Abstract
In vertebrate retinal progenitor cells, the proneural factor Atoh7 exhibits a dynamic tissue and cellular expression pattern. Although the resulting Atoh7 retinal lineage contains all seven major cell types, only retinal ganglion cells require Atoh7 for proper differentiation. Such specificity necessitates complex regulation of Atoh7 transcription during retina development. The Notch signaling pathway is an evolutionarily conserved suppressor of proneural bHLH factor expression. Previous in vivo mouse genetic studies established the cell autonomous suppression of Atoh7 transcription by Notch1, Rbpj and Hes1. Here we identify four CSL binding sites within the Atoh7 proximal regulatory region and demonstrate Rbpj protein interaction at these sequences by in vitro electromobility shift, calorimetry and luciferase assays and, in vivo via colocalization and chromatin immunoprecipitation. We found that Rbpj simultaneously represses Atoh7 transcription using both Notch-dependent and –independent pathways.
Introduction
During vertebrate embryonic development, multipotent retinal progenitor cells (RPCs) undergo a prolonged period of differentiation during which six neuronal (retinal ganglion, cone and rod photoreceptor, amacrine, horizontal and bipolar) and one glial (Müller) cell type are produced and assembled into a highly laminated tissue. Multiple signaling pathways and transcription factors, including proneural basic helix-loop-helix (bHLH) transcription factors, regulate retinal cell fate, as reviewed in1. Among these factors, Atoh7 (Atonal homolog 7, Math5, Ath5) is dynamically expressed by subsets of embryonic RPCs and required for retinal ganglion cell (RGC) formation2–6. Without Atoh7 function, essentially all RGCs fail to differentiate and adult animals lack optic nerves3,5,6. Although the Atoh7 retinal lineage includes all seven major cell types, gene activity is only required for RGC genesis. To understand how Atoh7 acts as an RGC competence factor requires deeper understanding of its mode of action as a DNA-binding protein, and the mechanisms tightly regulating its mRNA and protein. Here we focus on particular aspects of Atoh7 transcriptional regulation.
Previous studies of vertebrate Atoh7 genomic architecture defined two enhancer regions on the 5′ side of the lone Atoh7 coding exon, each containing multiple conserved noncoding elements (CNEs)7–10. In mice, the distant, shadow enhancer is 9.5 kb and the proximal, primary enhancer is 1.5Kb upstream of the Atoh7 ATG start codon. The primary enhancer is further subdivided into two CNEs, termed distal and proximal. The distal CNE contains validated Pax6 (paired domain) and Neurog2 (E box) binding sites, through which Pax6 activates transcription, and Neurog2 drives the initial wave of retinal neurogenesis2,10–12. However, the activities of these two factors cannot account for the rapid downregulation of Atoh7 in subsets of RPCs at differentiation.
Given that Notch signaling regulation of bHLH factors occurs widely, including throughout the vertebrate nervous system, we wish to understand how this pathway controls Atoh7 expression at the molecular level, in the context of retinogenesis. Canonical Notch signaling initiates with a Delta-like or Jagged/Serrate ligand on one cell binding to a Notch receptor on an adjacent cell. This triggers proteolytic cleavage of the receptor to ultimately release its intracellular domain (termed NICD). The NICD complexes with a CSL protein (CBF-1, RBPJ-κ, Recombination Signal Binding Protein for immunoglobulin kappa J region) in human/mouse, Su(H) (Suppressor of Hairless) in Drosophila, Lag-1 (lin12 and glp-1 phenotype) in C. elegans) and the Maml (Mastermind-like) co-activating factor. This protein complex activates downstream target gene transcription by binding to a variety of CSL sites within noncoding DNA13–16. Well-characterized effector genes of the Notch pathway include the Hes (Hairy/Enhancer of Split (E(Spl)) and Hey gene families, which encode transcriptional repressor proteins17–19. Intriguingly, during Drosophila retinal development, Notch signaling reiteratively regulates Atonal transcription20,21. In undifferentiated cells anterior to the eye disc morphogenetic furrow (a moving differentiation boundary), Notch signaling activates Atonal in a continuous stripe of cells. But more posteriorly, the Notch complex, via E(Spl) activity, suppresses Atonal expression in cells adopting non-R8 photoreceptor fates. This latter activity embodies the classic neurogenic role of Notch signaling. Importantly, these phases of Atonal regulation are separated in both time and space, and utilize distinct Atonal enhancers, as reviewed in22.
Predicted CSL binding sites are interspersed throughout metazoan genomes, but it is challenging to know which are in vivo targets. However, the probability of any gene being a direct target is enhanced when its expression is affected by Notch pathway loss- and gain-of-function mutants. Previous examinations of mouse retinal mutants showed that loss of Notch1, Rbpj or Hes1 derepresses Atoh7 mRNA expression and RGC neurogenesis23–29. By contrast, overexpression of activated Notch1 (NICD1) stimulates proliferation, thereby blocking Atoh7LacZ expression and RGC formation26,30. Yet, the mechanism(s) underlying Notch pathway regulation of Atoh7 remain unresolved.
A classical view of Notch regulation of bHLH factors holds that an activated Notch protein complex transcriptionally activates Hes1, which in turn would repress Atoh7 transcription. But, other models are possible, including Rbpj direct regulation of Atoh7, either in a corepressor complex (repression), or in a Notch complex (activation)21,31,32. However, all genetic data consistently support Rbpj suppression of Atoh7 transcription. In this study, we characterize four CSL binding sites situated in Atoh7 5′ regulatory DNA. We found that at the peak of Atoh7 retinal expression, Rbpj occupies one CSL site, R3 within the distal primary enhancer, and this binding site mediates transcriptional repression. We also discovered that simultaneous loss of all four binding sites reduced Atoh7 transcription, suggesting a distinct mode of Rbpj regulation, consistent with locus priming for efficient transcriptional regulation by other factors33. Importantly, both Rbpj-mediated repression at site R3, and activation via multiple sites are Notch-independent, although Notch1/3 signaling has a measurable impact on Atoh7 mRNA levels. Overall our data are suggestive of three separate, yet simultaneous modes of Rbpj regulation: locus priming, direct repression and canonical Notch pathway suppression. We propose that integrated regulatory inputs are important for precise control of Atoh7 pulsatile expression in the developing retina.
Results
Predicted Rbpj binding sites in Atoh7 regulatory DNA
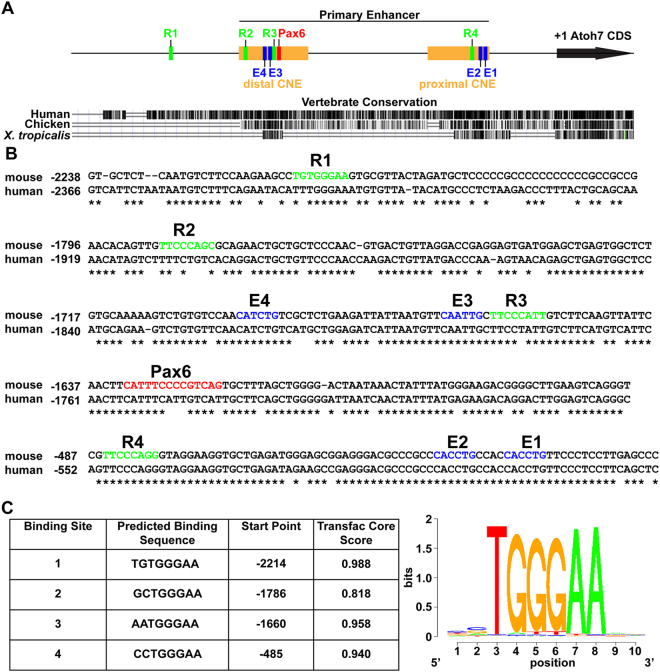
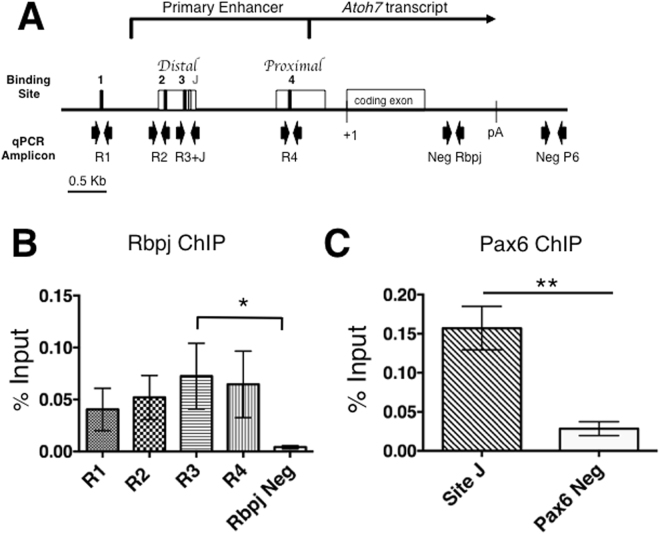
The goal of this study was to identify and test putative Rbpj consensus (CSL) binding sites relative to Atoh7 transcriptional activity. Because the Atoh7 primary enhancer recapitulates endogenous mRNA expression in frog, chick, zebrafish and mouse transgenic studies, and contains two CNEs, it was the strongest candidate to contain CSL binding sites8,10,11,34. However, we used the CSL consensus TRANSFAC MATCH algorithm (5′-[C/T]GTG[G/T]GAA-3′) to perform an unbiased search of 6 Kb of noncoding DNA surrounding the Atoh7 coding exon13–15. Four putative CSL sites (termed R1–R4) were identified; all positioned 5′ to the ATG codon (Fig. 1A). Three of these sites (R2–R4) reside in the primary enhancer CNEs, while the other site (R1) is 300 bp upstream of the primary enhancer (Fig. 1A,B). Of the four sites, R1, R3, and R4 are highly conserved between mouse and human (Fig. 1B), with only R3 and R4 having analogous sequences in the chick and frog Atoh7/Ath5 locus (not shown). Interestingly, site R3 is situated in between previously characterized Pax6 and Neurog2 (E4/E3) binding sites (Fig. 1A,B)8,10,11. Site R4 lies within the proximal CNE, very close to the TATAA box and two highly conserved Ebox binding sites (Fig. 1A,B). In the adult frog retina, this CNE is critical for maintaining Atoh7/Ath5 expression in the ciliary marginal zone8.
Figure 1.
Predicted Rbpj CSL binding sites in mouse Atoh7 5′ regulatory DNA. (A) Diagram of the primary enhancer and the distal and proximal CNEs, indicating the position of four predicted Rbpj binding sites (R1–R4, green boxes), a previously characterized Pax6 binding site (red box) and four E-boxes binding sites (E1–E4, blue boxes). UCSC Genome browser view of a 3 kb 5′ noncoding region of mouse Atoh7 (+1 = A of ATG start codon) using mm10 assembly and vertebrate evolutionary conservation tracks, with mouse as the reference genome. (B) ClustalW alignments of mouse and human Atoh7 genomic sequence, with asterisks indicating nucleotide identity diagrammed in (A,C) The four predicted Rbpj binding site sequences, identified by the Transfac CSL consensus matrix.
Rbpj and Atoh7 antibody validation and protein colocalization
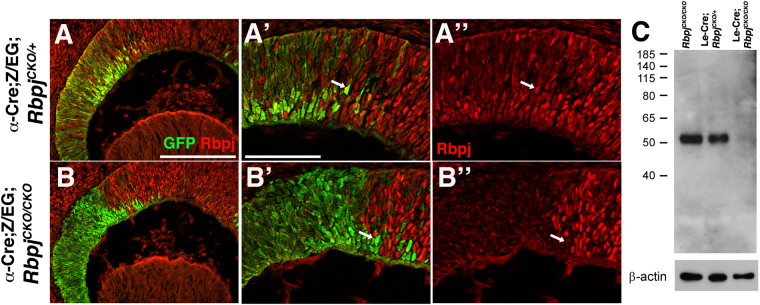
Both commercial and academic laboratory-made antibodies have been generated for detecting mammalian Rbpj or Atoh7 proteins. In a separate project, we rigorously tested a polyclonal antibody with high specificity for human and mouse Atoh7 proteins35. Previously, a rat anti-Rbpj antibody was used in our exploration of Notch signaling during mouse lens development36,37. Here we further confirmed the specificity of this monoclonal antibody, during mouse retinogenesis, and evaluated its usefulness in biochemical assays (Fig. 2). We immunolabeled E13.5 retinal cryosections from α-Cre;Z/EG;RbpjCK°/+ control and α-Cre;Z/EG;RbpjCKO/CKO mutant embryos (Fig. 2A,B). The α-Cre transgene induces Cre-mediated recombination in the distal retina38 and Cre-mediated recombination is permanently marked by GFP expression from the Z/EG transgene39. In α-Cre;Z/EG;RbpjCKO/+ controls, Rbpj protein is ubiquitous, including within the Cre lineage (GFP+ cells) (Fig. 2A). However, the same α-Cre cells lacking Rbpj showed a cell autonomous loss of protein expression (Fig. 2B). We also used this antibody for Western blotting, using denatured protein extracts from genotyped and pooled embryonic lenses, including from Rbpj conditional mutants40,41. A single immunoreactive band of 56 kDa was recognized in the wild type and heterozygous western lanes, but completely missing from homozygous mutant lens extracts (Fig. 2C).
Figure 2.
Rbpj antibody specificity. (A,B) GFP-positive cells (green) indicate the E13.5 α-Cre retinal lineage. A-A”) Anti-Rbpj labeling of α-Cre;Z/EG;RbpjCKO/+ control cryosections shows ubiquitous expression of the nuclear Rbpj protein (red), including within GFP+ cells (arrow). (B-B”) Cell autonomous loss of Rbpj expression (red) in conditionally mutant α-Cre;Z/EG;RbpjCKO/CKO cells (arrow). n = 3 biologic replicates embryos/genotype. (C) Western blot of E14.5 lens protein extracts, collected from RbpjCKO/CKO, Le-Cre;RbpjCKO/+ and Le-Cre;RbpjCKO/CKO embryos, respectively. A single band of 56 kDa, the predicted size of Rbpj, is absent from mutant lenses, with β-actin loading control at bottom. The top panel is the uncropped blot ECL autorad exposure, which was stripped and reprobed with Actin and Jagged1 loading controls (see Supplemental Fig S3). (A-B”) apical is up. Bar in A for A,B = 20μm; in A’-for A’ to B” = 40 μm. White arrows point to GFP+ cells expressing Rbpj in controls (A-A”), but not in GFP+;Rbpj mutant cells (B-B”).
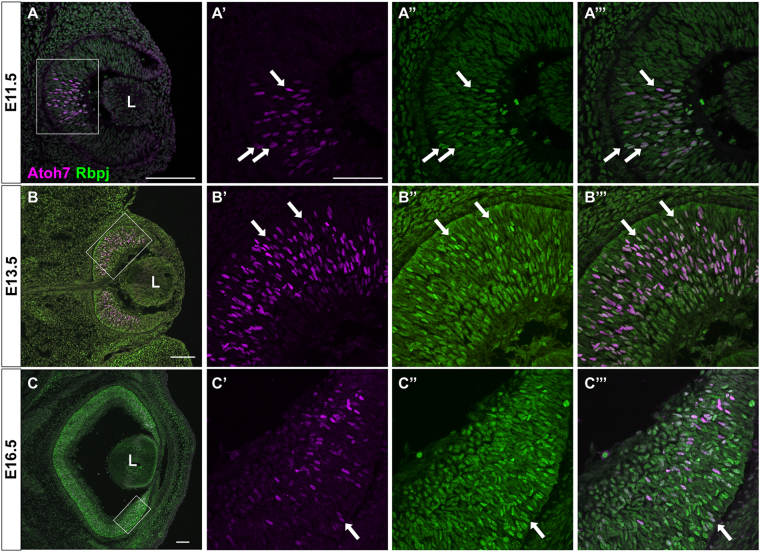
Next, we compared Atoh7 and Rbpj protein expression during in vivo embryonic retinal development. The spatiotemporal expression pattern of Atoh7 protein is highly dynamic, restricted to the central optic cup at E11.5 (Fig. 3A), but expressed by a broader group of E13.5 RPCs (Fig. 3B), and then confined to the periphery by E16.5 (Fig. 3C). Because Rbpj expression is ubiquitous, complete coexpression was expected. However, we noted that at every age almost all Atoh7+ cells exhibit brighter Rbpj expression than the Atoh7-negative cells. This is suggestive of differential Rbpj protein expression, although immunohistochemistry is a nonquantitative technique. To rule out non-specific secondary antibody cross-reactivity, we performed control experiments on retinal sections that were incubated with only one primary antibody (rabbit anti-Atoh7 or rat anti-Rbpj), prior to simultaneous application of both secondary antibodies (Fig. S1).
Figure 3.
Colocalization of Rbpj and Atoh7 proteins during retinal neurogenesis. (A–C) Double antibody labeling of retinal sections highlights essentially complete nuclear co-localization. The boxed area in each panel is shown at higher magnification to the right. (A-A’”) At E11.5 Atoh7 protein is restricted to a subset of central RPCs (A’). (B-B’”) By E13.5 the initial wave of neurogenesis has reached the periphery and Atoh7 is expressed more broadly throughout the apical neuroblast layer. (C-C’”) Consistent with mRNA expression studies, Atoh7+ cells are localized to the peripheral retina (C’). Panel C is a composite stitched together from 4 overlapping 10× image fields. Colocalization of Rbpj (green) and Atoh7 (purple) is shown as white. Rostral is up in all panels. L = lens; Bar in A,C,E = 100 μm; in B,D,F = 50 μm. In (A’-C”’) white arrows point to Atoh7+ cells that also contain high levels of Rbpj.
Rbpj binding to Atoh7 5′ regulatory DNA in vitro and in vivo
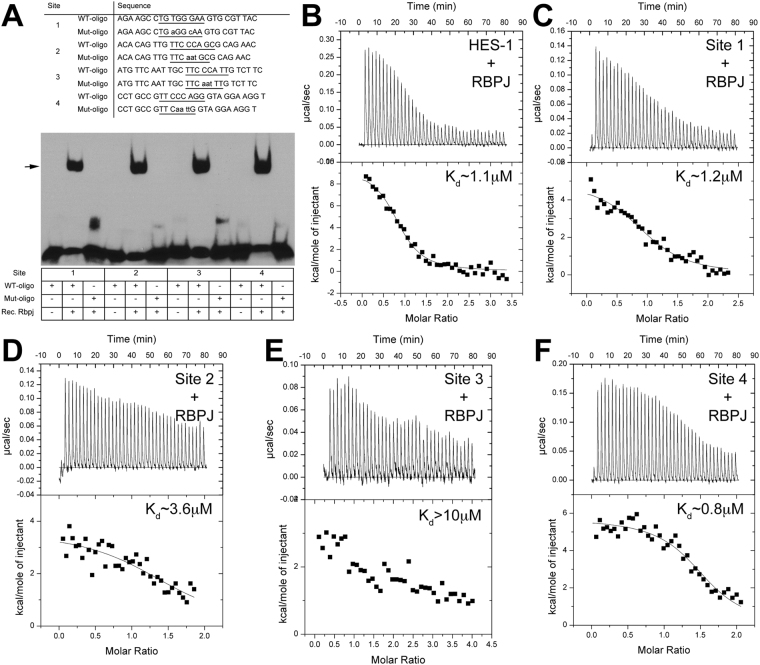
For initial assessment of Rbpj binding to the four putative CSL sites, we conducted in vitro electromobility shift assays (EMSA), using bacterially expressed and purified mouse Rbpj protein (residues 53–474), previously shown to bind DNA42. Rbpj protein was incubated with biotin-labeled double stranded oligonucleotides, in which the putative binding site is centrally located (Fig. 4A, Supplemental Table S4). We noted that all four sites shifted upon incubation with Rbpj protein (Fig. 4A). When key nucleotides within each CSL site were mutated, based on previous CSL-DNA structural studies, binding was abolished. We conclude that Rbpj protein specifically binds to each CSL binding site in vitro.
Figure 4.
In vitro binding of Rbpj to CSL consensus sites in Atoh7 5′ DNA. (A) Oligonucleotides with CSL consensus sites (underlined) identified in 5′Atoh7 noncoding DNA, synthesized and used for testing Rbpj protein-DNA binding by EMSA (WT, wild type; Mut, mutated). Lower case indicates a mutated nucleotide. Recombinant Rbpj protein and biotin-labeled oligonucleotide complexes undergo specific mobility shifts (arrow). See uncropped autorad exposure in Supplemental Fig. 3. n = 4 independent assays. (B–F) Representative thermograms (raw heat signal and nonlinear least squares fit to the integrated data) for Hes1 CSL consensus site43 (positive control in (B) and Atoh7 CSL sites R1–R4, all shown with their dissociation constants (Kd).
We also performed isothermal titration calorimetry (ITC) to quantitate binding, using purified Rbpj with the oligomeric DNA duplexes that correspond to the four putative CSL binding sites (Fig. 4B–F and Supplemental Table S5). As positive and negative controls, we tested Rbpj with the CSL binding site from the Hes1 proximal promoter element (GTTACTGTGGGAAAGAAAG) and the non-specific sequence (GCTACTCATACCTAGAACG), respectively, and detected binding from the Hes1 site (~1 μM Kd), but did not detect binding from the non-specific site (data not shown). The results showed Rbpj bound to three of the four putative CSL sites with comparable affinity to the well-characterized Hes1 site (Fig. 4B)43. However, we found that one consensus site, R3, displayed a lower binding affinity (Fig. 4E). While we were unable to separate any potential effects of nucleotide variation and flanking sequence, interestingly, R3 lies in between validated Pax6 and Neurog2 binding sites10,11.
Next, we wished to determine which CSL consensus sites are occupied by Rbpj during in vivo retinal development. Previously we demonstrated that Pax6 occupies consensus site J in the human ATOH7 gene, using chromatin from Ad12Her10 retinal cell line10. As a positive control, we performed Pax6 ChIP in parallel here, using mouse E14.5 retinal chromatin. This age was selected because it is the peak of Atoh7 expression44. Dissected retinas from individual embryonic litters were pooled and frozen en masse (n = 3 litters). After lysis, cross-linking, quantification and sheering, chromatin was immunoprecipitated with rat anti-Rbpj, rabbit anti-Pax6 or relevant IgG controls (see Methods). The antibody-bound chromatin complexes were purified, crosslinks reversed, and the isolated genomic DNA used as a template for real-time PCR. Each primer set amplified an amplicon specific for each putative binding site (Fig. 5A, Supplemental Table S4). As expected, Pax6 occupies site J in the Atoh7 primary enhancer (Fig. 5C). Although there was measurable occupancy of Rbpj at all four sites (R1–R4) relative to 3′UTR, only site R3 shows statistically significant enrichment (Fig. 5B). We conclude that Rbpj directly regulates Atoh7 transcription, via site R3. Although occupancy of the other three sites at E14.5 was not statistically significant, we hypothesized there could be more robust enrichment at other developmental ages, or that Rbpj simultaneous occupancy of multiple sites might contribute to a local Atoh7 chromatin configuration33.
Figure 5.
In vivo occupancy of Rbpj at Atoh7 locus in E14.5 retinal chromatin. (A) Diagram of mouse Atoh7 genomic locus indicating CSL binding sites and qPCR amplicons evaluated, with Pax6 site J site serving as positive control. (B,C). Real-time PCR analyses of DNA fragments amplified after Rbpj or Pax6 ChIP, displayed as the mean ± SEM of three biologic replicate assays, performed in PCR duplicate, with the preimmune IgG values subtracted. Only Rbpj site R3 is significantly enriched over negative control. *p ≤ 0.05; **p ≤ 0.01.
Rbpj differentially regulates Atoh7 transcription
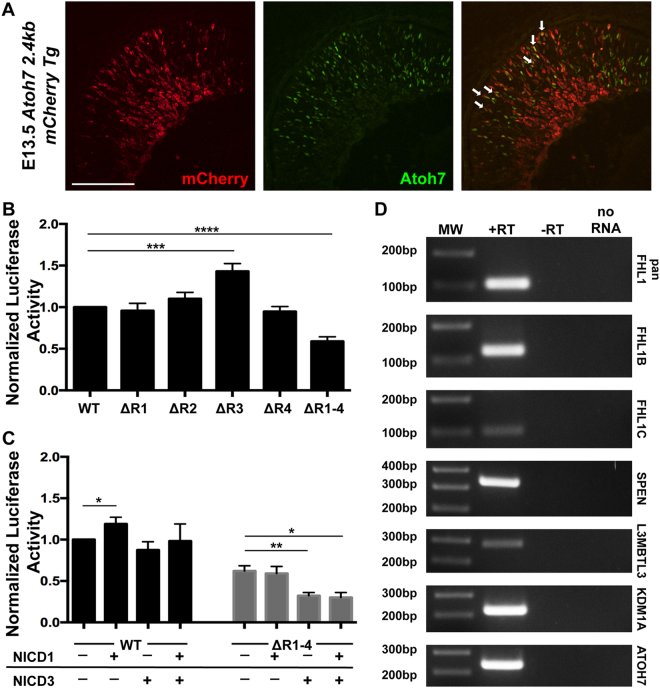
Although Rbpj binds to all four CSL sites in vitro and at least site R3 in vivo, it is unclear if it does so via the Notch complex (activation), or a corepressor complex (repression). To address these possibilities formally we used luciferase reporter assays to measure the activity and requirement for each site individually, versus simultaneous mutation of all 4 sites, within a previously identified primary gene Atoh7 enhancer7,10. However, first we compared Atoh7 primary gene enhancer expression to that of the endogenous protein, which had not been previously reported8,10,11,34,45,46. For this we created a transgenic construct with 2.4Kb of mouse Atoh7 5′ DNA joined to a minimal human β-globin promoter (BG) and red fluorescent monomeric Cherry reporter (mCherry)47. We then used antibody colabeling to evaluate mCherry expression in retinal cryosections from E13.5 transient transgenic embryos. We found that endogenous Atoh7 significantly overlaps with Cherry+ cells (Fig. 6A) in the proliferative neuroblast layer, but not in the differentiated ganglion cell layer (GCL). This difference can be attributed to the greater stability of the fluorophore protein compared to Atoh7. Coexpression here was roughly equivalent to what was previously reported for another mouse transgenic line in which the human ATOH7 shadow enhancer drives BG-mCherry expression7,35.
Figure 6.
Differential Notch-mediated regulation of Atoh7 transcription. (A) Colocalization of transient mCherry transgenic expression, driven by a 2.4 kb mouse Atoh7 enhancer, to endogenous Atoh7 protein in the E13.5 retina (White arrows point to colabeled cells). Both cytoplasmic and nuclear Cherry expression are seen, presumably due to the inefficiency of a synthetic nuclear localization sequence and reporter antibody sensitivity, which detected both nascent Cherry protein in the cytoplasm and its accumulation in the nucleus. Scale bar = 100 μm (B) Comparison of mouse Atoh7 transcriptional activity in HEK293T cells following single versus quadruple CSL site mutation. Transcriptional activity of individual CSL site mutants, and quadruple mutant within Atoh7–2.6Kb luciferase/pGL2 construct. Only site R3 in the distal CNE is required to suppress Atoh7 transcription. However, loss of sites R1–4 caused significant downregulation of Atoh7 transcription. n = 9 biological replicates (each performed in technical triplicate). (C) Cotransfection of activated NICD1, NICD3, or both constructs with Atoh7 wild type or RΔ1–R4 mutant luciferase constructs. n ≥ 3 biological replicates (each in technical triplicate). All luciferase experiments were normalized to a co-transfected Renilla control. A two-tailed, unpaired t-test with equal standard deviation and Gaussian distribution was used to determine p-value. (D) RT-PCR analysis of E13.5 retinal cDNA showing expression of all four co-repressor gene mRNAs. Distinct PCR primers for the Fhl1 (KyoT) gene were used that amplified an exon common to all splice products (pan Fhl1), and the specific splice variants Fhl1b (KyoT3) and Fhl1c (KyoT2) that uniquely contain the Rbpj-interaction domain78. All PCR reactions were run on a single gel, uncropped image provided in Supplemental Fig. S3. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p < 0.0001 and Figure S2 ***p ≤ 0.001; ****p < 0.0001.
Next, we performed luciferase assays in both HEK293T kidney-derived and AD12Her10 retinal-derived human cell lines, under identical conditions48,49. This strategy was chosen because our previous study of Pax6 regulation of Atoh7 transcription suggested that retinal-specific context influences assay output10. Yet we found no differences between these cell lines, although HEK293T cells endogenously express RBPJ, but not ATOH750,51, whereas AD12Her10 cells express both genes (Supplemental Fig. S2). Individual CSL site mutations (ΔR1, ΔR2, or ΔR4) did not affect transcriptional output, relative to wild type (Fig. 6A). By contrast, our mutation of CSL site R3 (ΔR3) derepressed luciferase activity over wild type levels, in both HEK293T and AD12Her10 cells, in the absence of exogenous Notch intracellular domains (Fig. 6A, Supplemental Fig. S2). We concluded that this particular binding site normally represses Atoh7 transcription. By contrast, when all four sites (ΔR1–4) were simultaneously mutated, there was a significant decrease in Atoh7 transcriptional levels (Fig. 6B, Supplemental Fig. 2). This is suggestive of coordinated, Rbpj-mediated transcriptional activation, among multiple (possibly all four) binding sites.
Rbpj occupancy of site R3 represses Atoh7, presumably reflecting corepressor complex activity. However, the coordinated enhancement via multiple sites might be attributable to direct regulation of Atoh7 transcription, via a Notch complex. Therefore, we tested for Notch-dependence by coexpressing the intracellular domains of Notch1 (NICD1) or Notch3 (NICD3) in our luciferase assays. Each receptor alone, as well as in combination, was previously shown to be required in vivo for particular aspects of Atoh7 expression26. Plasmids containing the NICD1, NICD3, or a mixture of the two, were cotransfected with either the Atoh7 wild type or ΔR1–R4 mutant luciferase constructs52,53. Increasing NICD1 levels stimulated Atoh7 transcription but had no impact on the ΔR1–R4 mutant (Fig. 6B). NICD3 or NICD1 + NICD3 coexpression did not affect wild type Atoh7 activity, but further suppressed transcription in the ΔR1-R4 mutant (Fig. 6B). We interpret these outcomes to mean that multiple CSL binding sites help maintain Atoh7 basal level transcription. The elevated luciferase activity seen after NICD1 overexpression might represent Notch-dependent regulation of sites R1, R2, and R4, or an ectopic effect of NICD1 acting as a Rbpj sink, de-repressing R3 similar to R3 mutagenesis. In addition, Notch-mediated regulation of Atoh7 must utilize other noncoding sequences based on the decrease of transcriptional activity with NICD3 overexpression, potentially through Hes consensus N-boxes16,43,54,55.
Multiple mammalian “co-repressor” genes encode proteins that interact with Rbpj via CSL binding sites56. These genes are unrelated at the primary sequence level, but predicted to link Rbpj to HDAC machinery, and include Smrt/Ncor2, Spen/Mint/Sharp, Fhl1b//KyoT2, Rita, Skip, L3mbtl3 and Kdm1a/Lsd157–59. Interestingly, Kdm1a/Lsd1 is expressed in the retina from E17-P15, and its pharmacologic inhibition in retinal explants induced an upregulation of bHLH factor expression60. Because nothing further is known about the retinal expression other Rbpj co-repressor genes, we used RT-PCR to test for transcription of four genes (Spen; Fhl1b; L3mbtl3; Kdm1a) in the E13.5 mouse retina (Fig. 6C). The Fhll/KyoT gene encodes multiple splice products, so we assayed for one exon common to all splice variants, as well as specific Fhl1b and Fhl1c exons that uniquely contain Rbpj-interaction domains61–63. We found that all of these co-repressor mRNAs are expressed during embryonic retinal neurogenesis (Fig. 6C). Given that at least four of seven putative co-repressors are present in the mammalian retina, elucidation of their specific mechanisms of action will require in-depth, future studies.
Discussion
Previous studies of Notch signaling in the vertebrate retina genetically linked Rbpj activity to Atoh7 expression and retinal ganglion cell differentiation24–29. Notch activity normally suppresses both Atoh7 and RGC neurogenesis, but the molecular mechanisms for this remain unresolved. Here, we explored the possibility that Rbpj can directly regulate Atoh7 transcription.
Bioinformatic analysis of noncoding sequences surrounding the mouse Atoh7 gene identified four putative Rbpj-CSL consensus binding sites. While a CSL consensus sequence is useful for predicting Rbpj target genes13–15, it is not the only nucleotide motif this protein can bind16,43. One particular binding site arrangement, called SPS (Su(H)-paired site), consists of two CSL binding sites in a head to head configuration separated by ~16 nucleotides64. These SPS elements are occupied by dimeric NICD/Rbpj complexes, to regulate transcription65–68. Although the Atoh7 upstream CSL binding sites lack a canonical SPS arrangement, there remains some possibility for such a mechanism, since a cryptic CSL element in the Hes5 promoter acts in a dimeric SPS complex65.
Among the CSL sites analyzed here for the embryonic retina, R3 stands out as unique. Counterintuitively, CSL has the weakest affinity for R3 in vitro, but was the only site that ChIPped CSL in vivo with statistical significance. Similar phenomenon has been observed for Su(H), the fly CSL ortholog, binding of the sparkled (spa) enhancer in Drosophila, whereby the low affinity Su(H) sites are critical for proper gene expression and patterning driven by spa69. The location of R3 within the distal CNE is flanked by a Neurog2-dependent Ebox (30 bp upstream) and a Pax6 binding site (20 bp downstream). Such spacing allows one or two helical turns to separate each of these sites. In the developing pancreas, this same distance is permissive for Rbpj physical interaction with the bHLH transcription factor Ptf1a70. Interestingly, Ptf1a is also expressed in the developing retina and influences neurogenesis of several retinal cell types71–73. In Drosophila and Xenopus there are other examples of CSL/Rbpj protein interactions with bHLH factors that activate transcription66,74,75. Hence, we cannot discount the possibility that Neurog2 (or another bHLH factor) may physically interact with Rbpj. Alternatively, Rbpj occupancy of site R3 might affect local chromatin architecture, thereby displacing activating factors. Because we detected both Rbpj and Pax6 occupancy in E14.5 retinal chromatin (within the same preparation), a mutually exclusive binding mechanism would seem implausible. One caveat was the use of whole retina chromatin, which could obscure distinct configurations of transcription factor binding at the Atoh7 primary enhancer, among a heterogenic population of retinal cells. For example, in mitotically-active RPCs that do not express Atoh7, Rbpj could act as a repressor at site R3. When these cells enter their terminal mitosis, they may activate Atoh7 expression, via Pax6 or Neurog2 binding, which would also displace nearby Rbpj-corepressor complexes. Conversely, during terminal differentiation, this relationship could be reversed, with Rbpj-corepressor binding at site R3 dislodging either Pax6 or Neurog2. Only the generation of single-cell genomic datasets can map the occupancy of particular enhancer binding site to the developmental status of cells, at distinct stages of retinogenesis.
Here we also provided some insight into in vivo context for our biochemical data, via direct comparison of Rbpj and Atoh7 protein expression patterns. Rbpj is well established as ubiquitously expressed, so co-localization with Atoh7 protein was already predicted. But, transcription factors that are coexpressed with their target genes typically activate transcription, not repress it. Indeed, Hes1, a known repressor of neurogenesis, displays mutually exclusive expression with βgal in Atoh7LacZ/+ eyes26. Yet, our transcriptional activity data clearly indicate that Rbpj repression of Atoh7 is the major mode of regulation, which correlates with all previous genetic findings25,29. However, we also note that Rbpj does not appear uniformly expressed, with brighter anti-Rbpj labeling coinciding with Atoh7 expression. The significance of this observation remains unclear. Better understanding will require the determination of which Rbpj regulation activities at work during distinct stages of retinal cell development. Moreover, we must clarify whether Notch1/3 signaling invokes canonical pathway regulation, namely Notch-Rbpj-Maml binding to Hes gene promoters, which may in turn directly repress Atoh7 transcription.
While highly speculative, we propose that lower levels of Rbpj protein expression are sufficient for binding to multiple CSL sites, which contributes to keeping the Atoh7 locus open and primed (yet transcriptionally silent)33, until its rapid, pulsatile expression is needed. Higher levels of Rbpj protein may subsequently be required to also engage in the activities of Notch and co-repressor complexes, which act at different regulatory sequences, and possibly at different rates to shut down Atoh7 transcription. Clearly additional transcription factors must simultaneously regulate Atoh7 (positively or negatively) since brighter-labeled Rbpj cells are capable of Atoh7 co-expression. The integration of these multiple modes of regulation allows for more precise modulation of target gene mRNA levels, particularly during highly dynamic developmental processes.
Experimental Methods
Ethics Statement
All mice were housed and cared for in accordance with the guidelines provided by the National Institutes of Health, Bethesda, Maryland, and the Association for Research in Vision and Ophthalmology, and conducted with approval and oversight from the Cincinnati Children’s Hospital Research Foundation and UC Davis Institutional Animal Care and Use Committees.
Animals
A Rbpjtm1Hon conditional allele (termed RbpjCKO) were maintained on a 129/SvJ background and genotyped as described41. α-Cre transgenic mice were maintained on a CD-1 background and genotyped as described38. Le-Cre mice were maintained on an FVB/N background and genotyped as described40. Z/EG lineage tracing mice (Tg(CAG-Bgeo/GFP)21Lb3/J) use the CMV enhancer/chicken actin promoter to constitutively express lacZ, which is replaced with eGFP expression upon Cre activation39. These mice were acquired from Jackson Labs (Stock Number 003920), maintained on a CD-1 background and genotyped as in39. Embryonic gestational age was determined by timed matings, with the date of the vaginal plug as E0.5.
The upstream, noncoding 2.4 Kb of mouse Atoh7 genomic DNA (nucleotides −3032 to −503 containing the primary enhancer but lacking the TATAA box) was PCR amplified using primers with engineered XbaI and BglII restriction sites, cloned into the Xba I-Bam HI sites of the pBGnCherry vector47 in the normal transcriptional orientation, and verified by Sanger sequencing. The Atoh7 fragment was PCR amplified (EXPAND Hi-Fidelity polyermase) from a previously subcloned 6.5 Kb mouse Atoh7 genomic DNA template3, digested with BglII and XbaI and then purified. The pBGnCherry vector47 contains a minimal human β-globin promoter and monomeric Cherry red fluorescent protein reporter cassette (mCherry), as well as a synthetic amino terminal nuclear localization signal (MAPKKKRKVEDV) downstream of the BamHI site. There is no intrinsic activity of this vector in transgenic mice47. Linearized DNA was microinjected into CD-1 mouse pronuclei by the CHRF Transgenic Core Facility. F0 embryos were collected at E13.5 and screened for live mCherry fluorescence with a Leica MZ12 dissecting scope equipped with a Texas-Red filter. We harvested 4 Cherry +/44 embryos and each Cherry-positive embryonic head was cryoembedded, sectioned and analyzed using immunohistochemistry and confocal imaging (see below).
Bioinformatics of Rbpj binding sites
Three kilobases of 5′ and three kilobases of 3′ noncoding genomic DNA from the mouse and human Atoh7 genes (Gene IDs 53404 and 220202) were aligned using the MacVector Clustal W algorithm (v. 12). CNEs were identified in multiple vertebrate genomes using the UCSC genome browser MultiZ alignment and conservation features and mm10 genome assembly (http://genome.ucsc.edu). Putative CSL/Rbpj binding sites were identified using the TRANSFAC MATCH program with matrices M01111 (V$RBPJK_Q4) and M01112 (V$RBPJK_01). Previously defined Pax6 paired domain and E-box binding sites within the mouse Atoh7 primary distal CNE are included for reference8,10,11.
Immunohistochemistry
Embryonic heads were fixed in 4% paraformaldehyde/PBS for 1 hour at 4 °C, processed through a sucrose/PBS series, cryoembedded and sectioned at 10 μm. Immunohistochemistry using our lab protocol2 to label with chick anti-GFP (Abcam, 1:1000, AB13970), rat anti-Rbpj (CosmoBio, 1:100, SIM-2ZRBP-1), rabbit anti-Atoh7 (Novus Biologicals, 1:500, NBP1-88639), or goat anti-mCherry polyclonal (SICGEN, 1:500, AB0040-200) primary antibodies. Secondary antibodies were directly conjugated to Alexa Fluor 488 (Invitrogen, A21208), Alexa Fluor 594 (Jackson ImmunoResearch, 712-586-153), Alexa 594 (Invitrogen, A11058), Alexa Fluor 647 (Invitrogen, A21244), or Dylight 649 (Jackson ImmunoResearch, 711-495-152). Microscopic imaging used either a Zeiss Axioplan fluorescent microscope with a black and white camera, Apotome deconvolution device, and Axiovision (v. 7.0) software, or a Leica DM5500 microscope equipped with a SPEII solid state confocal and Leica LASAF software. All digital micrographs were electronically adjusted equivalently for brightness, contrast and pseudocoloring using Adobe Photoshop CS5 software.
Western blotting
Pairs of lenses were harvested from E14.5 RbpjCKO/CKO, Le-Cre;RbpjCKO/+ and Le-Cre;RbpjCKO/CKO embryos and flash frozen. Twenty lenses (10 pairs) of the same genotype were pooled and lysed in RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP40) containing Complete protease inhibitors (Sigma 11697498001). Total protein concentrations were determined by Bradford assay (Biorad, 500-0006). NuPAGE 4–12% Bis-Tris gels (Invitrogen, NP0322BOX) were loaded with 20ug of total lens protein per gel lane, electrophoresed and transferred onto nitrocellulose membranes (Invitrogen, LC2000). Standard western blotting was performed, using rat anti-Rbpj (Cosmo Bio Co, 1:500 SIM-2ZRBP-2) or mouse anti-β-actin (Sigma, 1:3000, A1978) primary antibodies, followed by HRP-conjugated anti-rat IgG or mouse IgG secondary antibodies (Jackson Immunoresearch, Rat 112-035-175 1:5000, Mouse 315-035-003, 1:10,000). Blots were developed using a Supersignal West Pico Chemiluminescent substrate kit (Thermo Scientific, 34078), Kodak standard x-ray film and film developer.
Electrophoretic Mobility Shift Assay (EMSA)
Single-stranded complementary oligonucleotides containing predicted CSL binding sites were labeled using a Biotin 3′ end DNA labeling kit (Thermo Scientific, 89818). Double-stranded DNA probes were made by annealing biotin-labeled complementary oligonucleotide pairs at room temperature for one hour. 0.5 μM purified mouse Rbpj protein (residues 53–474; Friedmann et al., 2008) and 1 nM of labeled oligonucleotide complexes, in the presence of 1.9 ng/μl poly[d(I-C)] (Sigma, 10108812001), were resolved on a 6% DNA retardation gels (Invitrogen, EC63652BOX) in 0.5 × TBE buffer and then transferred to nylon membranes. The LightShift Chemiluminescent EMSA kit assay (Thermo Scientific, 20148) was performed on the blots, which were developed using the Chemiluminescent Nucleic Acid Detection kit (Thermo Scientific, 89880), Kodak x-ray film and film developer.
Isothermal titration calorimetry of CSL-DNA complexes
The production and purification of bacterially expressed Rbpj protein, residues 53–474, has been described16,42. Oligonucleotides from Integrated DNA Technologies (IDT) (Fig. 4A) were hydrated, purified, quantified and annealed as in16. All purified components were degassed, buffer matched and quantified as previously described16. A typical experiment was performed at 5 °C using a MicroCal VP-ITC microcalorimeter with the oligomeric duplex (~100 μM) in the syringe and Rbpj (~10 μM) in the cell and consisted of 40 injections of 7 μl each. Data analysis used the ORIGIN software and was fitted to a one-site binding model, with binding data representing the average of n = 3 experiments.
Chromatin immunoprecipitation and Real-time PCR
ChIP was performed as described10,76 with several modifications. 30 E14.5 CD-1 pooled embryonic retinas were crosslinked with 1% formaldehyde and the reaction stopped by addition of 125 mM final concentration of glycine. Chromatin was sheered to 300–1000 bp size range with a Bioruptor UCD-200 sonicator + chiller (Diagenode), for 20 minutes at high power with 15 sec ON/30 sec OFF cycles. Either 3 μg rat anti-Rbpj antibody (Cosmo Bio Co, SIM-2ZRBP1) or rat IgG (Jackson ImmunoResearch, 012-000-003) were incubated with 40 μg sonicated chromatin overnight at 4 °C. Immune complexes were collected with Protein G agarose beads (Sigma, P7700), washed several times and eluted using 0.5 M NaHCO3, 1% SDS elution buffer. Pax6 ChIP was run in parallel from each retinal chromatin prep, by incubating 20 μg of sheared chromatin with 1 μg anti-Pax6 (Covance, PRB-278P), or rabbit IgG (Jackson ImmunoResearch, 011-000-003), coupled to Protein A sepharose beads (GE Healthcare, 17-0780-01). Input and immunoprecipitated chromatin samples were initially analyzed by performing 30 cycles of PCR amplification and agarose gel electrophoresis, then quantified by real time PCR, using Table S4 primers, fast SYBR Green master mixes and a StepOnePlus PCR system (Applied Biosystems, 4385612 and 4376600). A standard curve using serial dilutions of 1% input chromatin was used to calculate the percent input of each sample. The p-values were determined by ANOVA and a Bonferroni posthoc test (Rbpj) or a student’s unpaired, 2-tailed t-test (Pax6) with GraphPad Prism software (v6).
Luciferase Assay
The upstream 2.6 Kb noncoding DNA from the mouse Atoh7 locus was previously cloned into the pGL2 luciferase vector10. Rbpj binding site mutations were generated using PCR-based site directed mutagenesis77 and verified by Sanger Sequencing. Either 3.5 × 105 HEK293T or 5 × 105 AD12HER10 cells were plated per well of a 6-well tissue culture plate. After 48 hours (~60% confluency) cultures were transfected according to the Fugene6 (Promega, E2692) protocol with a 5:1 Fugene6 to DNA ratio, with the DNA constituting 500 ng of luciferase plasmid and 50 ng of Renilla control plasmid (pRL). In Notch ICD overexpression experiments, 100 ng of NICD1/pBK-CMV, 100 ng of NICD3/p3XFLAG-CMV-7TM, or a mixture of 50 ng of each plasmid were cotransfected with the luciferase and Renilla plasmids. Cells were washed in PBS, harvested 48 hours after transfection in 500 μL 1 × PLB (Promega) and cell pellets stored at −80 °C. Cell extracts were assayed in technical triplicate using the Dual Luciferase Assay System (Promega, E1980) on a Perkin Elmer Victor X5 workstation. Luciferase activity levels were normalized to the control Renilla activity, and p-values determined with GraphPad Prism (v6) software, using a two-tailed, unpaired t-test with equal standard deviation and assuming a Gaussian distribution.
RT-PCR
Total RNA was extracted using the RNeasy micro kit (Qiagen, Cat No 74004) from 1 pair of dissected E13.5 retinas, or using Trizol (Invitrogen Cat No 15596026) for HER-10 cells. For embryonic retinal RNA 100 ng was reverse transcribed into cDNA using the iScript Synthesis kit and product protocol (BioRad, Cat No. 178891). For HER-10 cells, 6.5 μg of total RNA was first treated with 1U of 10 U/μL of DNase (Roche Cat No. 04716728001) by incubating at 37 °C × 35 min, 80 °C × 5 min, 90 °C × 3 min. Then 2 μg of treated RNA was used for cDNA synthesis, with Superscript III and manufacturer protocol (Invitrogen/ThermoFisher, Cat No. 18080093). Both experiments included a mock synthesis (lacking total RNA) performed in parallel. For embryonic retinas, 1 μL of cDNA was combined with individual primer sets (Supplemental Table S4) and Go-Taq polymerase (Promega, cat # M7122) for 35 cycles of PCR at 95 °C × 30 sec, 55 °C × 30 sec, 72 °C × 30 sec. PCR products were electrophoresed on a 2% TAE agarose gel. Alternatively, 1 μL HER-10 cDNA was combined with individual primer sets (Supplemental Table S4), 1 × PCR Buffer and dNTPs, 1 × Masteramp (Epicentre/Illumina, ME81210) and 1U of Taq polymerase (5U/μL Roche/Sigma, Cat No. 11146173001) for 35 cycles of PCR at 95 °C × 30 sec, 60 °C × 30 sec, 72 °C × 30 sec. PCR products were electrophoresed on a 1% TBE agarose gel.
Data availability
All data generated or analyzed during this study are included within this published article and its Supplementary Information files.
Electronic supplementary material
Acknowledgements
The authors thank Tasuku Honjo for Rbpj flox mice, Ruth Ashery Padan for α-Cre and Le-Cre transgenic lines, Shiming Chen and Guang-Hua Peng for guidance on ChIP assays, Brad VanderWielen for purified Rbpj protein, Jane Johnson for pBGnCherry vector, Malgorzata Quinn, Yuqi Cai and Tien Le for technical support, Joo-Seop Park and Tom Glaser for fruitful discussions and critical feedback. This work was supported by NIH R01 grants EY13612 to NLB, CA178974 to RAK, and NEI Training Grants T32EY015387 to JBM and T32ES007250 to ANC.
Author Contributions
J.B.M., M.-S.M., R.A.K. and N.L.B. conceived and designed the experiments; J.B.M., M.-S.M., A.N.R. and A.N.C. performed the experiments; J.B.M., M.-S.M., R.A.K. and N.L.B. analyzed the data and wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Joel B. Miesfeld and Myung-soon Moon contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28420-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain research. 2008;1192:90–98. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Brown NL, et al. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- 3.Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanekar S, et al. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–994. doi: 10.1016/S0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- 5.Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal Homolog. Neuron. 2001;30:725–736. doi: 10.1016/S0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- 6.Wang SW, et al. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghiasvand NM, et al. Deletion of a remote enhancer near ATOH7 disrupts retinal neurogenesis, causing NCRNA disease. Nat Neurosci. 2011;14:578–586. doi: 10.1038/nn.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutcheson DA, et al. bHLH-dependent and -independent modes of Ath5 gene regulation during retinal development. Development. 2005;132:829–839. doi: 10.1242/dev.01653. [DOI] [PubMed] [Google Scholar]
- 9.Prasov L, Nagy M, Rudolph DD, Glaser T. Math5 (Atoh7) gene dosage limits retinal ganglion cell genesis. Neuroreport. 2012;23:631–634. doi: 10.1097/WNR.0b013e328355f260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riesenberg AN, et al. Pax6 regulation of Math5 during mouse retinal neurogenesis. Genesis. 2009;47:175–187. doi: 10.1002/dvg.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skowronska-Krawczyk D, et al. Conserved regulatory sequences in Atoh7 mediate non-conserved regulatory responses in retina ontogenesis. Development. 2009;136:3767–3777. doi: 10.1242/dev.033449. [DOI] [PubMed] [Google Scholar]
- 12.Hufnagel RB, Le TT, Riesenberg AL, Brown NL. Neurog2 controls the leading edge of neurogenesis in the mammalian retina. Developmental biology. 2010;340:490–503. doi: 10.1016/j.ydbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tun T, et al. Recognition sequence of a highly conserved DNA binding protein RBP-J kappa. Nucleic acids research. 1994;22:965–971. doi: 10.1093/nar/22.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecourtois M, Schweisguth F. The Neurogenic Suppressor of Hairless DNA-Binding Protein Mediates the Transcriptional Activation of the Enhancer of Split Complex Genes Triggered by Notch Signaling. Gene Dev. 1995;9:2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- 15.Christensen S, Kodoyianni V, Bosenberg M, Friedman L, Kimble J. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H) Development. 1996;122:1373–1383. doi: 10.1242/dev.122.5.1373. [DOI] [PubMed] [Google Scholar]
- 16.Torella R, et al. A combination of computational and experimental approaches identifies DNA sequence constraints associated with target site binding specificity of the transcription factor CSL. Nucleic acids research. 2014;42:10550–10563. doi: 10.1093/nar/gku730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarriault S, et al. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi M, et al. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 19.Ohtsuka T, et al. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. The EMBO journal. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker NE, Yu SY. Proneural function of neurogenic genes in the developing Drosophila eye. Current biology: CB. 1997;7:122–132. doi: 10.1016/S0960-9822(06)00056-X. [DOI] [PubMed] [Google Scholar]
- 21.Bray S, Furriols M. Notch pathway: making sense of suppressor of hairless. Current biology: CB. 2001;11:R217–221. doi: 10.1016/S0960-9822(01)00109-9. [DOI] [PubMed] [Google Scholar]
- 22.Baker, N. E. & Brown, N. L. All in the Family: proneural bHLH genes and neuronal diversity. Development145, 10.1242/dev.159426 (2018). [DOI] [PMC free article] [PubMed]
- 23.Jadhav AP, Mason HA, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006;133:913–923. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- 24.Yaron O, Farhy C, Marquardt T, Applebury M, Ashery-Padan R. Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development. 2006;133:1367–1378. doi: 10.1242/dev.02311. [DOI] [PubMed] [Google Scholar]
- 25.Riesenberg AN, Liu Z, Kopan R, Brown NL. Rbpj cell autonomous regulation of retinal ganglion cell and cone photoreceptor fates in the mouse retina. J Neurosci. 2009;29:12865–12877. doi: 10.1523/JNEUROSCI.3382-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer KA, Riesenberg AN, Brown NL. Notch signaling differentially regulates Atoh7 and Neurog2 in the distal mouse retina. Development. 2014;141:3243–3254. doi: 10.1242/dev.106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HY, et al. Multiple requirements for Hes 1 during early eye formation. Developmental biology. 2005;284:464–478. doi: 10.1016/j.ydbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomita K, et al. Mammalian hairy and Enhancer of split homolog 1 regulates differentiation of retinal neurons and is essential for eye morphogenesis. Neuron. 1996;16:723–734. doi: 10.1016/S0896-6273(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 29.Zheng MH, et al. The transcription factor RBP-J is essential for retinal cell differentiation and lamination. Molecular brain. 2009;2:38. doi: 10.1186/1756-6606-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadhav AP, Cho SH, Cepko CL. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc Natl Acad Sci USA. 2006;103:18998–19003. doi: 10.1073/pnas.0608155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene. 2008;27:5099–5109. doi: 10.1038/onc.2008.223. [DOI] [PubMed] [Google Scholar]
- 32.Dou S, et al. The recombination signal sequence-binding protein RBP-2N functions as a transcriptional repressor. Mol Cell Biol. 1994;14:3310–3319. doi: 10.1128/MCB.14.5.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lake RJ, Tsai PF, Choi I, Won KJ, Fan HY. RBPJ, the major transcriptional effector of Notch signaling, remains associated with chromatin throughout mitosis, suggesting a role in mitotic bookmarking. PLoS Genet. 2014;10:e1004204. doi: 10.1371/journal.pgen.1004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kay JN, Link BA, Baier H. Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development. 2005;132:2573–2585. doi: 10.1242/dev.01831. [DOI] [PubMed] [Google Scholar]
- 35.Miesfeld JB, Glaser T, Brown NL. The dynamics of native Atoh7 protein expression during mouse retinal histogenesis, revealed with a new antibody. Gene Expr Patterns. 2017;27:114–121. doi: 10.1016/j.gep.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le TT, Conley KW, Brown NL. Jagged 1 is necessary for normal mouse lens formation. Developmental biology. 2009;328:118–126. doi: 10.1016/j.ydbio.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le TT, et al. Requirements for Jag1-Rbpj mediated Notch signaling during early mouse lens development. Dev Dyn. 2012;241:493–504. doi: 10.1002/dvdy.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marquardt T, et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/S0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 39.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. doi: 10.1002/1526-968X(200011/12)28:3/4<147::AID-GENE90>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 40.Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han H, et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. International immunology. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- 42.Friedmann DR, Wilson JJ, Kovall RA. RAM-induced allostery facilitates assembly of a notch pathway active transcription complex. The Journal of biological chemistry. 2008;283:14781–14791. doi: 10.1074/jbc.M709501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedmann DR, Kovall RA. Thermodynamic and structural insights into CSL-DNA complexes. Protein Sci. 2010;19:34–46. doi: 10.1002/pro.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brzezinski JAt, Prasov L, Glaser T. Math5 defines the ganglion cell competence state in a subpopulation of retinal progenitor cells exiting the cell cycle. Developmental biology. 2012;365:395–413. doi: 10.1016/j.ydbio.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hufnagel RB, Riesenberg AN, Saul SM, Brown NL. Conserved regulation of Math5 and Math1 revealed by Math5-GFP transgenes. Mol Cell Neurosci. 2007;36:435–448. doi: 10.1016/j.mcn.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willardsen MI, et al. Temporal regulation of Ath5 gene expression during eye development. Developmental biology. 2009;326:471–481. doi: 10.1016/j.ydbio.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meredith DM, Masui T, Swift GH, MacDonald RJ, Johnson JE. Multiple transcriptional mechanisms control Ptf1a levels during neural development including autoregulation by the PTF1-J complex. J Neurosci. 2009;29:11139–11148. doi: 10.1523/JNEUROSCI.2303-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grabham PW, Grand RJ, Byrd PJ, Gallimore PH. Differentiation of normal and adenovirus-12 E1 transformed human embryo retinal cells. Exp Eye Res. 1988;47:123–133. doi: 10.1016/0014-4835(88)90029-2. [DOI] [PubMed] [Google Scholar]
- 49.DuBridge RB, et al. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/MCB.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li, X. et al. Proteomic analyses reveal distinct chromatin-associated and soluble transcription factor complexes. Mol Syst Biol11 (2015). [DOI] [PMC free article] [PubMed]
- 51.Prasov L, et al. ATOH7 mutations cause autosomal recessive persistent hyperplasia of the primary vitreous. Hum Mol Genet. 2012;21:3681–3694. doi: 10.1093/hmg/dds197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ong CT, et al. Target selectivity of vertebrate notch proteins. Collaboration between discrete domains and CSL-binding site architecture determines activation probability. The Journal of biological chemistry. 2006;281:5106–5119. doi: 10.1074/jbc.M506108200. [DOI] [PubMed] [Google Scholar]
- 53.Hald J, et al. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Developmental biology. 2003;260:426–437. doi: 10.1016/S0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 54.Ohsako S, Hyer J, Panganiban G, Oliver I, Caudy M. Hairy function as a DNA-binding helix-loop-helix repressor of Drosophila sensory organ formation. Genes Dev. 1994;8:2743–2755. doi: 10.1101/gad.8.22.2743. [DOI] [PubMed] [Google Scholar]
- 55.Van Doren M, Bailey AM, Esnayra J, Ede K, Posakony JW. Negative regulation of proneural gene activity: hairy is a direct transcriptional repressor of achaete. Genes Dev. 1994;8:2729–2742. doi: 10.1101/gad.8.22.2729. [DOI] [PubMed] [Google Scholar]
- 56.Kovall RA, Blacklow SC. Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol. 2010;92:31–71. doi: 10.1016/S0070-2153(10)92002-4. [DOI] [PubMed] [Google Scholar]
- 57.Contreras-Cornejo H, et al. The CSL proteins, versatile transcription factors and context dependent corepressors of the notch signaling pathway. Cell Div. 2016;11:12. doi: 10.1186/s13008-016-0025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu T, et al. RBPJ/CBF1 interacts with L3MBTL3/MBT1 to promote repression of Notch signaling via histone demethylase KDM1A/LSD1. The EMBO journal. 2017;36:3232–3249. doi: 10.15252/embj.201796525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oswald F, et al. SHARP is a novel component of the Notch/RBP-J kappa signalling pathway. Embo Journal. 2002;21:5417–5426. doi: 10.1093/emboj/cdf549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Popova EY, Pinzon-Guzman C, Salzberg AC, Zhang SS, Barnstable CJ. LSD1-Mediated Demethylation of H3K4me2 Is Required for the Transition from Late Progenitor to Differentiated Mouse Rod Photoreceptor. Mol Neurobiol. 2016;53:4563–4581. doi: 10.1007/s12035-015-9395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang L, et al. KyoT3, an isoform of murine FHL1, associates with the transcription factor RBP-J and represses the RBP-J-mediated transactivation. Biochim Biophys Acta. 2008;1779:805–810. doi: 10.1016/j.bbagrm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Collins KJ, Yuan Z, Kovall RA. Structure and function of the CSL-KyoT2 corepressor complex: a negative regulator of Notch signaling. Structure. 2014;22:70–81. doi: 10.1016/j.str.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taniguchi Y, Furukawa T, Tun T, Han H, Honjo T. LIM protein KyoT2 negatively regulates transcription by association with the RBP-J DNA-binding protein. Mol Cell Biol. 1998;18:644–654. doi: 10.1128/MCB.18.1.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bailey AM, Posakony JW. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- 65.Arnett KL, et al. Structural and mechanistic insights into cooperative assembly of dimeric Notch transcription complexes. Nature structural & molecular biology. 2010;17:1312–1317. doi: 10.1038/nsmb.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cave JW, Loh F, Surpris JW, Xia L, Caudy MA. A DNA transcription code for cell-specific gene activation by notch signaling. Current biology: CB. 2005;15:94–104. doi: 10.1016/j.cub.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 67.Nam Y, Sliz P, Pear WS, Aster JC, Blacklow SC. Cooperative assembly of higher-order Notch complexes functions as a switch to induce transcription. Proc Natl Acad Sci USA. 2007;104:2103–2108. doi: 10.1073/pnas.0611092104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis RL, Turner DL, Evans LM, Kirschner MW. Molecular targets of vertebrate segmentation: two mechanisms control segmental expression of Xenopus hairy2 during somite formation. Developmental cell. 2001;1:553–565. doi: 10.1016/S1534-5807(01)00054-5. [DOI] [PubMed] [Google Scholar]
- 69.Swanson CI, Schwimmer DB, Barolo S. Rapid evolutionary rewiring of a structurally constrained eye enhancer. Current biology: CB. 2011;21:1186–1196. doi: 10.1016/j.cub.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masui T, Long Q, Beres TM, Magnuson MA, MacDonald RJ. Early pancreatic development requires the vertebrate Suppressor of Hairless (RBPJ) in the PTF1 bHLH complex. Genes Dev. 2007;21:2629–2643. doi: 10.1101/gad.1575207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dullin JP, et al. Ptf1a triggers GABAergic neuronal cell fates in the retina. BMC Dev Biol. 2007;7:110. doi: 10.1186/1471-213X-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujitani Y, et al. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development. 2006;133:4439–4450. doi: 10.1242/dev.02598. [DOI] [PubMed] [Google Scholar]
- 73.Nakhai H, et al. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development. 2007;134:1151–1160. doi: 10.1242/dev.02781. [DOI] [PubMed] [Google Scholar]
- 74.Castro B, Barolo S, Bailey AM, Posakony JW. Lateral inhibition in proneural clusters: cis-regulatory logic and default repression by Suppressor of Hairless. Development. 2005;132:3333–3344. doi: 10.1242/dev.01920. [DOI] [PubMed] [Google Scholar]
- 75.Lamar E, Kintner C. The Notch targets Esr1 and Esr10 are differentially regulated in Xenopus neural precursors. Development. 2005;132:3619–3630. doi: 10.1242/dev.01937. [DOI] [PubMed] [Google Scholar]
- 76.Peng GH, Chen S. Chromatin immunoprecipitation identifies photoreceptor transcription factor targets in mouse models of retinal degeneration: new findings and challenges. Visual neuroscience. 2005;22:575–586. doi: 10.1017/S0952523805225063. [DOI] [PubMed] [Google Scholar]
- 77.Liu H, Naismith JH. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 2008;8:91. doi: 10.1186/1472-6750-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Domenighetti AA, et al. Loss of FHL1 induces an age-dependent skeletal muscle myopathy associated with myofibrillar and intermyofibrillar disorganization in mice. Hum Mol Genet. 2014;23:209–225. doi: 10.1093/hmg/ddt412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included within this published article and its Supplementary Information files.