amsl), Khabrar (2200 m amsl), Satbunga (2300 m amsl), Mukhwa (2780 m amsl)] during July to October 2015 (Supplementary Fig. 1) and brought to laboratory. All the trees of selected cultivars were managed by similar agricultural practices for commercial production, such as pruning, thinning, irrigation, pest and disease control.
Morphological analysis
The target apple cultivars (Golden Delicious, Red Delicious, and Royal Delicious) were assessed on the basis of quantitative characters of fruit. Pertaining to morphological variability, the data from individual were recorded in the standard descriptor for quantitative characters like fruit weight, fruit height, fruit diameter, depth and width of stalk cavity and depth and width of eye basin. The fruit character like fruit weight was measured using weighing scale (Citizen, model CY510). The fruit height and fruit diameter were measured using digital Vernier Callipers (Mitutoyo, Japan). The depth and width of eye and stalk cavity were measured using a measuring scale.
Chemicals
The following standards were used for quantification of polyphenolic compounds: chlorogenic acid, procyanidin B2, epicatechin, phloridzin, and gallic acid purchased from Sigma-Aldrich (Germany). Folin-Ciocalteu’s reagent, acetonitrile, methanol, acetic acid, sodium carbonate, aluminium chloride, potassium acetate, 2,2- Azinobis-3-ethyl benzothiazoline-6-sulphonic acid (ABTS) and 2,4,6-tri- 2-pyridyl-1,3,5-triazine (TPTZ) were purchased from Merck (Darmstadt, Germany). 2,2-Diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, sodium carbonate, potassium persulphate, ferric chloride, sodium acetate, glacial acetic acid and hydrochloric acid from Qualigens (Mumbai, India). All the chemicals were of HPLC grade.
Extract preparation
The fruits collected were divided into three groups, i.e., whole fruit, peel, and pulp to study the polyphenolics content in different fruit portions. The pooled samples (peel, pulp, and whole fruit) from 30 mature apple fruits were cut into thin uniform slices and then grounded in mortar and pestle to a fine texture with the help of liquid nitrogen (to prevent enzymatic browning), and precisely 20 g of each sample was weighed into a test tube and extracted with 200 ml methanol (80%) containing 0.1% (v/v) of hydrochloric acid to obtain phenolics (Schieber et al. 2001). The mixture was sonicated using a sonicator (40 Hz; 10 min) (Model ANIS 09,001, Toshiba, India). The homogenized mixture was kept at 45 °C in a water bath for 1 h. The homogenate was stored in tightly capped bottles for 24 h at 4 °C and centrifuged (10,000 rpm) at room temperature for 20 min. The supernatant was removed from each tube and filtered through Whatman filter paper no. 2 and stored in a separate amber screw-cap glass vials at − 20 °C prior to analysis. All samples were prepared and analysed in 3 replicates.
Total phenolic content, flavonoids, flavonols and tannins
Total phenolic content in the methanolic extract of pulp, peel and whole fruit of selected cultivars was determined by Folin–Ciocalteu’s colorimetric method (Singleton et al. 1999). Quantification of total phenols at 765 nm using UV–Vis spectrophotometer (U-2001, Hitachi, Japan) was done on the basis of standard curve of gallic acid prepared in 80% (v/v) methanol and results were expressed in mg gallic acid equivalent per gram of fresh weight (mg GAE/g fw). Flavonoid content in the methanolic extract of fruit portion was determined by aluminium chloride colorimetric method (Chang et al. 2002). The absorbance of resulting reaction mixture was measured at 415 nm UV–VIS spectrophotometer. Total flavonol was estimated using the method of Kumaran and Karunakaran (2007). The absorbance of the resulting solution was read at 440 nm after 2.5 h incubation at 20 °C. Total flavonoid and flavonol content were calculated as mg quercetin equivalent per gram fresh weight (mg/g QE fw). Total tannin content was estimated following the method of Nwinuka et al. (2005) with minor modifications. The quantification of tannin was performed at 700 nm on the basis of a standard curve of tannic acid prepared in 80% (v/v) methanol. Results were expressed in mg tannic acid equivalent per gram fresh weight (mg TAE/g fw).
Antioxidant activity
2-2-Azinobis (3 benzylthiazole)-6-sulphonic acid (ABTS) assay
The antioxidant activity was measured by following improved ABTS method (Cai et al. 2004). Absorbance was recorded at 734 nm using UV–VIS spectrophotometer. A standard curve of various concentrations of ascorbic acid was prepared in 80% (v/v) methanol for the equivalent quantification of antioxidant potential with respect to ascorbic acid. Results were expressed in mM ascorbic acid equivalent per 100 g fresh weight (mM AAE/100 g fw).
2-2-Diphenyl-1-picrylhydrazyl (DPPH) assay
DPPH assay as described in Brand-Williams et al. (1995) was used. Absorbance at 520 nm was recorded by UV–VIS spectrophotometer. Results were expressed in mM ascorbic acid equivalent per 100 g fresh weight (mM AAE/100 g fw).
Ferric reducing antioxidant power (FRAP) assay
FRAP assay was performed following Faria et al. (2005). Absorbance was recorded at 593 nm for the preparation of standard curve of ascorbic acid and sample analysis. Results were expressed in millimole ascorbic acid equivalent per 100 g fresh weight (mM AAE/100 g fw).
HPLC analysis of polyphenols
High-performance liquid chromatography (HPLC) analysis was carried out on a Merck Hitachi HPLC system equipped with L-7100 series pump, L-7400 series detector, C18 analytical column (250 mm × 4.6 mm i.d., 5 µm, Purosphere ®) (Merck Pvt Ltd., Germany). The mobile phase A: 2.5% glacial acetic acid and B: acetonitrile (70:30 ratio) with 0.8 ml/min flow rate and 20 µl injection volume was used for screening of phenolics and flavonoids. The compounds (chlorogenic acid, procyanidin B2, epicatechin, phloridzin, gallic acid) spectrum was recorded at 280 nm wavelength. The concentration of each compound was calculated with the help of a corresponding external standard and results expressed in mg/100 g fresh weight.
Statistical analysis
Data on measured parameters were subjected to analysis of variance, Pearson correlation coefficient and multivariate analysis using SPSS version 16.0. The significance level was determined at p < 0.05. The coefficient of variation, range and mean was determined as indicators of variability. Data is presented as mean values ± standard error (SE) of 3 replicates for each analysis. Principal Component Analysis was performed using PAST software (Hammer et al. 2001) to examine the patterns in morphological and phytochemical profile of different cultivars at different altitudes. Mean values were used to create correlation matrix from were standardized PC scores extracted. Scatter plot of two major components were created using same software.
Results and discussion
Morphological variation
The morphological traits provide basic information for breeding programs, management of genetic resources, protection of cultivars and selection of candidates to diversify local production. Therefore, the study of the variance in morphological traits can be used for diversity analysis and establish a phylogenetic relationship within and between the cultivars (Rana et al. 2015). Apart from other morphological parameters, fruit size is considered one of the important parameter for selection of superior genotypes in breeding. The present study of variability for the morphological parameters associated with the fruit size (weight, height, diameter, depth and width of stalk cavity, depth and width of eye cavity) across an altitudinal gradient of 1771–2780 m amsl records morphological variation among target commercial cultivars of Western Himalaya. Among cultivars, the coefficient of variation (CV%) for fruit weight (27.05%) and fruit height (7.54%) was highest in Royal Delicious. While the CV% for fruit diameter and depth of stalk cavity was highest in Red Delicious (18.98 and 12.94% respectively), the width of stalk cavity CV% was highest in Golden Delicious (46.07%). The depth and width of eye basin showed highest CV% in Red Delicious cultivar (41.34, 22.6% respectively). Within tested parameters, highest CV% was recorded for the width of stalk cavity i.e. 46.07%. The range of variation in fruit weight and height was highest in Royal Delicious (44.91–108.91 g and 44.25–55.89 mm, respectively), while the mean fruit weight and height were highest in Red Delicious (103 ± 5.2 g) and Golden Delicious cultivar (56.8 ± 2.8 mm), respectively. The range of fruit diameter was highest in Red Delicious (40.12–62.91 mm) with mean being highest in Golden Delicious (62.35 ± 2.3 mm). The Depth of stalk cavity range and mean was highest in Red Delicious cultivar (0.98–1.43 cm and 1.16 ± 0.8 cm). Range and mean of the width of stalk cavity were highest in Golden Delicious (1.12–5.13 cm and 2.80 ± 1.2 cm). The depth and width of eye basin range were highest in Red Delicious (0.24–1.56 and 1.21–2.45 cm), with highest mean in Royal Delicious (1.32 ± 0.8 cm) and Golden Delicious cultivar (2.13 ± 1.1 cm) (Supplementary Table 1). A similar type of variation in morphological parameters has been reported in wild and cultivated varieties of pear and many other temperate fruits (Paganová 2003).
The PCA analysis of morphological parameters revealed two major components, which exhibited major variance among the populations/groups. PC1 revealed 84.5% variation and PC2 revealed 13.35% variance. The fruit weight and diameter were positively correlated with PC1 and PC2, respectively. The variables mainly responsible for ordination are fruit weight (PC1: 0.98; PC2: − 0.01); fruit diameter (PC1: 0.01; PC2: 0.99). The scatter plot divided into four quadrates wherein the different parameters clustered different cultivars at different locations together. In Q1, the width of eye basin and stalk cavity clustered Golden Delicious (Mukhwa location), Royal Delicious (Satbunga location), Golden Delicious and Red Delicious (Shitlakhet location), Golden Delicious (Naugaoun location), Golden Delicious and Red Delicious (Khabrar location). In Q2, the fruit diameter and fruit height clustered Golden Delicious (Chaubatiya location), Red Delicious (Mukhwa location), Royal Delicious (Khabrar location), Royal Delicious (Shitlakhet location), Golden Delicious (Satbunga location) and Red Delicious (Naugaoun location). In Q3, Fruit weight and depth of stalk cavity clustered Royal Delicious (Mukhwa location), Red Delicious (Chaubatiya and Satbunga location; Supplementary Fig. 2). Such variation in morphological parameters within and among cultivars may be attributed to the fact that the morphological traits are greatly influenced by environmental conditions such as rainfall and solar radiation, etc. (Mandić et al. 2011). The fruit traits (size) range of variability in different commercial cultivars from Western Himalaya can be taken into consideration for future breeding programs, thereby enhancing the health and livelihood options.
Phytochemical variation
Variation among cultivars
As plant’s genotype is considered of prime importance in the determination of its phytochemical profile, often surpassing the impacts of cultural and agricultural practices (Farneti et al. 2015); the study of phytochemical variation among different cultivars becomes utmost necessary. The concentration and composition of phenolic compounds have been reported to vary among the apple cultivars (Šavikin et al. 2014). In our study, among cultivars (whole fruit), total phenolics, flavonol, flavonoids, tannins, phenolic compounds (chlorogenic acid, procyanidin B2, epicatechin, phloridzin and gallic acid) and antioxidant activity (ABTS, DPPH, and FRAP) varied significantly (p < 0.05). The Golden Delicious was rich in chlorogenic acid; Red Delicious in total phenol and epicatechin and the Royal Delicious fruits in total tannin, flavonoids, flavonols, antioxidant activity (ABTS, DPPH, FRAP), procyanidin B2, phloridzin and gallic acid.
In consideration to variance (Whole fruit), the Golden Delicious and Red Delicious had the highest coefficient of variation (CV%) for total phenol (63.27 and 67.18%) while Royal Delicious had higher CV% for total flavonols (76.35%) and flavonoids (76.77%). In all the studied cultivars, the ABTS assay (antioxidant activity) was found to have a higher coefficient of variation [Golden Delicious (56.03%), Red Delicious (62.12%), and Royal Delicious (58.67%)] in comparison to DPPH and FRAP activity, highest being in Red Delicious. Likewise, in individual polyphenols category, phloridzin [a unique dihydrochalcone and chemo-taxonomical marker of apple (Guo et al. 2015) was found to have the highest coefficient of variation [Golden Delicious (62.74%), Red Delicious (56.41%), and Royal Delicious (68.34%)], highest being in Royal Delicious (Supplementary Table 2). The recorded variation of phloridzin among cultivars in the present study seems to be more similar to what Kalinowska et al. (2014) had reported for their studied cultivars.
The range and mean content was also found to vary among cultivars. For instance, the epicatechin range and mean content (as compared with other studied parameters) was highest in all three cultivars [Golden Delicious (16.54–27.55 mg/100 g fw; 21.46 ± 0.04 mg/100 g fw), Red Delicious (22.44–40.55 mg/100 g fw; 32.69 ± 0.03 mg/100 g fw), and Royal Delicious (22.68–43.64 mg/100 g fw; 31.01 ± 0.09 mg/100 g fw)], with Royal Delicious having higher range of variation and mean content (Supplementary Table 2). Van der Sluis et al. (2001) has also reported variation in flavonoid and chlorogenic acid concentration and antioxidant activity between the individual apple from different cultivars and the maximum variations in epicatechin and phloridzin concentration. This wide variation of phytochemicals among cultivars especially phloridzin and epicatechin might aid in identifying apple cultivars suitable for apple-derived products, as phloridzin in combination with epicatechin may be involved in the formation of orange oxidation products that account for about half of juice coloration (Oszmianski and Lee 1991) and may contribute significantly to the quality of apple juice and cider (Sanoner et al. 1999).
Variation within cultivars: effect of altitude
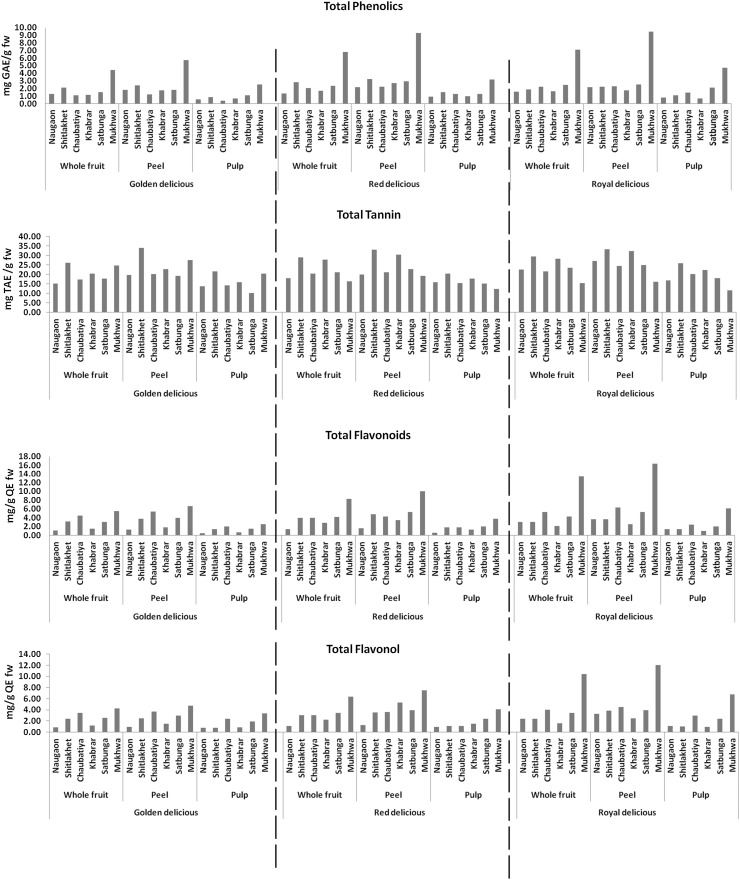
Within cultivars, the total phenolics, tannins, flavonols, flavonoids, antioxidant activity and phenolic compounds (chlorogenic acid, procyanidin B2, epicatechin, phloridzin and gallic acid) varied significantly (p < 0.05) among different locations. The Mukhwa location (2780 m amsl; highest altitude) was found to have a higher content of total phenol, total flavonol and flavonoid content, while the Shitlakhet location (2000 m amsl) was found to have a higher content of total tannin as compared to other locations in all the cultivars studied (Fig. 1).
Fig. 1.
Variation of total phenolics, tannins, flavonols, flavonoids among and within Golden Delicious, Red Delicious and Royal Delicious cultivars and fruit portions
The antioxidant activity was also found to be higher in the Mukhwa location (ABTS assay) and in Khabrar location (2200 m amsl) (FRAP assay) in all the cultivars. Although the DPPH assay showed higher antioxidant activity in Mukhwa location (Red and Royal Delicious), it exhibited higher antioxidant activity in Shitlakhet location (Golden Delicious) (Fig. 2). The individual phenolic compounds like chlorogenic acid, procyanidin B2, epicatechin were also higher in the Mukhwa location (in all three cultivars studied). Gallic acid was higher in Chaubatiya location (Golden Delicious and Royal Delicious) and in Mukhwa location (Red Delicious). Phloridzin content was higher in Satbunga location (Golden Delicious) and in Mukhwa location (Red Delicious and Royal Delicious) (Fig. 3).
Fig. 2.
Variation of antioxidant activity among and within Golden Delicious, Red Delicious and Royal Delicious cultivars and fruit portions
Fig. 3.
Variation of polyphenolics among and within Golden Delicious, Red Delicious and Royal Delicious cultivars and fruit portions
Pearson correlation analysis was performed between studied parameters (Morphological and phytochemicals) with altitude in order to study the variance. It was found that altitude was significantly correlated (p < 0.01) with total phenol (R = 0.82), total flavonol (R = 0.74), total flavonoids (R = 0.74), ABTS activity (R = 0.86) and DPPH activity (R = 0.55). Chlorogenic acid (R = 0.57) and phloridzin content (R = 0.49) were also found correlated to altitude (p < 0.05) (Table 1). The Chlorogenic acid is the main phenolic acid present in the apple, has a great ability to scavenge free radicals (Panzella et al. 2013). The morphological parameters were not found correlated with the altitude. The variation in the phytochemical profile of apple cultivars along with different altitudes is indicative of the ecological adaptations from the cultivars and genetic plasticity within the species.
Table 1.
Correlation among polyphenolics profile in apple cultivars with altitude
| Altitude | TP | TT | TFl | TFd | ABTS | DPPH | FRAP | CA | PB | EC | PD | GA | FW | FH | FD | DSC | WCS | DEB | WEB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude | 1.00 | |||||||||||||||||||
| TP | 0.82** | 1.00 | ||||||||||||||||||
| TT | − 0.15 | − 0.25 | 1.00 | |||||||||||||||||
| TFl | 0.74** | 0.90** | − 0.33 | 1.00 | ||||||||||||||||
| TFd | 0.74** | 0.90** | − 0.32 | 1.00** | 1.00 | |||||||||||||||
| ABTS | 0.86** | 0.89** | − 0.12 | 0.75** | 0.74** | 1.00 | ||||||||||||||
| DPPH | 0.55* | 0.74** | 0.12 | 0.61** | 0.60** | 0.82** | 1.00 | |||||||||||||
| FRAP | 0.15 | − 0.14 | 0.24 | − 0.06 | − 0.06 | 0.07 | − 0.08 | 1.00 | ||||||||||||
| CA | 0.57* | 0.35 | − 0.17 | 0.29 | 0.29 | 0.39 | 0.04 | − 0.09 | 1.00 | |||||||||||
| PB | 0.24 | 0.42 | − 0.02 | 0.49* | 0.49* | 0.34 | 0.31 | 0.41 | − 0.26 | 1.00 | ||||||||||
| EC | 0.33 | 0.64** | 0.11 | 0.55* | 0.56* | 0.48* | 0.59* | 0.01 | − 0.07 | 0.57* | 1.00 | |||||||||
| PD | 0.49* | 0.68** | − 0.36 | 0.75** | 0.75** | 0.67** | 0.55* | 0.10 | 0.20 | 0.56* | 0.64** | 1.00 | ||||||||
| GA | 0.01 | 0.03 | − 0.14 | 0.06 | 0.06 | − 0.01 | − 0.06 | 0.15 | − 0.33 | 0.31 | 0.08 | 0.12 | 1.00 | |||||||
| FW | 0.12 | 0.08 | − 0.34 | 0.13 | 0.12 | 0.09 | 0.01 | − 0.03 | − 0.16 | − 0.08 | 0.09 | 0.19 | 0.54* | 1.00 | ||||||
| FH | 0.21 | − 0.03 | − 0.22 | − 0.03 | − 0.04 | − 0.04 | − 0.21 | − 0.09 | 0.05 | − 0.31 | − 0.19 | − 0.18 | 0.18 | 0.71** | 1.00 | |||||
| FD | 0.10 | − 0.01 | 0.16 | − 0.16 | − 0.15 | 0.12 | − 0.03 | − 0.14 | 0.50* | − 0.33 | − 0.21 | − 0.19 | 0.06 | 0.02 | 0.04 | 1.00 | ||||
| DSC | 0.43 | 0.38 | 0.21 | 0.28 | 0.29 | 0.25 | 0.08 | 0.02 | 0.38 | 0.12 | 0.24 | − 0.04 | 0.30 | 0.02 | 0.15 | 0.40 | 1.00 | |||
| WCS | − 0.10 | − 0.11 | 0.07 | 0.00 | 0.01 | − 0.24 | − 0.30 | − 0.34 | 0.20 | − 0.21 | − 0.24 | − 0.28 | 0.26 | 0.04 | 0.14 | 0.53* | 0.51* | 1.00 | ||
| DEB | 0.26 | 0.53* | − 0.29 | 0.57* | 0.58* | 0.30 | 0.18 | − 0.28 | 0.36 | 0.24 | 0.46 | 0.52* | − 0.08 | 0.15 | − 0.03 | 0.17 | 0.21 | 0.23 | 1.00 | |
| WEB | − 0.09 | 0.09 | − 0.05 | − 0.01 | 0.01 | − 0.18 | − 0.24 | − 0.42 | 0.08 | − 0.10 | 0.06 | − 0.31 | 0.21 | 0.20 | 0.28 | 0.40 | 0.57* | 0.61** | 0.50* | 1.00 |
TP total phenolics, TT total tannin, TFl total flavonol, TFd total flavonoids, ABTS ABTS activity, DPPH DPPH activity, FRAP FRAP activity, CA Chlorogenic acid, PB procyanidin B2, EC epicatechin, PD phloridzin, GA gallic acid, FW fruit weigh, FH fruit height, FD fruit diameter, DSC depth of stalk cavity, WSC width of stalk cavity, DEB depth of eye basin, and WEB width of eye basin
Level of significance **p < 0.01, *p < 0.05 level
PCA analysis revealed two major components, which exhibited major variance among the populations/groups. In the phytochemical analysis, PC 1 revealed 51.16% variation and PC 2 revealed 22.49% variance. Procyanidin B2 and epicatechin were positively correlated with PC 1. PC 2 was positively correlated with total flavonoid, chlorogenic acid and epicatechin and negatively with total tannin. The variables mainly responsible for ordination are epicatechin (PC 1: 0.70; PC 2: 0.24); Procyanidin B2 (PC 1: 0.63; PC 2: − 0.09); chlorogenic acid (PC 1: − 0.18; PC 2: 0.78); Total flavonoid (PC 1: 0.15; PC 2: 0.22) and Total Tannin (PC 1: 0.07; PC 2 − 0.44). On the basis of scatter plot, it is observed that in quadrate 1 (Q1) Golden Delicious at different altitudes clustered together due to high content of chlorogenic acid whereas high content of epicatechin, Total flavonoid, Total flavonol, ABTS, and phloridzin in Red Delicious and Royal Delicious at Mukhwa location grouped together (Q2). In quadrate Q3, due to the high content of gallic acid, procyanidin B2 and total tannin in Red Delicious and Royal Delicious at Khabrar, Red Delicious and Royal Delicious at Shitlakhet location, Red Delicious at Naugaoun, Red Delicious and Royal Delicious at Chaubatiya, and Royal Delicious at Satbunga location grouped together. In Q4, FRAP content led to group of Royal Delicious at Naugaoun and Satbunga location (Fig. 4).
Fig. 4.
PCA analysis of phytochemical variation in Delicious group of apple cultivars in Western Himalaya
The variation in the phytochemical profile may be of great importance regarding the stability of antioxidant pool and processing of apples. There is defined stability order among the apple antioxidants e.g., catechin < epicatechin < procyanidins < dihydrochalcones < chlorogenic acid (Lavelli et al. 2011). Hence apple in a different growing location, which is higher in chlorogenic acid is more stable upon processing as compared to apple rich in catechin or epicatechin. Also, as already shown owing to the ability to interact strongly with proteins and polysaccharides, epicatechin and procyanidin B2 is involved in the formation of hazes and precipitates in the beverage (Beveridge and Wrolstad 1997). This diversity of phytochemicals is of paramount importance for the biological functions of phenolics in apples and apple-based food and beverages (Chinnici et al. 2004).
Variation among fruit parts (peel and pulp)
Nature and distribution pattern of phenolics varies considerably between apple pulp and peel. This range of difference is also cultivar dependent (Holderbaum et al. 2014). The present study reports cultivar and location-dependent variation of total phenol, flavonoid, flavonol, tannin, antioxidant activity and phenolic compounds in peel and pulp portions. All the studied parameters were found to have a higher range of variation and mean in peel portion as compared to pulp. Like in whole fruit, in Golden Delicious and Red Delicious, epicatechin content was found to have higher range and mean in peel [Golden Delicious (20.37–41.24 mg/100 g; 32.14 ± 0.03 mg/100 g fw), Red Delicious (34.74–52.97 mg/100 g; 43.74 ± 0.02 mg/100 g fw)] and pulp [Golden Delicious (10.36–22.21 mg/100 g; 15.22 ± 0.05 mg/100 g fw), Red Delicious (13.21–22.45 mg/100 g; 18.26 ± 0.02 mg/100 g fw)], when compared with other parameters studied, higher being in peel portion. For peel, present values of the study are in agreement and fall within the lower range of Kalinowska et al. (2014) who reported the epicatechin content between 0.30 and 233 mg/100 g fw in peel samples. In the case of Royal Delicious, the maximum range of variation and mean were recorded for total tannin content [peel (16.03–33.29; 26.33 ± 0.01), pulp (11.64–25.83 mg TAE/g FW; 19.11 ± 0.06 mg TAE/g FW), again higher being in peel portion. However, among fruit portion, the same consistent pattern of variation across locations (as whole fruit) was determined in the peel and pulp samples in all the cultivars tested, with significantly greater amounts in peels of all the cultivars tested. The results of the present study are in agreement with studies, which invariably reported that peel portion has higher phenolics and flavonoids than the pulp (Kalinowska et al. 2014). On average of three cultivars from six locations, the peels contained 2.24, 1.45, 1.97, 2.63, 1.94, 5.12, 2.49, 3.47, 1.57 fold higher total phenol, tannin, flavonol, flavonoid, chlorogenic acid, procyanidin B2, epicatechin, phloridzin and gallic acid, respectively than pulp portion. Wolfe and Liu (2003) also showed that total phenolics, flavonoids content were higher in the peels, followed by whole fruit and pulp. Drogoudi et al. (2008) reported 1.2–3.3 times higher content of phenolics in peel than the pulp extracts. A 3.5-110 fold variation on the basis of fresh matter in phloridzin content of apple peel and pulp has also been recorded (Veberic et al. 2005).
The antioxidant activity (ABTS, DPPH and FRAP) was also found to be higher in peel extracts. In the present study, the peel samples had 1.42, 1.62, 2.08 fold (ABTS, DPPH, FRAP) higher content of the antioxidant activity. Study elsewhere on peel extracts showed 1.5-9.2 times greater total antioxidant activity (Drogoudi et al. 2008). Also, the study of D’ Abrosca et al. (2007) has revealed variation in DPPH radical scavenging of apple peel and pulp. The radical scavenging activity of apple extracts depends on their phenolic composition in a qualitative and quantitative way (Chinnici et al. 2004). Additionally, the radical scavenging activity depends on the synergistic interactions between antioxidants. This is important selection criterion when considering the potential biological implications for apple plants, agriculture, and human health.
The higher phenolics in peel than pulp extracts might be due to the fact that phenolic compounds tend to accumulate in the dermal tissues of plant body because of their potential roles in protection against UV radiation, to act as attractants in fruit dispersal and as defence chemicals against pathogens and predators (D’Abrosca et al. 2007). The result of this study finds strong agreement with the study of Holderbaum et al. (2014), which suggested the existence of tissue-specific regulation of biosynthesis of phenolic compounds and important genotype-specific characteristics in regulatory mechanism.
Factorial analysis revealed a significant (p < 0.05) effect of cultivars, fruit portion, and locations individually or in combination on the total phenolics, flavonoids, flavonols, tannins, antioxidant activity, and phenolic compounds like chlorogenic acid, epicatechin, gallic acid, phloridzin and procyanidin B2 (Table 2). These factors might be responsible for variation among phytochemical content in the selected apple cultivars of the present study. The result of the present study showed that an environmental effect that varies between growing regions at different altitude had a significant effect on polyphenolics content. As the cultivars grown in different altitudes are exposed to different kinds of stress like the variation in UV radiation, chilling period, frost light intensity, photoperiod, temperature, and rainfall, which influence the synthesis of secondary metabolites, it could be a mechanism of plant response to stress (Veberic et al. 2005).
Table 2.
Multivariate analysis on total phenolics, tannins, flavonols, flavonoids, antioxidant activity and different phenolic compounds in studied Delicious group cultivars
| Source | df | MS | F | MS | F | MS | F | MS | F |
|---|---|---|---|---|---|---|---|---|---|
| Total phenol | Total tannin | Total flavonol | Total flavonoid | ||||||
| Cultivar | 2.00 | 10.58 | 61,480.00 | 92.36 | 235,200.00 | 26.57 | 119,700.00 | 38.07 | 149,500.00 |
| Fruit part | 2.00 | 42.89 | 249,300.00 | 826.46 | 2,105,000.00 | 52.01 | 234,300.00 | 135.68 | 532,800.00 |
| Location | 5.00 | 77.44 | 450,000.00 | 282.40 | 719,200.00 | 86.65 | 390,300.00 | 135.57 | 532,300.00 |
| Cultivar * fruit part | 4.00 | 0.83 | 4793.00 | 4.01 | 10,210.00 | 2.88 | 12,960.00 | 2.92 | 11,450.00 |
| Cultivar * location | 10.00 | 2.38 | 13,830.00 | 129.30 | 329,300.00 | 11.02 | 49,640.00 | 16.68 | 65,510.00 |
| Fruit part * location | 10.00 | 4.88 | 28,360.00 | 9.91 | 25,240.00 | 2.20 | 9906.00 | 9.31 | 36,550.00 |
| Cultivar * fruit part * location | 17.00 | 0.35 | 2026.00 | 10.21 | 26,000.00 | 0.87 | 3927.00 | 1.41 | 5541.00 |
| ABTS | DPPH | FRAP | Chlorogenic acid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cultivar | 2.00 | 4.33 | 18,050.00 | 1.01 | 6352.00 | 2.84 | 16,720.00 | 2.00 | 992.04 |
| Fruit part | 2.00 | 7.70 | 32,070.00 | 2.03 | 12,810.00 | 13.12 | 77,180.00 | 2.00 | 1503.51 |
| Location | 5.00 | 42.62 | 177,600.00 | 2.28 | 14,350.00 | 9.19 | 54,040.00 | 5.00 | 225.52 |
| Cultivar * fruit part | 4.00 | 0.25 | 1030.00 | 0.05 | 331.90 | 0.47 | 2760.00 | 4.00 | 14.83 |
| Cultivar * location | 10.00 | 1.39 | 5788.00 | 0.07 | 424.45 | 0.46 | 2686.00 | 10.00 | 127.69 |
| Fruit part * location | 10.00 | 1.09 | 4525.00 | 0.06 | 388.19 | 0.48 | 2847.00 | 10.00 | 48.84 |
| Cultivar * fruit part * location | 17.00 | 0.06 | 236.42 | 0.06 | 362.94 | 0.06 | 375.97 | 17.00 | 37.03 |
| Procyanidin B2 | Epicatechin | Phloridzin | Gallic acid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cultivar | 154,000.00 | 1250.28 | 159,000.00 | 1090.69 | 112,200.00 | 8.47 | 4137.00 | 196.73 | 26,750.00 |
| Fruit part | 233,400.00 | 3186.12 | 405,100.00 | 8892.37 | 914,900.00 | 26.70 | 13,040.00 | 777.87 | 105,800.00 |
| Location | 35,000.00 | 168.88 | 21,470.00 | 217.82 | 22,410.00 | 3.45 | 1684.00 | 21.46 | 2918.00 |
| Cultivar * fruit part | 2301.00 | 199.16 | 25,320.00 | 407.87 | 41,960.00 | 2.30 | 1124.00 | 49.73 | 6761.00 |
| Cultivar * location | 19,820.00 | 93.00 | 11,830.00 | 186.91 | 19,230.00 | 2.45 | 1198.00 | 108.81 | 14,790.00 |
| Fruit part * location | 7581.00 | 39.18 | 4982.00 | 71.90 | 7397.00 | 0.50 | 244.92 | 4.85 | 659.64 |
| Cultivar * fruit part * location | 5747.00 | 76.40 | 9714.00 | 104.05 | 10,700.00 | 0.28 | 135.57 | 19.04 | 2589.00 |
df degree of freedom, ms mean of sum
Level of significance ***p < 0.05
Conclusion
The three commercial apple cultivars studied in this comparative analysis varied to their phenolic composition and concentration with respect to growing locations at a different altitude. The higher altitudinal region in Western Himalaya can be promoted for apple cultivation so as to harness the maximum benefits of apple phytochemicals. It is also suggested that choice of cultivar should be made carefully before processing of apple or fresh consumption as phytochemicals were found varying between the cultivars. Higher concentration of measured phenolic compounds in peel portion emphasizes the importance of consumption and/or use of the whole fruit including the peel. The information from this study on the phytochemical diversity of important commercial apple cultivars across different altitudes may help small and medium scale industries in maintaining the batch consistency of their products.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Director GBPNIHESD for facilities and encouragement. We also thank colleagues of Biodiversity Conservation and Management Thematic group for the support and help. Partial Financial support by the Department of Biotechnology, Government of India (Project No: BT/PR11040/PBD/16/812/2008) is greatly acknowledged.
Compliance with ethical standards
Conflict of interest
All authors declares that there is no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3179-x) contains supplementary material, which is available to authorized users.
References
- Beveridge T, Wrolstad RE. Haze and cloud in apple juices. Crit Rev Food Sci Nutr. 1997;37(1):75–91. doi: 10.1080/10408399709527768. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Kalia K, Sharma M, Singh B, Ahuja PS. Processing of apple pomace for bioactive molecules. Crit Rev Biotechnol. 2008;28(4):285–296. doi: 10.1080/07388550802368895. [DOI] [PubMed] [Google Scholar]
- Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3(5):12. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoids content in Propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chinnici F, Bendini A, Gaiani A, Riponi C. Radical scavenging activities of peels and pulps from cv Golden Delicious apples as related to their phenolic composition. J Agric Food Chem. 2004;52(15):4684–4689. doi: 10.1021/jf049770a. [DOI] [PubMed] [Google Scholar]
- D’Abrosca B, Pacifico S, Cefarelli G, Mastellone C, Fiorentino A. ‘Limon-cella’ apple an Italian apple cultivar: phenolic and flavonoid contents and antioxidant activity. Food Chem. 2007;104(4):1333–1337. doi: 10.1016/j.foodchem.2007.01.073. [DOI] [Google Scholar]
- de Jesús Ornelas-Paz J, Quintana-Gallegos BM, Escalante-Minakata P, Reyes-Hernández J, Pérez-Martínez JD, Rios-Velasco C, Ruiz-Cruz S. Relationship between the firmness of Golden Delicious apples and the physicochemical characteristics of the fruits and their pectin during development and ripening. J Food Sci Technol. 2018;55(1):33–41. doi: 10.1007/s13197-017-2758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing DL, editor. Processed apple products. Berlin: Springer; 2012. [Google Scholar]
- Drogoudi PD, Vemmos S, Pantelidis G, Petri E, Tzoutzoukou C, Karayiannis I. Physical characters and antioxidant sugar and mineral nutrient contents in fruit from 29 apricot (Prunus × armeniaca L) cultivars and hybrids. J Agric Food Chem. 2008;56(22):10754–10760. doi: 10.1021/jf801995x. [DOI] [PubMed] [Google Scholar]
- Escarpa A, Gonzalez M. High-performance liquid chromatography with diode-array detection for the performance of phenolic compounds in peel and pulp from different apple varieties. J Chromatogr A. 1998;823(1–2):331–337. doi: 10.1016/S0021-9673(98)00294-5. [DOI] [PubMed] [Google Scholar]
- Faria A, Oliveira J, Neves P, Gameiro P, Santos-Buelga C, de Freitas V, Mateus N. Antioxidant properties of prepared blueberry (Vaccinium myrtillus) extracts. J Agric Food Chem. 2005;53(17):6896–6902. doi: 10.1021/jf0511300. [DOI] [PubMed] [Google Scholar]
- Farneti B, Masuero D, Costa F, Magnago P, Malnoy M, Costa G, Mattivi F. Is there room for improving the nutraceutical composition of apple. J Agric Food Chem. 2015;63(10):2750–2759. doi: 10.1021/acs.jafc.5b00291. [DOI] [PubMed] [Google Scholar]
- Guo S, Guan L, Cao Y, Li C, Chen J, Li J, Wu B. Diversity of polyphenols in the peel of apple (Malus sp) germplasm from different countries of origin. Int J Food Sci Technol. 2015;51(1):222–230. doi: 10.1111/ijfs.12994. [DOI] [Google Scholar]
- Hammer Ø, Harper DA, Ryan PD. PAST-paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4(1):9. [Google Scholar]
- Holderbaum DF, Kon T, Guerra MP. Dynamics of total phenolic content in different apple tissues and genotypes: impacts and relevance for breeding programs. Sci Hortic. 2014;168:58–63. doi: 10.1016/j.scienta.2014.01.020. [DOI] [Google Scholar]
- Jindal KK, Mankotia MS. Impact of changing climatic conditions on chilling units, physiological attributes and productivity of apple in Western Himalayas. Acta Hortic. 2004;662:111–117. doi: 10.17660/ActaHortic.2004.662.13. [DOI] [Google Scholar]
- Kalinowska M, Aleksandra B, Hanna LS, Waldemar P. Apples: content of phenolic compounds vs variety part of Apple and cultivation model extraction of phenolic compounds Biological Properties. Plant Physiol Biochem. 2014;84:169–188. doi: 10.1016/j.plaphy.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Khanizadeh S, Tsao R, Rekika D, Yang R, Charles MT, Vasantha Rupasinghe HP. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. J Food Compos Anal. 2008;21(5):396–401. doi: 10.1016/j.jfca.2008.03.004. [DOI] [Google Scholar]
- Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci Technol. 2007;40(2):344–352. doi: 10.1016/j.lwt.2005.09.011. [DOI] [Google Scholar]
- Lavelli V, Corey M, Kerr W, Vantaggi C. Stability and anti-glycation properties of intermediate moisture apple products fortified with green tea. Food Chem. 2011;127(2):589–595. doi: 10.1016/j.foodchem.2011.01.047. [DOI] [PubMed] [Google Scholar]
- Mandić D, Durašinović G, Savić B, Kikić S. Nova bosanka—new variety of winter wheat. Genetika. 2011;43(3):569–574. doi: 10.2298/GENSR1103569M. [DOI] [Google Scholar]
- Nwinuka NM, Ibeh GO, Ekeke GI (2005) Proximate composition and levels of some toxicants in four commonly consumed spices. J Appl Sci Environ Mgt 9(1):150–155. http://hdl.handle.net/1807/6437
- Oszmianski J, Lee CY. Enzymic oxidation of phloretin glucoside in model system. J Agric Food Chem. 1991;39(6):1050–1052. doi: 10.1021/jf00006a009. [DOI] [Google Scholar]
- Paganová V. Taxonomic reliability of leaf and fruit morphological characteristics of the Pyrus L taxa in Slovakia. Hortic Sci (Prague) 2003;30(3):98–107. doi: 10.17221/3869-HORTSCI. [DOI] [Google Scholar]
- Panzella L, Petriccione M, Rega P, Scortichini M, Napolitano A. A reappraisal of traditional apple cultivars from Southern Italy as a rich source of phenols with superior antioxidant activity. Food Chem. 2013;140(4):672–679. doi: 10.1016/j.foodchem.2013.02.121. [DOI] [PubMed] [Google Scholar]
- Rana JC, Chahota RK, Sharma V, Rana M, Verma N, Verma B, Sharma TR. Genetic diversity and structure of Pyrus accessions of Indian Himalayan region based on morphological and SSR markers. Tree Genet Genomes. 2015;11(1):1–14. doi: 10.1007/s11295-014-0821-2. [DOI] [Google Scholar]
- Sanoner P, Guyot S, Marnet N, Molle D, Drilleau JP. Polyphenol profiles of French cider Apple varieties (Malus Domestica Sp) J Agric Food Chem. 1999;47(12):4847–4853. doi: 10.1021/jf990563y. [DOI] [PubMed] [Google Scholar]
- Šavikin K, Živković J, Zdunić G, Gođevac D, Đorđević B, Dojčinović B, Đorđević N. Phenolic and mineral profiles of four Balkan indigenous apple cultivars monitored at two different maturity stages. J Food Compos Anal. 2014;35(2):101–111. doi: 10.1016/j.jfca.2014.05.004. [DOI] [Google Scholar]
- Schieber A, Keller P, Carle R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J Chromatogr A. 2001;910(2):265–273. doi: 10.1016/S0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- Shalini R, Gupta DK. Utilization of pomace from apple processing industries: a review. J Food Sci Technol. 2010;47(4):365–371. doi: 10.1007/s13197-010-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenolic and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Van der Sluis A, Dekker M, de Jager A, Jongen WM. Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar harvest year and storage conditions. J Agric Food Chem. 2001;49(8):3606–3613. doi: 10.1021/jf001493u. [DOI] [PubMed] [Google Scholar]
- Veberic R, Trobec M, Herbinger K, Hofer M, Grill D, Stampar F. Phenolic compounds in some apple (Malus domestica Borkh) cultivars of organic and integrated production. J Sci Food Agric. 2005;85(10):1687–1694. doi: 10.1002/jsfa.2113. [DOI] [Google Scholar]
- Wolfe KL, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51(3):609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.