Abstract
Due to its bioactive compounds, blue maize flour is a valuable ingredient for developing gluten-free products. The incorporation of alternative flours into gluten-free pasta is a challenge as it usually results in products that, despite their enhanced nutraceutical features, show reduced quality characteristics. Composite pasta was prepared with variable (25, 50 and 75%) contents of flours from white and blue maize, chickpea and unripe plantain, following a laboratory-scale process. The composite pasta exhibited acceptable cooking loss (9–11%); pasta with blue maize showed lower hardness and chewiness, but higher adhesiveness than its white maize-based counterpart. Blue maize-based pasta presented dark color. The addition of blue maize flour at 75% conveyed the highest total phenolic content retention after extrusion (80%) and cooking (70%). Pasting profile of blue maize pasta (50 and 75%) showed a defined second viscosity peak due to re-arrangement of starch components upon cooling. The observed retention of phenolic compounds with antioxidant capacity after cooking will be useful for further development (selection of ingredients, formulations and conditions of operation) of gluten-free products with potential health benefits.
Keywords: Blue maize flour, Gluten-free pasta, Processing conditions, Antioxidant properties
Introduction
Pasta, a wheat-based product, is a commonly consumed food either as a ready-to-eat option or requiring minimal preparation at home. Nowadays, there is an increasing demand for gluten-free products, so it is the interest of both celiac patients and the general public to purchase gluten-free products, particularly those exhibiting sensory and nutritional characteristics similar to conventional gluten-containing foods. Different ingredients have been used to replace the functionality of wheat gluten in food matrix formation, including formulations that contain cereals, pseudo-cereals and pulses.
The use of non-conventional flours in gluten-free pasta production obeys mainly to the need of mimicking the role of the gluten network in conventional products; however, non-conventional flours also contribute to higher bioactive compounds and indigestible carbohydrates contents than whole wheat flour, a feature that may provide health benefits (Bello-Pérez et al. 2014). Our research group has used a mixture of white maize, chickpea and green plantain flours in order to prepare different gluten-free products, such as spaghetti (Flores-Silva et al. 2014) and snacks (Flores-Silva et al. 2015), with acceptable quality characteristics and important nutritional values. Other non-colored grains, such as sorghum, have been used for preparing gluten-free pasta, evaluating the effects and interactions of diverse ingredients (e.g. pregelatinized starch, gums) on quality characteristics (cooking, color and texture). An optimized blend of these ingredients has been used to produce gluten-free sorghum pasta (Palavecino et al. 2017).
Red sorghum was used to prepare noodles and evaluate its quality characteristics; in general, cooked noodles containing red sorghum showed higher firmness than those prepared with white sorghum, but the products did not differ in tensile strength, cooking loss and water uptake values (Liu et al. 2012). That study, however, did not evaluate the impact of the anthocyanins in sorghum on the quality characteristics of the pasta. The properties of non-gluten free pastas containing different levels of red or white sorghum have also been investigated, showing that texture and cooking quality were not influenced by the type of sorghum, but the rapidly digestible starch content of red sorghum pasta was lower than in its white counterpart. Interestingly, no difference in slowly digestible starch content was found between the two sorghum-based products; the authors suggested an inhibitory effect of red sorghum polyphenols on digestive enzyme activity (Khan et al. 2013, 2014). Here, we suggest the use of blue maize as a way to increase the nutraceutical value of gluten-free pasta, an approach that has been recently explored for blue maize tortilla (Bello-Pérez et al. 2015) and chips (Del Pozo-Insfran et al. 2007). Blue maize exhibits antioxidant power (Aguayo-Rojas et al. 2012) and potential anti-cancer properties (Urias-Lugo et al. 2015), associated with its anthocyanins content. The bioactive properties of blue maize have not been tested in the matrix of gluten free pasta. The aim of this study was to evaluate the effect of blue maize on physical characteristics, pasting properties, total phenolic content and antioxidant capacity during gluten-free pasta processing. The influence of varying the blue-to-white maize ratio was also evaluated.
Materials and methods
Plant material
Unripe plantain (Musa paradisiaca L.), chickpea (Cicer arietinum L.), white and blue maize (Zea mays L.) flours were used to make gluten-free spaghettis. Unripe (stage 2) plantain was purchased from a local market at Cuautla, Morelos, Mexico, and the flour was obtained from the pulp of the fruit following the procedure described by Ovando-Martinez et al. (2009). Chickpeas were provided by Universidad de Sinaloa (Mexico) and milled by the procedure proposed by Flores-Silva et al. (2015). Commercial whole white maize flour was from Naturelo (Queretaro, Mexico) and pigmented (blue) maize was provided by the experimental field of INIFAP Texcoco, (México) during the 2012–2013 season. Blue maize flour was prepared according to Camelo-Méndez et al. (2017).
Spaghetti formulation and processing
Six gluten-free formulations containing unripe plantain, chickpea, white and blue maize flours were prepared (Table 1). Water (32 mL) was added to the homogenized blend of flours (100 g) and mixed for 5 min to allow hydration. The obtained dough was extruded (1.5-mm die) using a laboratory single screw extruder (Beutelspacher, México, D.F) at 50 °C in every the three heating zone to 72 rpm. The six pasta formulations were dried at 45 °C for 16 h in a forced air oven; for same analysis the dry pasta was milled and sieved (mesh 40, particle size 0.420 mm). Two batches of each formulation were prepared for further evaluation.
Table 1.
Gluten-free pasta formulation
| Ingredients (%) | Pasta (sample identification) | |||||
|---|---|---|---|---|---|---|
| WP25 | WP50 | WP75 | BP25 | BP50 | BP75 | |
| White maize | 25 | 50 | 75 | 0 | 0 | 0 |
| Unripe plantain | 37.5 | 25 | 12.5 | 37.5 | 25 | 12.5 |
| Chickpea | 37.5 | 25 | 12.5 | 37.5 | 25 | 12.5 |
| Blue maize | 0 | 0 | 0 | 25 | 50 | 75 |
| CMC* | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
*CMC carboxymethylcellulose
Cooking quality
Cooking tests were performed for each pasta sample in order to determine the optimum cooking time (OCT), according to AACCI Approved Method 66-50.01 (AACC 2000). Cooking loss was determined gravimetrically by weighing the residues after evaporating the cooking water. Water absorption capacity (WAC) measurements were performed under previously reported conditions (Flores-Silva et al. 2015); WAC percentages were calculated as [(weight of cooked drained pasta - weight of raw pasta)/weight of raw pasta] × 100. The diameter of dried and cooked spaghettis was determined based on a standard protocol (Petitot et al. 2010). The midpoint of 20 individual strands of spaghetti taken randomly was measured using a digital caliper (Model CD-6′′, Mitutoyo Corp., Kawasaki, Japan). Cooked pasta was freeze-drying, milled and sieved (mesh 40) and used in the different analysis.
Texture
Twelve measurements per sample were evaluated by texture profile analysis (TPA) using the texture analyzer (Brookfield CT3, Middleboro, MA 02346-1031 USA) within 5 min after cooking with the OCT. Different variables were determined: hardness, cohesiveness, adhesiveness, and chewiness. For all the measurements, the equipment with a 10-kg load cell. All the samples were prepared and kept until measured according to the approved AACC method (66-50 pasta cooking quality– firmness; AACC 2000).
Color analysis
Color measurements on fresh blends (before extrusion), raw samples (dried spaghetti) and cooked spaghetti samples were carried out according to Islas-Rubio et al. (2014) using a Minolta colorimeter model BC-10 (Konica Minolta Sensing Americas, Inc., Ramsey, NJ). The color data obtained were averages of thirty measurements. Results were expressed in the CIELAB coordinates. The values of chroma (C*ab) and hue (hab) for each sample were also calculated using the following formulas:
Phenolic extraction
Phenolics were extracted using the method reported by Sumczynski et al. (2016) with minor modifications. Briefly, five hundred mg per sample (blends, raw pasta and cooked pasta) were treated twice with 8 mL of methanol 80% [acidified with HCl 5% (1 N)] in an ultrasonic bath at 35 °C for 1 h. The supernatants were combined after centrifugation at 4000 g for 25 min and used to phenolic quantification.
Determination of total and phenol contents
Total phenolic content was determined by the Folin-Ciocalteu procedure (Bello-Pérez et al. 2015) expressed as milligrams of gallic acid equivalents per gram of dry matter by using a calibration curve.
Antioxidant Capacity
Antioxidant capacity was evaluated by of free radicals inhibition (ABTS assays) (Re et al. 1999). Ferric reducing antioxidant power (FRAP method) was quantified by the methodology proposed by Benzie and Strain (1996). Three independent experiments in triplicate were performed for each assayed extract and results were expressed in milligram equivalent of Trolox per gram of dry weight, using a Trolox standard n curve.
Pasting properties
Dispersions of the pasta at 10% (w/w, dry basis) were prepared in distilled water and analyzed in a rheometer with cell pasting starch (AR 1500ex-TA Instruments) with the experimental conditions reported (Chávez-Salazar et al. 2017).
Statistical analysis
Statistical analysis was done using the Statgraphics Plus version 5.1® (Manugistics, Inc., Rockville, MA, USA) software. Mean (at least three replicates) and standard deviations were determined for all measurements. One-way analysis of variance (ANOVA) was employed to establish significant differences. In all cases, statistically significant level was considered at p < 0.05.
Results and discussion
Physical and texture characteristics
In general, an increase in the maize flour (white and blue) level in the pasta formulation did not affect the cooking quality variables (Table 2). Slight differences were found between white and blue maize pastas with the same substitution level. These results show that replacing white maize by blue maize did not affect cooking quality of gluten-free pasta containing also a blend of chickpea and unripe plantain flours. Although slight differences in amylose, starch and protein contents between blue and white maize have been found (Bello-Pérez et al. 2016; Camelo-Méndez et al. 2017), they did not have noticeable impact on the cooking quality of the pasta samples studied here.
Table 2.
Cooking quality and texture parameters of gluten free-pasta made with the blends white maize-chickpea-green plantain and blue maize-chickpea-green plantain
| Parameters | Samples | |||||
|---|---|---|---|---|---|---|
| WS25 | WS50 | WS75 | BS25 | BS50 | BS75 | |
| Cooking quality* | ||||||
| OCT (min) | 6.67 ± 0.58a | 6.67 ± 0.58a | 6.67 ± 0.58a | 7.33 ± 0.58a | 6.67 ± 0.58a | 7.67 ± 0.58a |
| CL (%) | 10.32 ± 2.64a | 11.07 ± 0.31a | 9.16 ± 0.16a | 9.57 ± 0.49a | 9.76 ± 2.37a | 10.68 ± 1.11a |
| WAC (%) | 130.83 ± 17.98a,b | 110.25 ± 5.41b | 126.10 ± 13.91a,b | 148.04 ± 28.75a | 132.23 ± 6.11a,b | 131.18 ± 10.78a,b |
| DRS (mm) | 2.02 ± 0.06c,d | 2.12 ± 0.08a,b | 1.98 ± 0.06d | 2.13 ± 0.08a,b | 2.07 ± 0.05b,c | 2.19 ± 0.03a |
| DCS (mm) | 2.83 ± 0.15a | 2.80 ± 0.22a | 2.80 ± 0.12a | 2.82 ± 0.11a | 2.80 ± 0.12a | 2.84 ± 0.13a |
| Texture** | ||||||
| Hardness (N) | 21.59 ± 4.69a | 16.85 ± 3.62c | 21.12 ± 4.18a,b | 14.98 ± 2.72c,d | 13.04 ± 2.61d | 17.96 ± 6.59b,c |
| Cohesiveness | 0.86 ± 0.09a | 0.78 ± 0.09a,b | 0.75 ± 0.09a,b | 0.72 ± 0.22b | 0.77 ± 0.07a,b | 0.72 ± 0.21b |
| Adhesiveness (N*s) | 1.41 ± 0.94b | 1.60 ± 0.40b | 1.39 ± 0.64b | 1.79 ± 0.58a,b | 1.85 ± 0.69a,b | 2.30 ± 1.24a |
| Chewiness (N) | 18.62 ± 5.09a | 13.02 ± 2.54b,c | 16.12 ± 4.48a,b | 11.01 ± 4.14c | 10.07 ± 1.24c | 13.10 ± 6.16b,c |
Values are presented by mean ± SD
OCT optimum cooking time, CL cooking loss, WAC water absorption capacity, DRS diameter of raw spaghetti, DCS diameter of cooked spaghetti. For sample identification, see Table 1
The same superscript letter per row indicates no significant difference (p <0.05), LSD tests. *n = 5, **n = 1
Cooking loss (CL) values, a parameter that is connected to the acceptability by consumers, were 9–11% for gluten-free pasta. Those CL values were lower than the 12% limit suggested for gluten-free pasta of good quality (Fu 2008). Therefore, the gluten-free spaghetti prepared with unripe plantain, chickpea and maize (blue and white) falls within the good cooking quality range.
Texture characteristics have also an important role in the final acceptance of pasta products (Tudorica et al. 2002). Overall, no differences were found after increasing the maize flour level in the formulation, but some differences between blue and white pasta were observed when comparing equivalent substitution levels. Pasta containing blue maize flour had lower hardness, lower chewiness and higher adhesiveness than the corresponding white maize-based samples, a pattern that is related to the lower starch content of blue maize (Camelo-Méndez et al. 2017). The cohesiveness index gives an indication on how the sample holds together upon cooking. Gluten-free pastas showed cohesiveness values (0.72–0.86) similar to those reported by Flores-Silva et al. (2015) for composite gluten-free spaghettis (0.63–0.83) and those by Sözer and Kaya (2003) (0.88) for commercial wheat spaghetti evaluated at different cooking times and salt concentrations.
Color characteristics of pasta
Color measurements were performed on blends (before extrusion), extruded (uncooked) and cooked pasta. Figure 1 shows the color of raw pasta, where those with blue maize were darker than those containing white maize. Figure 2 shows the location of the samples in the (a*b*)-plane and their L* values. All samples were located in the first quadrant of the plane (positive values for a* and b*). The various pasta samples showed similar color values. This is not surprising, since they were prepared with the same ingredients blended in different proportions.
Fig. 1.
Photographs of raw gluten-free pastas made with blue maize, chickpea and unripe plantain (a) and white maize, chickpea and unripe plantain (b). For sample identification, see Table 1
Fig. 2.
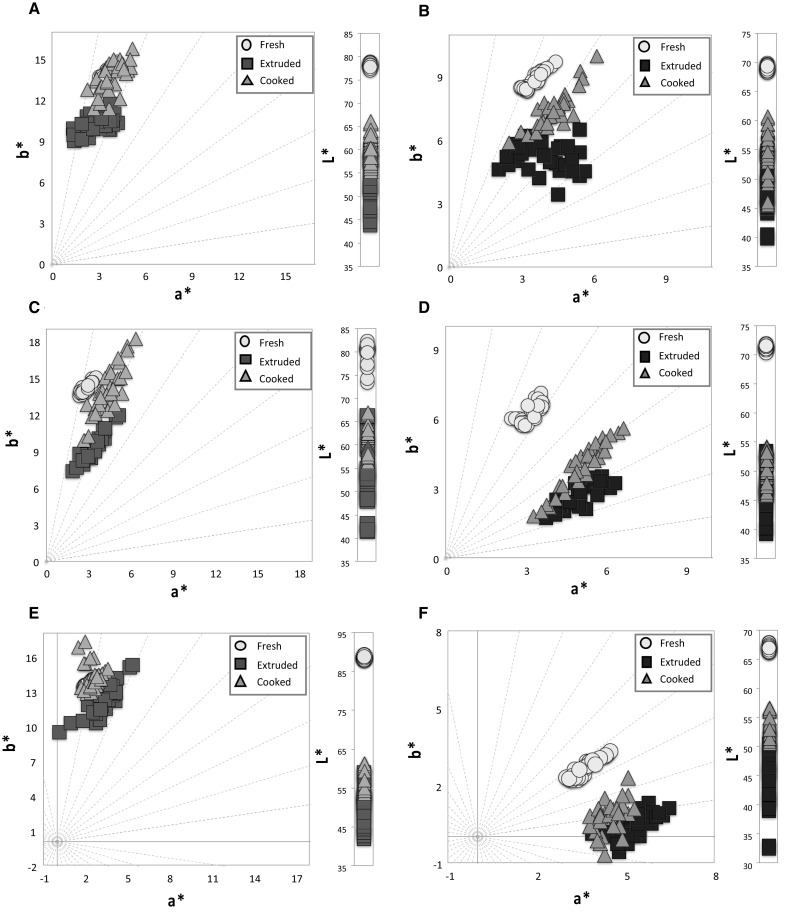
CIELAB color space (a*b*)-plane and lightness (L*) of gluten-free pastas prepared with white and blue maize flours at 25% (a, b), 50% (c, d) and 75% (e, f) respectively, at different processing stages
In dry pasta (uncooked), color is an important quality parameter from the consumer’s point of view. Recently, the market for functional pasta, prepared with diverse gluten-free ingredients (grains, fruits and pigment-containing vegetables) has grown considerably (Gallegos-Infante et al. 2010) due to the potential health benefits associated with their consumption. In general, the process followed for preparing pasta with white maize flour (extrusion) and the subsequent cooking of the extruded material results in a dark-colored product; this pattern is related to Maillard reactions favored by the relatively high temperatures reached during those processes, which increase the rate of reaction between protein and carbohydrates present in the formulation. Pasta with blue maize exhibited lower lightness than the white maize-based product, and the effect of processing was not directly evident, due to the dark color associated to its anthocyanins and their oxidation derivatives formed during processing (extrusion and cooking). Additionally, the reduction observed in L* values may be explained by the production of pigments through the polyphenol oxidase–mediated browning reaction upon the phenolic compounds present in the ingredients (Khan et al. 2013; Islas-Rubio et al. 2014).
The color differences in samples containing white and blue maize flours are attributed to the original color and the presence of pigments in the blue maize. The color changes in the gluten-free pasta may not hinder their acceptability by consumers, given that pasta products with various colors (green, black, brown, etc.) are commercially available. For this reason, and considering also that sauce (e.g. tomato) is normally added before pasta consumption, the dark color of the gluten-free pasta may be only of relative importance. In addition, some consumers may accept dark pasta if it contains nutraceutical compounds associated with potential health benefits (Islas-Rubio et al. 2014).
Effect of processing on total phenolic content and antioxidant capacity
Figure 3 shows the retention index of total phenolics in during extrusion and cooking of the pastas. Samples containing 75% blue maize presented the highest total phenolic content retention after extrusion and cooking (≈ 80% and 70%, respectively). This pattern is related to the higher total phenolics content of blue maize compared to white maize, which seems to be significantly retained after extrusion. Extrusion decreased the total phenolic content of pasta between 20 and 30%, while cooking produced an additional 10% loss. It can thus be proposed that during the first thermal treatment (extrusion), where a network is produced, the interactions among the different components of the flour (proteins, starch, non-starch polysaccharides and lipids) may protect the phenolic compounds (physical barrier), as it was shown in hard-to-cook kidney beans due to reduction in the intercellular spaces which decreses the release of phenolic compounds (Parmar et al. 2017); additionally, the formation of polyphenol-protein and polyphenol-starch complexes (Bordenave et al. 2014) may produce structures with greater thermal stability. The development of such network and complexes may explain why polyphenols were not extensively degraded upon cooking. Blue maize-based pasta showed high retention of phenolic compounds after cooking; this may have potential health benefits due to the antioxidant characteristics of these natural compounds.
Fig. 3.
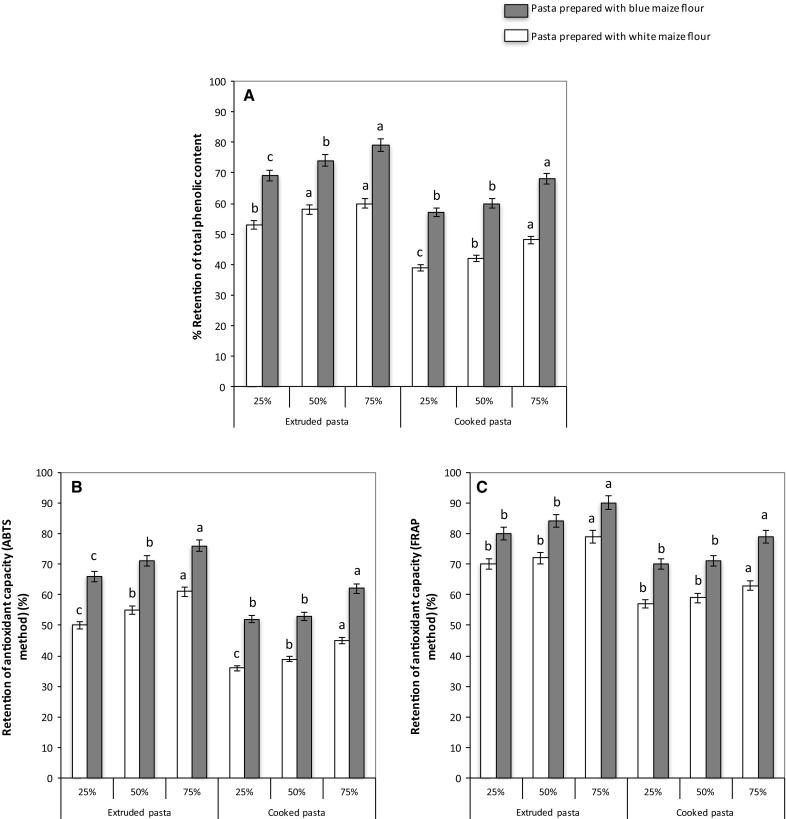
Retention (%) of total phenolic content (a) and retention (%) of antioxidant capacity (b) ABTS method and (c) FRAP of gluten-free pastas prepared with white and blue maize flours at different processing stages (extruded and cooking). Retention (%) was calculated from initial concentration in the formulations (before extrusion)
These results are in agreement with those reported by Fares et al. (2010), Verardo et al. (2011) and Khan et al. (2013), showing cooking-associated reductions in free phenolic acids, but the phenolic compounds that are retained after extrusion are bound phenolics with antioxidant capacity (Thakur et al. 2017). Unlike bound phenolic acids, free phenolic species are not physically trapped in the protein network (Naczk et al. 2011) which explains the leaching of these compounds into the cooking water. Here-recorded differences in polyphenols losses along processing between maize-containing pastas, may be due to the fact that anthocyanins interact with other macromolecules (e.g. proteins and carbohydrates) and are thus more strongly attached to the food matrix. The reduction in total phenolic content of pasta after cooking might reflect the leaching of phenolic compounds, particularly free phenolic acids and anthocyanins, into the cooking water and their concomitant thermal degradation (Khan et al. 2013).
The study of changes in antioxidant properties associated to manufacturing and preparation of processed foods, such as gluten-free pasta, has gained interest, considering the potential benefits associated to this biochemical feature. Figure 3 shows the retention of the antioxidant capacity of samples after extrusion and cooking. Overall, a higher antioxidant capacity (p < 0.05) was observed in pastas prepared with blue maize, a pattern that agrees with the phenolic compounds retention (Fig. 3). The data on antioxidant capacity retention indicates that the phenolic compounds remained after extrusion and cooking, and may thus be active in the ready-to-eat products, particularly in the blue maize pasta. Similar results were reported by Del Pozo-Insfran et al. (2007), who analyzed the phenolic content and antioxidant capacity in white and blue maize flours along the production process of tortillas and chips. Combined extrusion and cooking treatment decreased antioxidant capacity and total phenolic contents in all samples analyzed.
Pasting profile of gluten free pasta
Results of the pasting profile of the unprocessed flour blends and extruded pasta samples are depicted in Fig. 4. The raw blends showed a typical pasting profile, i.e. similar to that of isolated starch, where maximum viscosity, breakdown and setback points are evident. This pattern is due to the presence of essentially intact starch granules in the flours used in the pasta formulations. The blends with higher levels of chickpea and unripe plantain flours (Fig. 4a) showed the highest maximum peak viscosity, indicating that starch present in these flours has greater swelling properties than maize starch (Vamadevan and Bertoft 2015).
Fig. 4.
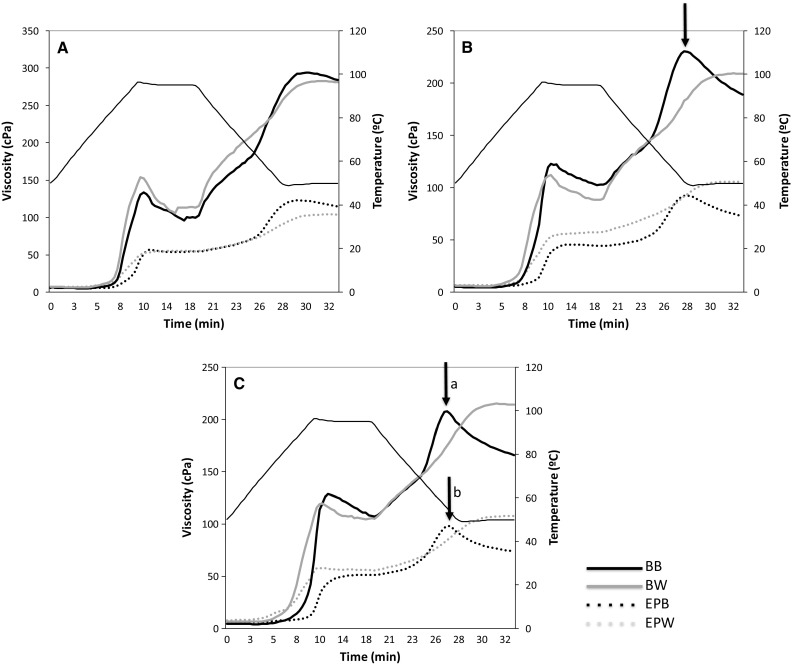
Pasting properties of blends and extruded gluten free pasta prepared with maize at 25% (a), 50% (b) and 75% (c)
The blends with high blue maize content (50 and 75%) presented a defined second viscosity peak (see arrow in Fig. 4b), which suggests that the starch granules (ghosts) and lineal alpha-glucan chains dispersed during heating undergo structural re-arrangement upon cooling (Vamadevan and Bertoft 2015). Rheological studies of starch isolated from field pea showed two peaks during heating, like to the pattern found in the blends with the highest blue maize level before extrusion; this pattern was attributed to swelling of the granules and formation of a network of swollen granules, which agrees with the marked retrogradation level observed (Singh et al. 2008). This pasting profile indicates the effect of blue maize components that are not present in white maize (mainly anthocyanins), which may explain some differences in cooking quality and texture characteristics among the pastas. Although the particle size of the flours was similar (0.038 mm), the composition (i.e. relative content of starch, proteins and non-starch polysaccharides) of each raw material has influence on the pasting profile (Thakur et al. 2015).
Extruded pasta samples showed lower peak viscosity than their raw counterparts, a behavior that may reflect restricted swelling due to starch disorganization during extrusion. The maximum peak viscosity was maintained during heating and the first phase of cooling, increasing in the late cooling step. Comparison of white and blue maize pasta evidenced higher peak and final viscosity for the former, but blue maize pasta showed a less intense second viscosity peak upon cooling than the white maize product (see arrows a and b in Fig. 4c), suggesting differences in the networks produced in the two pastas. This pattern may be related to the retention of total phenolics. However, further studies are necessary for better understanding of this phenomenon.
Conclusion
The use of extrusion for producing blue maize-containing pasta and its subsequent cooking induced a moderate reduction of the total phenolic content and antioxidant capacity, suggesting that the network produced along processing protected the phenolic compounds against thermal degradation. In this sense, gluten-free pasta containing blue maize flour has potential nutraceutical effect. The presence of an appreciable physical network in these composite gluten-free pasta products was evidenced by the pasting profile. The cooking quality (assessed as cooking loss) and texture characteristics of pasta with blue maize suggest that this ingredient can replace the role of gluten in pasta products.
Acknowledgements
The authors thank the support from CONACYT, México, SIP-IPN, COFAA-IPN, and EDI-IPN. One of the authors (GACM) also acknowledges a scholarship from CONACYT, Mexico.
References
- AACC (2000). Approved methods of the American Association of Cereal Chemists, 10th ed. (vols 1 and 2)
- Aguayo-Rojas J, Mora-Rochín S, Cuevas-Rodríguez EO, Serna-Saldivar SO, Gutierrez-Uribe JA, Reyes-Moreno C, Milán-Carrillo J. Phytochemicals and antioxidant capacity of tortillas obtained after lime-cooking extrusion process of whole pigmented Mexican maize. Plant Foods Hum Nutr. 2012;67:178–185. doi: 10.1007/s11130-012-0288-y. [DOI] [PubMed] [Google Scholar]
- Bello-Pérez LA, Flores-Silva PC, Camelo-Mendez GA. Non-conventional flours in the development of gluten-free food products. In: Langdon RT, editor. Gluten-free diets: food sources, role in celiac disease and health benefits. New York: Nova Publishers; 2014. pp. 223–237. [Google Scholar]
- Bello-Pérez LA, Flores-Silva PC, Camelo-Méndez GA, Paredes-López O, Figueroa-Cárdenas JD. Effect of the nixtamalization process on the dietary fiber content, starch digestibility, and antioxidant capacity of blue maize tortilla. Cereal Chem. 2015;92(3):265–270. doi: 10.1094/CCHEM-06-14-0139-R. [DOI] [Google Scholar]
- Bello-Pérez LA, Camelo-Méndez GA, Agama-Acevedo E, Utrilla-Coello RG. Nutraceutic aspects of pigmented maize: digestibility of carbohydrates and anthocyanins. Agrociencia. 2016;50:1041–1063. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bordenave N, Hamaker BR, Ferruzzi MG. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014;5:18–34. doi: 10.1039/C3FO60263J. [DOI] [PubMed] [Google Scholar]
- Camelo-Méndez GA, Agama-Acevedo E, Tovar J, Bello-Pérez LA. Functional characterization of raw and cooked blue maize flour: starch digestibility, total phenolic content and antioxidant activity. J Cereal Sci. 2017;76:179–185. doi: 10.1016/j.jcs.2017.06.009. [DOI] [Google Scholar]
- Chávez-Salazar A, Bello-Pérez LA, Agama-Acevedo E, Castellanos-Galeano FJ, Álvarez-Barreto CI, Pacheco-Vargas G. Isolation and partial characterization of starch from banana cultivars grown in Colombia. Int J Biol Macromol. 2017;98:240–246. doi: 10.1016/j.ijbiomac.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Del Pozo-Insfran D, Serna-Saldivar SO, Brenes CH, Talcott ST. Polyphenolics and antioxidant capacity of white and blue corns processed into tortillas and chips. Cereal Chem. 2007;84:162–168. doi: 10.1094/CCHEM-84-2-0162. [DOI] [Google Scholar]
- Fares C, Platani C, Baiano A, Menga V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010;119(3):1023–1029. doi: 10.1016/j.foodchem.2009.08.006. [DOI] [Google Scholar]
- Flores-Silva PC, Berrios JDJ, Pan J, Osorio-Díaz P, Bello-Pérez LA. Gluten-free spaghetti made with chickpea, unripe plantain and maize flours: functional and chemical properties and starch digestibility. Int J Food Sci Tech. 2014;49(9):1985–1991. doi: 10.1111/ijfs.12529. [DOI] [Google Scholar]
- Flores-Silva PC, Berrios JDJ, Pan J, Agama-Acevedo E, Monsalve-González A, Bello-Pérez LA. Gluten-free spaghetti with unripe plantain, chickpea and maize: physicochemical, texture and sensory properties. CYTA-J Food. 2015;13(2):159–166. doi: 10.1080/19476337.2014.929178. [DOI] [Google Scholar]
- Fu BX. Asian noodles: history, classification, raw materials, and processing. Food Res Int. 2008;41:888–902. doi: 10.1016/j.foodres.2007.11.007. [DOI] [Google Scholar]
- Gallegos-Infante JA, Rocha-Guzman NE, Gonzalez-Laredo FR, Ochoa-Martínez LA, Corzo N, Bello-Perez LA, Medina-Torres L, Peralta-Alvarez LE. Quality of spaghetti pasta containing Mexican common bean flour (Phaseolus vulgaris L.) Food Chem. 2010;119:1544–1549. doi: 10.1016/j.foodchem.2009.09.040. [DOI] [Google Scholar]
- Islas-Rubio AR, Calderón de la Barca AM, Cabrera-Chávez F, Cota-Gastélum AG, Beta T. Effect of semolina replacement with a raw: popped amaranth flour blend on cooking quality and texture of pasta. LWT Food Sci Technol. 2014;57(1):217–222. doi: 10.1016/j.lwt.2014.01.014. [DOI] [Google Scholar]
- Khan I, Yousif A, Johnson SK, Gamlath S. Effect of sorghum flour addition on resistant starch content, phenolic profile and antioxidant capacity of durum wheat pasta. Food Res Int. 2013;54:578–586. doi: 10.1016/j.foodres.2013.07.059. [DOI] [Google Scholar]
- Khan I, Yousif AM, Johnson SK, Gamlath S. Effect of sorghum flour addition on in vitro starch digestibility, cooking quality, and consumer acceptability of durum wheat pasta. J Food Sci. 2014;79(8):S1560–S1567. doi: 10.1111/1750-3841.12542. [DOI] [PubMed] [Google Scholar]
- Liu L, Herald TJ, Wang D, Wilson JD, Bean SR, Aramouni FM. Characterization of sorghum grain and evaluation of sorghum flour in a chinese egg noodle system. J Cereal Sci. 2012;55(1):31–36. doi: 10.1016/j.jcs.2011.09.007. [DOI] [Google Scholar]
- Naczk M, Towsend M, Zadernowski R, Shahidi F. Protein-binding and antioxidant potential of phenolics of mangosteen fruit (Garcinia mangostana) Food Chem. 2011;128(2):292–298. doi: 10.1016/j.foodchem.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Ovando-Martinez M, Sáyago-Ayerdi S, Agama-Acevedo E, Goñi I, Bello-Pérez LA. Unripe banana flour as an ingredient to increase the undigestible carbohydrates of pasta. Food Chem. 2009;113:121–126. doi: 10.1016/j.foodchem.2008.07.035. [DOI] [Google Scholar]
- Palavecino PM, Bustos MC, Heinzmann Alabí MB, Nicolazzi MS, Penci MC, Ribotta PD. Effect of ingredients on the quality of gluten-free sorghum pasta. J Food Sci. 2017;82(9):2085–2093. doi: 10.1111/1750-3841.13821. [DOI] [PubMed] [Google Scholar]
- Parmar N, Singh N, Kaur A, Thakur S. Comparison of color, antinutritional factors, mineral, phenolic profile and protein digestibility between hard-to-cook and easy-to-cook grains from different kidneay bean (Phaseolus vulgaris) accessions. J Food Sci Technol. 2017;54(4):1023–1034. doi: 10.1007/s13197-017-2538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitot M, Boyer L, Minier C, Micard V. Fortification of pasta with split pea and faba bean flours: pasta processing and quality evaluation. Food Res Int. 2010;43:634–641. doi: 10.1016/j.foodres.2009.07.020. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Singh N, Nakura Y, Inouchi N, Nishinari K. Structure and viscoelastic properties of starches separated from different legumes. Starch/Stärke. 2008;60:349–357. doi: 10.1002/star.200800689. [DOI] [Google Scholar]
- Sözer N, Kaya A. Changes in cooking and textural properties of spaghetti cooked with different levels of salt in the cooking water. J Texture Stud. 2003;34(4):381–390. doi: 10.1111/j.1745-4603.2003.tb01070.x. [DOI] [Google Scholar]
- Sumczynski D, Kotásková E, Družbíková H, Mlček J. Determination of contents and antioxidant activity of free and bound phenolics compounds and in vitro digestibility of commercial black and red rice (Oryza sativa L.) varieties. Food Chem. 2016;211:339–346. doi: 10.1016/j.foodchem.2016.05.081. [DOI] [PubMed] [Google Scholar]
- Thakur S, Kaur A, Singh N, Virdi AS. Successive reduction dry milling of normal and waxy corn: grain, grift, and flour properties. J Food Sci. 2015;80(6):C1144–C1155. doi: 10.1111/1750-3841.12895. [DOI] [PubMed] [Google Scholar]
- Thakur S, Singh N, Kaur A, Singh B. Effect of extrusion on physicochemical properties, digestibility, and phenolic profiles of grift fractions obtained from dry millingof normal and waxy corn. J Food Sci. 2017;82(5):1101–1109. doi: 10.1111/1750-3841.13692. [DOI] [PubMed] [Google Scholar]
- Tudoricǎ CM, Kuri V, Brennan CS. Nutritional and physicochemical characteristics of dietary fiber enriched pasta. J Agric Food Chem. 2002;50(2):347–356. doi: 10.1021/jf0106953. [DOI] [PubMed] [Google Scholar]
- Urias-Lugo DA, Heredia JB, Muy-Rangel MD, Valdez-Torres JB, Serna-Saldívar SO, Gutiérrez-Uribe JA. Anthocyanins and phenolic acids of hybrid and native blue maize (Zea mays L.) extracts and their antiproliferative activity in mammary (MCF7), liver (HepG2), colon (Caco2 and HT29) and prostate (PC3) Cancer Cells. Plant Foods Hum Nutr. 2015;70:193–199. doi: 10.1007/s11130-015-0479-4. [DOI] [PubMed] [Google Scholar]
- Vamadevan V, Bertoft E. Structure-function relationships of starch components. Starch Stärke. 2015;67(1–2):55–68. doi: 10.1002/star.201400188. [DOI] [Google Scholar]
- Verardo V, Arraez-Roman D, Segura-Carretero A, Marconi E, Fernandez-Gutierrez A, Caboni MF. Determination of free and bound phenolic compounds in buckwheat spaghetti by RP-HPLC–ESI-TOF-MS: effect of thermal processing from farm to fork. J Agric Food Chem. 2011;59(14):7700–7707. doi: 10.1021/jf201069k. [DOI] [PubMed] [Google Scholar]



