Abstract
Background
The stress in the perioperative period is compounded by unpredictable and un-physiological changes in sympathetic tone, cardiovascular performance, coagulation and inflammatory responses, all of which in turn lead to alterations in plaque morphology predisposing to perioperative myocardial infarction (PMI). PMI has a considerable morbidity and mortality in patients undergoing not only high risk surgery, but also even with minor surgical interventions.
Objective
To study the incidence of PMI and its predictors in patients undergoing non-cardiac surgery in a tertiary care hospital.
Materials and methods
Patients undergoing non-cardiac surgery were included in this prospective single-center observational study. The revised cardiac risk index (RCRI) was used for risk stratification. ECG monitoring was done for all patients. For patients suggestive of acute myocardial ischemia, echocardiography and serum troponin were evaluated. The patient was labeled as having a PMI if there was raised troponin level along with any one evidence of myocardial ischemia (symptoms, ECG changes or imaging results) and in these patients the factors predisposing to PMI were evaluated. All patients in the study were followed up to 30 days.
Results
Of the 525 patients analyzed, 33 patients (6.28%) had a PMI. Twelve out of the 33 (36.36%) PMI patients died within 30 days following surgery. Patients undergoing high risk surgery, smokers and patients with a past history of ischemic heart disease (IHD) were found to be at higher risk of developing PMI. The ASA physical status classification and the RCRI proved to be good predictors of PMI. Most of the PMI events (72.7%) occurred within 48 hours of surgery.
Conclusion
PMI is a dreaded complication associated with a very high mortality. High risk surgery, smoking and past history of ischemic heart disease were independent predictors of PMI. The RCRI is a useful tool in pre-operative risk stratification of patients.
Keywords: Perioperative myocardial infarction, Troponin, Lee’s revised cardiac risk index
1. Introduction
Despite 40 years of cumulative interest in the cardiovascular management of the patient undergoing noncardiac surgery, the subject matter remains as relevant as ever. The overall perioperative care of the patient has improved with the introduction of newer surgical techniques, combined with improved monitoring and patient safety during anesthesia. However the age and co-morbidities of patients undergoing non-cardiac surgery has increased. The incidence of perioperative myocardial infarction (PMI) has been investigated in several large-scale studies giving variable results between 0.3% to 36%, depending on the target population, study design, and the PMI definition used.1, 2 In-hospital mortality rates are between 12% to 40% after PMI have been reported.1, 2, 3, 4, 5, 6 PMI is one of the most important predictors of short- and long-term morbidity and mortality, length and cost of hospitalization associated with non-cardiac surgery.6 Given the silent nature and high mortality rate of PMI, prevention of a PMI is thus a prerequisite for an improvement in overall postoperative outcome.
2. Materials and methods
After the approval by the institutional ethics committee a total of 525 patients, meeting the inclusion criteria, not having any exclusion criteria posted for elective, non-cardiac surgery of at least 30 min duration were evaluated. Informed consent was taken. The sample size was calculated as 525 on the basis of an earlier study by Rao et al.,7 considering incidences of PMI in patients undergoing non-cardiac surgery and anticipated odds ratio as 5, level of significance 5%, with multiple correlation co-efficient as 0.5. Demographic data were collected from the patient and anaesthesia record. Preoperative ECG and 2D echocardiography findings were noted if obtained. The patients were risk stratified based on the Revised Cardiac Risk Index (RCRI) as well as the American Society of Anesthesiologists (ASA) physical status classification.
Perioperative data collected were type of surgery, type of anaesthesia, intra-operative blood loss as well as perioperative events. Perioperative events were defined as: tachycardia (heart rate greater than 100 per minute for greater than 5 minutes), bradycardia (heart rate less than 60 per minute), hypotension (systolic blood pressure less than 30% of baseline), or hypoxemia (SpO2 less than 90% for greater than 5 minutes).8 Intraoperatively, all patients had continuous ECG monitoring (with ST analysis) as well as repeat ECG done 24 h and 48 h following surgery. ECG signs of myocardial ischemia were defined as ST segment elevation or depression ≥ 1-mm or presence of new Q waves lasting ≥ 0.04 s and ≥ 1-mm deep in at least two contiguous leads and new onset left bundle branch block (LBBB).8 For patients with symptoms, hemodynamic disturbance or ECG changes suggestive of acute myocardial ischemia, cardiology consultation, bedside echocardiography and serum troponin levels were advised. Troponin-T was determined by the Cobas immunoassay analyzer (Roche Diagnostics, Mannheim, Germany). The normal range of Troponin-T values in our laboratory was 0.0001–0.0249 ng/ml. The diagnosis of PMI was made in patients with troponin levels higher than the normal laboratory range, along with any one evidence of myocardial ischemia (symptoms, ECG changes or RWMA). In these subsets of patients, the factors predisposing to MI were evaluated. All the patients were followed up to 30 days following surgery via a telephonic call to assess the mortality rate.
3. Inclusion criteria
Patients undergoing non-cardiac surgery of at least 30 min duration, aged more than 45 years and not having exclusion criteria were included in the study.
4. Exclusion criteria
The following group of patients was excluded from the study
-
■
Age <45 years
-
■
Patients with LBBB or hemi-block (on preoperative ECG)
-
■
Patients with pericarditis
-
■
Valvular heart disease
-
■
Severe left ventricular dysfunction (Ejection Fraction <30%)
-
■
Patients with subarachnoid hemorrhage or patients with intracranial pathology.
-
■
Not willing to consent
5. Statistical analysis
Descriptive and inferential statistics were used in our study. Continuous variables were represented as mean ± standard deviation. Categorical variables are represented as numbers or percentage when appropriate. For comparing continuous variable between PMI and non-PMI patients Independent t-test was used. For comparing categorical data Chi square test was used. A p value less than 0.05 was considered significant. Univariate/multivariate logistic regression analysis was used to test the association between the variables and the outcome. The data were analyzed with IBM SPSS software version 22.0.
6. Observation and results
A total of 525 patients, meeting the inclusion criteria, posted for elective, non-cardiac surgery were enrolled in this prospective observational study. The demographic data are presented in (Table 1, Table 2). The mean age and gender distribution between the PMI and non PMI patients were comparable (p = 0.7 & 0.2, respectively). Of the total patients, 152 (29%) had diabetes mellitus, 62 (11.8%) had a history of ischemic heart disease (IHD), 210 (40%) were hypertensives, 20 (3.8%) had a history of CVA, and 130 (24.8%) patients were smokers. Past history of IHD and smoking increased the risk of patients developing MI which was statistically significant (p value 0.004 and 0.005 respectively), whereas a history of diabetes, hypertension, CVA and alcohol consumption were statistically not significant. (p values 0.08, 0.8, 0.09 and 0.08, respectively) (Table 1)
Table 1.
Demographic features of PMI and No PMI patients.
| Variables | All patients (N = 525) | % of N | PERIOPERATIVE MI |
p value | ||||
|---|---|---|---|---|---|---|---|---|
| No |
Yes |
|||||||
| Count | % | Count | % | |||||
| Age (years) | 40–59 | 228 | 43.4 | 213 | 93.4 | 15 | 6.6 | 0.7 |
| 60–79 | 264 | 50.3 | 247 | 93.6 | 17 | 6.4 | ||
| 80–95 | 33 | 6.3 | 32 | 97.0 | 1 | 3.0 | ||
| Sex | Female | 217 | 41.3 | 207 | 95.4 | 10 | 4.6 | 0.2 |
| Male | 308 | 58.7 | 285 | 92.5 | 23 | 7.5 | ||
| DM | No | 354 | 94.9 | 19 | 5.1 | 0.08 | ||
| Yes | 152 | 29 | 138 | 90.8 | 14 | 9.2 | ||
| IHD | No | 439 | 94.8 | 24 | 5.2 | 0.004 | ||
| Yes | 62 | 11.8 | 53 | 85.5 | 9 | 14.5 | ||
| HTN | No | 295 | 94.2 | 19 | 6.1 | 0.8 | ||
| Yes | 210 | 40 | 196 | 93.3 | 14 | 6.7 | ||
| CVA | No | 475 | 94.2 | 30 | 5.9 | 0.09 | ||
| Yes | 20 | 3.8 | 17 | 85.0 | 3 | 15.0 | ||
| Smoking | No | 376 | 95.7 | 18 | 4.6 | 0.005 | ||
| Yes | 130 | 24.8 | 115 | 88.5 | 15 | 11.5 | ||
| Alcohol | No | 476 | 94.3 | 30 | 5.9 | 0.08 | ||
| Yes | 19 | 3.6 | 16 | 84.2 | 3 | 15.8 | ||
| Anaemia | No | 430 | 94.1 | 27 | 5.9 | 0.4 | ||
| Yes | 68 | 12.9 | 62 | 91.2 | 6 | 8.8 | ||
| Preoperative ECG changes | No | 459 | 87.4 | 435 | 94.8 | 24 | 5.2 | 0.009 |
| Yes | 66 | 12.6 | 57 | 87.7 | 9 | 13.6 | ||
| Preoperative 2D Echo RWMA | No | 476 | 90.7 | 454 | 95.4 | 22 | 4.6 | <0.0001 |
| Yes | 49 | 9.3 | 38 | 79.2 | 11 | 22.4 | ||
| ASA grade | I | 118 | 22.5 | 113 | 95.8 | 5 | 4.2 | |
| II | 259 | 49.3 | 251 | 96.9 | 9 | 3.1 | <0.0001 | |
| III | 107 | 20.4 | 98 | 91.6 | 9 | 8.4 | ||
| IV | 41 | 7.8 | 30 | 73.2 | 11 | 26.8 | ||
| ACC/AHA categories for NCS | Low Risk | 187 | 35.6 | 180 | 96.3 | 7 | 3.7 | <0.0001 |
| Intermediate Risk | 274 | 52.2 | 260 | 94.9 | 14 | 5.1 | ||
| High Risk | 64 | 12.2 | 52 | 81.3 | 12 | 18.8 | ||
| RCRI | ||||||||
| High Risk Surgery | No | 357 | 95.5 | 18 | 4.8 | 0.02 | ||
| Yes | 148 | 28.2 | 133 | 89.9 | 15 | 10.1 | ||
| History of IHD | No | 439 | 94.8 | 24 | 5.2 | 0.004 | ||
| Yes | 62 | 11.8 | 53 | 85.5 | 9 | 14.5 | ||
| History of CHF | No | 483 | 94.2 | 31 | 6.0 | 0.1 | ||
| Yes | 11 | 2.1 | 9 | 81.8 | 2 | 18.2 | ||
| History of CVA | No | 475 | 94.2 | 30 | 5.9 | 0.09 | ||
| Yes | 20 | 3.8 | 17 | 85.0 | 3 | 15.0 | ||
| Preoperative Insulin Therapy | No | 376 | 94.9 | 21 | 5.3 | 0.09 | ||
| Yes | 128 | 24.4 | 116 | 90.6 | 12 | 9.4 | ||
| Preoperative Creatinine >2 mg/dL | No | 471 | 94.4 | 29 | 5.8 | 0.04 | ||
| Yes | 25 | 4.8 | 21 | 84.0 | 4 | 16.0 | ||
Chi square test, DM-Diabetes Mellitus; IHD-Ischemic Heart Disease; HTN-Hypertension; CVA-Cerebrovascular Accident, ASA- American Society of Anesthesiology, ACC- American College of Cardiology, AHA- American Heart Association, NCS-Non cardiac surgery, RCRI- Revised cardiac risk index.
Table 2.
Distribution of patients across various surgical specialties and the incidence of PMI in each specialty.
| Department | All patients N = 525 | % of N | PERIOPERATIVE MI |
|||
|---|---|---|---|---|---|---|
| No |
Yes |
|||||
| Count | % | Count | % | |||
| ENT | 36 | 6.9% | 35 | 97.2 | 1 | 2.8 |
| Gastro surgery | 6 | 1.1% | 5 | 83.3 | 1 | 16.7 |
| General Surgery | 184 | 35.0% | 174 | 94.6 | 10 | 5.4 |
| Neurosurgery | 11 | 2.1% | 9 | 81.8 | 2 | 18.2 |
| OBG | 67 | 12.8% | 66 | 98.5 | 1 | 1.5 |
| OMFS | 1 | 0.2% | 1 | 100.0 | 0 | 0 |
| Onco-surgery | 24 | 4.5% | 18 | 75.0 | 6 | 25.0 |
| Orthopedics | 90 | 17.1% | 86 | 96.6 | 4 | 4.4 |
| Urology | 86 | 16.4% | 83 | 96.5 | 3 | 3.5 |
| Vascular Surgery | 20 | 3.8% | 15 | 75.0 | 5 | 25.0 |
Chi-square test- p < 0.0001. ENT-Ear Nose Throat; OBG-Obstetrics and Gynaecology OMFS-Oral and Maxillofacial surgery.
Of the 525 patients, who were prospectively studied, 33 patients developed PMI. The incidence of PMI was 6.3%. The distribution of patients across various surgical departments and the incidence of PMI in each group is shown in Table 2. The incidence of PMI was highest in the oncosurgery and vascular surgery group, wherein 25% of patients undergoing these surgeries had PMI, which was statistically significant (p < 0.0001) (Table 2).
Revised cardiac risk index (Modified Lee’s index) and the ASA physical status was used for risk assessment of patients. As shown in Table 1, the incidence of PMI in ASA grade III and IV was 8.4% and 25% respectively and it was statistically significant. (p < 0.0001). The patients were also classified based on the type of surgery as per the ACC/AHA Cardiac Risk Stratification for Noncardiac surgeries (NCS)15 as high risk, intermediate risk and low risk surgeries (Table 1). The incidence of PMI in high risk surgeries was 18.8%, whereas that in low risk and intermediate risk surgeries was 3.2% and 5.1% respectively. The difference in incidence of PMI was statistically significant. (p < 0.0001).
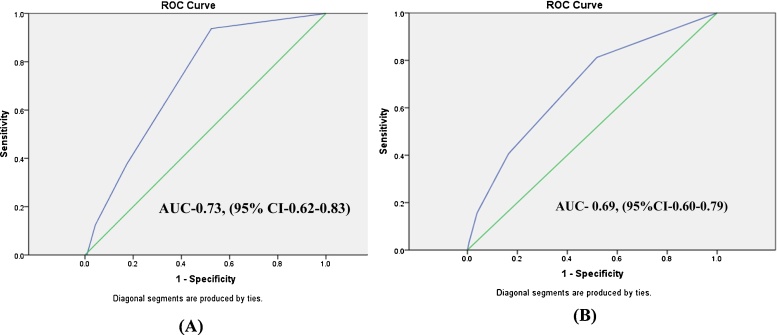
Risk stratification was also done based on the RCRI (Modified Lee’s Index, Table 1) shows the number of patients with each of the six variables/predictors of theRCRI. About 28.2% of the patients underwent a high risk surgery as per the definition in the RCRI. About 13.5% had a history of IHD while 24.4% were preoperatively on treatment with insulin for the control of DM. 4% had a previous history of CVA and 2.1% had a history of congestive heart failure. Also, 16% patients had a preoperative creatinine > 2 mg/dl. The incidence of PMI was 10.1% in patients undergoing a high risk surgery. 14.1% of patients with a history of IHD had a PMI. The incidence of PMI in patients with congestive heart failure, history of stroke, on preoperative insulin therapy was 18.2%, 14.3% and 9.4%, respectively. 16% of patients with preoperative serum creatinine greater than 2 mg/dl had PMI. Of all the parameters in RCRI, only high risk surgery, history of IHD and preoperative creatine greater than 2 mg/dl were independent predictors of PMI (p value 0.02, 0.01 and 0.04, respectively) in our study. (Table 1)
Patients were also grouped according to the number of RCRI variables/predictors they had preoperatively. Two hundred and forty three patients (46.2%) had none of the predictors in the RCRI, 188 patients (35.8%) had one predictor, 70 (13.3%) patients had two predictors and 24 patients had three or more than three predictors. After logistic regression analysis, we observed that the incidence of PMI increased as the number of predictors increased, the incidence of PMI with two, three and four predictors were 11.4%, 20% and 25%, respectively (p = 0.001). Based on the number of predictors, patients were classified into Classes I to IV, where Class I had zero predictors and class IV had three or more predictors. The PMI incidence in class IV was significantly higher than class III and II (p value 0.002, OR 10.395, 95% CI 2.9–37.2) (Table 3). To summarize ASA physical status classification, ACC/AHA Cardiac risk for NCS classification and RCRI are good tools to determine risk of PMI. Risk of PMI increases as the class in each of these tools increases. RCRI class IV had ten times higher risk of having PMI as compared to class I. In our study, the predictive value of the RCRI was fair both for PMI (AUC of 0.73) and for 30-day mortality (AUC of 0.60) according to the ROC curve (Fig. 1). We did not find any statistically significant association between pre-operative anemia (Hb < 10 g/dl) and the incidence of PMI. 13.6% of patients with PMI had preoperative ECG changes, and 22.4% of patients with PMI had preoperative 2D-Echocardiography with evidence of regional wall motion abnormality. The patients with preoperative ECG changes and RWMA on 2D-echocardiography were at a higher risk of developing PMI. (p = 0.009 and <0.0001 respectively). 172 patients were given general anesthesia (GA), 307 patients were given central neuraxial block (CNB) (spinal, epidural or combined spinal and epidural), while 30 patients were given combined GA with central neuraxial block. The incidence of PMI was highest in patients who were administered GA with central neuraxial block (16.7%), but this finding was not statistically significant. (p = 0.06). Combined technique is usually used for prolonged and high risk surgery so the incidence of PMI may be higher in this subset of patients. Prolonged tachycardia was seen in 7 of 33 (21.2%) PMI patients. Thirteen PMI patients (39.4%) had an intraoperative hypotension. Intraoperative ST-T changes were seen in 25 patients, of which 10 patients were diagnosed as PMI. These findings were found to be statistically significant for PMI. Only three out of the thirty three (9%) PMI patients had complained of chest pain. Postoperative hypotension was seen in 20 out of 33 patients. ST-T changes were seen in 20 out of the 33 PMI patients. Three patients who developed PMI were advised to undergo a coronary angiogram.
Table 3.
PMI rates in the study cohorts stratified by RCRI class.
| RCRI (Lee’s) Classes | PERIOPERATIVE_MI |
p value | OR | 95% CI | |||
|---|---|---|---|---|---|---|---|
| No |
Yes |
||||||
| Count | % | Count | % | ||||
| Class I | 237 | 97.5 | 6 | 2.5 | – | 1 | – |
| Class II | 174 | 92.6 | 14 | 7.4 | 0.02 | 3.2 | 1.200–8.4 |
| Class III | 62 | 88.6 | 8 | 11.4 | 0.004 | 5.097 | 1.705–15.232 |
| Class IV | 19 | 79.2 | 5 | 20.8 | 0.002 | 10.395 | 2.903–37.219 |
p < 0.0001, Logistic regression analysis, OR-Odds ratio; CI-Confidence interval.
Fig. 1.
Receiver operating characteristic curves (AUC) (with 95% CI) presenting the predictive value of the Revised cardiac risk index score and (A) 30-day mortality, (B) PMI (n = 525).
Of the 33 patients with PMI in our study, 24 patients (72.7%) developed PMI within 48 h of surgery. All patients were followed up to 30 days following surgery by a telephonic call to evaluate the mortality rate. Twelve out of 33 PMI patients (36.4%) died within 30 days, whereas four out of 492 non PMI patients (0.8%) had died during the follow up period. Two patients could not be followed up. Patients with PMI had a higher mortality than the non PMI patients (p < 0.0001).
7. Discussion
There are very few studies which have evaluated the incidence and predictors of PMI in our subset of population. In our study 525 patients undergoing elective, non-cardiac surgery in a tertiary care center were selected, and the incidence and predictors of PMI were evaluated. The incidence of PMI in our study was 6.3%. This is in agreement with earlier prospective large scale studies by Devereaux et al., namely the Perioperative Ischemic Evaluation (POISE study) 5%3 and Vascular events In non-cardiac Surgery patient cohort Evaluation (VISION study) 2.8%.2 In our study, 12 out of the 33 (36.36%) PMI patients died within 30 days, whereas in the POISE study it was 11.6%3 and 2% in the VISION study.2
Among high-risk patient, however the incidence of PMI may exceed, especially in patients undergoing vascular surgery where the incidence of PMI was 25% (p < 0.0001). IHD is more common in these subsets of patients and the hemodynamic stress often associated with vascular surgery accounts for their observed high risk of perioperative myocardial ischemic events.
Clinical presentation of PMI is most often either asymptomatic or non-specific and, thus relying only on classical symptoms alone will lead to missed diagnosis. Most cases of PMI are silent, primarily because of the effects of intraoperative anesthesia and postoperative analgesia. Moreover, in older and diabetic patients autonomic dysfunction may cause ambiguity in presentation. Similarly, in our study only 9% of patients reported precordial pain. Therefore, in the absence of pain, it is highly likely that PMI can be missed if cardiac markers and regular ECG monitoring are not routinely done. Although cardiac troponins are considered to be a specific marker for myocardial tissue damage, other conditions such as pulmonary embolism, sepsis, stroke, or acute kidney injury (AKI) can cause substantial troponin elevations.9, 10, 11, 12
Advanced age was found to be a significant predictor of cardiac events in most studies. In the POISE-II trial, at an age more than 75 years the risk for PMI was 23.5%.13, 14 However the relationship between age and PMI in our study was not found to be statistically significant (p = 0.7). Gender of the patient was not statistically significant in predicting adverse cardiac outcomes in our study, similar to POISE study. In our study, past history of IHD and smoking were found to be independent predictors of PMI. Certain conditions like diabetes mellitus, hypertension, impaired renal function, and prior CVA are known to be associated with higher perioperative cardiovascular events, however in our study these factors were not independent predictors of PMI. This could be attributed to the optimization of patients before elective surgeries and the number of patients with renal failure and CVA in our study were inadequate, hence a correlation could not be established.
Risk stratification for PMI should be an integral part of the preoperative evaluation. In order to stratify the preoperative risk of patients we relied on the RCRI, ASA physical status classification, and ACC/AHA Cardiac risk stratification.
According to ACC/AHA cardiac risk stratification for non-cardiac surgeries,15 patients undergoing high risk surgeries, had the highest adverse outcome. In our study, 18.8% of patients in the high risk category and also 2.3% of patients in the low risk category had a PMI. Various studies have proved ASA physical status classification to be a powerful predictor of postoperative MI and cardiac arrest, Gupta et al. showed a 9.9-fold increase in risk from ASA II to ASA IV.16 The findings were confirmed in our study where we observed an 8-fold increase in risk of PMI from ASA II to ASA IV (p < 0.0001). The RCRI, developed by Lee et al., is the most commonly used and broadly validated preoperative risk stratification tool.17 In our study, the predictive value of the RCRI was significant for PMI (p = 0.001). Though only IHD, high risk surgery and preoperative creatinine > 2 mg/dl were found to be independent risk predictors (p value 0.02, 0.01 and 0.04, respectively). The PMI event rates increased as the number of variables in RCRI increased. The event rate for PMI in Class IV RCRI in our study was 20.8%, and was significantly higher than class III and II (p value 0.002, OR 10.395, 95% CI 2.9–37.2). The continued value of the pre-operative ECG and 2D echocardiography has been demonstrated in our study also. We noticed a higher incidence of PMI (16.7%) in patients who received both GA with central neuraxial blockade; however, this finding was not statistically significant (p = 0.06). For most of the prolonged, extensive and high risk surgeries combined general and epidural anesthesia was given, therefore the results could be biased. In our study, the type of anesthesia as an independent predictor of PMI was not statistically significant.
Patients who developed perioperative hypotension associated with tachycardia had a high risk of developing PMI in our study. In a cohort study by Van Waes JA, et al. intraoperative hypotension was associated with an increased risk of postoperative myocardial injury.18 In the POISE-2 trial, hypotension was an independent predictor of MI.15 However, the most prominent mechanism is tachycardia, induced by autonomic imbalance, postoperative pain, hypovolemia, or reduction in heart rate limiting medications.19, 20 We observed that the majority of the PMI (72.7%) occurred within 48 h of surgery, which was similar to the finding in the POISE study (74.1% of PMIs occurred within 48 h of surgery).3
The strength of our study is that we have included patients undergoing non-cardiac surgical procedures across various specialties in a tertiary care, with a considerable heterogeneity in patient characteristics. The best available fifth generation high sensitivity Troponin-T assay was utilized in our study. Furthermore, to the best of our knowledge, no other previous study has evaluated the predictors of PMI prospectively in an Indian cohort.
The limitations of our study are that the number of patients studied were small, compared to other studies which were large and multi-centric. The number of patients with renal failure and CVA were less, because of which an adequate correlation could not be established. Troponin T was done only in patients with clinical and ECG evidence of PMI resulting in missing few cases of silent myocardial infarction.
8. Conclusion
The incidence of perioperative myocardial infarction (PMI) in our study was 6.28% with high mortality (36.36%). Past history of ischemic heart disease, and smoking were two independent predictors of PMI. The incidence of PMI was highest in patients undergoing high risk surgeries like vascular surgeries. The RCRI and ASA physical status classification were good preoperative tools for risk stratification of patients undergoing non-cardiac surgery.
Disclosure
No financial disclosure or grants.
Conflict of interest
No conflict of interest.
Author contributions
Jaison Chacha Sunny: Data collection and manuscript preparation.
Dinesh Kumar: Conceptalization of idea, Data analysis and manuscript preparation.
Nalini Kotekar: Conceptualization of idea, Data analysis and manuscript preparation.
Nagaraj Desai: Conceptualization of idea and manuscript preparation.
Acknowledgements
We acknowledge Dr Sumanth for his inputs on statistics. We also like to acknowledge the cooperation of patients and anaesthesiologist.
References
- 1.Devereaux P.J., Chan M., Eikelboom J. Evidence based Cardiology. 3rd ed. BMJ Books; London, England: 2009. Major vascular complications in patients undergoing noncardiac surgery: the magnitude of the problem, risk prediction, surveillance, and prevention; pp. 47–62. [Google Scholar]
- 2.Devereaux P.J., Chan M., Alonso-Coello P. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. The Vascular events In non cardiac Surgery patIents cOhort evaluatioN (VISION) Study Investigators. JAMA. 2012;307(21):2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 3.Devereaux P.J., Yang H., Yusuf S. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomized controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 4.Mackey W.C., Fleisher L.A., Haider S. Perioperative myocardial ischemic injury in high-risk vascular surgery patients: incidence and clinical significance in a prospective clinical trial. J Vasc Surg. 2006;43:533–538. doi: 10.1016/j.jvs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Adams 3rd J.E., Sicard G.A., Allen B.T. Diagnosis of perioperative myocardial infarction with measurement of cardiac troponin I. N Engl J Med. 1994;330:670–674. doi: 10.1056/NEJM199403103301003. [DOI] [PubMed] [Google Scholar]
- 6.Mangano D.T. Perioperative cardiac morbidity. Anesthesiology. 1990;72:153–184. doi: 10.1097/00000542-199001000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Rao J.Y., Yeriswamy M.C., Santhosh M.J. A look into Lee's score: peri-operative cardiovascular risk assessment in non-cardiac surgeries-usefulness of revised cardiac risk index. Indian Heart J. 2012;64:134–138. doi: 10.1016/S0019-4832(12)60047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landesberg G., Hillel Z. Electrocardiography, perioperative ischemia, and myocardial infarction. In: Miller R., editor. Miller's Anesthesia. 8th ed. Elsevier Saunders; Philadelphia: 2014. pp. 1429–1459. [Google Scholar]
- 9.Giannitsis E., Muller-Bardorff M., Kurowski V. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation. 2000;102:211–217. doi: 10.1161/01.cir.102.2.211. [DOI] [PubMed] [Google Scholar]
- 10.Konstantinides S., Geibel A., Olschewski M. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation. 2002;106:1263–1268. doi: 10.1161/01.cir.0000028422.51668.a2. [DOI] [PubMed] [Google Scholar]
- 11.Horwich T.B., Patel J., MacLellan W.R., Fonarow G.C. Cardiac troponin I is associated with impaired haemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation. 2003;108:833–838. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 12.Ooi D.S., Veinot J.P., Wells G.A., House A.A. Increased mortality in hemodialyzed patients with elevated serum troponin T: a one-year outcome study. Clin Biochem. 1999;32:647–652. doi: 10.1016/s0009-9120(99)00064-8. [DOI] [PubMed] [Google Scholar]
- 13.Devereaux P.J., Mrkobrada M., Sessler D.I. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–1503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 14.Devereaux P.J., Sessler D.I., Leslie K. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1504–1513. doi: 10.1056/NEJMoa1401106. [DOI] [PubMed] [Google Scholar]
- 15.Fleisher L.A., Fleischmann K.E., Auerbach A.D. ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol. 2014;130:2215–2245. doi: 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 16.Gupta P., Gupta H., Sundaram A. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387. doi: 10.1161/CIRCULATIONAHA.110.015701. [DOI] [PubMed] [Google Scholar]
- 17.Lee T.H., Marcantonio E.R., Mangione C.M. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 18.Van Waes J.A., van Klei W.A., Wijeysundera D.N., van Wolfswinkel L., Lindsay T.F., Beattie W.S. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35–44. doi: 10.1097/ALN.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 19.Landesberg G., Beattie W.S., Mosseri M., Jaffe A.S., Alpert J.S. Perioperative myocardial infarction. Circulation. 2009;119:2936–2944. doi: 10.1161/CIRCULATIONAHA.108.828228. [DOI] [PubMed] [Google Scholar]
- 20.Landesberg G., Mosseri M., Zahger D. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37:1839–1845. doi: 10.1016/s0735-1097(01)01265-7. [DOI] [PubMed] [Google Scholar]