Abstract
Air pollution is a global health threat and causes millions of human deaths annually. The late onset of respiratory diseases in children and adults due to prenatal or perinatal exposure to air pollutants is emerging as a critical concern in human health. Pregnancy and fetal development stages are highly susceptible to environmental exposure and tend to develop a long-term impact in later life. In this review, we briefly glance at the direct impact of outdoor and indoor air pollutants on lung diseases and pregnancy disorders. We further focus on lung complications in later life with early exposure to air pollutants. Epidemiological evidence is provided to show the association of prenatal or perinatal exposure to air pollutants with various adverse birth outcomes, such as preterm birth, lower birth weight, and lung developmental defects, which further associate with respiratory diseases and reduced lung function in children and adults. Mechanistic evidence is also discussed to support that air pollutants impact various cellular and molecular targets at early life, which link to the pathogenesis and altered immune responses related to abnormal respiratory functions and lung diseases in later life.
Keywords: Air pollutants, Polycyclic aromatic hydrocarbon, Particulate matter, Early disease origin, Respiratory diseases
Air pollution has become a major global threat to human health. Historically, multiple major episodes of air pollution occurred world wide in the early twentieth century have produced severe health outcomes. Most tragically, the “killer fog” in London in 1952 has caused 12,000 unexplained deaths and severe long-term effects in human health.1 Even in 2012, indoor and outdoor air pollution still caused an estimated 6.5 million deaths, which covers 11.6% of total global deaths.2 Exposure to air pollutants mostly occurs in industrial and rural areas due to various manufacturing, traveling, and living activities. Multiple review articles and meta-analyses have described a direct impact of air pollutants on respiratory responses and diseases.3, 4, 5, 6, 7 We will focus on the liaison of the later onset of respiratory diseases in childhood and adulthood with early life exposure to air pollutants at prenatal and perinatal stages.
Air pollutants
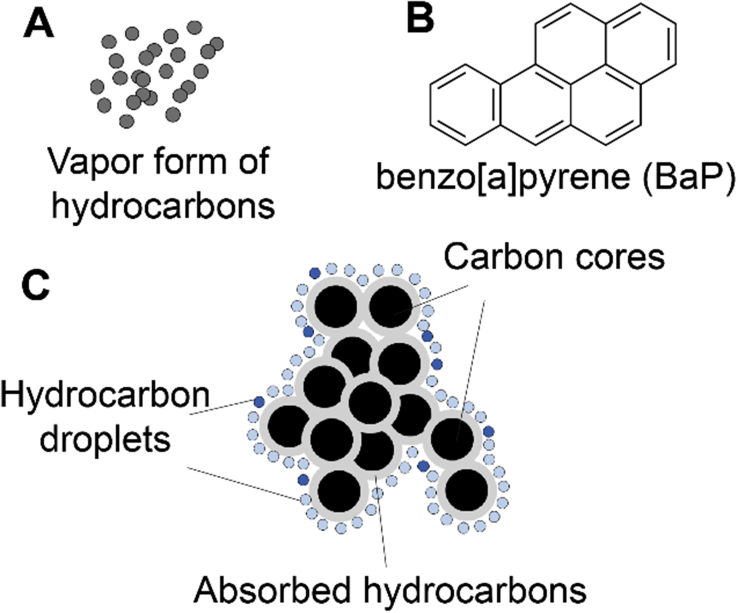
Air pollutants have complex chemical and physical features dependent on the sources of pollutants. Outdoor air pollutants are either derived from human activities, such as industrial emissions, road traffic, residential heating, shipping, air traffic, construction, agricultural activities, war and fire accidents, or from natural hazards, such as earthquake, tsunami, volcanic eruption, spontaneous forest fires, and extreme temperature.8, 9 Although natural hazards occur independent of human activities, they affect the living environment, health, and lives of humans as hazardous events.4, 10 Indoor air pollutants are generally released from smoking, building materials, air conditioning, house cleaning or air refreshing proucts, heating, lighting, and wood, fuel, or coal usage in cooking.6 Chemically, these pollutants can be presented as the vapor forms of inorganic pollutants, such as ozone (O3), carbon monoxide (CO), nitrogen dioxide (NO2), and sulfur dioxide (SO2), or as the vapor forms of organic pollutants, such as polycyclic aromatic hydrocarbons (PAHs), monocyclic hydrocarbons benzene, toluene, xylene, and aliphatic chemicals.4, 5 The particulate forms of air pollutants, however, usually consist of an inner carbon core with various organic pollutants and/or heavy metals on the surface (Fig. 1). The most harmful forms of particulate matter (PM) include PM10 (<10 μm in aerodynamic diameter), fine particles PM2.5 (<2.5 μm), and ultrafine particles (less than 0.1 μm or 100 nm), which can be released from diesel engines, volcanoes, asbestos, unpaved roads, plowing, burning fields, lint, pollens, and spores.11, 12 Detailed chemical components of these air pollutants have been summarized in multiple reviews4, 5, 6, 13, 14, 15 and we will focus on the health impact of these air pollutants with different chemical and physical natures.
Fig. 1.
Schematic demonstration of air pollutants: the vapor form (A) of organic air pollutants exemplified with the structure of benzo [a]pyrene (B) and the particulate form (diesel exhaust particles or particulate matters) of air pollutants (C).
Air pollution in respiratory diseases
Although the bronchopulmonary tract has multiple protective mechanisms, such as mucosal cilia and air-blood barrier, air pollutants are able to accumulate in or pass through lung tissues dependent on the size and chemical nature of pollutants.13 The vapor of air pollutants is prone to be absorbed by human tissues or dissolved in body fluids, mainly relying on their hydrophilicity and hydrophobicity. PM10 particles with larger size (∼10 μm) are able to reach the proximal airways and be mostly eliminated by mucociliary clearance. PM2.5, as a notable risk factor for health, can invade more deeply into the lungs.10, 16, 17 The ultrafine particles are capable of translocation through blood circulation to distal organs and tissues, such as liver tissue for detoxification and placental tissues during pregnancy.10 Negative health effects of air pollutants have been shown on multiple respiratory diseases, including respiratory infections,18, 19, 20 asthma,21, 22 chronic obstructive pulmonary disease (COPD),23 lung cancer, even in combination with stroke and heart diseases as reviewed.3, 24, 25, 26 We briefly outline these direct negative effects of air pollutants on major respiratory diseases as below.
Respiratory infections
Air pollution enhances the severity of respiratory infections, particularly in children.18, 19, 20 Especially, outdoor pollution in large cities is associated with a high burden of various acute respiratory infections, which together are responsible for nearly a third of all deaths in children under 5 years old.18 Exposure to NO2 and PMs in five German cities was associated with increasing cases of laryngo-tracheo-bronchitis mostly due to influenza viral infections.27 Another study conducted in three cities in Finland supported similar conclusions.28 However, indoor pollution contributes to high rates of chronic bronchitis of non-smoker cooking mothers in hilly regions of Nepal,29 suggesting that indoor pollution is likely more associated with respiratory infections in developing countries and rural areas. The adverse impact of air pollutants can be highlighted especially in individuals with pre-existing lung infections or other lung diseases, because they are likely at greater risk, and also in children, possibly because children have a relatively larger lung surface area and more outdoor physical activities with a greater chance to expose to air pollution.30
Asthma and COPD
Emergency visits for asthma are mostly related to the exacerbation effect of environmental exposure. Both major outdoor and indoor pollutants, including O3, CO, NO2, SO2, PM10, PM2.5, dust mite, pollen, pet dander, and smoke, contribute to more severe allergic responses. Specifically, allergic immunoglobulin E (IgE) responses to pollen or ovalbumin can be triggered by diesel exhaust particles (DEP) exposure31, 32 and airway responsiveness in asthmatic patients with house dust mite challenge can be potentiated by short-term exposure to nitrogen oxides.33 Similarly, long-term exposure to indoor air pollution from second-hand cigarette smoke and biomass fuel is able to induce chronic inflammation that contributes to COPD,23 while exposure to PMs is linked to the acute exacerbation-related hospitalization of COPD patients.34 Overall, more epidemiological associations have been reported to link the exposure to air pollutants with the development of asthmatic and chronic inflammation.3, 7, 24, 25, 35
Lung cancers
Although cigarette smoking is considered as a leading contributor to cancer-related death worldwide,36 multiple additional risk factors, including both indoor37 and outdoor38 air pollution, also contribute to the development of lung cancers. The air pollution exposure-induced pathogenesis of lung cancer is closely related to DNA injury, DNA adduct formation, chromosomal aberrations, and methylation modifications, some of which are under development as biomarkers of lung cancers relative to ambient air pollution exposure.39 It was established at least with intensive epidemiological evidence that various air pollutants are directly associated with the high incident rate of lung cancers.36, 37, 38, 39 In this review, we will emphasize the epidemiological and toxicological evidence for the delayed onset of respiratory diseases with prenatal or perinatal exposure to air pollutants.
Air pollution in pregnancy disorders
Exposure to ambient air pollution during the pregnancy is considered having a long-term impact on human health. The prenatal stage is characterized with an exquisite inflammatory homeostasis in mother and mysterious organogenesis in developing fetus, together presenting a highly susceptible window in human lives for adverse effects of environmental pollutants.40 Maternal exposure to air pollution can directly influence the fetus through the transfer of pollutant chemicals through amniotic fluid and placenta.41, 42 Evidence has been accumulated over past two decades to support the association of air pollutants with various adverse birth outcomes, including preterm birth (<37 weeks of gestational age), low birthweight (LBW) (<2500 g), small for gestational age (SGA), and intrauterine growth restriction (IUGR) (Table 1).43
Table 1.
Adverse pregnancy disorders and birth outcomes with prenatal exposure to air pollution.
| Air pollutants | Outcomes | Cohorts/analysis | Major findings | Reference |
|---|---|---|---|---|
| Traffic-related air pollutants | LBW SGA |
Vancouver, British Columbia, Canada | Decreased birth weight and short for gestational age LBW: 11%; 95%CI = 1.01 to 1.23 SGA: 26%; 95%CI = 1.07 to 1.49 |
Brauer et al48, 55 |
| CO | LBW Preterm birth |
Meta-analysisa | Decreased birth weight and increased risk of preterm birth LBW: 11.4 g; 95% CI = (−6.9 to 29.7) g Preterm birth: OR = 1.04; 95%CI = 1.02 to 1.06 |
Stieb et al56 |
| LBW | Northeastern cities in U.S.: Boston, Hartford, Philadelphia, Pittsburgh, Springfield, and Washington, DC | Decreased birth weight AOR = 1.31; 95% CI = 1.06 to 1.62 |
Maisonet et al59 | |
| LBW | Seoul, South Korea | Decreased birth weight AOR = 1.08; 95% CI = 1.04 to 1.12 |
Ha et al60 | |
| IUGR | Calgary, Edmonton, and Montreal, Canada |
Increased risk of intrauterine growth restriction First trimester: OR = 1.18; 95% CI = 1.14 to 1.23 Second trimester: OR = 1.15; 95% CI = 1.10 to 1.19 Third trimester: OR = 1.19; 95% CI = 1.14 to 1.24 |
Liu et al61 | |
| Cardiac ventricular septal defects | California Birth Defects Monitoring Program | Increased risk of cardiac ventricular septal defects 2nd quartile: OR = 1.62; 95% CI = 1.05 to 2.48 3rd quartile:OR = 2.09; 95% CI = 1.19 to 3.67 4th quartile:OR = 2.95; 95% CI = 1.44 to 6.05 |
Ritz et al62 | |
| NO2 | LBW | Meta-analysisa | Decreased birth weight: 28.1 g; 95% CI = (11.5 to 44.8) g | Stieb et al56 |
| LBW | Connecticut and Massachusetts | Decreased birth weight: 8.9 g; 95% CI = (7.0 to 10.8) g | Bell et al58 | |
| LBW Reduced birth length Smaller HC SGA |
INMA cohort in Valencia | Various birth outcomes including LBW: −40.3 g; 95% CI = (−96.3 to 15.6) g Birth length: −0.27 cm; 95% CI = (−0.51 to −0.03) cm HC: −0.17 cm; 95% CI = (−0.34 to −0.003) cm SGA: OR = 1.37; 95% CI = 1.01 to 1.85 |
Ballester et al63 | |
| LBW | Seoul, South Korea | Decreased birth weight AOR = 1.07; 95% CI = 1.03 to 1.11 |
Ha et al60 | |
| LBW Smaller HC |
Netherlands | Decreased birth weight and head circumference LBW: −3.4 g; 95% CI = (−6.2 to −0.6) g HC: −0.12 mm; 95% CI = (−0.17 to −0.06) mm |
van den Hooven et al47 | |
| LBW | European cohort study (ESCAPE) | Decreased birth weight OR = 1.06; 95% CI = 1.01 to 1.11 |
Pedersen et al64 | |
| IUGR | Calgary, Edmonton, and Montreal, Canada |
Increased risk of intrauterine growth restriction First trimester: OR = 1.16; 95% CI = 1.09 to 1.24 Second trimester: OR = 1.14; 95% CI = 1.06 to 1.21 Third trimester: OR = 1.16; 95% CI = 1.09 to 1.24 |
Liu et al61 | |
| SO2 | LBW | North eastern cities in US: Boston, Hartford, Philadelphia, Pittsburgh, Springfield, and Washington, DC | Decreased birth weight 25 to < 50th percentiles: AOR = 1.21; CI = 1.07 to 1.37 50 to < 75th percentiles: AOR = 1.20; CI = 1.08 to 1.35 75 to < 95th percentiles: AOR = 1.21; CI = 1.03 to 1.43 |
Maisonet et al59 |
| LBW | Beijing, China | Decreased birth weight: 7.3 g; OR = 1.11; 95% CI = 1.06 to 1.16 |
Wang et al65 | |
| LBW | Seoul, South Korea | Decreased birth weight AOR = 1.06; 95% CI = 1.02 to 1.10 |
Ha et al60 | |
| PM25 | LBW | Meta-analysisa | Decreased birth weight OR = 1.05; 95% CI = 0.99 to 1.12 |
Stieb et al56 |
| LBW | Connecticut and Massachusetts | Decreased birth weight: 14.7 g; 95% CI = (12.3 to 17.1) g | Bell et al58 | |
| LBW | European cohort study (ESCAPE) | Decreased birth weight OR = 1.18; 95% CI = 1.06 to 1.33 |
Pedersen et al64 | |
| LBW | International Collaboration on Air Pollution and Pregnancy Outcomes (ICAPPO) | Decreased birth weight OR = 1.10; 95% CI = 1.03 to 1.18 |
Dadvand et al66 | |
| IUGR | Calgary, Edmonton, and Montreal, Canada |
Increased risk of intrauterine growth restriction First trimester: OR = 1.07; 95% CI = 1.03 to 1.10 Second trimester: OR = 1.06; 95% CI = 1.03 to 1.10 Third trimester: OR = 1.06; 95% CI = 1.03 to 1.10 |
Liu et al61 | |
| PM10 | LBW Preterm birth |
Meta-analysisa | Decreased birth weight and increased risk of preterm birth LBW: OR = 1.10; 95% CI = 1.05 to 1.15 Preterm birth: OR = 1.06; 95% CI = 1.03 to 1.11 |
Stieb et al56 |
| LBW Preterm birth |
Los Angeles, California | Decreased birth weight and Increased risk of preterm birth LBW: OR = 1.21; 95% CI = 0.85 to 1.74 Preterm birth: OR = 1.17; 95% CI = 0.92 to 1.50 |
Wilhelm et al57 | |
| LBW Preterm birth Smaller HC SGA |
Netherlands | Various birth outcomes including, LBW: −3.6 g; 95% CI = (−6.7 to −0.4) g Preterm birth (3rd quartile): OR = 1.40; 95% CI = 1.03 to 1.89 Preterm birth (4th quartile): OR = 1.32; 95% CI = 0.96 to 1.79 HC: −0.18 mm; 95% CI = (−0.24 to −0.12) mm SGA: OR = 1.38; 95% CI = 1.00 to 1.90 |
van den Hooven et al47 | |
| LBW | European cohort study (ESCAPE) | Decreased birth weight OR = 1.16; 95% CI = 1.00 to 1.35 |
Pedersen et al64 | |
| LBW | International Collaboration on Air Pollution and Pregnancy Outcomes (ICAPPO) | Decreased birth weight OR = 1.03; 95% CI = 1.01 to 1.05 |
Dadvand et al66 | |
| LBW | Connecticut and Massachusetts | Decreased birth weight: 8.2 g; 95% CI = (5.3 to 11.1) g | Bell et al58 | |
| Polycyclic aromatic hydrocarbons (PAHs) | LBW Smaller HC |
Dominican and African-American residing in Washington Heights, Central Harlem, and the South Bronx, New York | Decreased birth weight and smaller head circumference LBW: P = 0.003 HC: P = 0.01 |
Perera et al67 |
| Preterm birth Increased IUGR SGA |
New York City, U.S. | Various birth outcomes including SGA: OR = 1.9; 95% CI = 1.1 to 3.5 IUGR: 4% decrease; P = 0.241 Preterm birth: 5-fold increase; 95% CI = 1.8 to 11.9; P = 0.001 |
Choi et al68 | |
| Total suspended particles (TSP) | LBW | Beijing, China | Decreased birth weight: 6.9 g; OR = 1.10; 95% CI = 1.05 to 1.14 |
Wang et al65 |
| LBW | Seoul, South Korea | Decreased birth weight AOR = 1.04; 95% CI = 1.00 to 1.08 |
Ha et al60 |
LBW: low birth weight; CI: confidence intervals; SGA: small for gestational age; OR: odds ratios; AOR: adjusted odds ratio; IUGR: intrauterine growth restriction; HC: head circumference.
Meta-analysis with EMBASE, MEDLINE, Scopus, Current Contents, Global Health, Cochrane, TOXLINE and the Canadian Research Index.
Assessing air pollution has involved in environmental monitoring at specific areas of interest, at a national or global scale.44, 45 As a result, U.S. Environmental Protection Agency (EPA) established an Air Quality System (AQS) database, which provides hourly or daily concentrations of pollutants measured from 1980 through 2009 for different geographic areas. Researchers can utilize this database to make a daily, monthly, or year-long estimation of air pollution exposure in a residence of study.46, 47 Alternatively, exposure to traffic-related air pollution has been simply estimated using distance, such as for the residence within 50 meters from highways, and further used to determine the association with the risk of adverse birth outcomes in Vancouver, Canada.48 However, we will focus on the common methods for monitoring individual exposure and pollutant metabolites, which are important to be considered in the pathogenesis of diseases. Personal air monitoring is one of the commonly used methods to measure individual exposure. The subjects are required to carry a personal monitor to collect vapors and particles of airborne pollutants on a microfiber filter.49 The individual exposure level during a monitoring period can be estimated by the calculation of accumulated pollutants, such as PM2.5, using device-specific parameters. The devices and methods to monitor personal exposure has also been comprehensively reviewed elsewhere.50 Urinary metabolites provide a convenient biological source to monitor both the amount of intake pollutants and the metabolites of individual pollutant chemicals. For example, the urinary pyrene metabolite 1-hydroxypyrene has been broadly used to reflect the individual exposure level to PAHs.51 Measuring urinary metabolites of pollutants further provide a sustainable approach to assess individual exposure at a long term process; thus, it is highly feasible in pre- and peri-natal exposure estimation.52 Thirdly, the pollutant levels in blood and tissues are also measured to show the specific level of pollutants or their metabolites that interact with cells and tissues, such as PAH-DNA adducts, including benzo[a]pyrene diol-epoxide DNA adducts, from blood and placenta tissues.53, 54 As the individual exposure levels are measured, the biological outcomes of exposure to specific pollutant chemicals can be further investigated and concluded.
Low birthweight and restrictions in fetal growth
LBW is a common indicator of adverse birth outcomes in the studies related to environmental exposure (Table 1) in meta-analyses based upon previously reported epidemiological studies, the decrease of birth weight (e.g., 10–30 g; 95% CI = −69 to 297) and increased odds ratio (OR) of LBW (e.g., OR = 105–110) are strongly associated with the exposure to outdoor CO, NO2, PM10, and PM2.5.56 In these studies, confident interval (CI) was used to show a range within which 95% of values lies and OR was calculated as a probability ratio of presented property to absent property. The impact of air pollution on LBW has become a critical global health concern. The researchers in Spain found that NO2 exposure during the pregnancy was associated with a reduction in birth weight (−40.3 g) and birth length (−0.27 cm) along with a smaller head circumference (−0.17 cm), showing a linear relationship to the risk of SGA.63 Other studies for exposure to PAHs in New York,67 and exposure to NO2, SO2, CO, PM10, and PM25 in Los Angeles,57 Connecticut, Massachusetts,58 and other northeastern cities59 have similarly supported an increase of the risk for LBW and preterm. In Beijing of China and Seoul of South Korea with high air pollution in Asia, researchers reported the increased risk of LBW associated with CO, total suspended particles, and SO2.60, 65 Additionally, European (ESCAPE) and international (ICAPPO) cohort studies combining multiple populations in different countries reported the effect of maternal exposure to air pollutants, including PM10 and PM2.5, on increased risk of LBW at term.64, 66 Trimester effects of air pollution exposure during pregnancy have been indicated in some studies (Table 1). Van den Hooven et al47 found that prenatal exposure to both PM10 and NO2 in the third trimester were inversely associated with birth weight (−3.6 g and −3.4 g) and fetal head circumference (−0.18 mm and −0.06 mm), and increased risk of SGA particularly with PM10. Some epidemiological studies also suggested certain unknown biological factors in different ethnical populations may influence the risk of environmental exposure in LBW. The association of PAH exposure with significantly reduced birth weight was observed in the Krakow Caucasians in Poland, while a 6-fold greater risk of LBW with PAH exposure was observed in New York City (NYC) African Americans than the risk in Krakow Caucasians. However, this association is missing in NYC Dominicans.68, 69 These results likely reflect that the impact of air pollution can be more susceptible to a certain ethnic group such as African Americans, suggesting the importance of gene and environmental interactions in the development of LBW. Additionally, numbers of researchers have investigated whether these abnormal birth outcomes can be reversed when air pollution is reduced, and interestingly, they were able to find that the risk of LBW could be prevented when air pollution was declined.64, 70
Preterm
Around 10% of all births are preterm with a gestation period less than 37 weeks in the United States71 and approximately 11% of births worldwide are preterm.72 Preterm birth, including 3% of very early preterm cases with gestation period less than 27 weeks, is connected to the maternal exposure to various environmental pollutants including smoking and air pollution containing PAHs73, 74, 75, 76, 77(Table 1). Both vapor and particulate pollutants, including CO, NO2, PM10, and PM2.5, had an adverse effect on preterm birth, for example, PM10 exposure in a Netherlands cohort study associated with the increased risk of preterm birth.47 However, results were less consistent for O3 and SO2, suggesting that there are variabilities of outcomes among each component of environmental chemicals.56 Risk of air pollution exposure to preterm birth may also be different in various human populations. Similar to LBW among African-American population, 5-fold greater risk of preterm birth also attributed to environmental tobacco smoke (ETS) in addition to the effect of prenatal PAH exposure on an increased risk of growth restriction.51 Moreover, preterm birth has been shown to have a general long-term effect on the lung function,78 which will be further discussed for its impact on later onset of respiratory diseases.
Deficit in respiratory system
While many studies have focused on the inadequate fetal growth and development, some studies have specifically related the birth defects of multiple organ systems with the exposure to the air pollution. Cigarette smoke impacts the development of multiple systems, including the respiratory, nervous, and cardiac systems, during pregnancy.79 For the respiratory system, the effect of air pollution on prenatal and perinatal lung disorders was mostly learned from the pregnancy with smokers or second-handed smokers. Pollutants derived from cigarette smoke are able to cross the placental barrier and causing multiple adverse effects on the fetal development, including chronic hypoxia, lung function, lung morphology such as branching and alveolarization.80 In animal models, such as rhesus monkeys, prenatal nicotine exposure alters pulmonary function in newborns.81 Both pulmonary functional and anatomic changes associated with maternal smoking and nicotine, which has most detrimental effects on the early development of the lung, are suggested to induce later respiratory illness79, 80, 81 and will be detailed below.
Epidemiological evidence for early origins of respiratory diseases
The Great Smog of London in 195282 built up a relationship between perinatal exposure to air pollution and the later development of respiratory diseases in life. A recent study analyzed the prevalence of asthma in the population exposed to the Great Smog in utero or in the first year of life. The results showed that exposure has increased the probability of asthma in both childhood (19.87%) and adulthood (9.53%)83 (Table 2), supporting that the early life and prenatal exposure to air pollution conveys a long-term effect in children and adults. Several other studies have similarly provided evidence supporting this interesting connection of early exposure to air pollutants and its long-term effect,84, 85, 86, 87 including studies in China that supported incidence of asthma, allergic rhinitis, and eczema in children was associated with maternal exposure to traffic-related pollutants during entire pregnancy.87 Multiple human cohort studies on tobacco smoking during the pregnancy period show the impact on placental function, fetal lung development, and further impact on the respiratory function of newborns and children. Symptomatically, maternal smoking will lead to an increased risk of pulmonary viral and bacterial infections, wheezing, reduced respiratory function reflected by low forced expiratory volume in 1 second (FEV1), asthma, and COPD.88, 89, 90
Table 2.
Prenatal exposure to air pollutants contributes to the onset of respiratory disorders in childhood and adulthood.
| Air pollutants | Respiratory disorders | Onset stage | Cohorts/analysis | Major findings | Reference |
|---|---|---|---|---|---|
| Maternal exposure to second-hand smoke (SHS); environmental tobacco smoke (ETS) | Asthma Wheeze |
Children Adolescents |
Meta-analysis with 79 cohorts | Increased risk of asthma and wheezing (>20%) wheeze: OR = 1.70; 95% CI = 1.24 to 2.35 asthma: OR = 1.85; 95% CI = 1.35 to 2.53 |
Burke et al91 |
| Asthma Wheeze |
Children | Meta-analysis with 43 papers | Increased risk of asthma and wheezing OR = 1.21; 95% CI = 1.13 to 1.31 |
Silvestri et al92 | |
| Respiratory symptoms Lower airway obstruction |
Children | United States (St. Louis, Missouri, US, and Cleveland, Ohio) and London, England | Increased respiratory symptoms and risk of lower airway obstruction FEV1/FVC: 18.9%; P < 0.001 Mid-expiratory phase/FVC ratio: 0.15; P = 0.001 Bronchodilator responsiveness: 12%; P = 0.03 |
Cohen et al93 | |
| Reduced pulmonary function COPD |
Adults | European Community Respiratory Health Survey | Reduced FEV1 and increased risk of COPD FEV1 (men): 95 mL; 95% CI = (67 to 124) mL FEV1 (women): 60 mL; 95% CI = (40 to 80) mL Increased COPD (men): 1 factor; OR = 1.7; 95% CI = 1.1 to 2.6 Increased COPD (women): >3 factors; OR = 1.6; 95% CI = 1.01 to 2.6 |
Svanes et al94, 95 | |
| Maternal exposure to smoke | Peripheral airflow obstruction | Infants | 105 infants from Louisville, KY | Altered lung function, and a response to a bronchodilator [FEF25]/PFEF = 0.119 ± 0.036 (P < 0.0005) |
Sheikh et al96 |
| Asthma | Children | 58,841 children born in Finland in 1987 | Increased risk of asthma: 25-36%; OR = 1.25; 95% CI = 1.09 to 1.44 | Jaakkola et al97 | |
| Reduced pulmonary function | Children Adults |
Meta-analysis with 692 articles from the Embase and Medline databases | Reduced FEV1 Mid-expiratory flow rates: 5.0% reduction; 95% CI = 3.3% to 6.6% End-expiratory flow rates: 4.3% reduction; 95% CI = 3.1% to 5.5% |
Cook et al98 | |
| Respiratory symptoms | Children | Kingston allergy birth cohort (KABC) | Decreased the rate of children without respiratory symptoms HR = 2.68; 95% CI = 1.48 to 4.84 |
North et al99 | |
| Asthma Reduced pulmonary function |
Children | 12 southern California communities | Reduced FEV1 and FEF25–75 FEV1 (boys): −13.6%; 95% CI = −18.9% to −8.2% FEV25-75 (boys): −29.7%; 95% CI = −37.8% to −20.5% FEV25-75 (girls): −26.6%; 95% CI = −36.4% to −15.1% |
Gilliland et al100 | |
| Asthma | Children | Childhood Asthma Management Program | Reduced FEV1 and increased risk of asthma in GSTM1-null children FEV1/FVC = 83.8%; P = 0.01 |
Rogers et al101 | |
| Reduced pulmonary function | Children | Meta-analysis with >20,000 children (aged 6–12 yrs.) from nine countries in Europe and North America | Reduced FEV1: 40%; 95% CI = 0.95% to 1.0% | Moshammer et al102 | |
| Reduced pulmonary function | Adults | Mater–University of Queensland Study of Pregnancy (MUSP) | Reduced FEV1 and FEF25–75 Regression coefficient = −0.16; 95% CI = −0.30 to −0.02 |
Hayatbakhshet al103 | |
| Reduced pulmonary function | Adults | Tucson Children's Respiratory Study | Reduced FEV1/FVC 2.8%; 95% CI = 0.9%–4.8%; P = 0.003 |
Guerra et al104 | |
| Asthma Wheeze |
Adolescents | Western Australian Pregnancy (Raine) Cohort | Increased risk of asthma and wheezing wheeze: OR = 1.77, 95% CI = 1.14–2.75 asthma: OR = 1.84, 95% CI = 1.16–2.92 |
Hollams et al105 | |
| Asthma | Adults | German Multicenter Allergy Study (MAS-90) | Increased risk of asthma HR = 1.79; 95% CI = 1.20 to 2.67 |
Grabenhenrichet al106 | |
| Asthma Wheeze |
Children | Meta-analysis with 43 papers | Increased risk of asthma and wheezing Asthma: OR = 1.22; 95% CI = 1.03 to 1.44 Wheezing: OR = 1.36; 95% CI = 1.19 to 1.55 |
Silvestri et al92 | |
| Asthma BHR |
Adolescents Adults | Göteborg, Sweden | Increased risk of asthma OR = 3.5; 95% CI = 1.1 to 11.3 |
Goksör et al107 | |
| Industrial-related air pollutants | Asthma | Children Adults |
Great smog exposed population in London | Increased risk of asthma Children: 19.87%; 95% CI = 3.37% to 36.38% Adult: 9.53%; 95% CI = −4.85% to 23.91% |
Bharadwaj et al83 |
| Traffic-related air pollutants | Asthma Wheeze Increased IgE |
Children | Columbia Center for Children's Environmental Health birth cohort | Positive associations between air pollution and asthma, wheeze, and IgE Asthma: OR = 1.43; 95% CI = 1.03 to 1.97 Wheeze: OR = 1.26; 95% CI = 1.01 to 1.57 Increased IgE: OR = 1.25; 95% CI = 1.09 to 1.42 |
Patel et al108 |
| Asthma Dyspnea Wheeze |
Children | Windsor Children's Respiratory Health Study |
Increased risk of asthma, dyspnea, and wheeze Wheeze: OR = 1.23; 95% CI = 1.07 to 1.41 Dyspnea: OR = 1.27; 95% CI = 1.05 to 1.52 Asthma: 8%; OR = 1.08; 95% CI = 1.012 to 1.149 |
Dales et al109 | |
| Asthma | Infants | Southwestern British Columbia (BC) | Increased risk of asthma: 12%; OR = 1.08; 95% CI = 1.04 to 1.12 | Clark et al110 | |
| NO2 | Reduced pulmonary function | Children | Environment and childhood (INMA) project | Reduced FEV1: −28.0 mL; 95% CI = (−52.9 to −3.2) mL | Morales et al111 |
| LRTI | Infants | Environment and childhood (INMA) project | Increased risk of respiratory illness including LRTI RR = 1.05; 95% CI = 0.98 to 1.12 |
Aguilera et al112 | |
| Pneumonia | Children | European cohort study (ESCAPE) | Increased risk of pneumonia OR = 1.30; 95% CI = 1.02 to 1.65 |
Maclntyre et al113 | |
| Asthma Rhinitis Eczema |
Children | 2598 preschool children aged 3–6 years in China | Increased risk of asthma, rhinitis, and eczema Asthma: 6.8%; OR = 1.69; 95% CI = 0.99 to 2.70 Allergic rhinitis: 7.3%; OR = 1.63; 95% CI = 1.03 to 2.77 Eczema: 28.6%; OR = 1.37; 95% CI = 1.04 to 1.80 |
Deng et al87 | |
| PM2.5 | Asthma | Children | 272 high-risk infants from Vancouver |
Increased risk of asthma: 50%; OR = 3.1; 95% CI = 1.3 to 7.4 | Carlsten et al114 |
| PM10 | Reduced pulmonary function | Children | BAMSE (Children, Allergy,Milieu, Stockholm, Epidemiological Survey) | Reduced FEV1: −59.3 mL; 95% CI = (−113 to −5.6) mL | Schultz et al115 |
| Pneumonia | Children | European cohort study (ESCAPE) | Increased risk of pneumonia OR = 1.76; 95% CI = 1.00 to 3.09 |
Maclntyre et al113 | |
| Reduced pulmonary function | Infants | Bern, Switzerland | Increase in minute ventilation 24.9 mL/min; 95% CI = (9.3 to 40.5) mL/min | Latzin et al116 | |
| Benzene | Reduced pulmonary function | Children | Environment and childhood (INMA) project | Reduced FEV1: −18.4 mL; 95% CI = (−34.8 to −2.1) mL | Morales et al111 |
| LRTI | Infants | Environment and childhood (INMA) project | Increased risk of respiratory illness including LRTI RR = 1.06; 95% CI = 0.94 to 1.19 |
Aguilera et al112 | |
| Diesel exhaust particles (DEPs) | Wheeze | Infants | The Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) | Increased risk of wheezing OR = 2.50; 95% CI = 1.15 to 5.42 |
Ryan et al117 |
| Polycyclic aromatic hydrocarbons (PAHs) | Respiratory symptoms | Infants | 333 newborns in Krakow, Poland | Increased respiratory symptoms Barking cough: RR = 4.80; 95% CI = 2.73 to 8.44 Wheezing without cold: RR = 383; 95% CI = 1.18 to 12.43 Sore throat: RR = 1.96; 95% CI = 1.38 to 2.78 Ear infection: RR = 1.82; 95% CI = 1.03 to 3.23 Cough irrespective of respiratory infections: RR = 1.27; 95% CI = 1.07 to 1.52 Cough without cold: RR = 1.72; 95% CI = 1.02 to 2.92 |
Jedrychowski et al118 |
| Asthma | Children | Columbia Center for Children's Environmental Health birth cohort | Increased risk of allergic sensitization RR = 1.15; P = 0.001 |
Perzanowski et al119 |
OR: odds ratios; CI: confidence intervals; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; COPD: chronic obstructive pulmonary disease; FEF: forced expiratory flow; PEF: peak expiratory flow; HR: hazard ratio; FEV25-75: forced expiratory volume at 25%–75%; BHR: bronchial hyper-responsiveness; IgE: immunoglobulin E; LRTI: lower respiratory tract infection.
Abnormal respiratory function
Abnormal respiratory function in late life is now known able to be induced by early exposure to air pollution120 and provoke long-term respiratory diseases.121 The early exposure in human life can first reduce the pulmonary function, which is usually measured by FEV1 and forced vital capacity (FVC), as summarized (Table 2). Several studies are detailed here. For example, reduced lung function with low FEV1 in children at preschool age was associated with exposure to high level of benzene and NO2 during pregnancy in a study from Spain.111 The FVC and FEV1 of asthmatic children with asthma diagnosis before age 2 and maternal smoking were negatively affected by the exposure to CO, PM10, and NO2 during the second-trimester of pregnancy in a California study.122 Another birth cohort study from Switzerland showed that prenatal exposure to PM10 was associated with higher minute ventilation and respiratory need in newborns.116 In addition to inorganic pollutants, perinatal exposure to ambient organic pollutants PAHs increases the risk of various respiratory symptoms during early infancy, including barking cough, wheezing, sore throat, ear infection, and cough irrespective of respiratory infections, independent of passive tobacco smoking.118
The impact of maternal smoking and related pregnancy complications on the lung function of offspring spans from infants96 to children,123 and to adolescents.124, 125 Maternal exposure to smoke as an example with mixed pollutants has been consistently associated with the reduction of FEV1 and maximal expiratory flow in children and young adults,98 together with a similar effect from E-cigarettes smoking.80 As a consequence, maternal smoking increases the susceptibility of fetus and newborns to illnesses with a greater deficit in lung function than those whose parents did not smoke,104 especially with respiratory diseases in children as reflected by an impaired lung function. In reported multiple birth cohort studies (Table 2), maternal smoking in pregnancy independently resulted in a 39–65% increased risk of wheezing and asthma at 4–6 years of age.126 A similar result with a 28–52% increased risk of wheezing in children was also shown from a meta-analysis of 79 cohorts.91 Additionally, a retrospective study with 20,000 children at the age of 6–12 years reported that maternal smoking is independently associated with a reduced lung function indicated by a lower FEV1.102 The outcomes induced by the effect of maternal smoking can be added up together with the other factors, such as viral infection, airway inflammation, and even epithelial metastasis. For example, the smokers with lower respiratory illnesses, caused by a viral infection from their early life, are more likely to develop asthma in adult.127
Respiratory infections
Because of the high prevalence of childhood respiratory infections in areas with severe air pollution,18, 19, 20 an epidemiological study on the association of later respiratory infections with maternal exposure to air pollutants is particularly meaningful. As summarized in Table 2, it was reported from a Spanish cohort that infants during the 12–18 months of age showed a relationship between the increased risk of respiratory illness, such as lower respiratory tract infection (LRTI) in this study, and exposure to NO2 (RR = 1.05) and benzene (RR = 1.06).112 More recent meta-analysis of 10 European birth cohorts (ESCAPE) study found a significant association between air pollution, measured with PM10, and NO2 levels, and pneumonia.113 Although it was well established that microbes are critical in the pathogenesis of respiratory diseases, air pollutants are able to interplay with both host and microbial factors and alter the disease course.
Allergy
Allergic respiratory diseases are induced by multiple genetic and environmental factors interlinked through IgE- and non-IgE-associated mechanisms.128 Allergens are necessary factors to stimulate allergic diseases, while air pollutants usually worsen allergic responses. Perinatal exposure to PAHs enhances the allergic responses to cockroach allergen as in a study with New York urban population.119 As distance to heavily trafficked intersections was used to indicate an exposure level to motor vehicle exhaustion and traffic-derived pollutants, children living closer to these intersections can be found with increased IgE levels.108 Exposure to diesel exhaust particles was associated with persistent wheeze by 36 months of age117 and enhanced allergic responses to allergens in human population.129, 130 In addition to vapor and particulate forms of PAHs, asthma in later life stages was also associated with maternal smoking.100, 131 Maternal tobacco smoking during the pregnancy increases the risk of developing asthma in children at the age of 7 years according to a study from Boston and Finnish cohorts.97 A similar effect was also observed in an exposure to second-hand tobacco smoke for the occurrence of asthma and wheeze symptoms132 or the onset of asthma and more severe airflow obstruction in the younger age of children.101 Overall, the association of later onset of asthma with maternal exposure to air pollutants is well supported by epidemiological evidence.
COPD
COPD has been considered as a disease that occurs mostly in aging groups, while increasing evidence recently supported that COPD may originate from environmental exposure in the maternal stage as recently reviewed.87, 95, 133 We have discussed that maternal exposure to air pollutants is associated with lower lung function in infancy, which can be further associated with adult lung function and COPD.94, 95 More evidence also shows postnatal or childhood exposure to air pollutants has vast impact on later development of COPD. For indoor exposure, around 35% of individuals with COPD in developing areas associated with the exposure to indoor smoke and biomass fuel combustion.134 In these cases, the biomass fuels, such as coal, straw, crop residues, wood, and animal dung, were used to heat and cook in the poorly ventilated household. For outdoor pollutants, the PM, O3, and SO2 from vehicle traffic and fuel combustion are also associated with respiratory disorders.135 Children living within 500 meters of a major freeway in southern California showed a deficit of FEV1 when compared with children who lived at least 1500 meters away from a freeway, as it is associated with COPD.135 For the particulate forms of chemical pollutants, increased PM10 exposure is associated with reduced lung function in children. While moved to areas with lower PM10 levels, these children showed an improved lung function.136 In the UK, higher carbon content in airway macrophages in induced sputum samples from the school children was associated with PM10 levels, which was further significantly correlated with lower respiratory function.137 The epidemiological evidence supporting the association of exposure to various air pollutants in childhood or adolescence with abnormal developmental outcomes of lung and later COPD diseases will facilitate the elucidation of molecular and cellular mechanisms contributing to the long-term adverse effects of air pollutants on COPD and other respiratory diseases.
Mechanistic evidence for prenatal disease origins
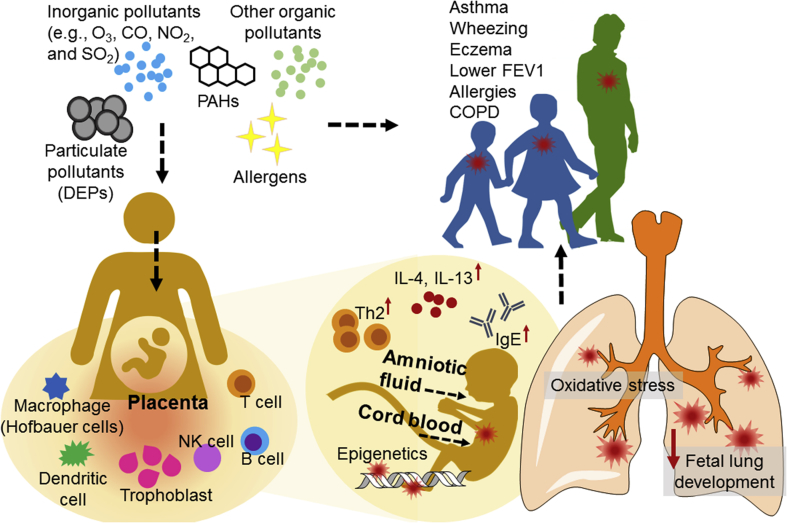
Although intensive epidemiological studies support the association of air pollution with abnormal birth outcomes, a high degree of variabilities among epidemiological studies requires more stringent control groups with less divergence in confounding factors.138 Findings in toxicological studies56 have started shedding some light on the prenatal origin of later respiratory diseases. Hypothetical mechanisms consider the impact of air pollutants on various myeloid, lymphoid, and stromal cells to produce: (1) transcription factors to control gene expression; (2) growth factors to control lung tissue development at early stages; (3) cytokines and chemokines to regulate tissue inflammatory responses (Fig. 2).
Fig. 2.
Schematic demonstration of prenatal origin of respiratory diseases. PAH: polycyclic aromatic hydrocarbon; FEV1: forced expiratory volume in 1 second; COPD: chronic obstructive pulmonary disease. DEP: diesel exhaust particle; IL: interleukin; Th2: type II helper T cells; IgE: immunoglobulin E; NK: natural killer cells.
Oxidation responses
Maternal smoking is one of the well-studied environmental factors altering lung function. Nicotine is able to cross placenta and exists in amniotic fluid, allowing nicotine to interact with the receptor expressed in the fetal airway and alter the development of fetal lung tissues.139 Meanwhile, nicotine was shown to increase the production of reactive oxygen species (ROS) and reduce the production of antioxidants, leading to an unbalanced oxidant-antioxidant environment and an adverse effect on cell integrity.140 This functional imbalance will further disturb lung development and cause air pollutant-exposed offsprings prone to developing respiratory diseases such as asthma and COPD in later life.141 The genetic variants and decreased expression of antioxidant genes, glutathione S-transferase mu 1 (GSTM1) and coenzyme NAD(P)H quinone dehydrogenase 1 (NQO1) were associated with pollutant-induced exacerbation of allergic and inflammatory diseases in humans.142, 143 The wild-type GSTM1 protein can prevent aggravation of allergic responses by second-hand smoke, while the wild-type glutathione S-transferase pi 1 (GSTP1) protein associated with enhanced nasal allergic responses challenged by DEPs.129, 130
Structural alterations
Structural changes more likely put a long-term effect of environmental exposure on respiratory diseases. The altered tissue structures and cellularity may last a longer period to reprogram respiratory functions. From animal studies, prenatal exposure to nicotine and maternal smoking caused abnormalities in lung development, including airway branching and dimensions. Continuous epithelial-cell growth and lung branching can be stimulated by prenatal nicotine exposure and result in longer and more torturous airways, leading to weaker respiratory function.84, 144 These outcomes likely attribute to the molecular interaction of nicotine with α7 nicotinic receptors in mice.139 Mouse studies have demonstrated that maternal smoking remodeled the airway tissue structures by: (1) increasing collagen deposition around the airways so that airway thickness is enhanced; (2) airway inflammatory responses become more severe in house dust mite (HDM)-challenged model due to the induction of goblet cell hyperplasia and the infiltration of neutrophils and mast cells.145 Smooth muscle thickness in the airway is a good predictor of altered airway responsiveness and respiratory function in smoke-exposed offspring. In animal studies, alveolar remodeling involving the alteration of smooth muscle is found in association with maternal smoking,146 and further leads to the increased volume of airway smooth muscle and the deposition of collagen, resulting in a subsequent reduction of airflow, reduced FEV1, and potential increase of airway hyperreactivity.147 Collagen deposition around the airways is observed also in monkeys148 with a compromised lung function, suggesting the formation of smaller airways and stiffer lungs.149 In humans, maternal smoking is able to cause thicker inner airway wall in the infants suffered from sudden infant death syndrome,150 suggesting the smoke pollutants contribute to the airway remodeling. Both human and animal studies supported an emphysema-like pathological change with thickening airways potentially due to the deposition of collagen and remodeled alveolar walls with the decreased surface area, lower capillary density, weaker respiratory function, and premature aging.146, 151
Monocytes, macrophages, and dendritic cells
Inflammatory responses mediated by hematopoietic cells in lung tissues have profound roles in regulating respiratory disease outcomes in asthma, COPD, cancers, and infectious diseases. Different subsets of immune cells are potentially impacted by air pollutants to post a long-term impact on respiratory diseases, although more studies are needed for a better understanding (Fig. 2). It is well known that endotoxin enhances the expression of antigen presenting molecules on airway macrophages and monocytes152 as used as a common stimulating factor to activate monocyte-derived dendritic cells and macrophages.153 The exposure to ozone enhances the expression of surface markers involved in innate immunity and antigen presentation on airway monocytes, such as human leukocyte antigen–antigen D related molecules (HLA-DR), the co-stimulatory molecule CD86, the co-receptor CD14 for binding bacterial endotoxin, and the antibody receptor CD16.154 The upregulation of these molecules will likely further enhance the activation of T cells and monocyte-mediated inflammatory responses. The functional upregulation of alveolar macrophage was shown in mouse acute lung inflammatory responses to carbon nanotube exposure.155 However, it remains poorly understood how innate immune responses in maternal exposure are translated to the impact on fetal lung development.
CD4+ T cells and cytokines
In response to the allergen and other environmental particles, dendritic cells can be activated156 to induce a type II helper T cell (Th2)-dominant immune response.157 The Th2-skewed response, as a regulatory mechanism in placenta and pregnancy, is interestingly connected to the adverse response caused by environmental pollutants, such as ozone that enhances Th2-skewed pulmonary inflammation in a rat pregnancy model.158 A Th2-mediated interleukin (IL)-13 production was indicated to enhance the allergic IgE level, in contrast to the monocyte function represented by CD14 gene expression. In tobacco smoke-influenced atopy in Dutch cohorts, the minor alleles of the IL-13 gene were significantly associated with elevated IgE levels and an increased risk of allergic sensitization in children, but minor alleles from the CD14 gene were associated with lower IgE and a decreased risk of allergic sensitization.159 As an organic air pollutant released from some manufacturer products such as polyurethane foam,160 the respiratory and skin sensitizer toluene diisocyanate (TDI) was known to induce a Th2 response.161, 162 In animal studies, application of TDI to mothers led to an enhanced respiratory allergy induced by OVA in offsprings.162 Further sensitized by allergens and aerosol challenge, the offsprings developed an increased Penh values, airway hyperresponsiveness, in which eosinophils and Th2 cytokines become unbalanced.161 Specifically, IL-13 but not IL-4 cytokine is required for allergic inflammation in the lung,163 supporting an exacerbating impact of TDI on lung allergic inflammation. In addition to the vapor forms of air pollutants, particulate pollutants DEPs containing both carbon cores and pollutant chemicals on the surface (Fig. 1) may have an adjuvant effect to protein allergens in humans31 and in rodents. Repeated exposure to low dose-DEP seemed resulting in an increased airway hyperresponsiveness and inflammatory cytokine expression,164, 165 while continuous exposure to DEP rapidly minimized this effect. Interestingly, the carbon particle cores without the surface organic pollutants also show a pro-asthmatic effect transferred from maternal exposure.165 In BALB/c mice treated with carbon black particles, lung inflammation in offsprings in responses to maternal exposure of inert particles is enhanced. The limited data virtually supported a disruption of the balanced of CD4+ T cells by multiple air pollutants, contributing to either hyperresponsiveness or pro-inflammatory alterations in respiratory pathology.
Innate lymphoid cells (ILCs)
ILCs are the newly identified family of innate immune cells, which lacks the antigen specific receptors unlike conventional T helper cells. ILCs can secrete various cytokines, including IL-5, IL-13, IL-17, and interferon (IFN)-γ, including multiple common cytokines produced by CD4+ T cells.166, 167 Due to their innate-like characteristics, ILCs are expected to more promptly respond to environmental factors and show different responding kinetics compared to T cells. ILCs have been reported to contribute to the respiratory diseases such as asthma.167, 168, 169 Recent studies in mice have shown that ILCs can be influenced by air pollutants to exacerbate the respiratory symptoms. For example, DEP-enhanced allergic airway inflammation can be attenuated by reduced number of functional ILCs with suppressed expression of the transcription factor GATA3, which promote the production of Th2-like cytokines.170 The other studies also showed important role of ILCs to mediate airway hyperresponsiveness induced by pollutants, such as O3 and multi-walled carbon nanotubes (MWCNT).171, 172 Whereas many functions of ILCs are unknown yet, accumulated evidence suggests a crucial role of ILCs to be further investigated in air pollution-exacerbated respiratory diseases.
Immunoglobulin E (IgE)
Particulate form of air pollutants has been shown to interestingly impact on the production of immunoglobulins in allergic responses. DEP was found to promote antibody production to neoantigens (keyhole limpet hemocyanin, KLH) with a mixed IgE and IgG responses. The production of IgE can be upregulated by Th2 cytokines, such as IL-4.173, 174, 175, 176 Interestingly, oxidative stress is also linked to an enhanced IgE response in environmental exposure, as supported by the observation that DEP-induced oxidative stress initiates a primary IgE response to subsequently encountered allergen based on the evidence of the inhibitory function of thiol antioxidants.177 Consistent to the role of oxidative stress in inducing a higher IgE response, the null mutation of anti-oxidant gene GSTM1 contributes to a larger increase of IgE in the exposure to air pollutants. PAHs also enhance the allergic responses to cockroach allergen, especially further augmented with the GSTM1 null genotype as in a study with New York urban population.119 A similar effect was also observed in GSTM1 null children in an exposure to second-hand tobacco smoke for the occurrence of asthma and wheeze symptoms.132 It remains far from full understanding of immune regulatory mechanisms. Immune pathways impacted by air pollutants may be previously known or unknown mechanisms, contributing to new discoveries of early environmental origins in respiratory diseases.
Epigenetics
Air pollutants may overwhelmingly influence the epigenetic regulators that determine the processes of DNA replication and transcription. The overall impact of environmental pollutants on DNA methylation has been comprehensively reviewed.77, 178, 179, 180 At a prenatal stage, mother and fetus are both exposed to air pollutants in different microenvironments (Fig. 2). Mothers are exposed to air pollutants through airway inhalation and body fluid transferring. Fetuses are exposed to air pollutants through the cross-placental transfer of the original or metabolized molecules of air pollutants. Although indirect passage of maternal epigenetic alteration to the fetus is possible, perhaps the direct epigenetic changes in fetus contribute more significantly to the later stage of respiratory diseases. The epigenetic alteration induced by pollutants usually includes DNA methylation and histone modification, which may carry through a long period181 and determine the initiation of gene expression through selective activation or inactivation of genes. The epigenetic regulation further contributes to controlling immune cell differentiation, inflammatory responses, cell growth, and apoptosis. In respiratory diseases, altered DNA methylation182, 183 is associated with the increased risk of childhood asthma related to maternal smoking,184 stress,185 and COPD.35 As an intensively investigated exposure model, tobacco smoking impacted DNA methylation in children, as shown with global and gene-specific methylation patterns in buccal cells from 348 to 272 children respectively. As a result, significant hypomethylation was observed in prenatal tobacco smoke at a DNA repetitive element, which is a marker of global DNA methylation. This observation is consistent with the protective function of GSTM1 supported by higher methylation in those children with GSTM1, but lower methylation in GSTM1 null children. However, methylation patterns are usually tissue- and cell-specific, as this may impact data interpretation in gene regulation.186 Besides DNA methylation, an increase of microRNA has also observed in a human acute challenge with ozone and exposure to ambient particulate pollutants.187, 188, 189, 190 Some microRNA such as mi-223 in both infant and maternal monocytes is associated with in utero exposure to tobacco smoke.191
Epigenetic regulations are involved in the differentiation of Th1, Th2,193, 192 and regulatory T (Treg) cells. For example, epigenetic regulation of the expression of the transcription factor Foxp3 determines Treg differentiation.194, 195 In a mice study, inhaled DEP exposure and intranasal Aspergillus fumigatus infection induced hypermethylation at the IFN-γ gene promoter and hypomethylation at the IL-4 promoter. This altered CpG methylation pattern of IFN-γ and IL-4 promoters mediated the differentiation of Th2 cells in vivo and correlated significantly with increased IgE production.196 The impact of trafficked air pollution and tobacco smoke has been shown on multiple immune regulatory genes, including Toll-like receptors (e.g., TLR2), multiple cytokines (e.g., IL-6, and IFN-γ, IL-4 as noted), nitric oxide synthase (NOS), and various transcription factors (e.g., Foxp3 and Runx3).178 The long-term effect of these early induced epigenetic alterations on the later onset of respiratory diseases remains elusive.
Conclusions and remarks
Air pollution increases the risk of respiratory diseases, such as asthma, respiratory infections, and COPD, in children and adults. Maternal exposure to air pollutants mediates both short-term and long-term effects on the respiratory system. As described, extensive epidemiologic and meta-analysis studies showed the association between prenatal air pollution exposure and the adverse birth outcomes, including preterm birth, intrauterine growth restriction, low birth weight, pregnancy loss, and defective fetal lung development. The maternal exposure and disorders further impact on fetal lung functional and structural development, leading to various late onset respiratory diseases. Abnormal lung development, disrupted immune responses, and altered epigenetic regulations were suggested as potential underlying mechanisms. Both comprehensive and in-depth mechanistic investigations are required for better understanding of causative pathways in the environmentally induced late onset of respiratory diseases.
Conflicts of interest
All authors have no conflicts of interest to disclose. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
This work was supported by National Institute of Environmental Health Sciences (ES006096), Center for Environmental Genetics (CEG), and National Institute of Allergy and Infectious Diseases (AI115358).
Edited by Yang Pan and Zhen-Wei Zhang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Davis D.L., Bell M.L., Fletcher T. A look back at the London smog of 1952 and the half century since. Environ Health Perspect. 2002;110:A734–A735. doi: 10.1289/ehp.110-a734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Orgnization . 2016. World Health Statistics, Monitoring Health for the Sustainable Development Goals.http://www.who.int/gho/publications/world_health_statistics/en/ [Google Scholar]
- 3.Guan W.J., Zheng X.Y., Chung K.F., Zhong N.S. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388:1939–1951. doi: 10.1016/S0140-6736(16)31597-5. [DOI] [PubMed] [Google Scholar]
- 4.Li J., Sun S., Tang R. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chronic Obstr Pulm Dis. 2016;11:3079–3091. doi: 10.2147/COPD.S122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloemsma L.D., Hoek G., Smit L.A. Panel studies of air pollution in patients with COPD: systematic review and meta-analysis. Environ Res. 2016;151:458–468. doi: 10.1016/j.envres.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Komalkirti A., Sundeep S. Household air pollution and its effects on health. F1000Res. 2016;5 doi: 10.12688/f1000research.7552.1. F1000 Faculty Rev-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiavoni G., D'Amato G., Afferni C. The dangerous liaison between pollens and pollution in respiratory allergy. Ann Allergy Asthma Immunol. 2017;118:269–275. doi: 10.1016/j.anai.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Curtis L., Rea W., Smith-Willis P., Fenyves E., Pan Y. Adverse health effects of outdoor air pollutants. Environ Int. 2006;32:815–830. doi: 10.1016/j.envint.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Koenig J.Q. Indoor and outdoor pollutants and the upper respiratory tract. J Allergy Clin Immunol. 1988;81:1055–1059. doi: 10.1016/0091-6749(88)90180-7. [DOI] [PubMed] [Google Scholar]
- 10.Falcon-Rodriguez C.I., Osornio-Vargas A.R., Sada-Ovalle I., Segura-Medina P. Aeroparticles, composition, and lung diseases. Front Immunol. 2016;7:3. doi: 10.3389/fimmu.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautam S., Yadav A., Tsai C.J., Kumar P. A review on recent progress in observations, sources, classification and regulations of PM2.5 in Asian environments. Environ Sci Pollut Res Int. 2016;23:21165–21175. doi: 10.1007/s11356-016-7515-2. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A.J., Brauer M., Burnett R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Amato G., Cecchi L., D'Amato M., Liccardi G. Urban air pollution and climate change as environmental risk factors of respiratory allergy: an update. J Investig Allergol Clin Immunol. 2010;20:95–102. [PubMed] [Google Scholar]
- 14.Li Q., Jiang J., Wang S. Impacts of household coal and biomass combustion on indoor and ambient air quality in China: current status and implication. Sci Total Environ. 2017;576:347–361. doi: 10.1016/j.scitotenv.2016.10.080. [DOI] [PubMed] [Google Scholar]
- 15.Li C., Fang D., Xu D. Main air pollutants and diabetes-associated mortality: a systematic review and meta-analysis. Eur J Endocrinol. 2014;171:R183–R190. doi: 10.1530/EJE-14-0287. [DOI] [PubMed] [Google Scholar]
- 16.D'Amato G., Liccardi G., D'Amato M., Holgate S. Environmental risk factors and allergic bronchial asthma. Clin Exp Allergy. 2005;35:1113–1124. doi: 10.1111/j.1365-2222.2005.02328.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang L., Pinkerton K.E. Air pollutant effects on fetal and early postnatal development. Birth Defects Res C Embryo Today. 2007;81:144–154. doi: 10.1002/bdrc.20097. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan A.J., Johnston S.L. Air pollution and infection in respiratory illness. Br Med Bull. 2003;68:95–112. doi: 10.1093/bmb/ldg022. [DOI] [PubMed] [Google Scholar]
- 19.Darrow L.A., Klein M., Flanders W.D., Mulholland J.A., Tolbert P.E., Strickland M.J. Air pollution and acute respiratory infections among children 0-4 years of age: an 18-year time-series study. Am J Epidemiol. 2014;180:968–977. doi: 10.1093/aje/kwu234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le T.G., Ngo L., Mehta S. Effects of short-term exposure to air pollution on hospital admissions of young children for acute lower respiratory infections in Ho Chi Minh City, Vietnam. Res Rep Health Eff Inst. 2012:5–72. discussion 73–83. [PubMed] [Google Scholar]
- 21.Jacquemin B., Siroux V., Sanchez M. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE) Environ Health Perspect. 2015;123:613–621. doi: 10.1289/ehp.1408206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.L., Wong W.H., Lau Y.L. Association between air pollution and asthma admission among children in Hong Kong. Clin Exp Allergy. 2006;36:1138–1146. doi: 10.1111/j.1365-2222.2006.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon S.B., Bruce N.G., Grigg J. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2:823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu G., Zhong N., Ran P. Air pollution and COPD in China. J Thorac Dis. 2015;7:59–66. doi: 10.3978/j.issn.2072-1439.2014.12.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurt O.K., Zhang J., Pinkerton K.E. Pulmonary health effects of air pollution. Curr Opin Pulm Med. 2016;22:138–143. doi: 10.1097/MCP.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L., Yang J., Song Y.F., Chen P.Y., Ou C.Q. The burden of COPD mortality due to ambient air pollution in Guangzhou, China. Sci Rep. 2016;6:25900. doi: 10.1038/srep25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz J., Spix C., Wichmann H.E., Malin E. Air pollution and acute respiratory illness in five German communities. Environ Res. 1991;56:1–14. doi: 10.1016/s0013-9351(05)80104-5. [DOI] [PubMed] [Google Scholar]
- 28.Jaakkola J.J., Paunio M., Virtanen M., Heinonen O.P. Low-level air pollution and upper respiratory infections in children. Am J Public Health. 1991;81:1060–1063. doi: 10.2105/ajph.81.8.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey M.R. Prevalence of chronic bronchitis in a rural community of the Hill Region of Nepal. Thorax. 1984;39:331–336. doi: 10.1136/thx.39.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saadeh R., Klaunig J. Children's inter-individual variability and asthma development. Int J Health Sci (Qassim) 2015;9:456–467. [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz-Sanchez D., Tsien A., Fleming J., Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997;158:2406–2413. [PubMed] [Google Scholar]
- 32.Muranaka M., Suzuki S., Koizumi K. Adjuvant activity of diesel-exhaust particulates for the production of IgE antibody in mice. J Allergy Clin Immunol. 1986;77:616–623. doi: 10.1016/0091-6749(86)90355-6. [DOI] [PubMed] [Google Scholar]
- 33.Tunnicliffe W.S., Burge P.S., Ayres J.G. Effect of domestic concentrations of nitrogen dioxide on airway responses to inhaled allergen in asthmatic patients. Lancet. 1994;344:1733–1736. doi: 10.1016/s0140-6736(94)92886-x. [DOI] [PubMed] [Google Scholar]
- 34.Tsai S.S., Chang C.C., Yang C.Y. Fine particulate air pollution and hospital admissions for chronic obstructive pulmonary disease: a case-crossover study in Taipei. Int J Environ Res Public Health. 2013;10:6015–6026. doi: 10.3390/ijerph10116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao H., Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl Pharmacol. 2011;254:72–85. doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathers C.D., Boerma T., Ma Fat D. Global and regional causes of death. Br Med Bull. 2009;92:7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- 37.Yu X.J., Yang M.J., Zhou B. Characterization of somatic mutations in air pollution-related lung cancer. EBioMedicine. 2015;2:583–590. doi: 10.1016/j.ebiom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen A.J., Ross A.H., Ostro B. The global burden of disease due to outdoor air pollution. J Toxicol Environ Health A. 2005;68:1301–1307. doi: 10.1080/15287390590936166. [DOI] [PubMed] [Google Scholar]
- 39.Demetriou C.A., Raaschou-Nielsen O., Loft S. Biomarkers of ambient air pollution and lung cancer: a systematic review. Occup Environ Med. 2012;69:619–627. doi: 10.1136/oemed-2011-100566. [DOI] [PubMed] [Google Scholar]
- 40.Selevan S.G., Kimmel C.A., Mendola P. Identifying critical windows of exposure for children's health. Environ Health Perspect. 2000;108(suppl 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luck W., Nau H., Hansen R., Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8:384–395. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- 42.Salvi S. Health effects of ambient air pollution in children. Paediatr Respir Rev. 2007;8:275–280. doi: 10.1016/j.prrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Olsen J. David Barker (1938–2013)–a giant in reproductive epidemiology. Acta Obstet Gynecol Scand. 2014;93:1077–1080. doi: 10.1111/aogs.12378. [DOI] [PubMed] [Google Scholar]
- 44.Kelly F.J., Fuller G.W., Walton H.A., Fussell J.C. Monitoring air pollution: use of early warning systems for public health. Respirology. 2012;17:7–19. doi: 10.1111/j.1440-1843.2011.02065.x. [DOI] [PubMed] [Google Scholar]
- 45.Wu Q., Wang X., Zhou Q. Biomonitoring persistent organic pollutants in the atmosphere with mosses: performance and application. Environ Int. 2014;66:28–37. doi: 10.1016/j.envint.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 46.Breton C.V., Mack W.J., Yao J. Prenatal air pollution exposure and early cardiovascular phenotypes in young adults. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Hooven E.H., Pierik F.H., de Kluizenaar Y. Air pollution exposure during pregnancy, ultrasound measures of fetal growth, and adverse birth outcomes: a prospective cohort study. Environ Health Perspect. 2012;120:150–156. doi: 10.1289/ehp.1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brauer M., Lencar C., Tamburic L., Koehoorn M., Demers P., Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116:680–686. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jedrychowski W., Perera F., Maugeri U. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int Arch Allergy Immunol. 2011;155:275–281. doi: 10.1159/000320376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKercher G.R., Salmond J.A., Vanos J.K. Characteristics and applications of small, portable gaseous air pollution monitors. Environ Pollut. 2017;223:102–110. doi: 10.1016/j.envpol.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 51.Kim H.Y., Kim H.R., Kang M.G. Profiling of biomarkers for the exposure of polycyclic aromatic hydrocarbons: lamin-A/C isoform 3, poly[ADP-ribose] polymerase 1, and mitochondria copy number are identified as universal biomarkers. BioMed Res Int. 2014;2014:605135. doi: 10.1155/2014/605135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peterson B.S., Rauh V.A., Bansal R. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatr. 2015;72:531–540. doi: 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pratt M.M., John K., MacLean A.B., Afework S., Phillips D.H., Poirier M.C. Polycyclic aromatic hydrocarbon (PAH) exposure and DNA adduct semi-quantitation in archived human tissues. Int J Environ Res Public Health. 2011;8:2675–2691. doi: 10.3390/ijerph8072675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whyatt R.M., Bell D.A., Jedrychowski W. Polycyclic aromatic hydrocarbon-DNA adducts in human placenta and modulation by CYP1A1 induction and genotype. Carcinogenesis. 1998;19:1389–1392. doi: 10.1093/carcin/19.8.1389. [DOI] [PubMed] [Google Scholar]
- 55.Brauer M., Hoek G., Smit H.A. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29:879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- 56.Stieb D.M., Chen L., Eshoul M., Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Wilhelm M., Ritz B. Local variations in CO and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environ Health Perspect. 2005;113:1212–1221. doi: 10.1289/ehp.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bell M.L., Ebisu K., Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maisonet M., Bush T.J., Correa A., Jaakkola J.J. Relation between ambient air pollution and low birth weight in the Northeastern United States. Environ Health Perspect. 2001;109(suppl 3):351–356. doi: 10.1289/ehp.01109s3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ha E.H., Hong Y.C., Lee B.E., Woo B.H., Schwartz J., Christiani D.C. Is air pollution a risk factor for low birth weight in Seoul. Epidemiology. 2001;12:643–648. doi: 10.1097/00001648-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 61.Liu S., Krewski D., Shi Y., Chen Y., Burnett R.T. Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction. J Expo Sci Environ Epidemiol. 2007;17:426–432. doi: 10.1038/sj.jes.7500503. [DOI] [PubMed] [Google Scholar]
- 62.Ritz B., Yu F., Fruin S., Chapa G., Shaw G.M., Harris J.A. Ambient air pollution and risk of birth defects in Southern California. Am J Epidemiol. 2002;155:17–25. doi: 10.1093/aje/155.1.17. [DOI] [PubMed] [Google Scholar]
- 63.Ballester F., Estarlich M., Iñiguez C. Air pollution exposure during pregnancy and reduced birth size: a prospective birth cohort study in Valencia, Spain. Environ Health. 2010;9:6. doi: 10.1186/1476-069X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pedersen M., Giorgis-Allemand L., Bernard C. Ambient air pollution and low birthweight: a European cohort study (ESCAPE) Lancet Respir Med. 2013;1:695–704. doi: 10.1016/S2213-2600(13)70192-9. [DOI] [PubMed] [Google Scholar]
- 65.Wang X., Ding H., Ryan L., Xu X. Association between air pollution and low birth weight: a community-based study. Environ Health Perspect. 1997;105:514–520. doi: 10.1289/ehp.97105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dadvand P., Parker J., Bell M.L. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121:267–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perera F.P., Rauh V., Tsai W.Y. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi H., Rauh V., Garfinkel R., Tu Y., Perera F.P. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008;116:658–665. doi: 10.1289/ehp.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi H., Jedrychowski W., Spengler J. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ Health Perspect. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rich D.Q., Liu K., Zhang J. Differences in birth weight associated with the 2008 beijing olympics air pollution reduction: results from a natural experiment. Environ Health Perspect. 2015;123:880–887. doi: 10.1289/ehp.1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin J.A., Hamilton B.E., Osterman M.J., Driscoll A.K., Mathews T.J. Births: final data for 2015. Natl Vital Stat Rep. 2017;66:1. [PubMed] [Google Scholar]
- 72.Blencowe H., Cousens S., Oestergaard M.Z. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 73.Jung K.H., Perzanowski M., Rundle A. Polycyclic aromatic hydrocarbon exposure, obesity and childhood asthma in an urban cohort. Environ Res. 2014;128:35–41. doi: 10.1016/j.envres.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melville J.M., Moss T.J. The immune consequences of preterm birth. Front Neurosci. 2013;7:79. doi: 10.3389/fnins.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Padula A.M., Noth E.M., Hammond S.K. Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth. Environ Res. 2014;135:221–226. doi: 10.1016/j.envres.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dejmek J., Solanský I., Benes I., Lenícek J., Srám R.J. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000;108:1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perera F., Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31:363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fellman V., Hellström-Westas L., Norman M. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301:2225–2233. doi: 10.1001/jama.2009.771. [DOI] [PubMed] [Google Scholar]
- 79.Campos M., Bravo E., Eugenín J. Respiratory dysfunctions induced by prenatal nicotine exposure. Clin Exp Pharmacol Physiol. 2009;36:1205–1217. doi: 10.1111/j.1440-1681.2009.05214.x. [DOI] [PubMed] [Google Scholar]
- 80.Spindel E.R., McEvoy C.T. The role of nicotine in the effects of maternal smoking during pregnancy on lung development and childhood respiratory disease. Implications for dangers of e-cigarettes. Am J Respir Crit Care Med. 2016;193:486–494. doi: 10.1164/rccm.201510-2013PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sekhon H.S., Keller J.A., Benowitz N.L., Spindel E.R. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164:989–994. doi: 10.1164/ajrccm.164.6.2011097. [DOI] [PubMed] [Google Scholar]
- 82.Bell M.L., Davis D.L., Fletcher T. A retrospective assessment of mortality from the London smog episode of 1952: the role of influenza and pollution. Environ Health Perspect. 2004;112:6–8. doi: 10.1289/ehp.6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bharadwaj P., Zivin J.G., Mullins J.T., Neidell M. Early-life exposure to the Great smog of 1952 and the development of asthma. Am J Respir Crit Care Med. 2016;194:1475–1482. doi: 10.1164/rccm.201603-0451OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinez F.D. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 85.Sly P.D., Bush A. From the cradle to the grave: the early-life origins of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193:1–2. doi: 10.1164/rccm.201509-1801ED. [DOI] [PubMed] [Google Scholar]
- 86.Sly P.D. The early origins of asthma: who is really at risk? Curr Opin Allergy Clin Immunol. 2011;11:24–28. doi: 10.1097/ACI.0b013e328342309d. [DOI] [PubMed] [Google Scholar]
- 87.Deng Q., Lu C., Li Y., Sundell J., Norbäck D. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res. 2016;150:119–127. doi: 10.1016/j.envres.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 88.Manoli S.E., Smith L.A., Vyhlidal C.A. Maternal smoking and the retinoid pathway in the developing lung. Respir Res. 2012;13:42. doi: 10.1186/1465-9921-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stocks J., Dezateux C. The effect of parental smoking on lung function and development during infancy. Respirology. 2003;8:266–285. doi: 10.1046/j.1440-1843.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 90.Stocks J., Sonnappa S. Early life influences on the development of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2013;7:161–173. doi: 10.1177/1753465813479428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burke H., Leonardi-Bee J., Hashim A. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 92.Silvestri M., Franchi S., Pistorio A., Petecchia L., Rusconi F. Smoke exposure, wheezing, and asthma development: a systematic review and meta-analysis in unselected birth cohorts. Pediatr Pulmonol. 2015;50:353–362. doi: 10.1002/ppul.23037. [DOI] [PubMed] [Google Scholar]
- 93.Cohen N.R., Brennan P.J., Shay T. Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nat Immunol. 2013;14:90–99. doi: 10.1038/ni.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Svanes C., Sunyer J., Plana E. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65:14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 95.Martinez F.D. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6:272–277. doi: 10.1513/pats.200808-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sheikh S., Goldsmith L.J., Howell L., Parry L., Eid N. Comparison of lung function in infants exposed to maternal smoking and in infants with a family history of asthma. Chest. 1999;116:52–58. doi: 10.1378/chest.116.1.52. [DOI] [PubMed] [Google Scholar]
- 97.Jaakkola J.J., Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health. 2004;94:136–140. doi: 10.2105/ajph.94.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cook D.G., Strachan D.P., Carey I.M. Health effects of passive smoking. 9. Parental smoking and spirometric indices in children. Thorax. 1998;53:884–893. doi: 10.1136/thx.53.10.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.North M.L., Brook J.R., Lee E.Y. The Kingston Allergy Birth Cohort: exploring parentally reported respiratory outcomes through the lens of the exposome. Ann Allergy Asthma Immunol. 2017;118:465–473. doi: 10.1016/j.anai.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Gilliland F.D., Berhane K., Li Y.F., Rappaport E.B., Peters J.M. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003;167:917–924. doi: 10.1164/rccm.200206-616OC. [DOI] [PubMed] [Google Scholar]
- 101.Rogers A.J., Brasch-Andersen C., Ionita-Laza I. The interaction of glutathione S-transferase M1-null variants with tobacco smoke exposure and the development of childhood asthma. Clin Exp Allergy. 2009;39:1721–1729. doi: 10.1111/j.1365-2222.2009.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moshammer H., Hoek G., Luttmann-Gibson H. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173:1255–1263. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- 103.Hayatbakhsh M.R., Sadasivam S., Mamun A.A., Najman J.M., Williams G.M., O'Callaghan M.J. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64:810–814. doi: 10.1136/thx.2009.116301. [DOI] [PubMed] [Google Scholar]
- 104.Guerra S., Stern D.A., Zhou M. Combined effects of parental and active smoking on early lung function deficits: a prospective study from birth to age 26 years. Thorax. 2013;68:1021–1028. doi: 10.1136/thoraxjnl-2013-203538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hollams E.M., de Klerk N.H., Holt P.G., Sly P.D. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med. 2014;189:401–407. doi: 10.1164/rccm.201302-0323OC. [DOI] [PubMed] [Google Scholar]
- 106.Grabenhenrich L.B., Gough H., Reich A. Early-life determinants of asthma from birth to age 20 years: a German birth cohort study. J Allergy Clin Immunol. 2014;133:979–988. doi: 10.1016/j.jaci.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 107.Goksör E., Amark M., Alm B., Gustafsson P.M., Wennergren G. The impact of pre- and post-natal smoke exposure on future asthma and bronchial hyper-responsiveness. Acta Paediatr. 2007;96:1030–1035. doi: 10.1111/j.1651-2227.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 108.Patel M.M., Quinn J.W., Jung K.H. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ Res. 2011;111:1222–1229. doi: 10.1016/j.envres.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dales R., Wheeler A.J., Mahmud M., Frescura A.M., Liu L. The influence of neighborhood roadways on respiratory symptoms among elementary schoolchildren. J Occup Environ Med. 2009;51:654–660. doi: 10.1097/JOM.0b013e3181a0363c. [DOI] [PubMed] [Google Scholar]
- 110.Clark N.A., Demers P.A., Karr C.J. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morales E., Garcia-Esteban R., de la Cruz O.A. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax. 2015;70:64–73. doi: 10.1136/thoraxjnl-2014-205413. [DOI] [PubMed] [Google Scholar]
- 112.Aguilera I., Pedersen M., Garcia-Esteban R. Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study. Environ Health Perspect. 2013;121:387–392. doi: 10.1289/ehp.1205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.MacIntyre E.A., Gehring U., Mölter A. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE Project. Environ Health Perspect. 2014;122:107–113. doi: 10.1289/ehp.1306755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carlsten C., Dybuncio A., Becker A., Chan-Yeung M., Brauer M. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med. 2011;68:291–295. doi: 10.1136/oem.2010.055152. [DOI] [PubMed] [Google Scholar]
- 115.Schultz E.S., Gruzieva O., Bellander T. Traffic-related air pollution and lung function in children at 8 years of age: a birth cohort study. Am J Respir Crit Care Med. 2012;186:1286–1291. doi: 10.1164/rccm.201206-1045OC. [DOI] [PubMed] [Google Scholar]
- 116.Latzin P., Röösli M., Huss A., Kuehni C.E., Frey U. Air pollution during pregnancy and lung function in newborns: a birth cohort study. Eur Respir J. 2009;33:594–603. doi: 10.1183/09031936.00084008. [DOI] [PubMed] [Google Scholar]
- 117.Ryan P.H., LeMasters G., Biagini J. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116:279–284. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 118.Jedrychowski W., Galas A., Pac A. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol. 2005;20:775–782. doi: 10.1007/s10654-005-1048-1. [DOI] [PubMed] [Google Scholar]
- 119.Perzanowski M.S., Chew G.L., Divjan A. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol. 2013;131:886–893. doi: 10.1016/j.jaci.2012.12.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoek G., Brunekreef B., Hofschreuder P., Lumens M. Effects of air pollution episodes on pulmonary function and respiratory symptoms. Toxicol Ind Health. 1990;6:189–197. [PubMed] [Google Scholar]
- 121.Gauderman W.J., Gilliland G.F., Vora H. Association between air pollution and lung function growth in southern California children: results from a second cohort. Am J Respir Crit Care Med. 2002;166:76–84. doi: 10.1164/rccm.2111021. [DOI] [PubMed] [Google Scholar]
- 122.Mortimer K., Neugebauer R., Lurmann F., Alcorn S., Balmes J., Tager I. Air pollution and pulmonary function in asthmatic children: effects of prenatal and lifetime exposures. Epidemiology. 2008;19:550–557. doi: 10.1097/EDE.0b013e31816a9dcb. discussion 561–562. [DOI] [PubMed] [Google Scholar]
- 123.Suresh S., O'Callaghan M., Sly P.D., Mamun A.A. Impact of childhood anthropometry trends on adult lung function. Chest. 2015;147:1118–1126. doi: 10.1378/chest.14-0698. [DOI] [PubMed] [Google Scholar]
- 124.Gauderman W.J., Avol E., Gilliland F. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 125.Gauderman W.J., Urman R., Avol E. Association of improved air quality with lung development in children. N Engl J Med. 2015;372:905–913. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Neuman Å., Hohmann C., Orsini N. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012;186:1037–1043. doi: 10.1164/rccm.201203-0501OC. [DOI] [PubMed] [Google Scholar]
- 127.Voraphani N., Stern D.A., Wright A.L., Guerra S., Morgan W.J., Martinez F.D. Risk of current asthma among adult smokers with respiratory syncytial virus illnesses in early life. Am J Respir Crit Care Med. 2014;190:392–398. doi: 10.1164/rccm.201311-2095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anto J.M., Bousquet J., Akdis M. Mechanisms of the development of allergy (MeDALL): introducing novel concepts in allergy phenotypes. J Allergy Clin Immunol. 2017;139:388–399. doi: 10.1016/j.jaci.2016.12.940. [DOI] [PubMed] [Google Scholar]
- 129.Gilliland F.D., Li Y.F., Gong H., Jr., Diaz-Sanchez D. Glutathione s-transferases M1 and P1 prevent aggravation of allergic responses by secondhand smoke. Am J Respir Crit Care Med. 2006;174:1335–1341. doi: 10.1164/rccm.200509-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gilliland F.D., Li Y.F., Saxon A., Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet. 2004;363:119–125. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- 131.Peters J.L., Boynton-Jarrett R., Sandel M. Prenatal environmental factors influencing IgE levels, atopy and early asthma. Curr Opin Allergy Clin Immunol. 2013;13:187–192. doi: 10.1097/ACI.0b013e32835e82d3. [DOI] [PubMed] [Google Scholar]
- 132.Kabesch M., Hoefler C., Carr D., Leupold W., Weiland S.K., von M.E. Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax. 2004;59:569–573. doi: 10.1136/thx.2003.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bush A. COPD: a pediatric disease. COPD. 2008;5:53–67. doi: 10.1080/15412550701815965. [DOI] [PubMed] [Google Scholar]
- 134.Lopez A.D., Mathers C.D., Ezzati M., Jamison D.T., Murray C.J. World Bank; Washington (DC): 2006. Global Burden of Disease and Risk Factors. [PubMed] [Google Scholar]
- 135.Gauderman W.J., Vora H., McConnell R. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369:571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 136.Avol E.L., Gauderman W.J., Tan S.M., London S.J., Peters J.M. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001;164:2067–2072. doi: 10.1164/ajrccm.164.11.2102005. [DOI] [PubMed] [Google Scholar]
- 137.Kulkarni N., Pierse N., Rushton L., Grigg J. Carbon in airway macrophages and lung function in children. N Engl J Med. 2006;355:21–30. doi: 10.1056/NEJMoa052972. [DOI] [PubMed] [Google Scholar]
- 138.Glinianaia S.V., Rankin J., Bell R., Pless-Mulloli T., Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology. 2004;15:36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- 139.Wongtrakool C., Wang N., Hyde D.M., Roman J., Spindel E.R. Prenatal nicotine exposure alters lung function and airway geometry through alpha7 nicotinic receptors. Am J Respir Cell Mol Biol. 2012;46:695–702. doi: 10.1165/rcmb.2011-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maritz G.S. Perinatal exposure to nicotine and implications for subsequent obstructive lung disease. Paediatr Respir Rev. 2013;14:3–8. doi: 10.1016/j.prrv.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 141.Maritz G.S., Harding R. Life-long programming implications of exposure to tobacco smoking and nicotine before and soon after birth: evidence for altered lung development. Int J Environ Res Public Health. 2011;8:875–898. doi: 10.3390/ijerph8030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu W., Peden D., Diaz-Sanchez D. Role of GSTM1 in resistance to lung inflammation. Free Radic Biol Med. 2012;53:721–729. doi: 10.1016/j.freeradbiomed.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Romieu I., Moreno-Macias H., London S.J. Gene by environment interaction and ambient air pollution. Proc Am Thorac Soc. 2010;7:116–122. doi: 10.1513/pats.200909-097RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wongtrakool C., Roser-Page S., Rivera H.N., Roman J. Nicotine alters lung branching morphogenesis through the alpha7 nicotinic acetylcholine receptor. Am J Physiol Lung Cell Mol Physiol. 2007;293:L611–L618. doi: 10.1152/ajplung.00038.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Blacquière M.J., Timens W., Melgert B.N., Geerlings M., Postma D.S., Hylkema M.N. Maternal smoking during pregnancy induces airway remodelling in mice offspring. Eur Respir J. 2009;33:1133–1140. doi: 10.1183/09031936.00129608. [DOI] [PubMed] [Google Scholar]
- 146.Petre M.A., Petrik J., Ellis R., Inman M.D., Holloway A.C., Labiris N.R. Fetal and neonatal exposure to nicotine disrupts postnatal lung development in rats: role of VEGF and its receptors. Int J Toxicol. 2011;30:244–252. doi: 10.1177/1091581810395332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sandberg K.L., Pinkerton K.E., Poole S.D., Minton P.A., Sundell H.W. Fetal nicotine exposure increases airway responsiveness and alters airway wall composition in young lambs. Respir Physiol Neurobiol. 2011;176:57–67. doi: 10.1016/j.resp.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 148.Sekhon H.S., Jia Y., Raab R. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest. 1999;103:637–647. doi: 10.1172/JCI5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hafström O., Milerad J., Sundell H.W. Altered breathing pattern after prenatal nicotine exposure in the young lamb. Am J Respir Crit Care Med. 2002;166:92–97. doi: 10.1164/rccm.2107082. [DOI] [PubMed] [Google Scholar]
- 150.Elliot J., Vullermin P., Robinson P. Maternal cigarette smoking is associated with increased inner airway wall thickness in children who die from sudden infant death syndrome. Am J Respir Crit Care Med. 1998;158:802–806. doi: 10.1164/ajrccm.158.3.9709055. [DOI] [PubMed] [Google Scholar]
- 151.Maritz G.S., Mutemwa M., Kayigire A.X. Tomato juice protects the lungs of the offspring of female rats exposed to nicotine during gestation and lactation. Pediatr Pulmonol. 2011;46:976–986. doi: 10.1002/ppul.21462. [DOI] [PubMed] [Google Scholar]
- 152.Schaumann F., Muller M., Braun A. Endotoxin augments myeloid dendritic cell influx into the airways in patients with allergic asthma. Am J Respir Crit Care Med. 2008;177:1307–1313. doi: 10.1164/rccm.200706-870OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sharma M., Zhang X., Zhang S. Inhibition of endocytic lipid antigen presentation by common lipophilic environmental pollutants. Sci Rep. 2017;7:2085. doi: 10.1038/s41598-017-02229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lay J.C., Alexis N.E., Kleeberger S.R. Ozone enhances markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. J Allergy Clin Immunol. 2007;120:719–722. doi: 10.1016/j.jaci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 155.Frank E.A., Birch M.E., Yadav J.S. MyD88 mediates in vivo effector functions of alveolar macrophages in acute lung inflammatory responses to carbon nanotube exposure. Toxicol Appl Pharmacol. 2015;288:322–329. doi: 10.1016/j.taap.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Porter M., Karp M., Killedar S. Diesel-enriched particulate matter functionally activates human dendritic cells. Am J Respir Cell Mol Biol. 2007;37:706–719. doi: 10.1165/rcmb.2007-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ohtani T., Nakagawa S., Kurosawa M., Mizuashi M., Ozawa M., Aiba S. Cellular basis of the role of diesel exhaust particles in inducing Th2-dominant response. J Immunol. 2005;174:2412–2419. doi: 10.4049/jimmunol.174.4.2412. [DOI] [PubMed] [Google Scholar]
- 158.Gunnison A.F., Weideman P.A., Sobo M. Enhanced inflammatory response to acute ozone exposure in rats during pregnancy and lactation. Fundam Appl Toxicol. 1992;19:607–612. doi: 10.1016/0272-0590(92)90100-v. [DOI] [PubMed] [Google Scholar]
- 159.Bottema R.W., Reijmerink N.E., Kerkhof M. Interleukin 13, CD14, pet and tobacco smoke influence atopy in three Dutch cohorts: the allergenic study. Eur Respir J. 2008;32:593–602. doi: 10.1183/09031936.00162407. [DOI] [PubMed] [Google Scholar]
- 160.Vangronsveld E., Berckmans S., Spence M. Toluene diisocyanate emission to air and migration to a surface from a flexible polyurethane foam. Ann Occup Hyg. 2013;57:650–661. doi: 10.1093/annhyg/mes105. [DOI] [PubMed] [Google Scholar]
- 161.Lim R.H., Arredouani M.S., Fedulov A., Kobzik L., Hubeau C. Maternal allergic contact dermatitis causes increased asthma risk in offspring. Respir Res. 2007;8:56. doi: 10.1186/1465-9921-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun L.Z., Elsayed S., Bronstad A.M. Airway inflammation and bronchial remodelling in toluene diisocyanate-exposed BALB/c mouse model. Scand J Immunol. 2007;65:118–125. doi: 10.1111/j.1365-3083.2006.01882.x. [DOI] [PubMed] [Google Scholar]
- 163.Herrick C.A., MacLeod H., Glusac E., Tigelaar R.E., Bottomly K. Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4. J Clin Invest. 2000;105:765–775. doi: 10.1172/JCI8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Matsumoto A., Hiramatsu K., Li Y. Repeated exposure to low-dose diesel exhaust after allergen challenge exaggerates asthmatic responses in mice. Clin Immunol. 2006;121:227–235. doi: 10.1016/j.clim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 165.Fedulov A.V., Kobzik L. Immunotoxicologic analysis of maternal transmission of asthma risk. J Immunotoxicol. 2008;5:445–452. doi: 10.1080/15476910802481765. [DOI] [PubMed] [Google Scholar]
- 166.Eberl G., Colonna M., Di Santo J.P., McKenzie A.N. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348 doi: 10.1126/science.aaa6566. aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yu S., Kim H.Y., Chang Y.J., DeKruyff R.H., Umetsu D.T. Innate lymphoid cells and asthma. J Allergy Clin Immunol. 2014;133:943–950. doi: 10.1016/j.jaci.2014.02.015. quiz 951. [DOI] [PubMed] [Google Scholar]
- 168.Marashian S.M., Mortaz E., Jamaati H.R. Role of innate lymphoid cells in lung disease. Iran J Allergy, Asthma Immunol. 2015;14:346–360. [PubMed] [Google Scholar]
- 169.Kim H.Y., Umetsu D.T., Dekruyff R.H. Innate lymphoid cells in asthma: will they take your breath away? Eur J Immunol. 2016;46:795–806. doi: 10.1002/eji.201444557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.De Grove K.C., Provoost S., Hendriks R.W. Dysregulation of type 2 innate lymphoid cells and TH2 cells impairs pollutant-induced allergic airway responses. J Allergy Clin Immunol. 2017;139:246. doi: 10.1016/j.jaci.2016.03.044. 257 e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Beamer C.A., Girtsman T.A., Seaver B.P. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 2013;7:1070–1081. doi: 10.3109/17435390.2012.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang Q., Ge M.Q., Kokalari B. Group 2 innate lymphoid cells mediate ozone-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2016;137:571–578. doi: 10.1016/j.jaci.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Diaz-Sanchez D., Dotson A.R., Takenaka H., Saxon A. Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms. J Clin Invest. 1994;94:1417–1425. doi: 10.1172/JCI117478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang M., Saxon A., Diaz-Sanchez D. Early IL-4 production driving Th2 differentiation in a human in vivo allergic model is mast cell derived. Clin Immunol. 1999;90:47–54. doi: 10.1006/clim.1998.4628. [DOI] [PubMed] [Google Scholar]
- 175.Finkelman F.D., Yang M., Orekhova T. Diesel exhaust particles suppress in vivo IFN-gamma production by inhibiting cytokine effects on NK and NKT cells. J Immunol. 2004;172:3808–3813. doi: 10.4049/jimmunol.172.6.3808. [DOI] [PubMed] [Google Scholar]
- 176.Diaz-Sanchez D., Garcia M.P., Wang M., Jyrala M., Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol. 1999;104:1183–1188. doi: 10.1016/s0091-6749(99)70011-4. [DOI] [PubMed] [Google Scholar]
- 177.Whitekus M.J., Li N., Zhang M. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol. 2002;168:2560–2567. doi: 10.4049/jimmunol.168.5.2560. [DOI] [PubMed] [Google Scholar]
- 178.Pacchierotti F., Spanò M. Environmental impact on DNA methylation in the germline: state of the art and gaps of knowledge. BioMed Res Int. 2015;2015:123484. doi: 10.1155/2015/123484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hew K.M., Walker A.I., Kohli A. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin Exp Allergy. 2015;45:238–248. doi: 10.1111/cea.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.de Planell-Saguer M., Lovinsky-Desir S., Miller R.L. Epigenetic regulation: the interface between prenatal and early-life exposure and asthma susceptibility. Environ Mol Mutagen. 2014;55:231–243. doi: 10.1002/em.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ji H., Biagini M.J.M., Brandt E.B., Brokamp C., Ryan P.H., Khurana H.G.K. Air pollution, epigenetics, and asthma. Allergy Asthma Clin Immunol. 2016;12:51. doi: 10.1186/s13223-016-0159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Terry M.B., Ferris J.S., Pilsner R. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:2306–2310. doi: 10.1158/1055-9965.EPI-08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oberlander T.F., Weinberg J., Papsdorf M., Grunau R., Misri S., Devlin A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 184.Li Y.F., Langholz B., Salam M.T., Gilliland F.D. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005;127:1232–1241. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 185.Cookson H., Granell R., Joinson C., Ben-Shlomo Y., Henderson A.J. Mothers' anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. 2009;123:847. doi: 10.1016/j.jaci.2009.01.042. 853.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang Y., Haitchi H.M., Cakebread J. Epigenetic mechanisms silence a disintegrin and metalloprotease 33 expression in bronchial epithelial cells. J Allergy Clin Immunol. 2008;121:1393–1399. doi: 10.1016/j.jaci.2008.02.031. 1399 e1391–1314. [DOI] [PubMed] [Google Scholar]
- 187.Fry R.C., Rager J.E., Bauer R. Air toxics and epigenetic effects: ozone altered microRNAs in the sputum of human subjects. Am J Physiol Lung Cell Mol Physiol. 2014;306:L1129–L1137. doi: 10.1152/ajplung.00348.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Baccarelli A., Wright R.O., Bollati V. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179:572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rager J.E., Bailey K.A., Smeester L. Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen. 2014;55:196–208. doi: 10.1002/em.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fossati S., Baccarelli A., Zanobetti A. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology. 2014;25:68–78. doi: 10.1097/EDE.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Herberth G., Bauer M., Gasch M. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J Allergy Clin Immunol. 2014;133:543–550. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 192.Fields P.E., Kim S.T., Flavell R.A. Cutting edge: changes in histone acetylation at the IL-4 and IFN-gamma loci accompany Th1/Th2 differentiation. J Immunol. 2002;169:647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 193.White G.P., Watt P.M., Holt B.J., Holt P.G. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 194.Kohli A., Garcia M.A., Miller R.L. Secondhand smoke in combination with ambient air pollution exposure is associated with increasedx CpG methylation and decreased expression of IFN-γ in T effector cells and Foxp3 in T regulatory cells in children. Clin Epigenet. 2012;4:17. doi: 10.1186/1868-7083-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Baron U., Floess S., Wieczorek G. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 196.Liu J., Ballaney M., Al-alem U. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci. 2008;102:76–81. doi: 10.1093/toxsci/kfm290. [DOI] [PMC free article] [PubMed] [Google Scholar]