Abstract
Loss-of-function mutations in dual oxidase (DUOX) 2 are the most common genetic variants found in congenital hypothyroidism (CH), and similar mutations have been recently reported in few very-early-onset inflammatory bowel disease (IBD) patients without CH. If DUOX2 variants indeed increase susceptibility for IBD, the enrichment of DUOX2 mutation carriers among CH patients should be reflected in higher risk for developing IBD. Using a database containing health insurance claims data for over 230 million patients in the United States, 42,922 subjects with CH were identified based on strict inclusion criteria using diagnostic codes. For subgroup analysis, CH patients with pharmacy records were stratified as transient or permanent CH based on the absence or presence of levothyroxine treatment, respectively. Patients were matched to an equal-sized, age- and gender-matched non-CH group. Compared to controls, CH patients had a 73% higher overall IBD prevalence (0.52% vs 0.30%; P < 0.0001). The CH-associated relative risk was higher for indeterminate or ulcerative colitis than Crohn’s disease. Patients with transient CH had higher odds for IBD (OR 2.39 (95% CI 1.77–3.23) than those with permanent CH (1.69 (95% CI 1.31–2.18). We conclude that patients with CH are at an increased risk of developing IBD. The risk was highest for patients with transient CH, for which partial defects in the DUOX2 system are a particularly common finding.
Introduction
The follicular cells of the thyroid gland constitutively express a homolog of the phagosomal NADPH oxidase, called dual oxidase 2 (DUOX2) that generates hydrogen peroxide at the apical membrane. By driving thyroid-peroxidase catalyzed iodide organification, this activity is rate limiting for the synthesis of thyroid hormone1. In fact, mutations in DUOX2, first described in 20022, are now regarded the most common known genetic risk factor in congenital hypothyroidism (CH)3–7 found in up to 83% of CH cohorts8. The severity of DUOX2-associated CH can range from transient neonatal hyperthyrotropinema to permanent profound hypothyroidism.
In addition to being constitutively expressed in thyrocytes, DUOX2 is robustly induced in the barrier epithelial cells of the gastrointestinal tract as a sentinel response to potential microbial threats. DUOX2 and its essential heterodimerization partner DUOXA2 are part of a “DUOX2 co-expression signature” most responsive to mucosal bacterial dysbiosis9 and mice with a defect in the DUOX2 system were found to have increased intestinal bacterial translocation to both commensals and pathogenic bacteria consistent with a role of DUOX2 in supporting immune homeostasis10, potentially by suppressing bacterial virulence11. By its function and regulation, DUOX2 is a candidate gene for inflammatory bowel diseases (IBD), chronic inflammatory disorders of the gastrointestinal tract. The most common forms of IBD are ulcerative colitis (UC), which is limited to colonic involvement, and Crohn’s disease (CD), which can involve the entire length of the intestine. The current understanding of IBD pathogenesis is that an altered luminal microbial composition with epithelial dysfunction results in increased bacterial translocation and immune stimulation12. Although no traditional pathogens are generally detected during active disease flares, increased virulence of commensal bacterial species, particularly Escherichia coli, can enhance their mucosal attachment, invasion, and intracellular persistence, leading to increased pathogen-recognition and mucosal inflammation12. Although there is no information from the published literature that any of the previously reported CH patients with DUOX2 mutations had GI symptoms, anecdotal support for the potential role of DUOX2 in IBD pathogenesis has been recently provided by reports of DUOX2 loss-of-function mutations in children with IBD but not CH13–15. However, DUOX2 lacks common allelic variants suitable for genome-wide association studies and whether DUOX2 variants have an impact on IBD risk in the general population is unknown.
Since a high proportion of patients identified in neonatal CH screening programs harbor DUOX2 variants that may also contribute to the susceptibility for IBD, we hypothesized that the prevalence of IBD would be increased for a CH cohort compared to a matched non-CH cohort. To test this hypothesis, we performed a retrospective cohort analysis using a national payer database in the United States comprising individual-level health care claims data for 230 million individuals.
Results
Patient demographics
Using the Truven Health MarketScan databases from 2009–2015, we identified 42,922 patients with CH and 42,922 age-and-gender matched non-CH individuals over the study period (Fig. 1). The mean (±standard deviation) age was 39.1 (25.5) years in each group. The percentage of females was 67.4% in either group. Additional baseline characteristics of patients are provided in Supplemental Table 1.
Figure 1.
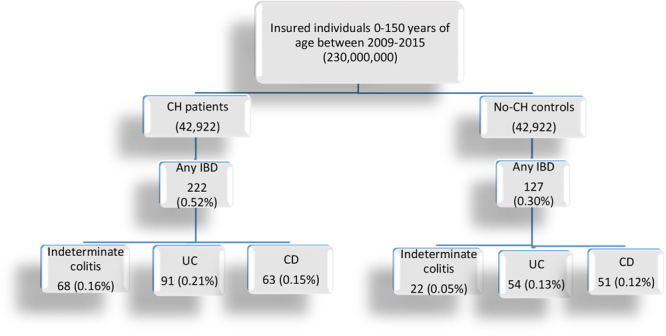
A diagram of the study cohort selection. CH = congenital hypothyroidism, IBD = inflammatory bowel disease, UC = ulcerative colitis, CD = Crohn’s disease.
Increased prevalence of IBD in subjects with congenital hypothyroidism
To determine whether individuals with CH are at increased risk of developing IBD, we compared the prevalence of IBD in CH vs. non-CH cohorts (n = 42,922). A significantly higher prevalence of patients having any IBD subtype was found in CH vs. non-CH cohorts (p < 0.0001, Table 1). The estimated prevalence of IBD per 100,000 individuals is 520 in CH and 300 in non-CH, indicating a 73% increased risk for developing IBD in CH patients. The higher prevalence was primarily due to increased risk for indeterminate colitis or UC, with only a trend towards more frequent occurrence of CD (Table 1). We found no significant difference in IBD severity based on the rate of hospitalization and IBD flare (data not shown).
Table 1.
The prevalence of IBD in patients with CH vs. non-CH controls.
| CH N = 42,922 | non-CH N = 42,922 | p value | |
|---|---|---|---|
| CD | 63 (0.15%) | 51 (0.12%) | 0.2607 |
| UC | 91 (0.21%) | 54 (0.13%) | <0.0021 |
| Indeterminate Colitis | 68 (0.16%) | 22 (0.05%) | <0.0001 |
| Any IBD | 222 (0.52%) | 127 (0.30%) | <0.0001 |
Sensitivity Analyses
To apply a stricter definition of CH, we applied a 3 ICD code requirement for CH and found similar results of an increased prevalence of any IBD in those with CH compared to those with no CH (0.52% vs. 0.28%; p < 0.0001) and stronger association with indeterminate colitis and UC (Supplemental Table 2).
Transient CH patients have the highest risk of developing IBD
Children with CH that regain normal thyroid function during early life are classified as transient CH. Whereas permanent CH is caused by a variety of genetic defects in addition to DUOX2 (e.g., TPO, TG, PAX8) as well as sporadic developmental abnormalities (e.g., ectopic thyroid gland), partial defects in the DUOX2 enzyme complex (DUOX2, DUOXA2) are the major known genetic risk factor for transient CH1,2,16–18. Compared to a cohort of patients with permanent CH, one limited to transient CH cases is likely enriched for DUOX2 defects. Thus, if the excess risk for IBD in CH patients is due to higher DUOX2 mutational load, IBD risk is anticipated to be even higher in transient CH (operationally defined as individuals diagnosed with CH not taking levothyroxine (T4) during the observation period) vs permanent CH (defined as those taking T4). Only patients in the CH cohort who had available pharmacy records were included in these analyses. Both permanent and transient CH cohorts had increased prevalence of IBD compared to non-CH controls (Tables 2 and 3), but the odds ratio was significantly higher for transient CH patients (OR 2.39 (95% CI 1.77–3.23) vs 1.69 (95% CI 1.31–2.18), respectively, Tables 4, 5). Compared to permanent CH patients, the prevalence of IBD in the transient CH group was 72% higher (0.81% vs 0.47%; p = 0.0003; non-CH: 0.30%) (Table 6). Similar to the findings for the total CH cohort, the strength of association between transient CH and IBD was highest for indeterminate colitis, followed by UC, and appeared to be weakest for CD.
Table 2.
The prevalence of IBD in patients with permanent CH (i.e., taking T4) vs. patients without CH (Bivariate comparison).
| Permanent CH N = 24,306 | Non-CH N = 42,922 | p value | |
|---|---|---|---|
| CD | 33 (0.14%) | 51 (0.12%) | 0.5501 |
| UC | 47 (0.19%) | 54 (0.13%) | 0.0298 |
| Indeterminate Colitis | 34 (0.14%) | 22 (0.05%) | 0.0001 |
| Any IBD | 114 (0.47%) | 127 (0.30%) | 0.0003 |
Table 3.
The prevalence of IBD in patients with transient CH (i.e., not taking T4) vs. patients without CH (Bivariate comparison).
| Transient CH N = 8,128 | Non-CH N = 42,922 | p value | |
|---|---|---|---|
| CD | 21 (0. 26%) | 51 (0.12%) | 0.0021 |
| UC | 28 (0. 34%) | 54 (0.13%) | <0.0001 |
| Indeterminate Colitis | 17 (0. 21%) | 22 (0.05%) | <0.0001 |
| Any IBD | 66 (0. 81%) | 127 (0.30%) | <0.0001 |
Table 4.
The primary endpoint (diagnosis of IBD) in patients with permanent CH vs. non-CH cohort (Multivariable Logistic Regression Models) - summarized models.
| Model* | Dependent variable | Odds Ratio** | 95% Confidence interval | p-value |
|---|---|---|---|---|
| 1 | UC | 1.64 | 1.11–2.43 | 0.0136 |
| 2 | Indeterminate Colitis | 2.90 | 1.23–2.38 | 0.0001 |
| 3 | Any IBD | 1.69 | 1.31–2.18 | <0.0001 |
*All three models adjusted for age and gender (see Supplemental Table 4 for the complete models).
**The odds of developing an endpoint in patients with CH taking T4 vs non-CH patients.
Table 5.
The primary endpoint (diagnosis of IBD) in patients with transient CH vs. non-CH cohort (Multivariable Logistic Regression Models) - summarized models.
| Model* | Dependent variable | Odds Ratio** | 95% Confidence interval | p-value |
|---|---|---|---|---|
| 1 | CD | 1.77 | 1.06–2.96 | 0.0291 |
| 2 | UC | 2.48 | 1.56–3.94 | 0.0001 |
| 3 | Indeterminate Colitis | 3.64 | 1.91–6.91 | <0.0001 |
| 4 | Any IBD | 2.39 | 1.77–3.23 | <0.0001 |
*All four models adjusted for age and gender (see Supplemental Table 5 for the complete models).
**The odds of developing an endpoint in patients with CH not taking T4 vs. non CH patients.
Table 6.
IBD prevalence in patients with transient CH (not taking T4) vs. permanent CH (taking T4).
| Transient CH N = 8,128 | Permanent CH N = 24,306 | p value | |
|---|---|---|---|
| CD | 21 (0.26%) | 33 (0.14%) | 0.0189 |
| UC | 34 (0.28%) | 47 (0.19%) | 0.0141 |
| Indeterminate Colitis | 22 (0.21%) | 34 (0.14%) | 0.1724 |
| Any IBD | 66 (0.81%) | 114 (0.47%) | 0.0003 |
Discussion
In a large cohort of the commercially insured U.S. population, we found an increased prevalence of IBD among individuals with CH compared to those without CH. To our knowledge, this is the first study providing evidence for the existence of a common risk factor for these conditions.
Hypothyroidism, more broadly defined and including common acquired causes, has previously been studied in IBD patients, and its frequency was either found to be unchanged or even lower than in non-IBD controls19. Thus hypothyroidism does not appear to be a risk factor in the pathogenesis of IBD. Rather, a suggestive link between IBD and hyperthyroidism, commonly due to autoimmune thyroid disorders, has been found in some studies20. This is potentially due to a shared immune pathology, which can lead to increased inflammation of distinct target organs. In light of these prior studies, the clear association between IBD risk and CH in the U.S. population reported here is notable and would indeed support the concept of a shared inborn susceptibility event, such as, variants in the DUOX2 system that are relevant for thyroid function as well as intestinal immune homeostasis. Interestingly, the association between CH and IBD risk was not related to the severity of CH but was higher for individuals with transient CH. Among the 32,434 CH cohort with available pharmacy claims data, 8,128 (25%) were apparently not treated with T4 and operationally defined as transient CH cases (Tables 2, 3, 6). Although these may include individuals with mild permanent CH in whom treatment had been inappropriately discontinued, the high prevalence of CH cases without ongoing T4 treatment is consistent with an earlier study estimating that by 36 months of age, 38% of US children diagnosed with CH had discontinued T4 treatment21. Common genetic defects that frequently manifest as transient-only CH are partial defects in the genes encoding the subunits of the rate-limiting enzyme in thyroid hormone synthesis, DUOX2 and DUOXA21,2,16,17.
DUOX2 loss-of-function variants appear to be the most common genetic abnormality in CH patients3–7. Since DUOX2 is rate-limiting for hormone synthesis, even mild heterozygous defects are anticipated to increase serum TSH level, which varies by one order of magnitude in the general population. However, minor defects are not necessarily sufficient to manifest haploinsufficiency detected by abnormal neonatal CH screening results. In fact, low genetic penetrance for CH is indicated by studies of families in which heterozygous mutation carriers other than the proband were consistently found to have normal thyroid function tests. Furthermore, by reviewing of DUOX2 variants found in the general population (ExAC database)22 we identified a wide spectrum of loss-of-function variants (Supplemental Table 3). While the frequency of individual variants is far below the threshold required for genome-wide association studies, the combined mutational load is substantial. For instance, over 3.2% of the general population harbor one of the previously in vitro tested loss-of-function variants (32 variants with in vitro activity ≤60% of the reference allele)23 with an even higher proportion of individuals carrying uncharacterized, but predicted to be deleterious variants, considerably higher than the prevalence of even mild CH (Supplemental Table 3). Similarly, among East Asians, over 2% carry the Y246X DUOXA2 complete loss-of-function allele, which has been linked to mild CH in the homozygous state24.
Additional risk factors presumably relevant for precipitating disease in carriers of DUOX2 mutations are distinct for CH and IBD. For CH, the function of the DUOX1 isoenzyme is likely critical for the potential to compensate partial DUOX2 defects25 and expressivity of the defects is presumably modulated by nutritional iodine intake1. Noteworthy, DUOX1 is expressed in the thyroid epithelial cells but not in the intestine, so compensated partial DUOX2 defects without associated CH might still have an impact on intestinal immune homeostasis leading to intestinal inflammation. For IBD, a different set of genetic (e.g., NOD2) and environmental (e.g., intestinal microbiota) factors are involved in its pathogenesis. It is thus not surprising that among the so-far studied pediatric CH cohorts with DUOX2 mutations no case of concurrent IBD has been reported, and that the recently described cases of DUOX2-associated IBD all had apparently normal thyroid function parameters13–15. Based on the high frequency of DUOX2 mutations in unselected CH cohorts of various ethnic origins3–7, our finding of a significantly increased risk of developing IBD among CH individuals lends support for the hypothesis of a shared susceptibility factor, specifically DUOX2 genetic variants that can affect both thyroid function as well as intestinal immune homeostasis.
An obvious strength of the current study is the large sample size that made testing the main hypothesis feasible. However, several limitations must also be noted. First, there are general limitations of claims based data not collected for research purposes, such as data entry errors and underreporting of diagnoses. To ameliorate the influence of misdiagnoses, we used a 2–3 code requirement in this study. Second, although we matched the study cohorts by age and gender, a generally acceptable method, it is not possible to fully account and correct for other possible confounders (e.g., smoking status, ethnicity).
In summary, we demonstrated a significant association between CH and the development of IBD providing evidence for a shared susceptibility factor. The risk was highest for patients with transient CH, for which partial defects in the DUOX2 system are a frequent genetic cause. Thus our results would seem to support the hypothesis that DUOX2 mutation carriers, common in the general population, are not only at increased risk for thyroid dysfunction but also impaired intestinal immune homeostasis.
Methods
Study Design and Data Source
We performed a retrospective cohort analysis to compare the prevalence of IBD in patients with and without CH. We queried the Truven Health MarketScan databases from January 1st, 2009 through December 31st, 2015 to identify patients with CH and a matched cohort of patients without CH. The MarketScan databases contain health insurance claims for over 230 million privately insured patients in the U.S. This study was considered exempt by the Institutional review board at the University of Michigan due to the de-identified nature of the dataset.
Study sample
The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes were used to identify patients with CH (243.x) and IBD (555.x and 556.x). In order to ensure that CH was confirmed, patients were selected for inclusion if they had at least 2 of the CH ICD-9-CM code in an outpatient setting in 2 different visits while for IBD diagnosis, patients were selected if they had at least 3 ICD-9 codes of IBD in an outpatient setting in 3 different visits between 2009–2015. This approach has a positive predictive value for CD of 0.84 and a positive predictive value for UC of 0.9126. We also required at least 1-year enrollment to adequately capture diagnoses and eliminate possible confounding resulting from loss of follow-up. Patients meeting the inclusion criteria for CH were matched in their birth year and sex, in a 1-to-1 fashion, to patients with no CH diagnosis selected from the pool of database participants. If a patient with CH had more than one match, a randomized algorithm was employed to select a match.
Primary Outcome
The primary endpoint was a diagnosis of IBD in patients with and without CH at any point of the study period. Given the congenital nature of CH, it is safe to assume that IBD manifested after the diagnosis of CH.
Statistical Analysis
We compared the demographics and prevalence of IBD diagnosis between the two cohorts using the Chi-square test for categorical data, and the Student t-test and Mann Whitney U test for continuous data. In sensitivity analyses, we used multivariable logistic regression models to adjust for age and gender in subgroup analyses. Variables (e.g., gender and age groups) were selected for inclusion in the multivariable model if they were significant in bivariate analyses (Supplemental Tables 4 and 5). A two-sided p < 0.05 was used to denote statistical significance. All analyses were performed using Statistical Analysis System (SAS) software version 9.4 (SAS Institute, Inc., Cary, NC). The 1-to-1 matching was performed with R version 3.3.2 (R foundation, Vienna, Austria).
Electronic supplementary material
Acknowledgements
This work was supported by Michigan Institute for Clinical & Health Research (MICHR), Clinical Translational Science Award NIH UL1TR000433 and UL1TR002240 and by NIH R01 DK117565-01 (JYK and HG).
Author Contributions
H.G. was involved in the interpretation of the data and critically revised the article’s intellectual content. M.N. contributed to the acquisition and analysis of data and was involved in the interpretation of the data and in the revision of the manuscript. T.D.K. contributed to the concept and design of the study, were involved in the interpretation of the data, and drafted the article. J.A., J.M.L., S.B. and M.E. critically revised the intellectual content of the manuscript. J.Y.K. contributed to the concept and design of the study, was involved in the interpretation of the data, and critically revised the article’s intellectual content. A.K.W. contributed to the concept and design of the study, oversaw the analysis and acquisition of data, was involved in the interpretation of the data, and critically revised the article’s intellectual content.
Competing Interests
J. M. L. consults for Unitio. Other authors have no disclosure to report.
Footnotes
Helmut Grasberger, Mohamed Noureldin and Timothy D. Kao contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28586-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
John Y. Kao, Email: jykao@umich.edu
Akbar K. Waljee, Email: awaljee@med.umich.edu
References
- 1.Grasberger H. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol Cell Endocrinol. 2010;322:99–106. doi: 10.1016/j.mce.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Moreno JC, et al. Inactivating mutations in the gene for thyroid oxidase 2 (thox2) and congenital hypothyroidism. N Engl J Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. [DOI] [PubMed] [Google Scholar]
- 3.Tan M, et al. The prevalence, clinical, and molecular characteristics of congenital hypothyroidism caused by duox2 mutations: A population-based cohort study in guangzhou. Horm Metab Res. 2016;48:581–588. doi: 10.1055/s-0042-112224. [DOI] [PubMed] [Google Scholar]
- 4.Park KJ, et al. Duox2 mutations are frequently associated with congenital hypothyroidism in the korean population. Ann Lab Med. 2016;36:145–153. doi: 10.3343/alm.2016.36.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu C, et al. Mutation screening of duox2 in chinese patients with congenital hypothyroidism. J Endocrinol Invest. 2015;38:1219–1224. doi: 10.1007/s40618-015-0382-8. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo K, et al. High prevalence of duox2 mutations in japanese patients with permanent congenital hypothyroidism or transient hypothyroidism. J Pediatr Endocrinol Metab. 2016;29:807–812. doi: 10.1515/jpem-2015-0400. [DOI] [PubMed] [Google Scholar]
- 7.de Filippis T, et al. A frequent oligogenic involvement in congenital hypothyroidism. Hum Mol Genet. 2017;26:2507–2514. doi: 10.1093/hmg/ddx145. [DOI] [PubMed] [Google Scholar]
- 8.Jiang, H., et al. High prevalence of duox2 gene mutations among children with congenital hypothyroidism in central china. Eur J Med Genet59:526-531 (2016). [DOI] [PubMed]
- 9.Haberman Y, et al. Pediatric crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest. 2014;124:3617–3633. doi: 10.1172/JCI75436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasberger, H., et al. Increased expression of duox2 is an epithelial response to mucosal dysbiosis required for immune homeostasis in mouse intestine. Gastroenterology149, 1849–1859 (2015). [DOI] [PMC free article] [PubMed]
- 11.Alvarez LA, et al. Nadph oxidase-derived h2o2 subverts pathogen signaling by oxidative phosphotyrosine conversion to pb-dopa. Proc Natl Acad Sci USA. 2016;113:10406–10411. doi: 10.1073/pnas.1605443113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 13.Hayes P, et al. Defects in nadph oxidase genes nox1 and duox2 in very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2015;1:489–502. doi: 10.1016/j.jcmgh.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine AP, et al. Genetic complexity of crohn’s disease in two large ashkenazi jewish families. Gastroenterology. 2016;151:698–709. doi: 10.1053/j.gastro.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parlato, M. et al. First identification of biallelic inherited duox2 inactivating mutations as a cause of very early onset inflammatory bowel disease. Gastroenterology (2017). [DOI] [PubMed]
- 16.Jin, H. Y. et al. High frequency of duox2 mutations in transient or permanent congenital hypothyroidism with eutopic thyroid glands. Horm Res Paediatr82, 252–260 (2014). [DOI] [PubMed]
- 17.Maruo Y, et al. Natural course of congenital hypothyroidism by dual oxidase 2 mutations from the neonatal period through puberty. Eur J Endocrinol. 2016;174:453–463. doi: 10.1530/EJE-15-0959. [DOI] [PubMed] [Google Scholar]
- 18.Enacan RE, et al. [transient congenital hypothyroidism due to biallelic defects of duox2 gene. Two clinical cases]. Arch Argent Pediatr. 2017;115:e162–e165. doi: 10.5546/aap.2017.e162. [DOI] [PubMed] [Google Scholar]
- 19.Shizuma T. Concomitant thyroid disorders and inflammatory bowel disease: A literature review. Biomed Res Int. 2016;2016:5187061. doi: 10.1155/2016/5187061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarnerot G, Azad Khan AK, Truelove SC. The thyroid in ulverative colitis and crohn’s disease. Ii. Thyroid enlargement and hyperthyroidism in ulcerative colitis. Acta Med Scand. 1975;197:83–87. doi: 10.1111/j.0954-6820.1975.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 21.Kemper AR, Ouyang L, Grosse SD. Discontinuation of thyroid hormone treatment among children in the united states with congenital hypothyroidism: Findings from health insurance claims data. BMC Pediatr. 2010;10:9. doi: 10.1186/1471-2431-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lek M, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muzza M, Fugazzola L. Disorders of h2o2 generation. Best Pract Res Clin Endocrinol Metab. 2017;31:225–240. doi: 10.1016/j.beem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Zamproni I, et al. Biallelic inactivation of the dual oxidase maturation factor 2 (duoxa2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008;93:605–610. doi: 10.1210/jc.2007-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aycan Z, et al. Digenic duox1 and duox2 mutations in cases with congenital hypothyroidism. J Clin Endocrinol Metab. 2017;102:3085–3090. doi: 10.1210/jc.2017-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou JK, et al. Accuracy of diagnostic codes for identifying patients with ulcerative colitis and crohn’s disease in the veterans affairs health care system. Dig Dis Sci. 2014;59:2406–2410. doi: 10.1007/s10620-014-3174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


