Abstract
Phyllanthus emblica L. is a tropical deciduous tree producing edible berries with potential medicinal value. In this study, a novel water-soluble phyllanthus emblica polysaccharide (PEP) from the berries was isolated by precipitation and purification, and analyzed for its structure features. The results showed that PEP was a α-pyran acidic heteropolysaccharide with a molecular weight of 1.31 × 105 Da, which included galacturonic acid, galactose, rhamnose, and arabinose with a molar ratio of 3.21:6.59:1:0.23. Furthermore, the antioxidant activities of PEP were determined and showed remarkable antioxidant capacities in DPPH, superoxide anion- and hydroxyl-radical scavenging, ferric-reducing antioxidant power, and lipid peroxidation inhibition. This work indicated that PEP as a natural antioxidant agent from the berries of Phyllanthus emblica L. had potential application for developing valuable nutraceutical in food industry.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3199-6) contains supplementary material, which is available to authorized users.
Keywords: Antioxidant activity, Indian gooseberry, Phyllanthus emblica, Polysaccharide
Introduction
Reactive oxygen species (ROS) such as hydroxyl radical (·OH), superoxide anion () and hydrogen peroxide (H2O2) are constantly produced during metabolism in aerobic organisms; their strong oxidative stress including lipid peroxidation, DNA damage, and denaturation of proteins, which cause a variety of diseases. Antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), ascorbic acid, and tertiary butylhydroquinone (TBHQ) have been used extensively for reducing oxidative stress resultant healthy problems. However, due to the potential toxicity and carcinogenicity to the human body, the uses of these synthetic antioxidants have raised significant concerns (Carocho and Ferreira 2013; Zeng et al. 2014). As a consequence, more attention has been focused on exploiting natural and safe antioxidants.
A particular activity of Phyllanthus emblica fruit is its antioxidant properties. The antioxidant activities of emblic fruit had been focused on the total phenolic compounds in crude extracts obtained through the use of solvents (water, methanol or ethanol). However, the active principle responsible for the antioxidant activity of this fruit has not been identified (Chatterjee et al. 2011). Polysaccharide from the crude extract of emblic fruit have revealed remarkable antitumor activities and can be used as antioxidants for the prevention of oxidative damage in living organisms (Chatterjee et al. 2011). Chatterjee et al. (2011) have shown that water soluble carbohydrate polymers from Phyllanthus emblica were glycoconjugates which contain phenolics, and the structural features and fluorescence quenching of the glycoconjugates have been analyzed. However, the investigations about the characterization and corresponding antioxidant activity of polysaccharides are rather limited. Therefore, our efforts have been made to characterize a novel antioxidant polysaccharide from Phyllanthus emblica, and its antioxidant activities were also determined in detail in the present study. The aim of this study was to gain valuable information about the structures and biological activities of the emblic polysaccharide.
Materials and methods
Materials and reagents
Fresh emblic fruits free of pest and disease without deformity were selected and collected from Quanzhou city, Fujian Province, China. The fruits were cleaned, pitted, and dried at 40 °C. The dry fruit pulp was ground into powder and passed through a 40-mesh sieve. The powder was pretreated with 80% ethanol at 70 °C twice to inactivate the hydrolyzing enzymes and to remove mono- and oligosaccharides, pigments and peptides. The powders were filtered and vacuum-dried, and the dry powder of fruit pulp was stored at − 40 °C until use.
Chemicals used in this study including galacturonic acid, glucuronic acid, the monosaccharide standard, hydroxylamine hydrochloride, n-propylamine, 3-phenylphenol, and trifluoroacetic acid were purchased from Sigma Chemical Co. (St. Louis, MO). Glucose, phenol, concentrated sulfuric acid, methanol, and ethanol were obtained from China National Pharmaceutical Group Co. (Beijing, China). All chemicals used were of analytical grade.
Extraction of crude polysaccharides
The extraction of polysaccharide was carried out using the method described by Cheng et al. (2016) with modifications. Briefly, the dry powder of emblic pulp was dissolved in hot distilled water at a ratio of 1:20 for 5 h and filtered. This procedure was repeated twice. The extracted polysaccharide was precipitated by the addition of fivefold volume of absolute alcohol, and the precipitants were centrifuged and freeze-dried.
Isolation and purification of polysaccharide
The extracted polysaccharides diluted to 5% (w/v) with deionized water were treated with Sevage reagent and dialyzed (MWCO5000, Sigma-Aldrich, USA). The crude polysaccharides were precipitated with fivefold volumes of absolute alcohol and were centrifuged and freeze-dried.
The crude polysaccharides (200 mg) were separated on a Sepharose CL-6B column (D3.5 × 90 cm) (GE Healthcare Bio-Sciences, Pittsburgh USA) and eluted using 100 mM NaCl solution at a flow rate of 0.5 mL/min to achieve homogeneity. The polysaccharide obtained at this stage was designated as PEP. The total saccharide, uronic acid, hexosamine, and total phenolic contents of PEP were determined according to the phenol–sulfuric acid assay (DuBois et al. 1956), meta-hydroxydiphenyl method (Blumenkrantz and Asboe-Hansen 1973), discriminating of galactosamine and glucosamine content assay (Wagner 1979), and Folin-Ciocalteu method (Chatterjee et al. 2011), respectively.
Determination of homogeneity and molecular weight
The homogeneity and molecular weight of PEP were determined by high-performance size exclusion chromatography (HPSEC) using a Hitachi L-2000 HPLC system (Hitachi, Ltd., Tokyo, Japan) and a size exclusion chromatography (SEC) column (Sepax Nanofilm SEC-250, 7.8 mm × 300 mm i.d., with a particle size of 5 μm, Sepax Technologies, Newark Delaware, USA) according to published analytical methods (Zeng et al. 2012). The presence of polysaccharide in the eluted fractions was determined by the phenol–sulfuric acid method (DuBois et al. 1956) and the presence of protein was analyzed by the Lowry method (Lowry et al. 1951).
Monosaccharide composition analysis
The monosaccharide composition of PEP was analyzed by gas chromatography-mass spectrometry (GC–MS) according to the method for quantitative determination of uronic acids (Blumenkrantz and Asboe-Hansen 1973). For the simultaneous detection of aldose and uronic acid, the equimolar concentrations (2 mol/L) of monosaccharide standards were mixed with 1 mL of 0.1 mol/L sodium carbonate solution and 1 mg inositol in the ampoule bottle. Acetic acid (25%) was used to neutralize the solution. The neutral solution was desalinated for 2 h by adding the cation exchange resin, then rinsed with 5 mL distilled water and filtered with quantitative filter paper to obtain the supernatant solution. The supernatant solution was concentrated under reduce pressure and dried after adding 3 mL methanol. This process was repeated five times to get rid of borate. The aldonic acid of the monosaccharide was converted into lactone by vacuum heating at 85 °C for 2 h. The residue was dissolved in 1 mL anhydrous pyridine and 1 mL n-propylamine and heated at 55 °C for 30 min. The reaction solution was concentrated and dried at 45 °C under reduced pressure. Anhydrous pyridine and acetic anhydride (0.5 mL each) were added to dissolve the residue and heated at 95 °C for 1 h, then concentrated and dried. The residue was dissolved into 1 mL anhydrous chloroform, and centrifuged. The supernatant was collected for GC–MS analysis (TRACE GC-POLARISQ, Thermo Fisher Scientific, USA).
For the detection of monosaccharide composition in samples, 5 mg PEP was dissolved in 2 mL 2 mol/L trifluoroacetic acid solution in the ampoule bottle. The bottle was filled with N2 gas and sealed. After the hydrolization at 110 °C for 2 h, the polysaccharide hydrolysate was dried under reduced pressure below 40 °C. The reaction system was added 3 mL methanol and evaporated. This process was repeated five times to remove trifluoroacetic acid. Then, the hydrolysate was reduced and acetylated following the method described above. The residue was dissolved into 5 mL anhydrous chloroform for GC–MS analysis.
FT-IR spectroscopy
Fourier-transform infrared (FT-IR) spectra of the polysaccharide was determined using an Avatar 360 FT-IR spectrometer (Thermo Nicolet Co., USA) in the wavenumber range of 400–4000 cm−1 with KBr pellets and referenced against air (Sun et al. 2008).
Evaluation of antioxidant activities in vitro
To assay the in vitro antioxidant activities, PEP was dissolved in distilled water at different concentrations (0.5–8 mg/mL). Antioxidant activities were evaluated by the testing free-radical scavenging activity, anti-lipid peroxidation activity, and ferric-reducing antioxidant power (FRAP) according to the methods described by Prieto et al. (1999), Dorman and Hiltunen (2004), and Zeng et al. (2012).
Statistical analysis
The data were presented as mean ± standard error of three determinations. Data were analyzed by an analysis of variance (P < 0.05) and the means separated by Duncan’s multiple range test using SPSS (version 15.0 for Windows, SPSS Inc., CO, USA). Multiple comparisons of means were done by the LSD (least significance difference) test.
Results and discussion
Isolation and purification of PEP
Through the repeated freeze–thaw cycle, depigmentation with 30% H2O2, dialysis, and freeze-drying, we obtained a yellowish powder. The gel filtration chromatography (GFC) technique was employed for further isolation and purification of polysaccharide from emblic fruit. As shown in supplementary A1, the crude polysaccharides were subjected to a Sepharose CL-6B column to yield a distinct peak. The recovery of pure emblic polysaccharide was found to be 10.33%. The eluted fractions were treated by desalination and freeze-drying to produce PEP that was a fluffy white powder. According to supplementary A2a, the purity of PEP was found to be 99.04% calculated based on the area normalization method of the high-performance size exclusion chromatography (HPSEC). This suggested that PEP was a homogeneous polysaccharide so that monosaccharide composition and polysaccharide structure could be further analyzed by GC–MS and infrared spectroscopy, respectively.
Physicochemical properties analysis
The crude polysaccharides were found to contain 31.13% of total sugar, 26.24% of protein, and 15.3 mg GAE/g of total phenolics. Further purification resultant PEP had a total sugar content of 61.67%. This content appeared to be low, which was probably due to a high uronic acid content (26.04%). It was reported that the phenol–sulfuric acid method was less reliable in detecting the polysaccharide content of samples with a high content of uronic acid and tended to produce a value which was lower than the real polysaccharide content (Sun et al. 2005). The phenolics were very low as total phenol contents of PEP were only 1.6 mg GAE/g of the sample. The ultraviolet spectra assay showed that PEP had no absorption at 280 or 260 nm, which indicated that PEP contained no protein or nucleic acid. Additionally, the content of protein of PEP tested by HPLC was nearly undetectable (only 0.01%) (supplementary A2b).
Homogeneity and molecular weight determination of PEP
The homogeneity and molecular weight of PEP was determined by high performance size exclusion chromatography (HPSEC) analysis. Supplementary A2 indicated that PEP was a homogeneous polysaccharide with a high purity, and its equivalent dextran molecular weight was estimated to 1.31 × 105 Da based on the equation of the standard curve made with a group of dextran standards.
Monosaccharide composition of PEP
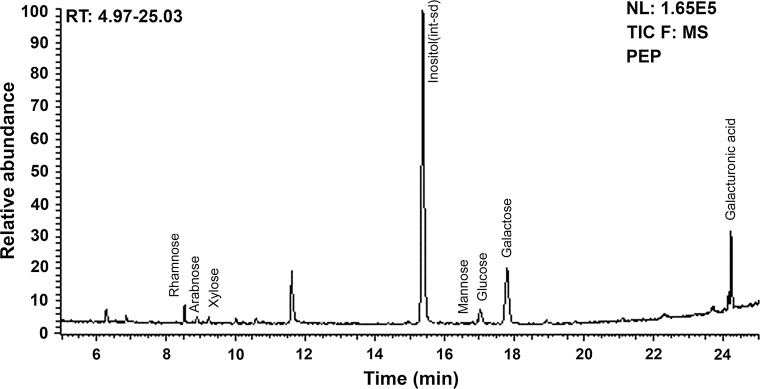
We used GC–MS method to further determine the composition of monosaccharide (Fig. 1), the results showed that the polysaccharide from P. emblica was composed of galacturonic acid (GalA), galactose (Gal), rhamnose (Rha), and arabinose (Ara) with a molar ratio of 3.21:6.59:1:0.23, together with smaller quantities of glucose, xylose and mannose.
Fig. 1.
Gas chromatography mass spectrometry (GC–MS) analysis of monosaccharide composition of a polysaccharide (PEP) isolated from Phyllanthus emblica fruits
In present study, we established a derivation method to simultaneously analyze aldose and uronic acid for the determination of monosaccharide compositions and their molar ratios of PEP. It is a simpler, quicker, more sensitive, and more specific method which could analyze the monosaccharide composition of emblic polysaccharide accurately and conveniently.
FT-IR spectral analysis
The FT-IR spectra of PEP ranged from 400 to 4000 cm−1 (supplementary A3). The bands at 3435 cm−1 were derived from the stretching vibration of O–H, which indicated that hydroxyl groups existed in PEP; the bands at 2929 cm−1 were derived from the stretching vibration of C-H; and the bands at 1425 cm−1 were derived from the bending vibration of C–OH (Santhiya et al. 2002). The band at 1146 cm−1 was attributed to galactose. The strong bands at 1745 and 1629 cm−1 were caused by the C=O stretching vibration, indicating the presence of ionic uronic acid. The signal at 1072 cm−1 represented the glycosidic linkages with a pyranose ring ether (Zhao et al. 2005). The signal close to 830 cm−1 was due to the C-H variable angle vibration of the α-anomer (Kačuráková et al. 2000; Shi et al. 2007). The FTIR spectroscopy results suggested that the configuration of PEP from P. emblica is α-pyran acidic heteropolysaccharide containing uronic acid.
Antioxidant activities of PEP
DPPH assay
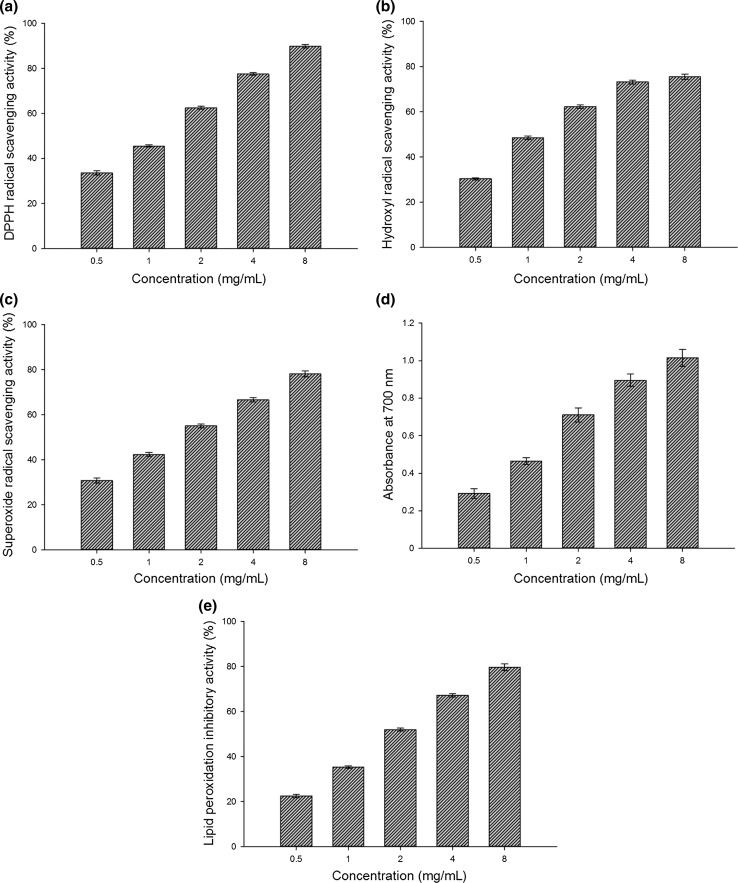
The DPPH scavenging ratio of PEP ranged from 33.54 to 89.85% with a concentration-dependent manner (Fig. 2a). The half effective concentration (EC50) of PEP was 1.26 ± 0.08 mg/mL, while the EC50 of BHT and ascorbic acid were 1.43 ± 0.07 and 0.91 ± 0.05 mg/mL, respectively (Table 1). The mechanism of scavenging DPPH may be associated with the hydroxyl group of a monosaccharide unit that donates a proton to combine with the unpaired electron of DPPH to form the non-radical DPPH-H (Zhang et al. 2014; Zhao et al. 2015).
Fig. 2.
Antioxidant activities of PEP on DPPH radicals (a), hydroxyl radicals (b), superoxide anion radicals (c), ferric-reducing antioxidant powers (d) and lipid peroxidation inhibition (e). Each value is expressed as the mean ± standard error (n = 3)
Table 1.
The half effective concentration (EC50) of an isolated polysaccharide (PEP) from Phyllanthus emblica along with chemical butylated hydroxytoluene (BHT) and ascorbic acid in scavenging free radicals analyzed using different methods
| Assays | EC50 (mg/mL) | ||
|---|---|---|---|
| PEP | BHT | Ascorbic acid | |
| DPPH scavenging activity | 1.26 ± 0.08 | 1.43 ± 0.07 | 0.91 ± 0.05 |
| Hydroxyl radical scavenging activity | 1.04 ± 0.14 | 2.18 ± 0.31 | 1.53 ± 0.15 |
| Superoxide radical scavenging activity | 1.61 ± 0.13 | 3.79 ± 0.39 | 1.55 ± 0.21 |
| FRAP assay | 1.11 ± 0.10 | 1.23 ± 0.14 | 1.42 ± 0.21 |
| Inhibition of lipid peroxidation | 1.92 ± 0.17 | 2.64 ± 0.31 | 1.68 ± 0.18 |
Data are represented as mean ± standard error (n = 3)
Hydroxyl radical scavenging activity
The scavenging effects of PEP increased with the increasing concentration, but the scavenging activity did not clearly increase after the concentration exceeded 4 mg/ml (Fig. 2b). The scavenging activities of PEP ranged from 30.35 to 75.57% of the hydroxyl radical; its EC50 was 1.04 ± 0.14 mg/mL, which was lower than that of BHT (2.18 ± 0.31 mg/mL) and ascorbic acid (1.53 ± 0.15 mg/mL) from Table 1. These results demonstrated that the hydroxyl radical scavenging activity of PEP was greater than both BHT and ascorbic acid. An explanation for the antioxidant activity might be due to the hydroxyl group in the polysaccharide that can donate one hydrogen to bind with the hydroxyl radical to achieve the scavenging effect (Zhao et al. 2015).
Superoxide radical-scavenging activity
PEP was observed to possess a strong ability to scavenge superoxide radical (Fig. 2c). The scavenging ratio was 30.76% at 0.5 mg/mL of PEP and reached 78.15% when the concentration increased to 8 mg/mL. The EC50 of PEP was 1.61 ± 0.13 mg/mL compared to 3.79 ± 0.39 and 1.55 ± 0.21 mg/mL for BHT and ascorbic acid, respectively (Table 1). The mechanism of the scavenging superoxide anion of PEP may be associated with the presence of some types of electrophilic groups such as ketones or aldehydes, facilitating liberation of hydrogen from the O–H bond and thus stabilizing the superoxide anion (Lin et al. 2009).
Ferric reducing antioxidant power (FRAP)
As shown in Fig. 2d, the reducing power of PEP determined at 700 nm was dose-dependent. The FRAP increased from 0.292 to 1.015 when the concentration of PEP increased from 0.5 to 8.0 mg/ml. Table 1 show that the EC50 values of PEP was 1.11 ± 0.10 mg/ml, while the EC50 values for BHT and ascorbic acid were 1.23 ± 0.14 and 1.42 ± 0.21 mg/ml, respectively.
Inhibitory effect on lipid peroxides
The present study showed PEP dose-dependent inhibition of lipid peroxides in the lecithin liposome system (LLS) (Fig. 2e). The lipid peroxidation inhibitory activity increased from 22.52 to 79.68% when the concentration of PEP increased from 0.5 to 8.0 mg/ml. The EC50 values of PEP, BHT, and ascorbic acid were 1.92 ± 0.17, 2.64 ± 0.31, and 1.68 ± 0.18 mg/ml, respectively (Table 1). The presence of uronic acid groups in PEP could activate the hydrogen atom of the anomeric carbon, which could block or slow down the process of lipid peroxidation and inhibits the activity of ROS (Sun et al. 2014).
Conclusion
The present study isolated a remarkable antioxidant polysaccharide (PEP) from fruits of Phyllanthus emblica. PEP was a α-pyran acidic heteropolysaccharide containing uronic acid with a molecular weight of 1.31 × 105 Da. It was composed of galacturonic acid, galactose, rhamnose, and arabinose in a molar ratio of 3.21:6.59:1:0.23. Antioxidant activities of PEP were concentration-dependent with activities stronger than BHT. Moreover, the antioxidant activities were related to the high uronic acid content. Our study showed that PEP might be a powerful natural antioxidant agent and could potentially be used for developing novel natural antioxidant products for nutriceutical in food industry. Further studies are warranted to determine the structure and the relationship between structure and antioxidant activities to provide more fundamental information.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant No. 31501694), Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20113515120016), Scientific Research Fund of Fujian Provincial Education Department (Grant No. JK2012016), and Foundation for University Key Teacher by Horticulture Department of Fujian Agriculture and Forestry University (Grant No. FAFU2012YYPY03). Yongyu Li was also funded by the China Scholarship Council.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3199-6) contains supplementary material, which is available to authorized users.
Contributor Information
Jianjun Chen, Phone: (407) 814-6161, Email: jjchen@ufl.edu.
Shaohua Wu, Phone: +86 591 83789241, Email: wsh6677@hotmail.com.
Liaoyuan Zhang, Phone: +86 591 83789492, Email: zliaoyuan@126.com.
References
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Carocho M, Ferreira ICFR. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Chatterjee UR, Bandyopadhyay SS, Ghosh D, Ghosal PK, Ray B. In vitro anti-oxidant activity, fluorescence quenching study and structural features of carbohydrate polymers from Phyllanthus emblica. Int J Biol Macromol. 2011;49:637–642. doi: 10.1016/j.ijbiomac.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Cheng H, Jia Y, Wang L, Liu X, Liu G, Li L, et al. Isolation and structural elucidation of a novel homogenous polysaccharide from Tricholoma matsutake. Nat Prod Res. 2016;30:58–64. doi: 10.1080/14786419.2015.1034711. [DOI] [PubMed] [Google Scholar]
- Dorman HJD, Hiltunen R. Fe(III) reductive and free radical-scavenging properties of summer savory (Satureja hortensis L.) extract and subfractions. Food Chem. 2004;88:193–199. doi: 10.1016/j.foodchem.2003.12.039. [DOI] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Kac̆uráková M, Capek P, Sasinková V, Wellner N, Ebringerová A. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohyd Polym. 2000;43:195–203. doi: 10.1016/S0144-8617(00)00151-X. [DOI] [Google Scholar]
- Lin CL, Wang CC, Chang SC, Inbaraj BS, Chen BH. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int J Biol Macromol. 2009;45:146–151. doi: 10.1016/j.ijbiomac.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Santhiya D, Subramanian S, Natarajan KA. Surface chemical studies on sphalerite and galena using extracellular polysaccharides isolated from Bacillus polymyxa. J Colloid Interface Sci. 2002;256:237–248. doi: 10.1006/jcis.2002.8681. [DOI] [PubMed] [Google Scholar]
- Shi Y, Sheng J, Yang F, Hu Q. Purification and identification of polysaccharide derived from Chlorella pyrenoidosa. Food Chem. 2007;103:101–105. doi: 10.1016/j.foodchem.2006.07.028. [DOI] [Google Scholar]
- Sun Y, Tang J, Gu X, Li D. Water-soluble polysaccharides from Angelica sinensis (Oliv.) Diels: preparation, characterization and bioactivity. Int J Biol Macromol. 2005;36:283–289. doi: 10.1016/j.ijbiomac.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang S, Li T, Li X, Jiao L, Zhang L. Purification, structure and immunobiological activity of a new water-soluble polysaccharide from the mycelium of Polyporus albicans (Imaz.) Teng. Biores Technol. 2008;99:900–904. doi: 10.1016/j.biortech.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Sun L, Wang L, Li J, Liu H. Characterization and antioxidant activities of degraded polysaccharides from two marine Chrysophyta. Food Chem. 2014;160:1–7. doi: 10.1016/j.foodchem.2014.03.067. [DOI] [PubMed] [Google Scholar]
- Wagner WD. A more sensitive assay discriminating galactosamine and glucosamine in mixtures. Anal Biochem. 1979;94:394–396. doi: 10.1016/0003-2697(79)90379-8. [DOI] [PubMed] [Google Scholar]
- Zeng WC, Zhang Z, Gao H, Jia LR, Chen WY. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohyd Polym. 2012;89:694–700. doi: 10.1016/j.carbpol.2012.03.078. [DOI] [PubMed] [Google Scholar]
- Zeng WC, Zhang Z, Jia LR. Antioxidant activity and characterization of antioxidant polysaccharides from pine needle (Cedrus deodara) Carbohyd Polym. 2014;108:58–64. doi: 10.1016/j.carbpol.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Zhang CM, Yu SH, Zhang LS, Zhao ZY, Dong LL. Effects of several acetylated chitooligosaccharides on antioxidation, antiglycation and NO generation in erythrocyte. Bioorg Med Chem Lett. 2014;24:4053–4057. doi: 10.1016/j.bmcl.2014.03.083. [DOI] [PubMed] [Google Scholar]
- Zhao G, Kan J, Li Z, Chen Z. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohyd Polym. 2005;61:125–131. doi: 10.1016/j.carbpol.2005.04.020. [DOI] [Google Scholar]
- Zhao ZY, Zhang Q, Li YF, Dong LL, Liu SL. Optimization of ultrasound extraction of Alisma orientalis polysaccharides by response surface methodology and their antioxidant activities. Carbohyd Polym. 2015;119:101–109. doi: 10.1016/j.carbpol.2014.11.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.