Abstract
Oxidation is a significant problem in processed foods affecting their physico-chemical, shelf life and health properties. Natural antioxidants could be viable alternatives to synthetic variants for safely improving antioxidation properties of processed foods. The aim of this study was to assess the singular and combined effects of beetroot and chocolate on the oxidative stability of a high fat and protein processed food (sponge cake) during storage and gastrointestinal digestion. Cakes were prepared and assessed for antioxidant potential, polyphenols, and oxidative stability, and macronutrient oxidation during simulated gastro-intestinal digestion. Beetroot significantly improved the antioxidant and polyphenol profiles of sponge cake which further improved with chocolate addition. Beetroot also significantly increased the oxidative stability and shelf-life of sponge cake, and these effects were enhanced when combined with chocolate. Chocolate significantly reduced lipid oxidation during the gastric phase of digestion. However, both chocolate and beetroot did not curtail lipid oxidation in the intestinal phase, nor protein oxidation at any of the phases. Promisingly, beetroot and chocolate addition did not affect textural parameters and delayed staling by up to 2 days. Overall, the benefits of beetroot and chocolate addition were manifested more in the food system than during its digestion. Beetroot improves the oxidative stability and shelf life of processed foods, and its effects could be enhanced through combining with other natural products.
Keywords: Beetroot, Chocolate, Oxidation, Antioxidants, Oxidative stability, Gastrointestinal digestion, Processed foods
Introduction
Processed foods remain popular and are widely consumed by all segments of the population. The high protein and fat contents often seen in processed foods increase their susceptibility to oxidation, affecting their physico-chemical characteristics and shelf life. End-products of macronutrient oxidation have been shown to also adversely affect health. For instance, aldehydes such as malondialdehyde resulting from the oxidation of fatty acids exert mutagenic and atherogenic effects, while protein oxidation products such as carbonyls promote cell ageing and age-related diseases (Miyata et al. 1998; Niedernhofer et al. 2003). Synthetic antioxidants are often added to processed foods to curtail macronutrient oxidation, although they have been implicated in exacerbating disease (Shahidi and Ambigaipalan 2015).
Food reformulation strategies for improving the health properties of processed foods are being increasingly adopted in response to consumer and public health demands for healthier diets (Leroy et al. 2015). Natural products in particular are being increasingly used in food reformulations for the multiple benefits they confer both to consumers and manufacturers. These include improved nutritional profiles and producing ‘clean label’ products. Our work has looked at the potential of beetroot in this regard as it is rich in phytochemicals with demonstrated nutritional and antioxidant properties (Clifford et al. 2015). Some of these effects were confirmed in our work on bread, burgers and mayonnaise where beetroot addition had several potentially beneficial product specific effects including decreasing fat and protein oxidation, and improving anti-oxidant potential, oxidative stability and shelf-life (Duthie et al. 2013; Raikos et al. 2015; Ranawana et al. 2016a). However, its effects appear to be product specific, possibly mediated by food composition and processing conditions.
Numerous studies have demonstrated the antioxidant properties of chocolate (Goya et al. 2016), however there is limited data on how its addition into processed foods affect oxidative stability and functional attributes. The objective of the present study was to further assess the effects of adding beetroot on functional and chemical antioxidant properties of processed foods, and to determine its combined effects with chocolate. Sponge cake was selected as the model as it is a high fat and protein food that is widely consumed. Beetroot and chocolate are ingredients conventionally used in cake, which further supports its suitability as a test model.
The specific aims of the study were to assess the singular and combined effects of beetroot and chocolate on the oxidative stability properties of sponge cake and during its gastro-intestinal digestion. The study hypothesised that the addition of beetroot or chocolate would improve the functional antioxidant properties of sponge cake and show cumulative benefits when combined.
Materials and methods
Chemicals and reagents
The reagents used were: Na2PO4, KH2PO4, NaCl, pancreatin, pepsin, mucin, α-amylase, Adenosin diphosphate, Trichloroacetic acid, folin and ciocalteu’s phenol reagent, Na2CO3, Gallic acid, 1,1,3,3-tetramethoxypropane (TMP), thiobarbituric acid, sodium hydroxide, glacial acetic acid, 300 mM acetate buffer, HCl, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), FeCl3, 0.2% 2,4-dinitrophenylhydrazine (DNPH), 6M guanidine hydrochloride,. All reagents were of analytical grade and sourced from Sigma-Aldrich Co Ltd. (Dorset, UK) unless otherwise stated.
Preparation of beetroot and cakes
Small to medium sized fresh beetroots (Globe var) were purchased from a local supermarket (Sainsbury’s Supermarkets Ltd, London, UK) and were washed and dried. The beetroots were pricked with a fork, sprinkled with water, placed covered in a microwavable dish and cooked for seven minutes at 60% power and three minutes at 80% power in a commercial microwave oven (1600 Watts; CF359, Buffalo Appliances, Bristol, UK). The cooled beetroot was peeled, ground to a puree and passed through a sieve to ensure no particles were present, and used immediately in the preparation of the cakes. The cocoa used was a standardised brand (Cadbury Bourneville, Cadbury, Birmingham, UK) and previously characterised for polyphenol content (Santos and Coe 2016).
The study evaluated four types of cakes; chocolate cake with beetroot (CB), chocolate cake without beetroot (CN), plain cake with beetroot (PB) and plain cake (PN). The cake formulation used is typical of what is used in commercial production (Campbell et al. 2016). For preparing the two beetroot cakes (Table 1) the sugar, oil and egg were beaten for 2 min using a hand-held beater until homogenous. The beetroot puree was folded in followed by the dry ingredients. For the beetroot-free cakes, the sugar, oil and egg were beaten for 2 min using a hand-held beater until homogenous. Then dry ingredients were folded in. Finally the water was added and the batter mixed until smooth. The cake batters were poured into greased and lined loaf tins (11 cm × 21 cm) and baked in a non-fan-assisted oven at 180 °C for 45 min. The cooked cakes were cooled in the tins for 5 min before unmoulding and cooling on a wire rack. For the antioxidant, polyphenol and oxidative stability experiments samples of the cooled cakes were immediately freeze dried (Model HS1, Frozen in Time Ltd., York, UK) and used. Fresh cake samples were used for the digestions and for texture measurement.
Table 1.
Ingredient composition of cakes
| Ingredients (g) | Source | Chocolate cake with beetroot | Chocolate cake without beetroot | Plain cake with beetroot | Plain cake without beetroot |
|---|---|---|---|---|---|
| Cocoa powder | Cadbury Bourneville | 50 | 50 | – | – |
| All-purpose flour | Tesco stores | 175 | 225 | 225 | 275 |
| Baking powder | Dr. Oetker | 9 | 9 | 9 | 9 |
| Caster sugar | Silver spoon | 200 | 200 | 200 | 200 |
| Beetroot | Sainsbury’s supermarkets, var. Globe | 250 | – | 250 | – |
| Eggs | Tesco stores | 130 | 130 | 130 | 130 |
| Rapeseed oil | Tesco organic | 225 | 225 | 225 | 225 |
| Salt | Saxa | 3 | 3 | 3 | 3 |
| Water | – | 0 | 200 | 0 | 200 |
| Total batter weight | 1042 | 1042 | 1042 | 1042 | |
| Weight reduction after baking (%) | 9.3 | 9.6 | 9.1 | 10.0 |
Measuring antioxidant capacity and total polyphenol content
Preparation of sample extracts
1 g of sample (ground freeze dried cake powders) was added to eppendorfs containing 10 mL of 0.9% NaCl. An aqueous isotonic extraction medium was selected as this was more physiologically relevant. The suspensions were mixed on a roller for 30 min, sonicated in a water bath for 30 min and centrifuged at 6000 rpm (CR312, Jouan, Thermo Fisher Scientific, Renfrew, UK) for 10 min. The procedure was repeated to ensure maximum extraction, combined and stored at − 70 °C until analysed for antioxidant potential and total polyphenol content.
Analysis of total antioxidant potential and polyphenol content
Antioxidant potential was measured using the Ferric ion Reducing Antioxidant Power (FRAP) and total phenolics in the extracts was estimated according to methods described earlier (Raikos et al. 2016).
Oxidative stability of cakes
The Rancimat method was used to measure susceptibility to lipid oxidation. The 743 Rancimat model (Metrohm Ltd, Herisau, Switzerland) measures the resistance of food products containing fats and oils to oxidative rancidity, and thus provides an indication of shelf life. Freeze dried cake samples (2.5 g) were transferred to the reaction vessels and subjected to an accelerated oxidation at 120 °C and an ambient air flow rate of 20L/h.
Simulated Gastro-intestinal digestions
The method used has been described elsewhere (Ranawana et al. 2016a, b). Cake samples were weighed into 15 mL black centrifuge tubes (LightSafe, Sigma-Aldrich, Dorset, UK) and 3 mL of cold simulated saliva was added. The digestion tubes were incubated at 37 °C for 5 min to complete the oral phase of digestion (pH 6.8) and the phase halted by the addition of 0.3M HCl. The gastric phase of digestion was subsequently initiated by the addition of SGF (pH 2.0) which contained 0.68 mg of ascorbic acid, 0.11 mg of FeSO4 and 6.8 mg of ADP to create a pro-oxidant environment. The gastric digestion phase was continued for 4 h, aliquots being transferred at 2 h to tubes containing SIF (pH 8.0) to simulate intestinal digestion phase for 2 h. At baseline, and during each of the digestion phases digesta were transferred into, (1) glass tubes containing 20% trichloroacetic acid for measuring concentrations of TBARS and, (2) glass tubes containing 0.2% dinitrophenylhydrazine (DNPH, in 3.5 M HCl) for measuring protein carbonyls (PCs). Digesta samples collected for TBARS quantification were analysed immediately following the digestions whilst those collected for PC analysis were stored at − 70 °C and analysed within 7 days. All of the cake samples were subjected to in vitro digestions in three independent runs and the data pooled for analysis.
Measurement of thiobarbituric acid reactive substances (TBARS) in digesta samples
Concentrations of TBARS were analysed by the method described previously (Duthie et al. 2013) using high performance liquid chromatography. One millilitre of freshly prepared thiobarbituric acid reagent (0.67 g thiobarbituric acid, 100 mL glacial Acetic acid in 200 mL solution) was added into digesta sample tubes and the contents heated for 30 min at 90–100 °C. Cooled samples were centrifuged and the supernatants analysed using HPLC using a Waters 2695 Separations Module (Waters Corporation, Milford, USA) equipped with a Waters 2475 fluorescence detector (Waters Ltd, Elstree, UK) and a Luna® 5 µm C18 (2) 100 Å, 100 × 4.6 mm column (Phenomenex, Cheshire, UK). TBARS were determined with isocratic elution at a flow rate of 0.6 mL/min, sample run was 15 min, injection volume was 20 µl and fluorescence detector wavelengths were set to 515 nm (excitation) and 546 nm (emission). The mobile phase consisted of 60% (v/v) KH2PO4 (50 mM, pH 7.0) and 40% (v/v) methanol. Standard solutions of TMP was used for constructing calibration curves and quantification of TBARS (concentration range: 0–2 mMol/L).
Measurement of protein carbonyls in digesta
Protein carbonyls in the digesta samples were analysed as previously described (Duthie et al. 2013). The digesta samples in 0.2% DNPH were heated at 45°c for 1 h and centrifuged at 13,000×g for 5 min. The supernatant was removed and discarded and ethanol:ethyl acetate (1:1 v/v) was added to re-dissolve the pellet. The samples were incubated at room temperature for 10 min whilst vortexing occasionally. The centrifuging, supernatant removal and washing procedure was repeated a further two times. The pellet was re-dissolved in 300 µL 6M guanidine hydrochloride and absorbance read at 370 nm (µQuant, Bio-Tek instruments Inc, Winooski, USA), and PC content quantified using the molar extinction coefficient of 22,000 M−1 cm−1.
Texture analysis of fresh cakes and during storage
Freshly baked and cooled cakes were cut into 20 mm thick slices and 3 randomly selected slices from each type were placed in air tight plastic containers. One set of samples was analysed on the day of baking for baseline measurements (day 0). The remainder were stored for 1, 2 and 4 days in a dark cupboard at ambient temperature (21 °C). Texture profile analysis of the cake crumb was carried out at 0, 1, 2 and 4 days of storage using a texture analyser (CT3, Brookefield Viscometers Ltd, Harlow, UK) equipped with a cylinder probe (TA25/1000, D = 25.4 mm). Sample cubes (20 mm × 20 mm × 20 mm) were prepared from the cake slices (in triplicate). The cubes were 50% compressed twice to give a two bite texture profile. Trigger load and test speed were 5 g and 0.5 mm/s respectively.
Statistical analysis
Statistical analysis was carried out using SPSS (version 22, IBM, Portsmouth, UK), and data processed using MS Excel software (Microsoft, Reading, UK). Total TBARS and PC formed during 4 h of in vitro gastric digestion and 2 h of intestinal digestion were quantified by calculating the Areas Under the digestion Curves (AUC) using the trapezoidal rule. Data on the TBARS and PC contents in the cakes, AUCs from gastrointestinal digestions, antioxidant capacity, total polyphenol content and Rancimat induction times were analysed using one-way ANOVA with cake type and parameters as the independent and dependent factors respectively. Texture data was analysed using a factorial ANOVA model. Post hoc tests were carried out using the Tukey, Ryan, Einot, Gabriel and Welsch Q procedure, and Dunnett’s test as appropriate. A P < 0.05 was considered significant. Data normality was assessed using the Kolmogorov–Smirnov test.
Results and discussion
Similar to chocolate the high prevalence of natural antioxidants in beetroot is well documented (Clifford et al. 2015). To our knowledge this is the first study comparing beetroot and chocolate and their combined effects on antioxidant and functional properties, particularly within a processed food model that is inherently high in fat and protein, and therefore prone to oxidation. Compositional analysis of the cakes using dietary software (NetWisp, Tinuviel Software, Warington, UK) indicated that addition of beetroot did not alter macronutrient contents (Table 2) but increased total fibre and micronutrient contents, particularly Potassium, Phosphorus, Iron and folate. The improvement in nutritional properties is unsurprising as beetroot and cocoa are rich in fibre, micronutrients and trace elements (Ninfali and Angelino 2013; Steinberg et al. 2003).
Table 2.
Nutrition composition of the cakes
| Nutrient | CB cake | CN cake | PB cake | PN cake |
|---|---|---|---|---|
| Protein (g) | 4.6 | 4.5 | 4.2 | 4.1 |
| Fat (g) | 24.3 | 24.3 | 23.3 | 23.3 |
| Carbohydrate (g) | 36.3 | 37.8 | 39.5 | 40.9 |
| AOAC fibre (g) | 2.8 | 2.5 | 1.4 | 1.1 |
| Potassium (mg) | 237 | 122 | 172 | 58 |
| Magnesium (mg) | 34 | 31 | 10 | 8 |
| Phosphorus (mg) | 176 | 161 | 150 | 135 |
| Iron (µg) | 1.33 | 1.23 | 0.92 | 0.82 |
| Folate (µg) | 19 | 6 | 19 | 6 |
| Water content (%) | 21.3 | 20.5 | 18.9 | 18.5 |
Compositions determined using nutritional analysis software NetWisp, Tinuviel Software, Warington, UK
CB chocolate beetroot cake, CN plain chocolate cake, PB beetroot cake, PN plain cake
Total antioxidant potential and polyphenol content of cakes
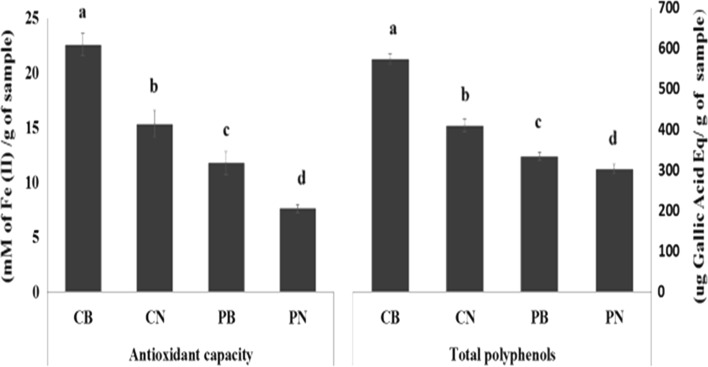
In agreement with previous observations (Li et al. 2015) the antioxidant potential of cakes showed a strong correlation with polyphenol contents (P < 0.001; r = 0.97). There was a significant effect of cake type on the antioxidant capacity (F (3, 8) = 873.38, P < 0.001), and post hoc analyses showed that the CB cake had a significantly higher antioxidant capacity (22.6 ± 1.0 µM Fe(II)/mL) when compared with the other three cakes (PN: 7.6 ± 0.4 µM Fe(II)/mL, PB: 11.8 ± 1.1 µM Fe(II)/mL and CN: 15.4 ± 1.2 µM Fe(II)/mL) (Fig. 1). This indicates the presence of both these natural products had cumulative effects on antioxidant status. The addition of beetroot (0.24 g/g) or cocoa (0.05 g/g) increased the antioxidant potential of the cake to similar degrees supporting evidence that cocoa has a greater antioxidant potential on a per-gram basis (Belščak et al. 2009; Wootton-Beard et al. 2011). The advantage of beetroot however is its bulkiness which could replace fat and carbohydrate ingredients whilst conferring antioxidant levels comparable to cocoa.
Fig. 1.
Antioxidant capacity and polyphenol content of cakes. CB chocolate and beetroot cake, CN chocolate cake, PB beetroot cake, PN plain cake. Columns with different letters are significantly different, One-way ANOVA, P < 0.05. Error bars are standard errors
A significant effect of cake type on total polyphenol content was also observed (F (3, 8) = 278.5, P < 0.001) (Fig. 1) with the four cakes showing significantly different levels. The chocolate cakes (CB and CN) had significantly higher levels of polyphenols (CB:574.9 ± 12.3 µg GAE/g of sample, CN: 410.7 ± 16.5 µg GAE/g of sample) when compared to the cakes without chocolate (PB: 334.7 ± 9.6 µg GAE/g of sample, PN: 303.1 ± 11.3 µg GAE/g of sample), and this further supports chocolate as a rich source of phytochemicals. No additive effects on antioxidant potential or total polyphenols were observed when chocolate and beetroot were combined, which may be due to masking of some polyphenols with proteins, carbohydrates and fats (Jakobek 2015). However, published data on this is equivocal as the degree of binding may depend on factors such as polyphenol chemistry, matrix, macronutrient characteristics and processing conditions. For instance, heating has been shown to increase the binding of polyphenols (Yazdi and Corredig 2012).
Oxidative stability of cakes
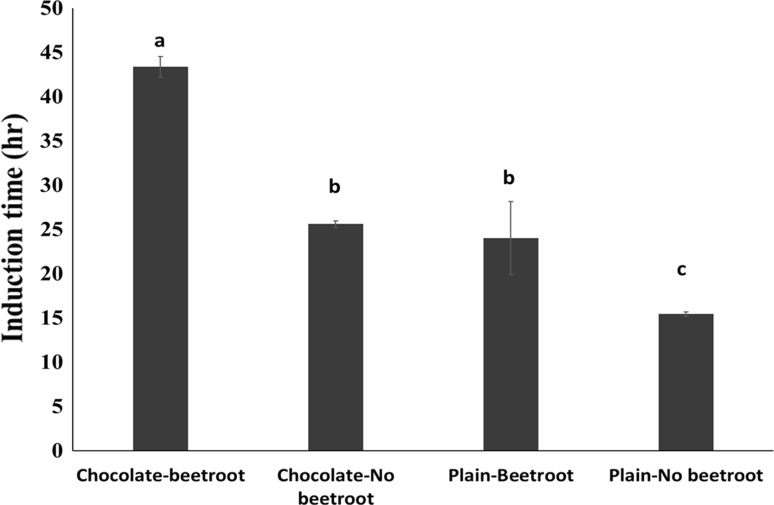
Reflecting antioxidant potential and total polyphenols, Rancimat determined induction times were also significantly affected by cake type (F(3,8) = 88.8; P < 0.001) (Fig. 2), where that of CB cake was almost three-fold longer (43.4 ± 1.2 h) than PN cake (15.5 ± 0.3 h), suggesting strong synergistic effects. Induction times for CN (25.6 ± 0.4 h) and PB (24.0 ± 4.1 h) cakes did not significantly differ (P > 0.05) indicating both these ingredients had comparable effects on product shelf life. However, their combination in the cake served to markedly increase oxidative stability as evidenced by the absolute increment seen in CB compared to PN (27.9 h) which was over two-fold higher than was seen for CN and PB (10.1 and 8.5 h respectively).
Fig. 2.
Oxidative stability of cake measured as Induction time. Columns with different letters are significantly different, One-way ANOVA, P < 0.05. Error bars are standard errors
Prolongation of induction times of cake with beetroot addition agrees with our previous findings with mayonnaise and bread (Raikos et al. 2015; Ranawana et al. 2016a). The present study indicated that comparable effects on oxidative stability occur with addition of chocolate, and this has not been previously reported. Induction time is a predictor of product shelf life (Farhoosh 2007) which suggest that the addition of beetroot and chocolate increases product longevity, possibly through the antioxidant effects of the inherent phytochemicals. Inclusion of these natural ingredients could allow reduction in usage of adversely perceived synthetic antioxidants (Shahidi and Ambigaipalan 2015) allowing manufacturers to limit problematical lipid and protein oxidation of commercially processed products while enhancing nutritional benefits.
Generation of thiobarbituric acid reactive substances (TBARS) during digestion
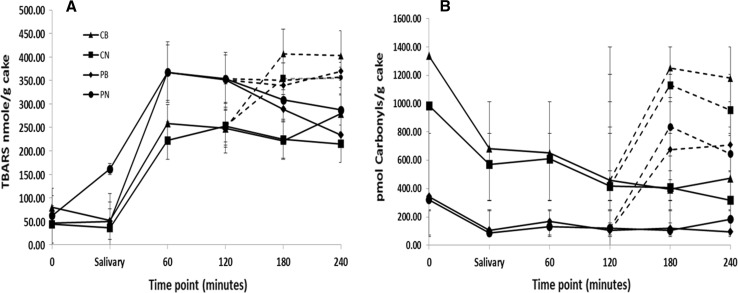
There was a significant effect of cake type on the total amount of TBARS generated during the gastric phase of digestion (F (3, 20) = 24.5, P < 0.001) (Fig. 3), and post hoc analyses showed that the PN cake contained a significantly higher amount of TBARS (75819.9 ± 1605.0 nmol/g.min, P < 0.001) when compared to the CN (49689.7 ± 6431.9 nmol/g.min) and CB cakes (53988.6 ± 7820.3 nmol/g.min), suggesting the addition of chocolate and beetroot reduced fat oxidation during this phase. The intestinal phase showed a general increase in the amount of TBARS produced in the chocolate cakes (CB and CN) when compared to the gastric phase, and showed significant differences (F (3, 20) = 3.69, P < 0.05). The CB and PN cakes showed similar levels of TBARS (44875.8 ± 2971.2 nmol/g.min and 40356.9 ± 4357.2 nmol/g.min respectively), however the former contained a significantly higher amount compared to the CN and PB cakes.
Fig. 3.
Changes in Thiobarbituric acid reactive substances (TBARS) (A) and Protein Carbonyls (B) in cakes during simulated gastro-intestinal digestion. Solid lines represent the baseline, oral and gastric phases, the broken lines represent the small intestinal phase. CB chocolate and beetroot cake, CN chocolate cake, PB beetroot cake, PN plain cake. Error bars are standard errors
The study provides a first comparative record of the effects of beetroot and cocoa addition on oxidation of macronutrients contained in a processed food during gastrointestinal digestion. The human alimentary tract can often be oxygen-rich showing gradients along its length and breadth (Espey 2013) and this could be exacerbated by mastication which aerates the chyme. Therefore, protecting macronutrients from oxidation during digestion could be an important role of dietary antioxidants. Chocolate and beetroot polyphenols have been shown to be stable during gastric transit (Rios et al. 2002; Wootton-Beard et al. 2011) suggesting their antioxidant properties should remain intact. We found that the chocolate-containing treatments lowered lipid oxidation (measured as TBARS) during the gastric phase and had equivocal effects during the intestinal phase and this agrees with evidence showing cocoa polyphenols are more stable in acidic pH (Andres-Lacueva et al. 2008). Beetroot did not curtail lipid oxidation during digestion and this too agrees with previous observations (Ranawana et al. 2016a). Interestingly however, the combination of beetroot and cocoa significantly increased TBARS in the intestinal phase compared to when they were alone. We are unable to propose a reason for this observed negative additive effect and warrants further study.
Generation of protein carbonyls during digestion
Protein carbonyls are formed during the oxidative cleavage of proteins, from the production of carbonyl groups during protein oxidation, and as secondary reactions of lipid oxidation. Therefore, the PC composition in a food would depend on the quality and quantity of macronutrients contained in it. Their relative stability makes them a useful measure of protein oxidation.
The amount of PCs observed during the gastric phase of digestion was significant (F (3, 8) = 17.08, P = 0.001), and post hoc analyses showed that the chocolate cakes (CB:130009.18 ± 29587.05 pmol/g.min and CN: 116711 ± 29372.07 pmol/g.min) contained significantly higher amounts than the others (PB: 30856.0 ± 11521.2 and PN: 30432.14 ± 12735.14 pmol/g.min) (Fig. 3). Similarly, protein carbonyls in the intestinal phase was significant (F (3, 8) = 16.88, P = 0.001) with the two chocolate-containing cakes produced similar but higher levels compared to the PB and PN cakes. The higher carbonyl levels at all digestion-phases for the chocolate-containing cakes suggest they originated from the cocoa, and indeed carbonyls are abundant in cocoa representing an important flavour group (Aprotosoaie et al. 2016). Carbonyls are a diverse family of compounds comprising both beneficial (flavour) and harmful (food degradation and oxidative stress) variants (Fedorova et al. 2014) and our method was unable to distinguish between them. Therefore it remains to be confirmed how the addition of cocoa may be affecting undesirable protein oxidation during digestion. In agreement with previous observations beetroot did not impact on PC generation at any of the digestion phases (Ranawana et al. 2016a).
Textural changes during storage of cakes
Texture has been shown to be affected by oxidation, and the addition of natural products rich in phytochemicals have been demonstrated to curtail related processes such as retrogradation (Patrignani et al. 2014). Therefore, texture was analysed as part of the study to assess how reformulation with beetroot and chocolate affected this physical attribute. The four cake types had significantly different levels of hardness with an interactive effect of treatment and day (F (15, 32) = 4.30, P = 0.001) (Table 3). All four cakes increased in hardness during storage, being similar on day 1 but increasing by day 2. Post hoc analyses showed the hardness of CB, CN and PB cakes initially were less than PN cake but these differences were not apparent by day 4. This suggests that the addition of chocolate and beetroot has beneficial short-term effects on hardness. Significant differences in the degree of adhesiveness were also observed between the four cake types but there were no discernible interactive effects of treatment and day. Adhesiveness tended to fluctuate in the cakes during storage with no discernible pattern (Table 3) although the PB cake showed the highest degree of adhesiveness overall (0.825 ± 0.4). This may be due to the greater water retention capacity of beetroot (Shyamala and Jamuna 2010), suggesting it could be used to improve the moistness of products.
Table 3.
Textural parameters of cakes when fresh and during storage
| Day 0 | Hardness (g) (cycle 1) | Hardness (g) (cycle 2) | Adhesiveness (mJ) | Fracturability (g) | Springiness (mm) |
|---|---|---|---|---|---|
| CB | 397.3 ± 103.2 | 306.7 ± 91.1 | 0.16 ± 0.1 | 397.3 ± 103.2 | 8.8 ± 0.5ab |
| CN | 218.7 ± 27.4 | 166.7 ± 23.1 | 0.36 ± 0.2 | 179.3 ± 47.7 | 10.1 ± 0.4c |
| PB | 391.7 ± 94.1 | 320.0 ± 64.2 | 0.90 ± 0.5 | 391.7 ± 94.1 | 9.3 ± 0.1bc |
| PN | 417.7 ± 108.6 | 308.3 ± 72.5 | 0.46 ± 0.1 | 417.7 ± 108.6 | 8.2 ± 0.4a |
| Day 1 | |||||
| CB | 546.3 ± 33.9 | 403.0 ± 25.2 | 0.26 ± 0.2 | 546.3 ± 33.9 | 9.0 ± 0.3 |
| CN | 464.7 ± 131.2 | 335.3 ± 112.6 | 0.13 ± 0.1 | 464.7 ± 131.2 | 9.8 ± 0.9 |
| PB | 359.7 ± 52.0 | 294.0 ± 31.8 | 0.46 ± 0.1 | 359.7 ± 52.0 | 10.0 ± 0.5 |
| PN | 555.0 ± 133.1 | 431.3 ± 95.8 | 0.60 ± 0.5 | 521.7 ± 183.2 | 9.0 ± 1.0 |
| Day 2 | |||||
| CB | 598.7 ± 37.4bc | 445.3 ± 33.5bc | 0.26 ± 0.3 | 598.66 ± 37.4bc | 9.1 ± 0.2 |
| CN | 376.3 ± 25.6a | 267.3 ± 12.7a | 0.43 ± 0.3 | 376.33 ± 25.6a | 8.6 ± 1.0 |
| PB | 500.0 ± 47.6ac | 386.3 ± 30.9ac | 1.13 ± 0.6 | 500.0 ± 47.6ac | 10.0 ± 0.4 |
| PN | 1081.0 ± 158.0d | 751.3 ± 95.3d | 0.40 ± 0.4 | 1081.0 ± 158.0d | 8.1 ± 0.4 |
| Day 4 | |||||
| CB | 723.0 ± 280.1 | 516.6 ± 208.7 | 0.76 ± 0.7 | 723.0 ± 280.1 | 8.98 ± 0.8 |
| CN | 501.3 ± 28.2 | 339.7 ± 24.6 | 0.40 ± 0.2 | 446.0 ± 47.9 | 7.9 ± 0.5 |
| PB | 624.3 ± 80.7 | 473.3 ± 63.5 | 0.80 ± 0.3 | 624.3 ± 80.7 | 8.6 ± 0.2 |
| PN | 844.7 ± 22.5 | 601.3 ± 13.2 | 0.70 ± 0.26 | 787.3 ± 97.2 | 7.6 ± 0.5 |
Day 0 represents fresh cakes; Values are means ± SD
Values within a column for each day with different superscript letters are significantly different, One-way ANOVA, P < 0.05. Columns with no superscripts denote statistically similar values for the four cakes
CB chocolate beetroot cake, CN plain chocolate cake, PB plain beetroot cake, PN plain cake
Fracturability was similar in all the cakes when fresh (day 0) and showed significant increases after 1, 2 and 4 days of storage (P < 0.001) (Table 3) with PN cake showing the greatest values by days 2 and 4. Therefore beetroot and chocolate appear to reduce fracturability during storage, which is desirable in baked products. Notably, the CN cake showed the overall lowest fracturability during storage. Springiness was significantly different in the four cake types when fresh ((F (3, 8) = 13.4, P = 0.002) with post hoc tests showing CN and PB cakes having significantly higher values than PN cake. However, all four cakes showed comparable springiness after 1, 2 and 4 days of storage. Overall, beetroot did not adversely alter the texture of cake and this agrees with previous data using bread (Ranawana et al. 2016a). Combining beetroot and chocolate does not appear to have adverse effects on textural parameters and this is promising from a sensory perspective.
Phytochemical stability during processing
The predominant phytochemicals in beetroot include betalains, ferulic acid derivatives, phenolic amides and flavonoids (Kujala et al. 2002) whilst cocoa contains a more complex mixture of catechins, procyanidins, anthocyanins and flavonols (Wollgast and Anklam 2000). Processing conditions have been shown to affect the antioxidant properties of phytochemicals (Kalt 2005) highlighting the need to assess their oxidative effects on a product-specific basis. In sponge cake baking core temperatures usually do not exceed 100 °C (Fehaili et al. 2010). Cocoa polyphenols have a relatively high thermal stability as they have withstood roasting temperatures around 150 °C (Ramli et al. 2006), and therefore would be stable within the cake matrix. The beetroot used in the cakes was microwaved as our work showed that mild heat processing improves betalain stability (Raikos et al. 2016). However, the temperature–time combination may be important for phytochemical stability.
The thermal treatment of betalain produces degradation products such as isobetanin and neobetanin which are found in high quantities in processed beetroot products (Herbach et al. 2004). Although we did not measure betalain degradation products in the cakes it is likely they were high due to the two thermal treatments the beetroot was subjected to. Limited work has been carried out to determine the functional properties of these degradation products. Wootton-Beard et al. (2014) found that the consumption of a beetroot juice predominating in neobetalins significantly reduced glycaemic and insulinaemic responses in volunteers, suggesting they may have functional properties. This study showed that the cooked beetroot cakes had high antioxidant potentials which is suggestive of antioxidant effects of heat degraded betalains. However this remains to be confirmed in future studies.
Conclusion
To our knowledge this is the first study assessing the singular and combined effects of beetroot and chocolate addition on oxidative stability of a processed food, both in the product and during simulated gastro-intestinal digestion. In response to consumer and public health demands, as protein and unsaturated fat contents increase in processed foods so does the importance of antioxidant ingredients for protecting them. The present study showed that beetroot increased the antioxidant and polyphenol profiles of sponge cake which further improved with the addition of chocolate. Beetroot also improved the oxidative stability and estimated shelf-life of sponge cake, and these effects were further enhanced when combined with chocolate. Chocolate was more promising in curtailing lipid oxidation during gastro-intestinal digestion while beetroot showed neutral effects on both lipid oxidation and protein oxidation. Textural parameters were not adversely affected by beetroot and chocolate addition, and both slowed staling suggesting positive effects. Overall, the results indicated that the benefits of beetroot and chocolate addition were manifested more in the food system through improving oxidative stability and shelf life, than during its digestion. However, their presence did not adversely affect macronutrient oxidation during digestion but served to marginally improve protection.
Acknowledgements
The authors are grateful to the Rural and Environment Science and Analytical Services (RESAS) Division of the Scottish government for funding the study. None of the authors declare any conflicts of interest.
References
- Andres-Lacueva C, Monagas M, Khan N, Izquierdo-Pulido M, Urpi-Sarda M, Permanyer J, Lamuela-Raventos R. Flavanol and flavonol contents of cocoa powder products: influence of the manufacturing process. J Agric Food Chem. 2008;56:3111–3117. doi: 10.1021/jf0728754. [DOI] [PubMed] [Google Scholar]
- Aprotosoaie AC, Luca SV, Miron A. Flavor chemistry of cocoa and cocoa products—an overview. Compr Rev Food Sci Food Saf. 2016;15:73–91. doi: 10.1111/1541-4337.12180. [DOI] [PubMed] [Google Scholar]
- Belščak A, Komes D, Horžić D, Ganić KK, Karlović D. Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Res Int. 2009;42:707–716. doi: 10.1016/j.foodres.2009.02.018. [DOI] [Google Scholar]
- Campbell L, Euston SR, Ahmed MA. Effect of addition of thermally modified cowpea protein on sensory acceptability and textural properties of wheat bread and sponge cake. Food Chem. 2016;194:1230–1237. doi: 10.1016/j.foodchem.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Clifford T, Howatson G, West DJ, Stevenson EJ. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015;7:2801–2822. doi: 10.3390/nu7042801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie G, Campbell F, Bestwick C, Stephen S, Russell W. Antioxidant effectiveness of vegetable powders on the lipid and protein oxidative stability of cooked turkey meat patties: implications for health. Nutrients. 2013;5:1241–1252. doi: 10.3390/nu5041241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Rad Biol Med. 2013;55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- Farhoosh R. The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J Am Oil Chem Soc. 2007;84:205–209. doi: 10.1007/s11746-006-1030-4. [DOI] [Google Scholar]
- Fedorova M, Bollineni RC, Hoffmann R. Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev. 2014;33:79–97. doi: 10.1002/mas.21381. [DOI] [PubMed] [Google Scholar]
- Fehaili S, Courel M, Rega B, Giampaoli P. An instrumented oven for the monitoring of thermal reactions during the baking of sponge cake. J Food Eng. 2010;101:253–263. doi: 10.1016/j.jfoodeng.2010.07.003. [DOI] [Google Scholar]
- Goya L, Martín MÁ, Sarriá B, Ramos S, Mateos R, Bravo L. Effect of cocoa and its flavonoids on biomarkers of inflammation: studies of cell culture, animals and humans. Nutrients. 2016;8:212–224. doi: 10.3390/nu8040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbach K, Stintzing F, Carle R. Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. J Food Sci. 2004;69:492–498. [Google Scholar]
- Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–567. doi: 10.1016/j.foodchem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Kalt W. Effects of production and processing factors on major fruit and vegetable antioxidants. J Food Sci. 2005;70:11–19. doi: 10.1111/j.1365-2621.2005.tb09053.x. [DOI] [Google Scholar]
- Kujala TS, Vienola MS, Klika KD, Loponen JM, Pihlaja K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur Food Res Technol. 2002;214:505–510. doi: 10.1007/s00217-001-0478-6. [DOI] [Google Scholar]
- Leroy P, Requillart V, Soler L, Enderli G. An assessment of the potential health impacts of food reformulation. Eur J Clin Nutr. 2015;70:694–699. doi: 10.1038/ejcn.2015.201. [DOI] [PubMed] [Google Scholar]
- Li X, Wasila H, Liu L, Yuan T, Gao Z, Zhao B, Ahmad I. Physicochemical characteristics, polyphenol compositions and antioxidant potential of pomegranate juices from 10 Chinese cultivars and the environmental factors analysis. Food Chem. 2015;175:575–584. doi: 10.1016/j.foodchem.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Miyata T, Inagi R, Asahi K, Yamada Y, Horie K, Sakai H, Uchida K, Kurokawa K. Generation of protein carbonyls by glycoxidation and lipoxidation reactions with autoxidation products of ascorbic acid and polyunsaturated fatty acids. FEBS Lett. 1998;437:24–28. doi: 10.1016/S0014-5793(98)01079-5. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- Ninfali P, Angelino D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia. 2013;89:188–199. doi: 10.1016/j.fitote.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Patrignani M, Conforti PA, Lupano CE. The role of lipid oxidation on biscuit texture during storage. Int J Food Sci Tech. 2014;49:1925–1931. doi: 10.1111/ijfs.12550. [DOI] [Google Scholar]
- Raikos V, Neacsu M, Morrice P, Duthie G. Anti-and pro-oxidative effect of fresh and freeze-dried vegetables during storage of mayonnaise. J Food Sci Tech. 2015;52:1–10. doi: 10.1007/s13197-015-1897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikos V, McDonagh A, Ranawana V, Duthie G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: effects on physical stability, texture and sensory attributes. Food Sci Hum Wellness. 2016;5:191–198. doi: 10.1016/j.fshw.2016.10.002. [DOI] [Google Scholar]
- Ramli N, Hassan O, Said M, Samsudin W, Idris NA. Influence of roasting conditions on volatile flavor of roasted Malaysian cocoa beans. J Food Process Preserv. 2006;30:280–298. doi: 10.1111/j.1745-4549.2006.00065.x. [DOI] [Google Scholar]
- Ranawana DV, Raikos V, Campbell F, Bestwick C, Nicol P, Milne L, Duthie G. Breads fortified with freeze-dried vegetables: quality and nutritional attributes. part 1: breads containing oil as an ingredient. Foods. 2016;5:19–32. doi: 10.3390/foods5010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranawana V, Campbell F, Bestwick C, Nicol P, Milne L, Duthie G, Raikos V. Breads fortified with freeze-dried vegetables: quality and nutritional attributes. Part II: breads not containing oil as an ingredient. Foods. 2016;5:62–76. doi: 10.3390/foods5030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios LY, Bennett RN, Lazarus SA, Remesy C, Scalbert A, Williamson G. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr. 2002;76:1106–1110. doi: 10.1093/ajcn/76.5.1106. [DOI] [PubMed] [Google Scholar]
- Santos M, Coe S (2016) The total polyphenol content of various commercial cocoa beverages, with and without the addition of cow’s milk. Proc Nutr Soc 75
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects–a review. J funct foods. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Shyamala B, Jamuna P. Nutritional content and antioxidant properties of pulp waste from daucus carota and beta vulgaris. Malays J Nutr. 2010;16:397–408. [PubMed] [Google Scholar]
- Steinberg FM, Bearden MM, Keen CL. Cocoa and chocolate flavonoids: implications for cardiovascular health. J Am Diet Assoc. 2003;103:215–223. doi: 10.1053/jada.2003.50028. [DOI] [PubMed] [Google Scholar]
- Wollgast J, Anklam E. Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res Int. 2000;33:423–447. doi: 10.1016/S0963-9969(00)00068-5. [DOI] [Google Scholar]
- Wootton-Beard PC, Moran A, Ryan L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Int. 2011;44:217–224. doi: 10.1016/j.foodres.2010.10.033. [DOI] [Google Scholar]
- Wootton-Beard PC, Brandt K, Fell D, Warner S, Ryan L. Effects of a beetroot juice with high neobetanin content on the early-phase insulin response in healthy volunteers. J Nutr Sci. 2014;3:1–9. doi: 10.1017/jns.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi SR, Corredig M. Heating of milk alters the binding of curcumin to casein micelles. A fluorescence spectroscopy study. Food Chem. 2012;132:1143–1149. doi: 10.1016/j.foodchem.2011.11.019. [DOI] [PubMed] [Google Scholar]