Abstract
Meiotic cells undergo genetic exchange between homologs through programmed DNA double-strand break (DSB) formation, recombination and synapsis. In mice, the DNA damage-regulated phosphatidylinositol-3-kinase-like kinase (PIKK) ATM regulates all of these processes. However, the meiotic functions of the PIKK ATR have remained elusive, because germline-specific depletion of this kinase is challenging. Here we uncover roles for ATR in male mouse prophase I progression. ATR deletion causes chromosome axis fragmentation and germ cell elimination at mid pachynema. This elimination cannot be rescued by deletion of ATM and the third DNA damage-regulated PIKK, PRKDC, consistent with the existence of a PIKK-independent surveillance mechanism in the mammalian germline. ATR is required for synapsis, in a manner genetically dissociable from DSB formation. ATR also regulates loading of recombinases RAD51 and DMC1 to DSBs and recombination focus dynamics on synapsed and asynapsed chromosomes. Our studies reveal ATR as a critical regulator of mouse meiosis.
ATR kinase is required for meiosis in non-mammalian model organisms. Here the authors demonstrate, using a tissue-specific knockout approach, that ATR is also essential for male meiosis in mouse, regulating meiotic recombination and synapsis.
Introduction
ATR is a serine–threonine kinase with ubiquitous functions in somatic genome stability and checkpoint control1. Studies on non-mammalian organisms have revealed that ATR is also essential for meiosis. ATR orthologs regulate meiotic double-strand break (DSB) resection2, stoichiometry of DSB-associated strand-exchange proteins RAD51 and DMC13, inter-homolog bias4, 5 and crossover formation6. They are also components of prophase I checkpoints that ensure centromere pairing7, timely repair of recombination intermediates8, 9 and correct coupling of DNA replication with DSB induction10, 11. In humans, hypomorphic Atr mutations cause Seckel syndrome, a pleiotropic, autosomal recessive disorder associated with dwarfism, craniofacial abnormalities, intellectual disability and cryptorchidism12. In human cancer cell lines, ATR haploinsufficiency impairs the DNA damage response13. Determining the functions of ATR in mouse meiosis has been challenging. Heterozygous Atr deletion compromises postnatal survival14 and homozygous deletion causes embryonic lethality14, 15. An inducible Cre-ERT2 approach recently revealed that ATR regulates meiotic sex chromosome inactivation (MSCI), the silencing of the X and Y chromosomes in male meiosis, via serine-139 H2AX phosphorylation (γH2AX)16. However, this method resulted in partial rather than complete ATR depletion.
Here we describe a superior conditional strategy for dissecting additional meiotic ATR functions. Using this approach, we show that ATR regulates homologous synapsis as well as multiple steps in recombination. By generating mutants deficient in both ATR and ATM, we identify shared and distinct functions for these kinases in mouse meiosis.
Results
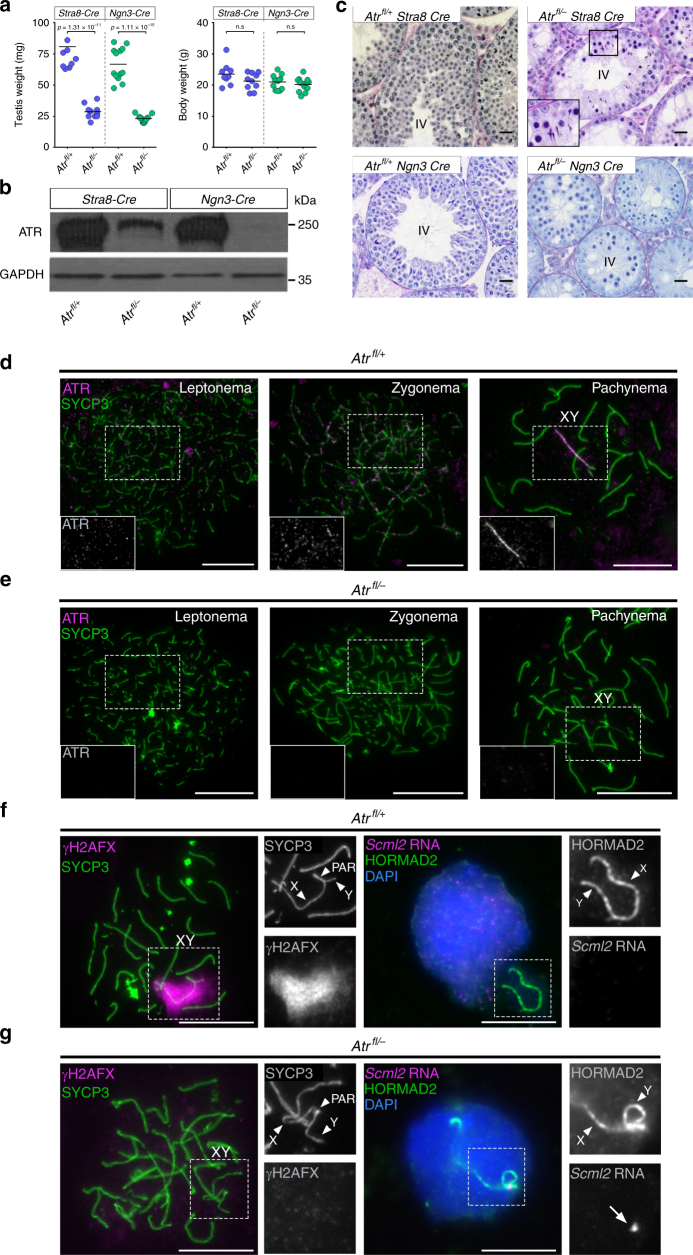
A strategy for efficient meiotic Atr depletion
For this purpose, we generated male mice carrying one Atr floxed (Atrfl) allele, in which the exon 44 kinase domain of Atr is flanked by loxP sites17, and one Atr-null (Atr–) allele, in which the first three coding exons of Atr are replaced by a neomycin selection cassette14. The resulting Atrfl/– males also carried a transgene expressing Cre recombinase under the control of either a Stra8 or Ngn3 promoter fragment. Stra8-Cre is expressed from P3 (postnatal day 3)18, while Ngn3-Cre is expressed from P719, 20. Testis weights at P30 were reduced three- to fourfold in Atrfl/– Stra8-Cre males and Atrfl/– Ngn3-Cre males relative to Atrfl/+ Cre-carrying (i.e., Atr heterozygous) controls, while body weights were unaffected (Fig. 1a). We observed no difference in testis weights between Atrfl/+ males carrying Cre transgenes and those not carrying Cre transgenes (Fig. 1 legend). Western blotting showed that ATR protein was reduced in Atrfl/– Stra8-Cre testes, and even more so in Atrfl/– Ngn3-Cre testes (Fig. 1b). This finding supports previous evidence that the majority of testis ATR expression occurs in spermatocytes16, 21. Testis histology revealed germ cell failure at seminiferous tubule stage IV, corresponding to mid pachynema of meiosis, in both Cre models (Fig. 1c), reminiscent of findings in Atrfl/– Cre-ERT2 mice16. However, the stage IV elimination was clearly less robust in Atrfl/– Stra8-Cre than Atrfl/– Ngn3-Cre males, because elongating spermatids were observed in some testis sections from the former but not latter genotype (Fig. 1c inset). We therefore focused on Atrfl/– Ngn3-Cre mice (hereafter Atrfl/–), with Atrfl/+ Ngn3-Cre (hereafter Atrfl/+) serving as controls.
Fig. 1.
A conditional strategy for efficient depletion of ATR during male mouse meiosis. P30 testis and body weights (a), testis western blots (b), and periodic acid–Schiff and hemotoxylin/eosin-stained stage IV testis sections (c) in Atrfl/+ Stra8-Cre males (n = 9 males), Atrfl/– Stra8-Cre males (n = 10 males), Atrfl/+ Ngn3-Cre males (n = 13 males) and Atrfl/– Ngn3-Cre males (n = 13 males; means and p values for a indicated; unpaired t-test). Testis weights in Atrfl/+ Stra8-Cre and Atrfl/+ Ngn3-Cre males are not significantly different from those in Atrfl/+ Cre negative males derived from the same crosses (n = 17 Atrfl/+ Cre negative males from the Stra8-Cre cross, p = 0.37, n = 11 Atrfl/+ Cre negative males from the Ngn3-Cre cross, p = 0.06). Inset in c shows presence of elongating spermatids in some tubules from Atrfl/– Stra8-Cre males. d, e ATR (magenta) and SYCP3 (green) staining in Atrfl/+ Ngn3-Cre (denoted Atrfl/+) and Atrfl/– Ngn3-Cre (denoted Atrfl/–) males. In Atrfl/+ males (n = 2 males) ATR is observed as foci (see insets) in 85% of leptotene cells (n = 20 cells) and 82% of zygotene cells (n = 28 cells), and on the asynapsed region of the XY bivalent in 100% of pachytene cells (n = 30 cells). In Atrfl/– males (n = 2 males) ATR is observed in no cells at these three stages (n = 21, 30 and 32 cells at leptonema, zygonema and pachynema, respectively). f Validation of ATR depletion in Atrfl/– by analysis of MSCI. In Atrfl/+ males, all pachytene cells show coating of the X and Y chromosome (arrowheads) but not the pseuodautosomal regions (PAR, arrowhead) by γH2AX (magenta; left panels; XY bivalent in box magnified in smaller panels). This coating causes silencing of the X-chromosome gene Scml2 (magenta; right panels) and compartmentalization of the XY bivalent (labeled with HORMAD2; green) in the sex body (n = one male, 29 cells; magnified in smaller panels). g In Atrfl/– males, XY chromosome γH2AX coating and sex body compartmentalization do not occur. As a result, Scml2 expression (arrow) persists in all early pachytene cells (n = one male; 30 cells). Scale bar in (c) 20 µm, other scale bars 10 µm
Combined immunofluorescence for ATR and the axial element protein SYCP322 confirmed that the characteristic ATR staining pattern observed in control leptotene, zygotene and pachytene spermatocytes (Fig. 1d) was absent in Atrfl/– males (Fig. 1e). Furthermore, MSCI, assayed at early pachynema by acquisition of γH2AX on the XY bivalent and RNA fluorescent in situ hybridization (FISH) to detect absence of expression of the X-chromosome gene Scml2, was present in control males (Fig. 1f) but abolished in Atrfl/– males (Fig. 1g). Thus, by multiple criteria, Atrfl/– males exhibited efficient ATR depletion.
At stage IV, when wild-type spermatocytes reach mid pachynema, Atrfl/– spermatocytes contained highly fragmented chromosome axes and nucleus-wide γH2AX staining (Supplementary Fig. 1a; see Methods for meiotic staging criteria used throughout this study). These mid-pachytene Atrfl/– cells were readily distinguishable from Atrfl/– cells at leptonema, in which axial elements were shorter and uniform in length, and γH2AX staining across the nucleus was more heterogeneous (Supplementary Fig. 1b). Mid-pachytene axis fragmentation and nucleus-wide γH2AX staining were also noted in Atm−/− males (Supplementary Fig. 1c), as described previously23. Neither phenotype was observed in Spo11−/−, Dmc1−/− and Msh5−/− males (Supplementary Fig. 1d-f), which display stage IV arrest. Instead, γH2AX in Spo11−/− spermatocytes was restricted to the transcriptionally inactive pseudosex body (Supplementary Fig. 1d), while in Dmc1−/− and Msh5−/− spermatocytes it formed axis-associated clouds (Supplementary Fig. 1e,f), consistent with published reports23–25. These findings suggested that mid-pachytene axis degeneration and nucleus-wide γH2AX staining are features of ATR and ATM (ataxia telangiectasia mutated) deletion, and not merely a consequence of stage IV germ cell death.
Mid-pachytene elimination in males deficient in Atr, Atm and Prkdc
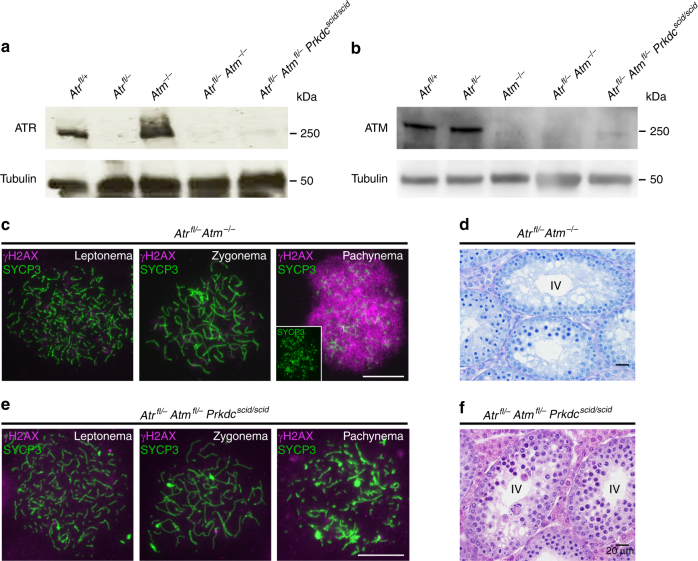
We investigated mechanisms driving mid-pachytene elimination of Atrfl/– spermatocytes. In male mice stage IV arrest in response to recombination defects is promoted by ATM26, whereas MSCI failure can cause stage IV elimination independently of ATM16, 27. Since Atrfl/– males exhibit defective MSCI, we predicted that mid-pachytene germ cell loss would be preserved in mice doubly deficient for ATR and ATM, irrespective of whether ATR is involved in recombination.
To test this prediction, we examined testis histology in Atrfl/– Atm−/− mutants. As expected, double-mutant testes exhibited greatly reduced ATR and ATM protein levels (Fig. 2a, b). At leptonema, axis morphology was grossly unaffected (Fig. 2c, left panel; Supplementary Fig. 1g), but most spermatocytes at later stages exhibited axial fragmentation (Fig. 2c, right panel inset; Supplementary Fig. 1g). Thus, axis morphology was more severely compromised in double mutants than in either single mutant. Leptotene and zygotene H2AX phosphorylation are catalyzed by ATM and ATR, respectively23, 24, 28, 29. In leptotene spermatocytes from Atrfl/– Atm−/− mutants, γH2AX was absent (Fig. 2c, left panel). Furthermore, zygotene spermatocytes that lacked axial fragmentation, and could thus be unambiguously staged, lacked γH2AX (Fig. 2c, middle panel). Thus, both phosphatidylinositol-3-kinase-like kinases (PIKKs) had been efficiently depleted in these double mutants. Consistent with our prediction, stage IV elimination was preserved in Atrfl/– Atm−/− testes (Fig. 2d). In these males we observed a population of spermatocytes with nucleus-wide γH2AX staining. Based on their advanced axial fragmentation pattern (Fig. 2c, right panel) and adlumenal location within seminiferous tubule sections (Supplementary Fig. 1h), these spermatocytes were inferred to be at mid pachynema.
Fig. 2.
Mid-pachytene germ cell elimination is preserved in mice deficient in the PIKKs. Western blot showing a ATR and b ATM depletion in mice with different PIKK mutations. c γH2AX (magenta) and SYCP3 (green) immunostaining (n = 2 males, 25 cells for each stage) and d stage IV elimination in Atrfl/– Atm−/− males. e γH2AX and SYCP3 immunostaining (n = 2 males, 25 cells for each stage) and f stage IV elimination in Atrfl/– Atm fl/– Prkdcscid/scid males. Scale bar 20 µm in d, f and 10 µm in c, e
Our findings were consistent with a checkpoint-independent mechanism, most likely defective MSCI, driving germ cell loss in Atrfl/– Atm−/− males. However, the remaining DNA damage-regulated PIKK, PRKDC, was still present in these mutants and could contribute a checkpoint function. We therefore examined germ cell progression in males deficient in all three DNA damage-regulated PIKKs. For this experiment we used the Prkdc scid mutation30 and an Atm flox allele31, because combined homozygosity for Prkdc scid and the Atm-null mutation causes embryonic lethality32. In Atrfl/– Atm fl/– Prkdcscid/scid males, testis ATM and ATR levels were depleted (Fig. 2a, b), and consequently ATM- and ATR-dependent γH2AX staining was absent (Fig. 2e, left and middle panels). Interestingly, pachytene nucleus-wide γH2AX staining was also abolished (Fig. 2e, right panel). Thus, PRKDC mediates pachytene serine-139 H2AX phosphorylation in Atrfl/– Atmfl/– males. Nevertheless, in the triple-mutant stage IV germ cell loss still occurred, and axis fragmentation was even more severe than in Atrfl/– Atmfl/– males (Fig. 2e, right panel; Fig. 2f). Thus, mid-pachytene elimination persists in mice deficient in all three DNA damage-regulated PIKKs.
Atr regulates homologous synapsis
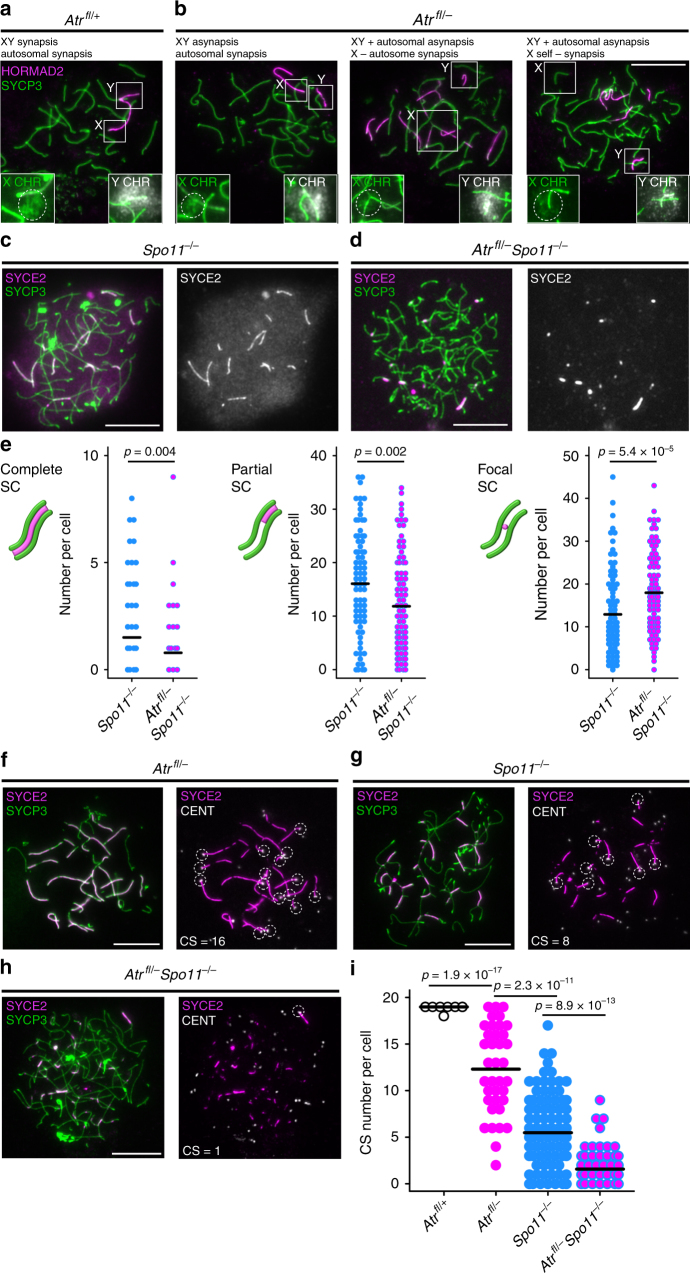
We also investigated synapsis and recombination in Atrfl/– males, and where possible we compared findings in this mutant to those in Atrfl/– Atmfl/– males. To address whether ATR regulates homologous synapsis, we immunostained for SYCP3 and the asynapsis marker HORMAD2. We focused on Atrfl/– cells at early pachynema, i.e., prior to extensive axial element fragmentation (Supplementary Fig. 1a). Synapsis was normal in 86% (n = 104) of early pachytene cells from control males, a frequency similar to that in Atrfl/+ males without the Ngn3-Cre transgene (89%; n = 100). As expected, in control cells HORMAD2 was absent on the autosomal bivalents and present on the non-homologous, asynapsed regions of the X and Y chromosome in these instances (Fig. 3a). Furthermore, in these cells, non-homologous synapsis between the sex chromosomes and the autosomes was not observed. However, in Atrfl/– males, only 20% (n = 107) of early pachytene cells achieved complete homologous synapsis. The remaining cells exhibited varying degrees of asynapsis affecting the XY pair and the autosomes (Fig. 3b; see below for details). In addition, X and Y self-synapsis and non-homologous synapsis between the sex chromosomes and autosomes were more common in Atrfl/– cells (Fig. 3b). In mice, the XY pseudoautosomal regions (PARs) undergo late synapsis and DSB formation33, and in yeast the homolog bias of late-forming DSBs is partially dependent on ATR2. We therefore determined whether asynapsis in Atrfl/– males more often affects the sex chromosomes than the autosomes. We identified the sex chromosomes using DNA FISH (Slx probe for X and Sly probe for Y; Fig. 3a, b; insets). In Atrfl/– males, 77% of early pachytene cells exhibited XY asynapsis, while 59% exhibited autosomal asynapsis (see legend for further details). In control males, 13% of early pachytene cells exhibited XY asynapsis and 13% autosomal asynapsis. Thus, ATR deletion has a more deleterious effect on XY than on autosomal synapsis.
Fig. 3.
ATR is required for homologous synapsis. Examples of early pachytene synaptic outcomes in a Atrfl/+ males (n = 3 males) and b Atrfl/– males (n = 3 males) assessed using HORMAD2 (magenta), SYCP3 (green) and subsequent DNA FISH using Slx and Sly probes (labeled in insets as X chromosome in green and Y chromosome in white). The Slx probe hybridizes to a sub-region of the X chromosome (circled), while the Sly probe coats the majority of the Y chromosome. In Atrfl/+ males (a), both the autosomes and the XY PARs are synapsed, while the non-homologous regions of the XY pair are asynapsed. In Atrfl/+ males (n = 104 cells, 2 males), 90 cells had normal synapsis, 12 cells had asynapsis of both the XY and autosomes, 1 cell had asynapsis only of the XY and 1 cell had asynapsis only of the autosomes. b Three examples of synaptic defects in Atrfl/– males, each described above respective image. In Atrfl/– males (n = 107 cells, 2 males), 21 cells had normal synapsis, 59 cells had asynapsis of both the XY and autosomes, 23 cells had asynapsis only of the XY, 4 cells had asynapsis only of the autosomes, 10 cells had X self-synapsis, 11 cells had Y self-synapsis and 7 had non-homologous synapsis between the X and/or Y and autosomes. c, d Comparison of pachytene synaptic outcomes in c Spo11−/− and d Atrfl/– Spo11−/− males using immunostaining for SYCE2 (magenta) and SYCP3 (green). e Quantitation of complete, partial and focal SC in Spo11−/− males (n = 2 males; 88 cells) and Atrfl/– Spo11−/− males (n = 2 males; 96 cells). Means and p values (unpaired t-test) indicated. f–h Epistasis analysis of SPO11 and ATR in synapsis using the same markers plus immunostaining for centromeres (CENT; white) to determine CS number. For each cell, colocalizing SYCE2-CENT signals are indicated with dashed circles, and resulting CS numbers are shown. i CS number in Atrfl/+ males (n = 2 males; 55 cells), Atrfl/– males (n = 2 males; 37 cells), Spo11−/− males (n = 2 males; 114 cells) and Atrfl/– Spo11−/− males (n = 2 males; 64 cells). Mean values and p values (Mann–Whitney test) indicated. Scale bars 10 µm
Asynapsis can result from defects in synaptonemal complex (SC) formation or recombination. To address whether ATR can promote SC formation independent of recombination, we used the Spo11-null mutation, which permits genetic dissociation of synapsis from recombination initiation34. Spo11−/− males do not form programmed DSBs, yet achieve extensive SC formation between non-homologs35, 36. If deletion of ATR impeded SC formation in Spo11 nulls, then ATR must have a role in SC formation. We therefore compared synapsis between Spo11−/− and Atrfl/– Spo11−/− males. Like each single mutant, Atrfl/– Spo11−/− males exhibited stage IV germ cell elimination (Supplementary Fig. 2a,b). We classified SC formation, assessed using SYCP3 and the SC central element component SYCE237, into three classes: (i) complete SC, encompassing the entire axis length, (ii) partial SC, extending along only part of an axis, and (iii) focal SC (Fig. 3c–e). Relative to Spo11−/− males (Fig. 3c), Atrfl/– Spo11−/− males (Fig. 3d) exhibited a decrease in complete and partial SC formation, and an increase in focal SC (Fig. 3c–e). These findings suggest that ATR promotes conversion of SC foci into longer SC stretches. Using Atrfl/– Spo11−/− males, we also demonstrated that formation of the pseudosex body in Spo11−/− males is ATR dependent (Supplementary Fig. 2c, d).
Our data suggested that ATR can promote synapsis independent of recombination. To strengthen these findings, we devised an additional method to quantify synapsis. We triple immunostained cells for SYCP3, SYCE2 and centromeres, and counted the number of centromeres that had achieved synapsis, i.e., that colocalized with SYCE2 signals (centromere-SYCE2, or CS number; Fig. 3f–i). The mean CS number was reduced in Atrfl/– relative to control males, confirming that ATR is required for normal levels of synapsis (Fig. 3f, i). In Atrfl/– Spo11−/− males, the mean CS number was reduced relative to that in Spo11−/− males (Fig. 3g–i). Thus, ATR promotes SC formation both in the presence and absence of SPO11-generated DSBs. We did not compare synapsis in Atrfl/– males with that in Atrfl/– Atm−/− males, because in the latter model chromosome axes were highly fragmented at early pachynema.
Atr deletion does not influence DSB abundance
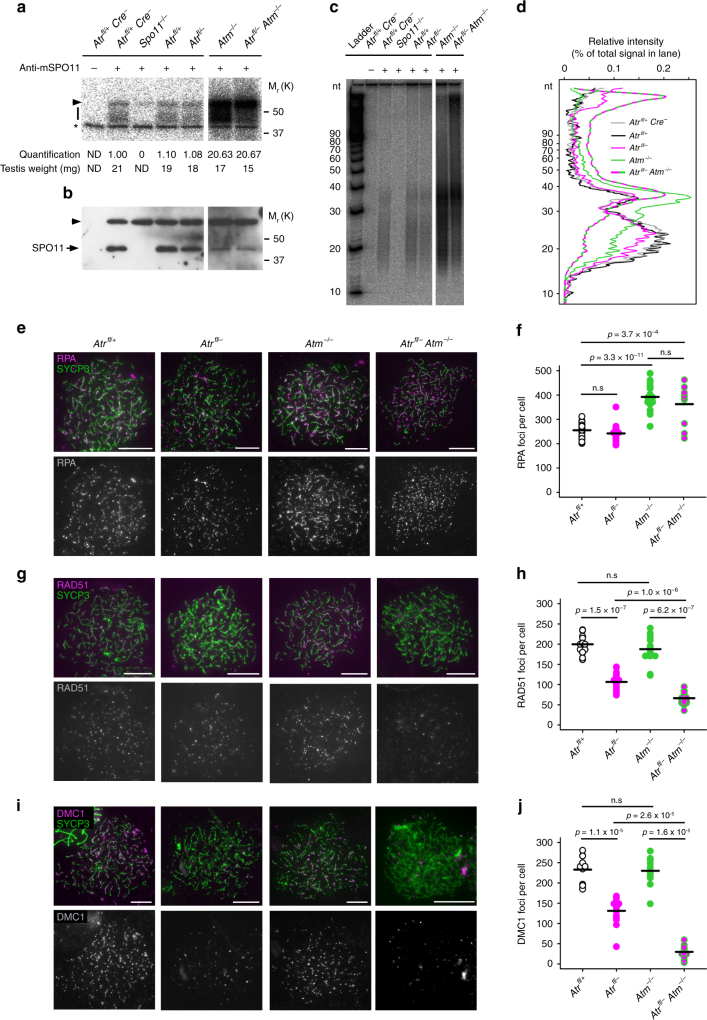
We further examined roles of ATR in DSB formation. Orthologs of ATM influence DSB homeostasis by acting as negative regulators of DSB induction38–42. In Saccharomyces cerevisiae ATR promotes DSB formation indirectly by increasing the length of prophase I43, but its impact on DSB levels in mammals is unknown. To address this question, we measured abundance of covalent SPO11-oligonucleotide (SPO11-oligo) complexes, by-products of DSB induction, in testis extracts44. Consistent with previous work40, in Atm−/− testes SPO11-oligo complex levels were elevated relative to those observed in wild-type mice (Fig. 4a, b). However, SPO11-oligo complex levels in Atrfl/– testes, as well as in Atrfl/+ males without the Ngn3-Cre transgene, were not detectably changed relative to controls (Fig. 4a, b). SPO11-oligo complex levels in Atrfl/– Atm−/− testes were similar to those in Atm−/− testes (p = 0.29; Fig. 4a, b). Thus, ATR and ATM have distinct functions with respect to DSB formation.
Fig. 4.
ATR ablation does not alter DSB levels but leads to reduction in leptotene recombinase counts. a–d Analysis of SPO11-oligo complexes in P13 testes. SPO11 was immunoprecipitated from whole-testis extracts and SPO11-associated oligos were end-labeled with terminal deoxynucleotidyl transferase and [α-32P] dCTP, then either separated on SDS-PAGE gels followed by autoradiography (a) and western blotting with anti-SPO11 antibody (b) or digested with proteinase K and resolved on denaturing polyacrylamide sequencing gels (c; background-subtracted lane traces in d). A representative experiment is shown. An additional Atrfl/+ Cre– control is shown to demonstrate that the Ngn3-Cre transgene does not influence SPO11-oligo levels. In a, b, the bar indicates SPO11-oligo complexes, arrowhead indicates the immunoglobulin heavy chain and asterisk marks non-specific labeling; ND not determined. Each panel shows lanes from the same exposure of a single western blot or autoradiograph, with intervening lanes omitted. For quantitation, SPO11-oligo complex signals were background-subtracted and normalized to Atrfl/+ Cre– (n = 2) controls. SPO11-oligo quantitation: Atrfl/+ (1.05 ± 0.07-fold, mean and s.d., n = 2 males), Atrfl/– (1.17 ± 0.22-fold, n = 4 males), Atm−/− (14.58 ± 5.24-fold, n = 3 males) and Atrfl/– Atm−/− males (11.38 ± 13.15-fold). The reduced SPO11 protein levels in Atm−/− were previously documented40, 79, but the molecular basis is not understood. e–j Analysis of leptotene focus counts using SYCP3 (green) and early recombination markers (magenta): RPA (e, f), RAD51 (g, h) and DMC1 (i, j) in Atrfl/+ males (n = 2 males; 27 cells for RPA, 19 cells for RAD51, 14 cells for DMC1), Atrfl/– males (n = 2 males; 21 cells for RPA, 19 cells for RAD51, 13 cells for DMC1), Atm−/− males (n = 2 males; 22 cells for RPA, 15 cells for RAD51, 13 cells for DMC1) and Atrfl/– Atm−/− males (n = 2 males; 10 cells for RPA, 20 cells for RAD51, 13 cells for DMC1). RPA counts are not significantly different between Atrfl/– males (mean 249 foci) and Atrfl/+ males without the Ngn3-Cre transgene (mean 227 foci, n = 2 males, 17 cells), and are thus not influenced by Atr haploinsufficiency. Mean and p values (Mann–Whitney test) indicated. Scale bars 10 µm
In addition to its role in DSB homeostasis, ATM is implicated in nucleolytic processing of DSBs40, 45–47. SPO11 oligos are longer in Atm−/− mice, with an increase in intermediate (~40–70 nucleotide (nt)) and large (>300 nt) species at the expense of the small (~15–27 nt) ones observed in wild type40. SPO11-oligo size distribution in Atrfl/– males and in Atrfl/+ males without the Ngn3-Cre transgene were not detectably changed relative to controls (Fig. 4c, d). However, in Atrfl/– Atm−/− testes, SPO11 oligos were on average even larger than in Atm−/− testes, with a decrease in the abundance of small and intermediate oligos and a substantial increase in the amount of high-molecular-weight species running near the top of the gel (Fig. 4c, d). We conclude that ATR is largely dispensable for initial nucleolytic processing of SPO11-generated DSBs, but that it can partially compensate when ATM is not present to promote these events.
Atr controls RAD51 and DMC1 abundance at DSBs in leptonema
During leptonema, resected DSBs are coated with RPA (replication protein A) and the recombinases RAD51 and DMC1, which carry out strand invasion and recombinational repair. We used immunostaining to determine whether these early recombination components are influenced by ATR. Leptotene focus counts for RPA subunit 2 (hereafter termed RPA) were similar between Atrfl/– and controls (Fig. 4e, f). This finding supports conclusions from SPO11-oligo complex quantification that ATR does not influence DSB abundance (Fig. 4a, b), and is consistent with RPA acting upstream of ATR48. In addition, RPA counts in controls did not differ from those in Atrfl/+ males without the Ngn3-Cre transgene (Fig. 4 legend). Importantly though, in Atrfl/– males, RAD51 counts were reduced by almost half relative to controls (Fig. 4g, h). DMC1 counts were lower by a similar magnitude (Fig. 4i, j). Thus, in Atrfl/– males, DSB abundance appears grossly unaffected, but recombinase localization is compromised.
We also quantified RPA2, RAD51 and DMC1 foci at leptonema in Atm−/− and Atrfl/– Atm−/− males. In both models, RPA counts were elevated twofold relative to Atrfl/– and control males (Fig. 4e, f). In Atm−/− males, RAD51 and DMC1 focus counts were similar to controls (Fig. 4g–j). The failure of recombination focus counts to fully account for the large increase in DSB formation in the absence of ATM has been proposed to reflect inability of cytological methods to resolve the clustered DSBs that form nearby on the same chromatid or pair of sister chromatids40, 42. However, in Atrfl/– Atm−/− males RAD51 and DMC1 counts were reduced to levels even lower than that observed in Atrfl/– males (Fig. 4g–j). Thus, RPA counts are increased by deleting ATM but not ATR, while RAD51 and DMC1 counts are decreased by deleting ATR, and even more so by deleting both ATR and ATM.
Atr regulates DSB dynamics during later prophase I
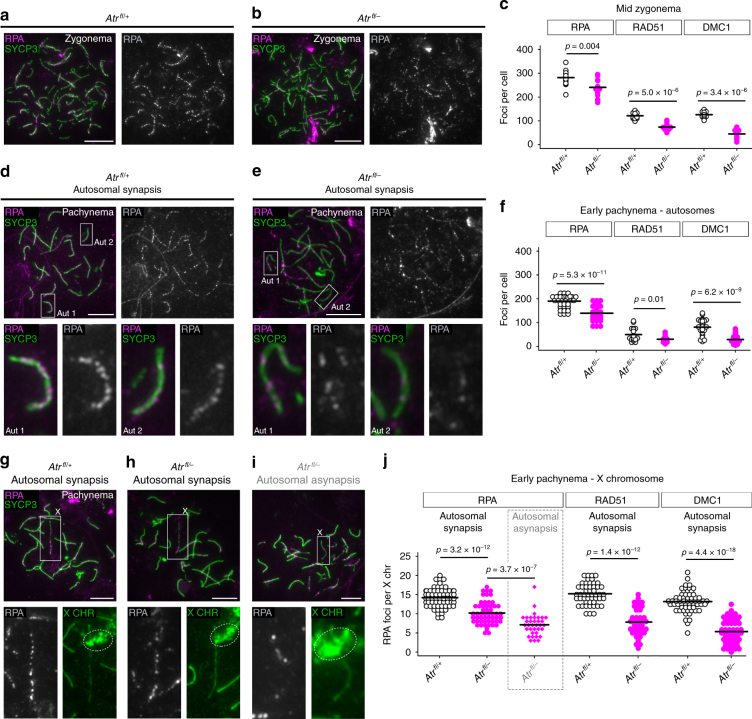
We also tested if ATR influences later stages of recombination by quantifying RPA, RAD51 and DMC1 foci throughout prophase I. We restricted our analysis to Atrfl/– males because the extensive chromosome fragmentation after leptonema in Atrfl/– Atm−/− testes prevented a comparison of ATR and ATM functions at these later stages. Interestingly, while RPA counts in Atrfl/– males were equivalent to those in controls at late leptonema (Fig. 4e, f), they were reduced at mid zygonema (Fig. 5a–c). RAD51 and DMC1 counts were also lower in Atrfl/– than control males at this stage (Fig. 5c; Supplementary Fig. 3a-d). At early pachynema, DSB markers were assayed both on the autosomes and on the X chromosome, focusing initially on cells with normal autosomal synapsis. Relative to control males, in Atrfl/– males RPA, RAD51 and DMC1 counts were reduced on the autosomes (Fig. 5d–f; Supplementary Fig. 3e-h) and on the X chromosome (Fig. 5g, h, j; Supplementary Fig. 3i-l). The recombination defect in Atrfl/– males therefore affects RAD51 and DMC1 at leptonema, and all three DSB markers at mid zygonema and early pachynema.
Fig. 5.
ATR regulates DSB marker counts during zygonema and pachynema. a, b RPA (magenta) and SYCP3 (green) immunostaining in Atrfl/+ and Atrfl/– males. c RPA, RAD51 and DMC1 counts at mid zygonema in Atrfl/+ males (n = 2 males; 16 cells for RPA, 15 cells for RAD51, 15 cells for DMC1) and Atrfl/– males (n = 2 males; 16 cells for RPA, 15 cells for RAD51, 15 cells for DMC1). d, e Examples of early pachytene cells from Atrfl/+ and Atrfl/– males with normal autosomal synapsis. Two representative autosomes (aut 1 and 2) are boxed in upper panels and magnified in lower panels. f autosomal RPA, RAD51 and DMC1 counts at early pachynema in Atrfl/+ males (n = 2 males; 49 cells for RPA, 19 cells for RAD51, 33 cells for DMC1) and Atrfl/– males (n = 2 males; 43 cells for RPA, 19 cells for RAD51, 35 cells for DMC1). g, h Early pachytene Atrfl/+ and Atrfl/– cells with normal autosomal synapsis and the X chromosome (boxed in upper panels) identified by Slx DNA FISH (dashed circles in lower panels). i Early pachytene Atrfl/– cell with autosomal asynapsis. j X-chromosome RPA, RAD51 and DMC1 counts at early pachynema in Atrfl/+ males (n = 2 males; 64 cells for RPA, 49 cells for RAD51, 59 cells for DMC1) and Atrfl/– males (n = 2 males; 62 cells for RPA in autosomal synapsis category, 35 cells for RPA in autosomal asynapsis category, 36 cells for RAD51, 53 cells for DMC1). Mean and p values (Mann–Whitney test) indicated. Scale bars 10 µm
In mice, synapsis is dependent upon recombination35, 36, 49, 50. Asynapsis in Atrfl/– males could therefore result not only from SC defects per se (Fig. 3), but also from aberrant recombination. We therefore asked whether early pachytene Atrfl/– spermatocytes with autosomal asynapsis exhibit alterations in DSB marker counts relative to Atrfl/– spermatocytes with normal autosomal synapsis. We used RPA as a representative DSB marker, and determined counts on the X chromosome as an indication of recombination levels. Since it can be obscured by asynapsed autosomes, the X chromosome was identified using Slx DNA FISH. In Atrfl/– cells with autosomal asynapsis (Fig. 5i) X-chromosome RPA counts were lower than those in Atrfl/– cells exhibiting normal autosomal synapsis (Fig. 5h, j). Greater alterations in recombination markers therefore correlate with the asynapsis phenotype in Atrfl/– males.
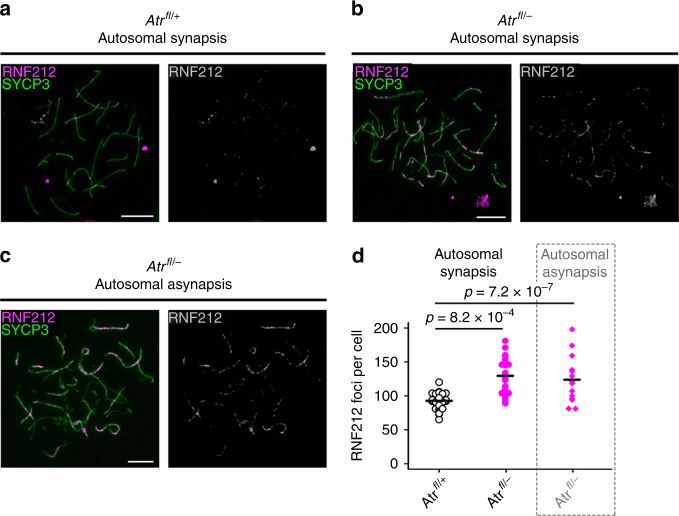
To further define ATR roles later in recombination, we examined the intermediate recombination marker RNF212, a RING-family E3 ligase that localizes to sites of synapsis and is implicated in designation of crossovers51. Interestingly, RNF212 focus counts were higher in Atrfl/– cells relative to controls (Fig. 6a–d). The elevation was observed in cells with normal synapsis as well as those with autosomal asynapsis. Thus, while deletion of ATR causes a reduction in RPA, RAD51 and DMC1 counts at early pachynema, it leads to an elevation in RNF212 counts at this stage.
Fig. 6.
ATR regulates RNF212 counts during pachynema. a–c RNF212 (magenta) and SYCP3 (green) immunostaining at early pachynema in Atrfl/+ and Atrfl/– males. d RNF212 counts in Atrfl/+ male (n = 1 male; 29 cells) and Atrfl/– male with normal autosomal synapsis (n = 1 male; 15 cells) or autosomal asynapsis (n = 1 males; 25 cells). Mean and p values (Mann–Whitney test) indicated. Scale bars 10 µm
Discussion
The functions of ATR in mammalian meiosis have been unclear. We show here that ATR is required during unperturbed meiosis to regulate chromosome axis integrity, synapsis and recombination. Atr haploinsufficiency compromises the DNA damage response during mitosis13. We did not observe haploinsufficiency phenotypes in male meiosis, possibly because they are too mild to detect by our approaches. Alternatively, since ATR expression in the testis far exceeds that in other tissues21, Atr haploinsufficiency may be better tolerated during meiosis than mitosis. As is the case in mitosis1, in meiosis ATR has roles that are both shared and distinct from ATM. Differences in ATR and ATM functions are likely explained by the contrasting substrate specificities1 and meiotic expression profiles24 of these kinases.
Deletion of Atr causes mid-pachytene germ cell elimination. This phenotype is also observed in mice deficient in all three DNA damage-regulated PIKKs. Multiple lines of evidence suggest that PIKK depletion in these mice is efficient. Nevertheless, the use of conditional alleles means that residual PIKK activity may be present and sufficient for checkpoint maintenance. Setting aside this caveat, our findings do not exclude a contribution of PIKKs to mid-pachytene elimination in synapsis and recombination mutants. However, they do confirm the existence of additional mechanisms that can trigger elimination. Under such circumstances, mid-pachytene failure is likely caused by defective MSCI27. We suggest that the coexistence of multiple overlapping surveillance mechanisms during prophase I in males explains why checkpoint responses are more robust than those in females52, 53.
Like ATM54–56, ATR regulates homologous synapsis. ATR can promote synapsis independently of meiotic DSB formation, presumably through modification of SC proteins. Among the many SC components, HORMAD1/2 and SMC3 are established ATR phosphortargets16, 57, 58. While additional SC candidates no doubt exist, HORMAD1 is of particular interest, because this protein can also promote synapsis in the absence of recombination34. We find that in wild-type males, asynapsis of the XY pair occurs at similar frequency to that of all autosomal pairs combined. This bias towards XY asynapsis may be attributable to the small length and terminal location of the PAR. Notably however, in Atr mutants, the bias is exaggerated, such that asynapsis of the XY bivalent occurs at higher frequency than that of all autosomes combined. The PAR is unusual, undergoing late DSB formation and synapsis33, and being enriched for repressive chromatin marks not observed at the termini of autosomal bivalents59, 60. We suggest that ATR promotes one or more of these unique properties to ensure successful XY interactions.
DSB frequency increases drastically when ATM is missing40, but ATR deficiency has little if any effect on SPO11-oligo complex amounts in either ATM-proficient or ATM-deficient backgrounds. Thus, we conclude that ATR does not contribute significantly to control of DSB numbers. Atm mutants also exhibit an increase in the lengths of SPO11 oligos40. This phenotype is not observed in Atr single mutants, but SPO11-oligos become even longer in Atr Atm double mutants. These findings suggest that ATR can partially substitute for ATM in promoting normal nucleolytic processing of DSBs. For example, ATR may influence the 3′→5′ exonuclease activity of MRE11, which is known to govern Spo11-oligo length in yeast61. CtIP and its yeast ortholog Sae2, MRE11 partners important for DSB processing, are PIKK phosphortargets62–64.
Although ATR deficiency does not appear to modulate DSB numbers, Atr mutants do display a substantial reduction in RAD51 and DMC1 focus counts. This finding suggests that ATR specifically promotes the assembly of RAD51 and DMC1 on resected DSBs. It has been suggested that ATR phosphorylates RAD5165. ATR also phosphorylates CHK1 during meiosis66, and CHK1 in turn promotes RAD51 loading at DSBs67. A nonexclusive alternative is that focus numbers are reduced in the mutant because ATR slows their turnover, for example by inhibiting use of the sister chromatid as a recombination partner5, 68. Atm single mutants do not display a reduction in RAD51 and DMC1 foci, but the interpretation of this result is complicated by the fact that the mutants make more DSBs that are thought to often occur in clusters that may not be cytologically distinguishable from single DSBs40, 42. Thus, whether and how ATM might influence RAD51 and DMC1 assembly has been unclear. We find that RAD51 and DMC1 focus counts are lower in the Atr Atm double mutants than in the Atr single mutant without an apparent change in DSB number. This finding suggests that ATM can indeed facilitate assembly of RAD51- and DMC1-containing recombination intermediates, at least in the absence of ATR. If so, ATM may promote RAD51 and DMC1 assembly directly, or may do so indirectly by fostering DSB resection, as its ortholog Tel1 does in yeast2, 46. ATM may also influence the lifespan of RAD51 and DMC1 foci via effects on sister chromatid recombination.
Later in prophase I, spermatocytes lacking ATR exhibit lower counts not only for RAD51 and DMC1 but also for RPA. In contrast, focus counts for the intermediate recombination marker RNF212 are elevated. These changes could imply additional functions for ATR in later processing of recombination intermediates and/or crossover designation. Alternatively, the reduction in RPA, RAD51 and DMC1 may indicate the presence of fewer unrepaired DSBs. This possibility is not precluded by the RNF212 findings, because RNF212 can localize to the SC in the absence of recombination51. Reduced DSB number in Atr mutants could result from premature repair using the sister chromatid5, or from a failure to induce new DSBs on asynapsed chromosomes69. Interestingly, the ATR substrates HORMAD1/2 are implicated both in inhibiting sister chromatid recombination70, 71 and in promoting ongoing DSB formation70, 72. Our data are less consistent with a defect in generating new DSBs on asynapsed chromosomes, because SPO11-oligo data showed no reduction in DSB formation in Atr mutants. Furthermore, in these mutants decreased RPA counts were also observed on synapsed autosomes, and the magnitude of the decrease was similar to that seen for the asynapsed X chromosome. If there are fewer DSBs during pachynema in the Atr mutant, then the increase in RNF212 foci seen at this stage could imply a role for ATR in regulating RNF212 abundance at the SC independently of recombination.
Whether ATR regulates crossover formation and designation is currently unclear. Analyses of male mice with a hypomorphic Atr mutation or with pharmacologically inhibited ATR suggest that this kinase is required for crossovers73. We could not analyze crossover formation in our Atr mutants because crossover markers appear after the point of germ cell elimination in these mice (Supplementary Fig. 4). Conditional deletion of Atr in the female mouse germline, where recombination defects incur germ cell elimination later in meiosis74, may help to resolve this point.
Methods
Animal experiments
All mice were maintained under UK Home Office Regulations, UK Animals (Scientific Procedures) Act 1986, and according to ethical guidelines at the National Institute for Medical Research (NIMR) and the Francis Crick Institute Mill Hill laboratory. Permission for animal experiments was granted by The Crick Biological Research Facility Strategic Oversight Committee (BRF-SOC) incorporating Animal Welfare and Ethical Review Body (AWERB) (Project Licence P8ECF28D9 granted by the Secretary of State). Genetically modified models are previously published and are maintained on a predominantly C57BL/6 background: Ngn3-Cre20, Stra8-Cre18, Atr14, Atr flox75, Atm76, Atm flox31, Spo1136 and Dmc149. Prkdcscid/scid mice were obtained from the Jackson Labs and are maintained on an NOD background. Littermate controls were used where possible.
Immunofluorescence, focus counting and western blotting
Immunofluorescence experiments on surface spread spermatocytes were carried out as previously described28. In brief, cells were permeabilized for 10 min in 0.05% Triton X-100 and fixed for 1 h minimum in 2% formaldehyde, 0.02% SDS in phosphate-buffered saline (PBS). Slides were rinsed in distilled water, air dried and blocked in PBT (0.15% bovine serum albumin, 0.10% TWEEN-20 in PBS) for 1 h. Slides were incubated with the following antibodies in a humid chamber overnight at 37 °C: guinea-pig anti-SYCP3 (made in-house) 1:500, rabbit anti-SYCP3 (Abcam ab-15092) 1:100, rabbit anti-ATR (Cell Signalling #2790) 1:50, mouse anti-γH2AX (Millipore 05–636) 1:100, rabbit anti-HORMAD2 (Tóth lab) 1:100, guinea-pig anti-SYCE2 (gift from Howard Cooke/Ian Adams) 1:800, human anti-centromere (CREST ab gift from Bill Earnshaw) 1:1000, rabbit anti-RPA (Abcam ab-2175) 1:100 anti-rabbit RAD51 (Calbiochem PC130) 1:25, goat anti-DMC1 (Santa Cruz sc-8973) 1:25. Western blotting was carried out as previously described77 (Supplementary Fig. 6). Counting of recombination protein foci was performed manually, considering only foci that colocalized with axial elements.
Meiotic staging
The presence of asynapsis can make discrimination between zygotene cells and pachytene cells with asynapsis challenging. We used the following criteria (Supplementary Fig. 5). (1) DAPI (4′,6-diamidino-2-phenylindole) staining: at zygonema DAPI staining is bright and centromeres are clustered in a few subdomains, while in pachynema DAPI staining is heterogeneous, with euchromatin stained faintly and centromeres stained brightly and forming multiple subdomains. (2) The length and thickness of axial elements/SCs: axial elements are long and thin in zygonema, and are not yet fully formed, but they are shorter and thicker and fully formed in pachynema. (3) Chromosomal asynchrony: in contrast to zygotene cells, pachytene cells with asynapsis exhibit asynchrony in synapsis between individual bivalents, i.e., the coexistence of completely asynapsed bivalents and multiple fully synapsed bivalents. For comparison of synapsis between Spo11−/− males and Atrfl/– Spo11−/− males, pachytene cells were identified by virtue of having fully developed axial elements.
RNA, DNA FISH and SPO11-oligo analysis
FISH was carried out with digoxigenin-labeled probes as previously described78. CHORI BAC probe, RP24-204O18 (CHORI) was used for Scml2 RNA FISH, RP23-470D15 for Slx DNA FISH, and RP24-502P5 for Sly DNA FISH. Analyses of abundance of SPO11-oligo complexes and sizes of SPO11 oligos were performed as previously described40, 44.
Microscopy
Imaging was performed using an Olympus IX70 inverted microscope with a 100-W mercury arc lamp. For chromosome spread and RNA FISH imaging, an Olympus UPlanApo 100×/1.35 NA oil immersion objective was used. For testis section imaging, an Olympus UPlanApo 40×/0.75 NA objective was used. A Deltavision RT computer-assisted Photometrics CoolsnapHQ CCD camera with an ICX285 Progressive scan CCD image sensor was utilized for image capture. Then, 8 or 16-bit (512 × 512 or 1024 × 1024 pixels) raw images of each channel were captured and later processed using Fiji software.
Statistics
Statistical calculations were performed using GraphPad Prism 6.0. For comparison of two genotypes, Mann–Whitney test or t-tests were performed.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Electronic supplementary material
Acknowledgements
We are grateful to Bill Earnshaw for providing the CREST anti-centromere antibody, Howard Cooke, Ian Adams for providing the SYCE2 antibody, Paula Cohen for providing the anti-MLH3 antibody, Neil Hunter for the anti-RNF212 antibody, Eric Brown for providing the Atr flox and null mice, members of the Turner laboratory for critical reading of the manuscript and the Francis Crick Institute Technology platforms for excellent assistance. This work was supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (FC001193), the UK Medical Research Council (FC001193) and the Wellcome Trust (FC001193). Work in the Keeney lab is supported in part by the US National Institutes of Health grant R35 GM118092; J.L. was supported in part by American Cancer Society fellowship PF-12-157-01-DMC. S.P., A.M.-L. and I.R. are supported by the Ministerio de Ciencia e Innovación (BFU2016-80370-P).
Author contributions
A.W., O.O., V.M. and J.M.A.T. performed animal generation and genotyping; A.W., S.K.M., M.S., S.P., A.M.-L. and J.M.A.T. performed immunofluorescence; A.W., E.E. and V.M. performed western blots; A.W. and S.K.M. performed RNA FISH; T.H. performed DNA FISH; J.L. and S.K. performed SPO11-oligo experiments; A.W. and D.d.R. performed histology; A.W. and J.Z. performed data plotting and statistics; A.T. supplied HORMAD2 antibody. J.M.A.T. wrote the manuscript with critical input from A.T., S.K., I.R. and Turner lab members.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-04850-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marechal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. 2013;5:pii: a012716. doi: 10.1101/cshperspect.a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi N, Brown MS, Bishop DK, Borner GV. Gradual implementation of the meiotic recombination program via checkpoint pathways controlled by global DSB levels. Mol. Cell. 2015;57:797–811. doi: 10.1016/j.molcel.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurzbauer MT, Uanschou C, Chen D, Schlogelhofer P. The recombinases DMC1 and RAD51 are functionally and spatially separated during meiosis in Arabidopsis. Plant Cell. 2012;24:2058–2070. doi: 10.1105/tpc.112.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grushcow JM, et al. Saccharomyces cerevisiae checkpoint genes MEC1, RAD17 and RAD24 are required for normal meiotic recombination partner choice. Genetics. 1999;153:607–620. doi: 10.1093/genetics/153.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carballo JA, Johnson AL, Sedgwick SG, Cha RS. Phosphorylation of the axial element protein Hop1 by Mec1/Tel1 ensures meiotic interhomolog recombination. Cell. 2008;132:758–770. doi: 10.1016/j.cell.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter AT. Recombination nodules and synaptonemal complex in recombination-defective females of Drosophila melanogaster. Chromosoma. 1979;75:259–292. doi: 10.1007/BF00293472. [DOI] [PubMed] [Google Scholar]
- 7.Falk JE, Chan AC, Hoffmann E, Hochwagen A. A Mec1- and PP4-dependent checkpoint couples centromere pairing to meiotic recombination. Dev. Cell. 2010;19:599–611. doi: 10.1016/j.devcel.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Abdu U, Brodsky M, Schupbach T. Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr. Biol. 2002;12:1645–1651. doi: 10.1016/S0960-9822(02)01165-X. [DOI] [PubMed] [Google Scholar]
- 9.Lydall D, Nikolsky Y, Bishop DK, Weinert T. A meiotic recombination checkpoint controlled by mitotic checkpoint genes. Nature. 1996;383:840–843. doi: 10.1038/383840a0. [DOI] [PubMed] [Google Scholar]
- 10.Murakami H, Nurse P. Meiotic DNA replication checkpoint control in fission yeast. Genes Dev. 1999;13:2581–2593. doi: 10.1101/gad.13.19.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blitzblau HG, Hochwagen A. ATR/Mec1 prevents lethal meiotic recombination initiation on partially replicated chromosomes in budding yeast. eLife. 2013;2:e00844. doi: 10.7554/eLife.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 13.O’Driscoll M, Dobyns WB, van Hagen JM, Jeggo PA. Cellular and clinical impact of haploinsufficiency for genes involved in ATR signaling. Am. J. Human Genet. 2007;81:77–86. doi: 10.1086/518696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 15.de Klein A, et al. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 2000;10:479–482. doi: 10.1016/S0960-9822(00)00447-4. [DOI] [PubMed] [Google Scholar]
- 16.Royo H, et al. ATR acts stage specifically to regulate multiple aspects of mammalian meiotic silencing. Genes Dev. 2013;27:1484–1494. doi: 10.1101/gad.219477.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–628. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadate-Ngatchou PI, Payne CJ, Dearth AT, Braun RE. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis. 2008;46:738–742. doi: 10.1002/dvg.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng K, Wang PJ. Blockade of pachytene piRNA biogenesis reveals a novel requirement for maintaining post-meiotic germline genome integrity. PLoS Genet. 2012;8:e1003038. doi: 10.1371/journal.pgen.1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Keegan KS, et al. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes Dev. 1996;10:2423–2437. doi: 10.1101/gad.10.19.2423. [DOI] [PubMed] [Google Scholar]
- 22.Lammers JH, et al. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol. Cell. Biol. 1994;14:1137–1146. doi: 10.1128/MCB.14.2.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barchi M, et al. Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol. Cell. Biol. 2005;25:7203–7215. doi: 10.1128/MCB.25.16.7203-7215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellani MA, Romanienko PJ, Cairatti DA, Camerini-Otero RD. SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm-/- spermatocytes. J. Cell Sci. 2005;118:3233–3245. doi: 10.1242/jcs.02466. [DOI] [PubMed] [Google Scholar]
- 25.Mahadevaiah SK, et al. Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J. Cell Biol. 2008;182:263–276. doi: 10.1083/jcb.200710195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacheco S, et al. The ATM signaling cascade promotes recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet. 2015;11:e1005017. doi: 10.1371/journal.pgen.1005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royo H, et al. Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr. Biol. 2010;20:2117–2123. doi: 10.1016/j.cub.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Turner JM, et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 2005;37:41–47. doi: 10.1038/ng1484. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Capetillo O, Liebe B, Scherthan H, Nussenzweig A. H2AX regulates meiotic telomere clustering. J. Cell. Biol. 2003;163:15–20. doi: 10.1083/jcb.200305124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Araki R, et al. Nonsense mutation at Tyr-4046 in the DNA-dependent protein kinase catalytic subunit of severe combined immune deficiency mice. Proc. Natl. Acad. Sci. USA. 1997;94:2438–2443. doi: 10.1073/pnas.94.6.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zha S, Sekiguchi J, Brush JW, Bassing CH, Alt FW. Complementary functions of ATM and H2AX in development and suppression of genomic instability. Proc. Natl. Acad. Sci. USA. 2008;105:9302–9306. doi: 10.1073/pnas.0803520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurley KE, Kemp CJ. Synthetic lethality between mutation in Atm and DNA-PK(cs) during murine embryogenesis. Curr. Biol. 2001;11:191–194. doi: 10.1016/S0960-9822(01)00048-3. [DOI] [PubMed] [Google Scholar]
- 33.Kauppi L, et al. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science. 2011;331:916–920. doi: 10.1126/science.1195774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel K, et al. Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat. Cell Biol. 2011;13:599–610. doi: 10.1038/ncb2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell. 2000;6:975–987. doi: 10.1016/S1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 36.Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell. 2000;6:989–998. doi: 10.1016/S1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 37.Costa Y, et al. Two novel proteins recruited by synaptonemal complex protein 1 (SYCP1) are at the centre of meiosis. J. Cell Sci. 2005;118:2755–2762. doi: 10.1242/jcs.02402. [DOI] [PubMed] [Google Scholar]
- 38.Joyce EF, et al. Drosophila ATM and ATR have distinct activities in the regulation of meiotic DNA damage and repair. J. Cell Biol. 2011;195:359–367. doi: 10.1083/jcb.201104121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Kim KP, Kleckner NE, Storlazzi A. Meiotic double-strand breaks occur once per pair of (sister) chromatids and, via Mec1/ATR and Tel1/ATM, once per quartet of chromatids. Proc. Natl. Acad. Sci. USA. 2011;108:20036–20041. doi: 10.1073/pnas.1117937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lange J, et al. ATM controls meiotic double-strand-break formation. Nature. 2011;479:237–240. doi: 10.1038/nature10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carballo JA, et al. Budding yeast ATM/ATR control meiotic double-strand break (DSB) levels by down-regulating Rec114, an essential component of the DSB-machinery. PLoS Genet. 2013;9:e1003545. doi: 10.1371/journal.pgen.1003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia V, Gray S, Allison RM, Cooper TJ, Neale MJ. Tel1(ATM)-mediated interference suppresses clustered meiotic double-strand-break formation. Nature. 2015;520:114–118. doi: 10.1038/nature13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray S, Allison RM, Garcia V, Goldman AS, Neale MJ. Positive regulation of meiotic DNA double-strand break formation by activation of the DNA damage checkpoint kinase Mec1(ATR) Open Biol. 2013;3:130019. doi: 10.1098/rsob.130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neale MJ, Pan J, Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436:1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terasawa M, Ogawa T, Tsukamoto Y, Ogawa H. Sae2p phosphorylation is crucial for cooperation with Mre11p for resection of DNA double-strand break ends during meiotic recombination in Saccharomyces cerevisiae. Genes Genet. Syst. 2008;83:209–217. doi: 10.1266/ggs.83.209. [DOI] [PubMed] [Google Scholar]
- 46.Mimitou EP, Yamada S, Keeney S. A global view of meiotic double-strand break end resection. Science. 2017;355:40–45. doi: 10.1126/science.aak9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohibullah N, Keeney S. Numerical and spatial patterning of yeast meiotic DNA breaks by Tel1. Genome Res. 2017;27:278–288. doi: 10.1101/gr.213587.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 49.Pittman DL, et al. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol. Cell. 1998;1:697–705. doi: 10.1016/S1097-2765(00)80069-6. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida K, et al. The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol. Cell. 1998;1:707–718. doi: 10.1016/S1097-2765(00)80070-2. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds A, et al. RNF212 is a dosage-sensitive regulator of crossing-over during mammalian meiosis. Nat. Genet. 2013;45:269–278. doi: 10.1038/ng.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morelli MA, Cohen PE. Not all germ cells are created equal: aspects of sexual dimorphism in mammalian meiosis. Reproduction. 2005;130:761–781. doi: 10.1530/rep.1.00865. [DOI] [PubMed] [Google Scholar]
- 53.Hunt PA, Hassold TJ. Sex matters in meiosis. Science. 2002;296:2181–2183. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- 54.Barchi M, et al. ATM promotes the obligate XY crossover and both crossover control and chromosome axis integrity on autosomes. PLoS Genet. 2008;4:e1000076. doi: 10.1371/journal.pgen.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y, et al. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 56.Barlow C, et al. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development. 1998;125:4007–4017. doi: 10.1242/dev.125.20.4007. [DOI] [PubMed] [Google Scholar]
- 57.Luo H, et al. Regulation of intra-S phase checkpoint by ionizing radiation (IR)-dependent and IR-independent phosphorylation of SMC3. J. Biol. Chem. 2008;283:19176–19183. doi: 10.1074/jbc.M802299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukuda T, et al. Phosphorylation of chromosome core components may serve as axis marks for the status of chromosomal events during mammalian meiosis. PLoS Genet. 2012;8:e1002485. doi: 10.1371/journal.pgen.1002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turner JM, Burgoyne PS, Singh PB. M31 and macroH2A1.2 colocalise at the pseudoautosomal region during mouse meiosis. J. Cell Sci. 2001;114:3367–3375. doi: 10.1242/jcs.114.18.3367. [DOI] [PubMed] [Google Scholar]
- 60.Peters AH, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/S0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 61.Garcia V, Phelps SE, Gray S, Neale MJ. Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1. Nature. 2011;479:241–244. doi: 10.1038/nature10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterson SE, et al. Activation of DSB processing requires phosphorylation of CtIP by ATR. Mol. Cell. 2013;49:657–667. doi: 10.1016/j.molcel.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cartagena-Lirola H, Guerini I, Viscardi V, Lucchini G, Longhese MP. Budding yeast Sae2 is an in vivo target of the Mec1 and Tel1 checkpoint kinases during meiosis. Cell Cycle. 2006;5:1549–1559. doi: 10.4161/cc.5.14.2916. [DOI] [PubMed] [Google Scholar]
- 64.Baroni E, Viscardi V, Cartagena-Lirola H, Lucchini G, Longhese MP. The functions of budding yeast Sae2 in the DNA damage response require Mec1- and Tel1-dependent phosphorylation. Mol. Cell. Biol. 2004;24:4151–4165. doi: 10.1128/MCB.24.10.4151-4165.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flott S, et al. Regulation of Rad51 function by phosphorylation. EMBO Rep. 2011;12:833–839. doi: 10.1038/embor.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fedoriw AM, Menon D, Kim Y, Mu W, Magnuson T. Key mediators of somatic ATR signaling localize to unpaired chromosomes in spermatocytes. Development. 2015;142:2972–2980. doi: 10.1242/dev.126078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sorensen CS, et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat. Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 68.Goldfarb T, Lichten M. Frequent and efficient use of the sister chromatid for DNA double-strand break repair during budding yeast meiosis. PLoS Biol. 2010;8:e1000520. doi: 10.1371/journal.pbio.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kauppi L, et al. Numerical constraints and feedback control of double-strand breaks in mouse meiosis. Genes Dev. 2013;27:873–886. doi: 10.1101/gad.213652.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wojtasz L, et al. Mouse HORMAD1 and HORMAD2, two conserved meiotic chromosomal proteins, are depleted from synapsed chromosome axes with the help of TRIP13 AAA-ATPase. PLoS Genet. 2009;5:e1000702. doi: 10.1371/journal.pgen.1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin YH, McGuire MM, Rajkovic A. Mouse HORMAD1 is a meiosis i checkpoint protein that modulates DNA double- strand break repair during female meiosis. Biol. Reprod. 2013;89:29. doi: 10.1095/biolreprod.112.106773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keeney S, Lange J, Mohibullah N. Self-organization of meiotic recombination initiation: general principles and molecular pathways. Annu. Rev. Genet. 2014;48:187–214. doi: 10.1146/annurev-genet-120213-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pacheco, S. et al. ATR is required to complete meiotic recombination in mice. Nat. Commun.10.1038/s41467-018-04851-z (2018). [DOI] [PMC free article] [PubMed]
- 74.Di Giacomo M, et al. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc. Natl. Acad. Sci. USA. 2005;102:737–742. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruzankina Y, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barlow C, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/S0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 77.Hamer G, Kal HB, Westphal CH, Ashley T, de Rooij DG. Ataxia telangiectasia mutated expression and activation in the testis. Biol. Reprod. 2004;70:1206–1212. doi: 10.1095/biolreprod.103.024950. [DOI] [PubMed] [Google Scholar]
- 78.Mahadevaiah SK, Costa Y, Turner JM. Using RNA FISH to study gene expression during mammalian meiosis. Methods Mol. Biol. 2009;558:433–444. doi: 10.1007/978-1-60761-103-5_25. [DOI] [PubMed] [Google Scholar]
- 79.Bellani MA, Boateng KA, McLeod D, Camerini-Otero RD. The expression profile of the major mouse SPO11 isoforms indicates that SPO11beta introduces double strand breaks and suggests that SPO11alpha has an additional role in prophase in both spermatocytes and oocytes. Mol. Cell. Biol. 2010;30:4391–4403. doi: 10.1128/MCB.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.