Abstract
The effect of maturity level on fruit quality properties, volatile composition and sensory attributes was investigated in two important apricot varieties (Hacıhaliloğlu and Kabaaşı). The soluble solid content was used as the maturity index for the classification of apricots according to their maturity levels as immature (14–20 °Brix), mature (20–24 °Brix) and over-mature (> 24°Brix). Changes in the volatile composition of samples at different maturity levels were characterized using headspace solid phase micro-extraction gas chromatography–mass spectrometry. The results showed that the quality attributes of the Kabaaşı were affected to a lesser extent by the maturity level than Hacıhaliloğlu. From the immature to over-mature, fruit weight, dry matter and pH increased while firmness and titratable acidity decreased (p < 0.05) in both varieties. Volatile composition was affected by both apricot variety and maturation levels. The main volatiles were aldehydes, alcohols, ketones, esters, terpenes and hydrocarbons. Compared to Kabaaşı, the concentrations of the volatile compounds were higher in Hacıhaliloğlu regardless of the maturity levels. Among the samples, Hacıhaliloğlu at over-mature level received the highest “overall liking” score. The principal component analysis made on the measured quality attributes allowed the discrimination of apricot varieties and their maturity levels.
Keywords: Apricot, Maturity, Volatile, Quality, Sensory, PCA
Introduction
The amount of apricots produced in the Malatya province of Turkey constitutes approximately 20% of the total global production, which makes Turkey the largest apricot producing country in the world (FAO 2013). Apricots from the Malatya province distinguish themselves from other apricot cultivars with their higher dry matter (DM) and sugar contents (Akin et al. 2008). Among several apricot varieties cultivated in Malatya province, ‘Hacıhaliloğlu’ and ‘Kabaaşı’ are the most widespread varieties, which are well adapted to the local climate (Durmaz et al. 2010). Hacıhaliloğlu is the variety of choice in the region due to its superior sensorial quality and accounts for 70% of the total production. Kabaaşı is mainly preferred due to its late blooming property, which allows it to escape from the risk of frost-bite.
Malatya apricots are mainly used for dried apricot production and in order to maximize the yield after drying, fruits are harvested when they have soluble solid content (SSC) in the range of 20–24 °Brix (Akin et al. 2008). Fruits having SSC higher than 24 °Brix are not preferred for drying because they tend to release their juice during drying which forms an undesirable sticky surface causing problem of clumping during storage. Therefore, the SSC is the major criterion used in practice for the determination of the maturity level of these varieties in Malatya province. However, there is no evidence that optimum harvest maturity for drying is also ideal for the fruits destined to fresh consumption.
In fresh fruit marketing, appearance (color, size, severity of defects), flavor (taste and aroma), texture and nutritional value are the major attributes which affect the consumers’ decision (Barrett et al. 2010). Regarding the flavor perception of fruits, both non-volatile (sugars and organic acids) and volatile compounds play a major role. Ethyl acetate, hexanal, (E)-2-hexenal, hexyl acetate, limonene, 6-methyl-5-hepten-2-one, linalool, menthone, geraniol, β-ionone and γ-decalactone were identified as the major volatile compounds related to characteristic apricot aroma (Greger and Schieberle 2007; Guillot et al. 2006; Takeoka et al. 1990).
The composition of the volatile constituents of apricots is largely dependent on the cultivar (Guillot et al. 2006). Previously, we have found significant differences in the composition and content of individual volatile compounds identified in ‘Hacıhaliloğlu’ and ‘Kabaaşı’ apricots harvested at horticultural maturity stage (Gokbulut and Karabulut 2012). The composition of the volatile constituents of apricots may exhibit important changes during fruit development as well. In a study carried out on 28 French varieties, it was found that the levels of volatiles increased greatly during postharvest ripening (Aubert and Chanforan 2007; Goliáš et al. 2011; Xi et al. 2016). Therefore, in order to determine the optimum harvest maturity for Malatya apricots destined to fresh consumption aroma profile and sensory attributes of the fruits at different maturity stages should be characterized on a cultivar specific basis. However, to the best of our knowledge, such a study has not been carried out yet for ‘Hacıhaliloğlu’ and ‘Kabaaşı’ apricots.
Therefore in the present study, we investigated the effect of maturity level on the physiochemical and sensorial quality properties and volatile composition of the two commercially important apricot varieties (Hacıhaliloğlu and Kabaaşı) cultivated in Malatya province. By this way, it was aimed at determining the optimum harvest maturity for these varieties for the fresh consumption.
Materials and methods
Chemicals
All chemicals standards and solvents were purchased from Sigma-Aldrich (Steinheim, Germany) unless otherwise specified.
Fruit samples
The fruits of two commercially important apricot cultivars, namely Hacıhaliloğlu and Kabaaşı, were obtained from the Malatya Apricot Research Institute orchard (38°19′24.28″ N latitude, and 38 17′07.09″ E longitude.) on July 2016. The fruits were hand-picked and immediately transported to the laboratory. Upon arrival to the laboratory, apricots were classified into three maturity classes on the basis of their SSC, which is the current approach used in practice in Malatya province. According to this approach, apricots were classified as immature (IM), mature (M) and overmature (OM) having SSC in the range of 14–20, 20–24 and > 24 °Brix, respectively. The SSC of the fruits were estimated by a FT-NIR spectrometer (MPA multipurpose FT-NIR, Bruker, Ettlingen, Germany). The advantage of using FT-NIR spectroscopy was that it enables non-destructive and rapid measurement of the apricot SSC. FT-NIR based measurement also allowed us to classify high number of fruits (200 fruit for each maturity level which makes 600 in total) rapidly within 3 h. For doing so we used an in-house predictive PLS-R model (n = 718, R2p = 0.861, RMSEP = 1.72 °Brix, RPD = 2.68) which relate SSC and NIR spectrum of the fruits. For each maturity level, at least 50 apricots were frozen at − 80 °C in an ultra low temperature laboratory freezer (NuAire, Minnesote, USA) for aroma analysis, while fresh fruits were used for quality analysis and sensory evaluation.
Fruit quality indices
Fruit weight (g) of apricot was measured in 10 randomly selected fruits. Fruit firmness was measured on opposite sides of each fruit without peeling by a handheld firmness tester (Agrosta®100 Touchscreen, Apollinaire Ltd., Serqueux, France). The instrument is equipped with a 10 mm cylindrical needle tip connected to a pressure sensor which measures the compression force when the tip was pressed manually towards the fruit. The mean of ten replicates (on two sides of one fruit) was reported as kg/cm2. For DM, the samples were dried at 70 °C under vacuum (0.07–0.08 MPa) until they reached a constant weight. The final and initial weight differences were used to calculate the DM. SSC was measured as °Brix by placing a small amount of apricot juice in a handheld refractometer (Atago, Tokyo, Japan). The pH of the apricot juice was measured by a pH meter (Thermo Fisher Scientific Inc., Germany). Titratable acidity (TA) was determined by titration of 10 ml apricot juice to pH 8.1 with 0.1 N NaOH and expressed as percentage of malic acid. DM, SSC, pH and TA analyses were carried out in triplicate. Color values on the peel of apricot samples were measured with a Minolta CM-700d spectrophotometer (Minolta, New Jersey, USA). The measurements were displayed in L*, a*, and b* values which represents light–dark spectrum with a range from 0 (black) to 100 (white), the green–red spectrum with a range from − 60 (green) to +60 (red), and the blue–yellow spectrum with a range from − 60 (blue) to + 60 (yellow) dimensions, respectively. Color of central region on both sides of ten apricots was measured for each treatment and average values were reported.
Extraction of volatiles
All of the frozen apricots were thawed at 4 °C and used for volatile analysis. The stone was discarded and the pulp was cut into cubic pieces. Apricot puree was obtained using a Warring blender (Model HGB2WTS3, Connecticut, USA) at room temperature for 5 min. Volatile compounds were extracted according to the procedure described by Gokbulut and Karabulut (2012). In brief, a total of 5 g of puree and 10 µL of internal standard solution (2-methyl-3-heptanone) were placed into a 15 ml headspace vial, sealed with a septum and an aluminum cap. Carboxen–polydimethylsiloxane (CAR/PDMS, 75 µm) fibre with manual holder from Supelco (Bellefonte, PA, USA) was used for the extraction of volatiles. The fibre was preconditioned before the analyses, according to the instructions of the manufacturer. For extraction of volatiles, the solid phase micro extraction (SPME) fibre was exposed to the headspace of the vial containing apricot puree and the volatiles were extracted onto fiber at 40 °C for 30 min. For thermal desorption the SPME fibre was immediately inserted into the gas chromatograph (GC) injection port. A desorption time of 3 min at 250 °C was used in splitless mode.
GC–MS conditions
Chromatographic analysis was performed in a GC (Shimadzu GC–2010)-MS (Shimadzu QP–2010) system fitted with a ZB-Wax (60 m, 0.25 mm inner diameter, 0.25 µm film thickness; Phenomenex, USA). The column temperature was held at 40 °C for 2 min and increased to 240 °C for 6 min at the rate of 5 °C/min. Helium was used as a carrier gas at flow rate of 1 ml/min. The mass detector operated in electron impact (EI)-mode at 70 eV in a range of 15–210 amu. The tentative identifications of the volatile compounds were achieved by retention indices (RI), comparing mass spectra of unknown compounds with those in Wiley 7 (7th edition) and NIST/EPA/NIH 02 mass spectral library. The identification of the volatile compounds was performed by calculation of RIs of each compound by using n-alkane series from C8 to C20 (Sigma-04070) under the same conditions The RI values were also compared with those described in literature determined under the same conditions. Semi-quantitative analysis was performed based on comparison of individual peak areas to that of internal standard on a GC–MS total ion chromatogram. Each sample was analyzed in triplicate.
Sensory evaluation
Sensory evaluation of fresh Hacıhaliloğlu and Kabaaşı fruits at three maturity levels was performed by 10 trained panelists during two different sessions. Panelists were asked to evaluate the colour intensity (1 = greenish-yellow, 9 = commercial colour), sweetness (1 = none, 9 = very sweet), sourness (1 = not sour, 9 = very sour), aroma (1 = none, 9 = much), flavour intensity (1 = none, 9 = much) and overall liking (1 = unacceptable, 9 = acceptable) by using 9 point hedonic scale. For each panelist, 3 whole apricot fruits from each maturity level were given for the evaluation. Data are expressed as mean ± SD (n = 10 well-trained panelists).
Statistical analysis
The effect of maturity levels on quantitative data were analyzed by analysis of variance (ANOVA).Regarding the sensory data, which do not show normal distribution according to Shapiro–Wilk test, group differences were analyzed by Kruskal–wallis test and Dunn’s multiple-comparison procedure was used as a tool for comparisons of means at a level of p < 0.05 using the SPSS package program version 16.0 (SPSS Inc., Chicago, IL, USA) and XLStat version 2014.1.01 (Addinsoft, Paris, France).
For the principal component analysis (PCA) 18 observations (apricot samples) and 55 variables were used in total. Prior to analysis, data was normalized to unit variance by subtracting the mean from each value in a group and dividing it by the standard deviation. By this way, the scale effect due to different units of variables was eliminated, which might otherwise could bias the results by increasing the contribution of the variables with high unit values on the explained variance. PCA was made by using XLStat software, version 2010.2.02 (Addinsoft, Paris, France).
Results and discussion
Fruit quality indices
The average fruit weight, firmness, DM, SSC, pH, TA and color values of the apricots at three maturity levels were given in Table 1. As described in material and method section, SSC of the fruits was used as the criterion for the determination of the maturity levels. Regarding Hacıhaliloğlu variety, significant differences (p < 0.05) were observed in fruit weight, firmness, DM and pH of the fruits of different maturity levels. For Kabaaşı variety, however, the differences in fruit weight and firmness of the fruits at different maturity levels were not significant (p > 0.05). For Hacıhaliloğlu, as expected, TA values were higher at IM (p < 0.05) than those of other maturity levels, whereas for Kabaaşı TA is at its highest value at M level. The decrease in TA during fruit ripening can be attributed to the utilization of organic acids as a substrate for respiration and production of the flavor compounds (Sangkasanya et al. 2014), which also results in an increase in pH. Kabaaşı distinguishes itself from Hacıhaliloğlu also with its peel color. As a yellow apricot variety, the green peel color of Hacıhaliloğlu at IM level turns gradually to vivid yellow as fruits get ripened and occasionally small red blushed areas can also be observed. On the other hand, the green peel color of the Kabaaşı apricot at IM level does not disappear completely at M and OM levels but turn to greenish-yellow with large red blushed areas. The objective color measurement results given in Table 1 also confirm these observations. As can be clearly seen from the table, L* and b* values were higher in Hacıhaliloğlu than Kabaaşı apricots at M and OM levels. On the other hand a* value of the Kabaaşı apricots were significantly higher than that of Hacıhaliloğlu regardless of the maturity level. The lower L* value reflects the darkening of the apricot varieties by carotenoid accumulation (Ruiz et al. 2005). This is in accordance with our previous findings, where we found that total carotenoid content of Kabaaşı was three times greater than that of Hacıhaliloğlu (Akin et al. 2008).
Table 1.
Changes in the quality indices of two apricot varieties over the maturity levels
| Hacıhaliloğlu | Kabaaşı | |||||
|---|---|---|---|---|---|---|
| Immature | Mature | Over-mature | Immature | Mature | Over-mature | |
| Weight (g) | 23.52 ± 1.10c | 29.42 ± 0.82b | 32.42 ± 1.33ab | 31.27 ± 1.40ab | 34.82 ± 3.82a | 34.38 ± 0.99a |
| Firmness (kg/cm2) | 8.69 ± 0.12a | 7.73 ± 0.88b | 3.83 ± 0.85d | 8.91 ± 0.14a | 8.72 ± 0.16a | 5.96 ± 0.26c |
| DM (%) | 18.34 ± 0.38f | 25.25 ± 0.20c | 30.89 ± 0.07a | 19.31 ± 0.19e | 23.68 ± 0.12d | 27.83 ± 0.32b |
| SSC ( °Brix) | 16.53 ± 0.12b | 23.50 ± 0.10c | 27.63 ± 0.06a | 18.73 ± 0.12e | 22.60 ± 0.20d | 26.20 ± 0.20b |
| pH | 4.82 ± 0.03e | 5.25 ± 0.02b | 5.32 ± 0.02a | 5.07 ± 0.01d | 5.14 ± 0.01c | 5.21 ± 0.04b |
| TA (%) | 0.38 ± 0.01a | 0.20 ± 0.01e | 0.29 ± 0.01b | 0.23 ± 0.20d | 0.26 ± 0.01c | 0.22 ± 0.01de |
| L* | 69.26 ± 0.88c | 68.09 ± 2.75c | 64.77 ± 2.39bc | 62.12 ± 2.46b | 65.55 ± 2.89bc | 55.98 ± 3.21a |
| a* | 4.57 ± 3.45a | 14.26 ± 1.47bc | 12.50 ± 0.91b | 22.15 ± 3.98de | 18.23 ± 0.78 cd | 22.74 ± 0.82e |
| b* | 44.41 ± 0.68a | 44.81 ± 1.92a | 43.29 ± 3.97a | 37.98 ± 1.26b | 40.58 ± 2.22ab | 32.31 ± 3.41c |
Data are expressed as mean ± SD
Mean ± SD followed by the same letter, within a line, are not significantly different (p > 0.05)
DM dry matter, SSC soluble solid content, TA titratable acidity
Volatile compounds
Table 2 shows the volatile aroma compounds of apricot samples at different maturity levels. In total, 38 volatile compounds were identified in various concentrations and they were grouped into 6 main groups according to their functional groups; (i) esters (11 compounds), (ii) alcohols (9 compounds), (iii) aldehydes (9 compounds), (iv) hydrocarbons (4 compounds), (v) ketones (4 compounds) and (vi) terpenes (1 compounds). We observed marked differences in the number of volatile compounds and their quantities in two apricot varieties at different maturity levels. Compared to Kabaaşı variety, the concentrations of the volatile compounds were higher in Hacıhaliloğlu variety regardless of the maturity levels.
Table 2.
Changes in the volatile compounds (µg/kg fresh weight) of two apricot varieties at different maturity levels
| Compounds | RI | Hacıhaliloğlu | Kabaaşı | ||||
|---|---|---|---|---|---|---|---|
| Immature | Mature | Over-mature | Immature | Mature | Over-mature | ||
| Esters | |||||||
| Methyl acetate | 807 | 6.64 ± 0.29a | 6.82 ± 0.68a | 6.68 ± 0.46a | 5.09 ± 1.07b | 6.26 ± 0.36ab | 5.73 ± 0.70ab |
| Ethyl acetate | 871 | 0.05 ± 0.01c | nd | 1.52 ± 0.20a | 0.36 ± 0.04b | 0.52 ± 0.09b | 0.36 ± 0.10b |
| Methyl isobutanoate | 914 | nd | 0.18 ± 0.01b | 0.19 ± 0.01b | 0.18 ± 0.07b | 0.20 ± 0.05b | 0.27 ± 0.03a |
| Propyl acetate | 969 | nd | 0.12 ± 0.00b | 0.44 ± 0.06a | nd | nd | nd |
| Methyl butanoate | 982 | nd | 0.55 ± 0.03a | 0.21 ± 0.05c | 0.48 ± 0.07a | 0.41 ± 0.05b | 0.42 ± 0.02b |
| Methyl 2-methylbutyrate | 1008 | 0.12 ± 0.01c | 0.19 ± 0.01ab | 0.21 ± 0.01a | 0.20 ± 0.02ab | 0.19 ± 0.04ab | 0.17 ± 0.02b |
| Butyl acetate | 1069 | nd | 3.38 ± 0.59b | 17.23 ± 3.25a | 0.22 ± 0.03a | 0.59 ± 0.10c | 0.84 ± 0.28c |
| Isobutyl butanoate | 1087 | nd | 0.09 ± 0.01c | nd | 0.15 ± 0.01a | 0.11 ± 0.01b | 0.13 ± 0.01a |
| Butyl butylate | 1214 | 7.35 ± 0.40a | 0.22 ± 0.03c | 0.52 ± 0.16b | nd | nd | nd |
| Hexyl acetate | 1272 | 0.17 ± 0.04 cd | 0.47 ± 0.08b | 1.94 ± 0.41a | 0.34 ± 0.07c | nd | 0.09 ± 0.03d |
| 3-Hexen-1-ol acetate | 1320 | 0.50 ± 0.09b | 0.12 ± 0.02c | nd | 1.36 ± 0.31a | nd | nd |
| Alcohols | |||||||
| 2-Propanol | 921 | 0.17 ± 0.02c | 0.23 ± 0.05b | 0.40 ± 0.02a | 0.11 ± 0.02d | nd | nd |
| Ethanol | 930 | 0.41 ± 0.08c | 1.97 ± 0.30c | 10.76 ± 1.82a | 2.65 ± 0.52cb | 4.03 ± 1.12b | 4.49 ± 1.81b |
| 1-Hepthanol | 1037 | 0.09 ± 0.02b | 0.16 ± 0.03a | nd | 0.10 ± 0.01b | nd | 0.14 ± 0.01a |
| 1-Butanol | 1154 | nd | nd | 1.15 ± 0.24a | nd | nd | 0.25 ± 0.02b |
| 1-Hexanol | 1356 | 17.92 ± 3.97a | 8.20 ± 1.02c | 13.47 ± 2.87b | 1.71 ± 0.29d | 2.66 ± 0.54d | 3.14 ± 0.24d |
| 3-Hexen-1-ol | 1316 | 0.18 ± 0.03c | 0.30 ± 0.05b | 0.39 ± 0.09a | 0.21 ± 0.03c | nd | nd |
| (E)-2-Hexen-1-ol. | 1410 | 15.35 ± 2.68a | 3.70 ± 0.46c | 7.88 ± 0.51b | 1.04 ± 0.16d | 1.12 ± 0.23d | 1.47 ± 0.03d |
| 2-Ethylhexanol | 1491 | 0.46 ± 0.08ab | 0.43 ± 0.02b | 0.44 ± 0.08ab | 0.49 ± 0.15ab | 0.65 ± 0.18ab | 0.66 ± 0.11a |
| Linalool-L | 1547 | 0.31 ± 0.08a | 0.17 ± 0.02b | 0.26 ± 0.06ab | 0.21 ± 0.05ab | 0.15 ± 0.03b | 0.21 ± 0.07ab |
| Aldehydes | |||||||
| Acetaldehyde | 721 | 5.91 ± 0.68d | 15.58 ± 3.08c | 23.20 ± 2.16b | 10.70 ± 2.85 cd | 25.66 ± 7.27ab | 31.12 ± 2.96a |
| Butanal | 858 | 0.26 ± 0.05c | 0.44 ± 0.03b | 0.55 ± 0.03a | nd | nd | nd |
| 2-Methylbutanal | 906 | 0.22 ± 0.05c | 0.26 ± 0.02c | 0.17 ± 0.01c | 1.00 ± 0.39a | 0.39 ± 0.09bc | 0.75 ± 0.37ab |
| 3-Methylbutanal | 910 | 4.91 ± 0.63b | 0.80 ± 0.12c | 1.24 ± 0.12a | 4.49 ± 1.32b | 7.89 ± 2.02a | 4.67 ± 2.11b |
| Hexanal | 1080 | 158.92 ± 32.72a | 43.68 ± 4.21b | 39.37 ± 9.59bc | 17.08 ± 1.79 cd | nd | 5.85 ± 0.34d |
| (Z)-3-Hexenal | 1135 | 1.44 ± 0.25a | 0.36 ± 0.06b | 0.27 ± 0.01b | nd | nd | nd |
| (E)-2-Hexenal | 1225 | 288.26 ± 56.74a | 95.23 ± 12.90b | 75.86 ± 5.27bc | 39.56 ± 4.56 cd | 13.94 ± 2.78d | 15.53 ± 1.02d |
| 2,4-Hexanedial | 1413 | 0.18 ± 0.03a | 0.17 ± 0.04a | nd | nd | nd | nd |
| 2,4-Heptadienal | 1474 | 0.41 ± 0.06a | 0.14 ± 0.02bc | 0.17 ± 0.05b | nd | 0.08 ± 0.04 cd | 0.06 ± 0.02d |
| Hydrocarbons | |||||||
| 1,1-Dimethoxypropane | 859 | nd | nd | nd | 0.21 ± 0.07a | 0.26 ± 0.08a | 0.29 ± 0.07a |
| Decane | 993 | 2.21 ± 0.27b | 2.56 ± 0.34ab | 2.93 ± 0.12a | 2.15 ± 0.07b | 2.57 ± 0.38ab | 2.70 ± 0.68ab |
| Pentadecane | 1059 | nd | 0.11 ± 0.01b | nd | 0.10 ± 0.01b | 0.11 ± 0.02b | 0.13 ± 0.00a |
| Undecane | 1084 | nd | 1.22 ± 0.06a | 1.22 ± 0.26a | 0.65 ± 0.16b | 0.74 ± 0.12b | 0.75 ± 0.15b |
| Ketones | |||||||
| 2-Propanone | 795 | 4.21 ± 0.76bc | 3.74 ± 0.86c | 8.78 ± 1.71a | 3.95 ± 1.11bc | 6.51 ± 1.69ab | 4.29 ± 1.62bc |
| 6-Methyl-5-hepten-2-one | 1344 | 0.10 ± 0.02a | 0.11 ± 0.02a | 0.15 ± 0.03a | 0.11 ± 0.03a | 0.12 ± 0.03a | 0.13 ± 0.03a |
| Β-ionone | 1669 | nd | 1.25 ± 0.23b | 2.93 ± 0.36a | nd | 0.36 ± 0.08c | 0.14 ± 0.01d |
| Dihydro-β-ionone | 1855 | 0.62 ± 0.13c | 5.74 ± 1.28b | 9.33 ± 1.32a | nd | 1.09 ± 0.22c | 1.21 ± 0.07c |
| Terpenes | |||||||
| I-Limonene | 1196 | 0.77 ± 0.10b | 0.94 ± 0.15ab | 1.05 ± 0.14a | 0.30 ± 0.06c | 0.40 ± 0.20c | 0.39 ± 0.04c |
Data are expressed as mean ± SD
Mean ± SD followed by the same letter, within a row, are not significantly different (p > 0.05)
nd not detected
Several forms of acetates were identified. In Hacıhaliloğlu apricots, except for methyl acetate and butyl butylate, they tend to increase with increasing maturity level. Compared to the other forms of acetates, the increase in butyl acetate concentration was the most marked. For Kabaaşı, on the contrary, concentrations of the acetates tend to decrease as the fruits get mature. Acetates, especially ethyl acetate and hexyl acetate, are considered as fruity odor descriptors in apricots (Guillot et al. 2006; Solís-Solís et al. 2007) and hexyl acetate was previously reported to be one of the major contributors of the apricot aroma (El Hadi et al. 2013; González-Agüero et al. 2009; Guichard and Souty 1988). Butyl acetate, 2-methylbutyl acetate and hexyl acetate are considered as characteristic apple-like aroma contributors in ripe fruits and their presence indicates that amino acids were served as substrates for acetate esters via β-oxidation (Dixon and Hewett 2000). Among several forms of esters identified in Hacıhaliloğlu variety (Table 1), butyl butylate (butyl butanoate) which possesses strong pear-like aroma (El Hadi et al. 2013) was the most abundant and its concentration decreased (p < 0.05) with increasing maturity.
In Hacıhaliloğlu variety, 1-hexanol and (E)-2-hexen-1-ol were the most abundant alcohols identified in IM fruits and their concentration showed decreasing (p < 0.05) tendency as fruits maturity level increases. These compounds were also reported as the major contributors to apricot aroma (Gokbulut and Karabulut 2012; González-Agüero et al. 2009). However, in Kabaaşı apricots, no significant differences were observed in their concentrations among fruits of different maturity levels. Regardless of the variety, ethanol was the most abundant alcohol identified in the fruits and its concentration increased with maturity. This may be due to the conversion of the aldehydes to alcohols in ripening fruit with the presence of alcohol dehydrogenase and alcohol acyl co-A reductase (González-Agüero et al. 2009).
In agreement with the results of a previous study (Gokbulut and Karabulut 2012), aldehydes including acetaldehyde, hexanal and (E)-2-hexenal were the most abundant volatile aroma compounds found in the both apricot variety regardless of the maturity level. Hexanal and (E)-2-hexenal are known as being green and grassy odor descriptors in fruits (Xi et al. 2016), while acetaldehyde, which is also suggested as an important contributor to apricot flavor (Greger and Schieberle 2007), has a pungent, solvent-like aroma (Obenland et al. 2012). In accordance with the literature (El Hadi et al. 2013; Xi et al. 2016), the concentrations of hexanal and (E)-2-hexenal were low in M and, even lower in OM fruits, while the concentration of acetaldehyde was 3 and 4 folds higher in OM than IM fruits of Kabaaşı and Hacıhaliloğlu varieties, respectively. Similar findings were reported in a study carried out with avocados (Obenland et al. 2012) and authors found that hexanal and (E)-2-hexenal were the most abundant volatile compounds present in immature avocados. González-Agüero et al. (2009) suggested that during fruit growth tissues are disrupted and the membrane lipids become more accessible to lipoxygenase enzymes which are converted into saturated and unsaturated volatile C6 aldehydes and alcohols.
We also identified 4 hydrocarbon compounds in low quantities. Among these, decane in Hacıhaliloğlu and pentadecane in Kabaaşı decreased with increasing maturity, while there were no significant differences in the concentrations of others in fruits of different maturity level. Limonene, a terpenoid hydrocarbon, was reported to be responsible for the fruity and citrus character of the fruit aroma (Guillot et al. 2006). In the present study, limonene was present in low quantities in the fruits of both varieties. However, no relation was found between the concentration of these volatiles and the fruit maturity level. In general, ketones in Hacıhaliloğlu such as 2-propanone, β-ionone and dihydro-β-ionone increased with increasing maturity. However, for Kabaaşı variety, there were no significant differences between (p > 0.05) their concentrations in the fruits at different maturity levels, except for β-ionone. 6-methyl-5-hepten-2-one and β-ionone were reported to be responsible for the floral character of the apricot aroma (Greger and Schieberle 2007; Guillot et al. 2006; Takeoka et al. 1990) and these compounds were detected previously in the same apricot varieties (Gokbulut and Karabulut 2012). According to El Hadi et al. (2013), most of the ketones such as β-ionone are synthesized from carotenoids by carotenoid cleavage dioxygenase enzymes.
Sensory evaluation
As it was presented in Table 3 the sensory evaluation results indicated that all the sensory scores except “sourness” increased with increasing maturity level. Hacıhaliloğlu variety received higher sensory scores than that of Kabaaşı at M and OM levels (p < 0.05). Among all the samples analyzed, Hacıhaliloğlu apricots at OM level received the highest “overall liking” score. However, there were no significant differences (p > 0.05) between the sensory scores of M and OM levels of Kabaaşı except “color” attribute.
Table 3.
Sensory analysis results of two apricot varieties at different maturity levels
| Sensory attributes | Hacıhaliloğlu | Kabaaşı | ||||
|---|---|---|---|---|---|---|
| Immature | Mature | Over-mature | Immature | Mature | Over-mature | |
| Colour | 2.3 ± 0.6c | 7.1 ± 1.1ab | 7.9 ± 0.9a | 4.2 ± 0.9bc | 6.8 ± 0.9abc | 7.9 ± 1.1a |
| Sweetness | 3.6 ± 0.9b | 7.7 ± 0.5a | 8.7 ± 0.4a | 3.4 ± 1.1b | 6.8 ± 1.1ab | 7.1 ± 1.1a |
| Sourness | 4.9 ± 1.1a | 1.6 ± 0.8b | 1.1 ± 0.3b | 2.3 ± 1.3ab | 1.4 ± 0.7b | 1.5 ± 0.7b |
| Aroma | 3.2 ± 0.9bc | 7.6 ± 0.6ab | 8.4 ± 0.8a | 3.4 ± 1.1c | 6.3 ± 1.0abc | 6.1 ± 1.1abc |
| Flavour | 3.6 ± 1.0c | 7.0 ± 1.0ab | 7.9 ± 1.1a | 3.0 ± 0.9c | 5.8 ± 1.1bc | 6.0 ± 1.3bc |
| Overall liking | 4.8 ± 1.0b | 7.4 ± 0.7a | 8.2 ± 0.8a | 4.8 ± 0.8b | 6.9 ± 0.8ab | 7.3 ± 0.9a |
Data are expressed as mean ± SD (n = 10 well-trained panellists)
Mean ± SD followed by the same letter, within a row, are not significantly different according to Kruskal–wallis test (p > 0.05)
PCA analysis
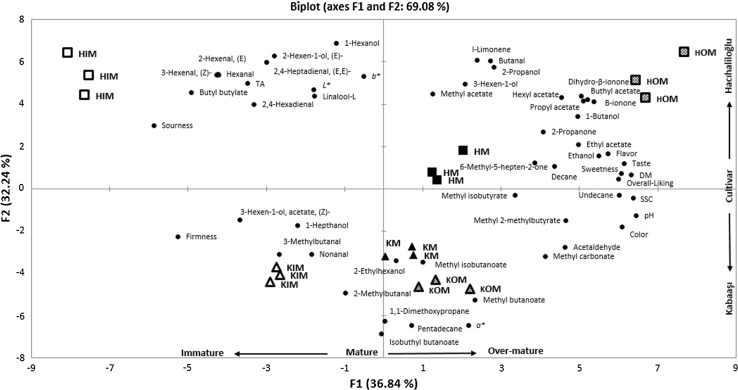
In order to better visualize the correlations between all the variables obtained from the physical, sensory and volatile compounds analysis and their relation with the type of apricot variety and maturity stage, PCA was carried out. As it can be seen from the biplot projection of the loadings and scores values of the PCA (Fig. 1), 69.08% of the total variance of the whole data set could be explained by selecting the first two principle components. The results of the PCA showed that apricots at different maturity levels clearly separated from each other on the basis of their volatile aroma compounds, sensory evaluation scores and physical quality parameters. First principal component (PC1) axis can be designated as the maturity axis, on which the maturity increases from left to right, whereas PC2 is discriminative of the variety. As it can be seen from the Fig. 1 Kabaaşı variety clustered on the lower part and Hacıhaliloğlu variety was clustered on the upper part of the PC2 axis. Regarding sensory evaluation scores “overall liking” is positively correlated with “sweetness” (r = 0.953) and negatively correlated with “sourness” (r = –0.721). As expected, sensory evaluation scores for “sweetness and sourness” were strongly correlated with SSC (r = 0.934 and r = −0.805) and TA (r = −0.435 and r = 0.754) of the apricots, respectively. For Hacıhaliloğlu variety, overall liking scores increase as the maturity changed from IM to OM. However, this tendency is less marked for Kabaaşı for which the difference between M and OM fruits sensory scores did not show any significant difference. In this regard, the apricots of Hacıhaliloğlu variety at OM level were the most appreciated by the panelists whereas the apricots of the same variety at IM were the least appreciated. Besides, it is clearly seen that apricot varieties showed distinct volatile profiles which could be used to discriminate the varieties and maturity levels. The most abundant volatile compounds discriminating OM Hacıhaliloğlu variety apricots from other groups, which had the highest sensory scores, were ethyl acetate, hexyl acetate, propyl acetate, butyl acetate, isobutyl acetate, 1-butanol, β-ionone, dihydro-β-ionone, 2,3-octanedione and undecane. These aroma compounds were either absent or less noticeable in immature fruits in which the aroma compounds associated to grassy green notes such as hexenal, (E)-2-hexenal, 3-hexenal, 2,4-hexanedial, butyl butylate and 3-butene-2-ol were prevalent. Kabaaşı variety showed completely different aroma profile compared to Hacıhaliloğlu. It is interesting to note that for Kabaaşı variety, the composition of the volatile compounds only slightly changed as fruits get mature which was also the case for the other quality parameters related to fruit maturation such as firmness and pH. Nevertheless, at IM level, nonanal and 1,8-cineole were the predominant volatile compounds whereas as fruits get mature the content of the volatile compounds such as isobutylbutonoate, pentadecane, 1,1-dimethoxypropane, methyl butanoate, 2-butanone 3 methyl increased.
Fig. 1.
Biplot of the principal component analysis carried on the physical quality parameters, sensory evaluation scores and volatile composition data of Hacıhaliloğlu and Kabaaşı apricot varieties classified as immature, mature and over-mature on the basis of soluble solid contents measured nondestructively by the FT-NIR spectroscopy technique. The variables used for the analysis were indicated in black circle and apricot samples were indicated with different symbols according to their maturity level. The first letter in the sample labels designates variety (H Hacıhaliloğlu, K Kabaaşı) and last two letters designate maturity level (IM immature, M mature, OM over-mature). Percentages in brackets correspond to the portion total variance explained by the corresponding principal component
Conclusion
The results of the present study showed that volatile composition and sensorial quality of the Hacıhaliloğlu and Kabaaşı apricots differentially evolved during maturation. For Hacıhaliloğlu variety, sensory analysis results revealed that OM fruits (> 24% Brix) were appreciated more than IM (< 20% Brix) and M fruits (20–24% Brix) due to their appealing bouquet of aroma and high degree of sweetness. Regarding Kabaaşı variety, M (20–24% Brix) and OM fruits (> 24% Brix) were equally appreciated by the panelists. Therefore it can be concluded that for Hacıhaliloğlu variety current practice of harvesting fruits having SSC in the range of 20–24 °Brix does not reflect the “optimum harvest maturity” for fresh consumption. However, for Kabaaşı variety currently used “optimum harvest maturity” criterion for selecting apricots for drying can be used for fresh consumption as well.
Acknowledgements
The authors are grateful for the financial support of the Scientific and Technological Research Council of Turkey (TUBITAK Research Project Number 112 G 018).
References
- Akin EB, Karabulut I, Topcu A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008;107:939–948. doi: 10.1016/j.foodchem.2007.08.052. [DOI] [Google Scholar]
- Aubert C, Chanforan C. Postharvest changes in physicochemical properties and volatile constituents of apricot (Prunus armeniaca L.). Characterization of 28 cultivars. J Agric Food Chem. 2007;55:3074–3082. doi: 10.1021/jf063476w. [DOI] [PubMed] [Google Scholar]
- Barrett DM, Beaulieu JC, Shewfelt R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: desirable levels, instrumental and sensory measurement, and the effects of processing. Crit Rev Food Sci. 2010;50:369–389. doi: 10.1080/10408391003626322. [DOI] [PubMed] [Google Scholar]
- Dixon J, Hewett EW. Factors affecting apple aroma/flavour volatile concentration: a review. N Z J Crop Hortic. 2000;28:155–173. doi: 10.1080/01140671.2000.9514136. [DOI] [Google Scholar]
- Durmaz G, Cam M, Kutlu T, Hisil Y. Some physical and chemical changes during fruit development of five common apricot (Prunus armeniaca L.) cultivars. Food Sci Technol Res. 2010;16:71–78. doi: 10.3136/fstr.16.71. [DOI] [Google Scholar]
- El Hadi MAM, Zhang F-J, Wu FF, Zhou C-H, Tao J. Advances in fruit aroma volatile research. Molecules. 2013;18:8200–8229. doi: 10.3390/molecules18078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2013) FAO statistical database. http://fao.org. Accessed June 2017
- Gokbulut I, Karabulut I. SPME–GC–MS detection of volatile compounds in apricot varieties. Food Chem. 2012;132:1098–1102. doi: 10.1016/j.foodchem.2011.11.080. [DOI] [Google Scholar]
- Goliáš J, Letal J, Dokoupil L. Influence of maturity on volatile production and chemical composition of fruits of six apricot cultivars. J Appl Bot Food Qual. 2011;84:76–84. [Google Scholar]
- González-Agüero M, Troncoso S, Gudenschwager O, Campos-Vargas R, Moya-León MA, Defilippi BG. Differential expression levels of aroma-related genes during ripening of apricot (Prunus armeniaca L.) Plant Physiol Biochem. 2009;47:435–440. doi: 10.1016/j.plaphy.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Greger V, Schieberle P. Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. J Agric Food Chem. 2007;55:5221–5228. doi: 10.1021/jf0705015. [DOI] [PubMed] [Google Scholar]
- Guichard E, Souty M. Comparison of the relative quantities of aroma compounds in fresh Apricot (Prunus armeniaca) from six different varieties. Z Lebensm Unters Forsch. 1988;186:301–307. doi: 10.1007/BF01027031. [DOI] [Google Scholar]
- Guillot S, Peytavi L, Bureau S, Boulanger R, Lepoutre JP, Crouzet J, Schorr-Galindo S. Aroma characterization of various apricot varieties using headspace-solid phase microextraction combined with gas chromatography–mass spectrometry and gas chromatography–olfactometry. Food Chem. 2006;96:147–155. doi: 10.1016/j.foodchem.2005.04.016. [DOI] [Google Scholar]
- Obenland D, Collin S, Sievert J, Negm F, Arpaia ML. Influence of maturity and ripening on aroma volatiles and flavor in ‘Hass’ avocado. Postharvest Biol Technol. 2012;71:41–50. doi: 10.1016/j.postharvbio.2012.03.006. [DOI] [Google Scholar]
- Ruiz D, Egea J, Gil MI, Tomás-Barberán FA. Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. J Agric Food Chem. 2005;53:9544–9552. doi: 10.1021/jf051539p. [DOI] [PubMed] [Google Scholar]
- Sangkasanya S, Lertsiri S, Meenune M. Changes in fruit quality and volatile flavor compounds during on-tree maturation of longkong. Int Food Res J. 2014;21:1659–1665. [Google Scholar]
- Solís-Solís HM, Calderón-Santoyo M, Schorr-Galindo S, Luna-Solano G, Ragazzo-Sánchez JA. Characterization of aroma potential of apricot varieties using different extraction techniques. Food Chem. 2007;105:829–837. doi: 10.1016/j.foodchem.2007.01.061. [DOI] [Google Scholar]
- Takeoka G, Flath R, Mon T, Teranishi R, Guentert M. Volatile constituents of apricot (Prunus armeniaca L.) J Agric Food Chem. 1990;38:471–477. doi: 10.1021/jf00092a031. [DOI] [Google Scholar]
- Xi W, Zheng H, Zhang Q, Li W. Profiling taste and aroma compound metabolism during apricot fruit development and ripening. Int J Mol Sci. 2016;17:998–1020. doi: 10.3390/ijms17070998. [DOI] [PMC free article] [PubMed] [Google Scholar]