To the Editor:
Autosomal-recessive mutations in genes required for secretory lysosome-mediated lymphocyte cytotoxicity cause primary hemophagocytic lymphohistiocytosis (HLH), an early-onset, life-threatening hyperinflammatory syndrome.1 Similarly, mutations in RAB27A and LYST are associated with HLH, yet manifest hypopigmentation, because Rab27a and LYST also facilitate trafficking of pigment-containing lysosomes in melanocytes.2 We detail individuals from 5 families from the Baltic area with a novel structural variant at the 5′ untranslated region (UTR) of RAB27A associated with an atypical form of Griscelli syndrome type 2 (GS2) manifesting as late-onset HLH, marked neuroinflammation, skin granulomas, lymphoma, and normal pigmentation (see Table E1 in this article's Online Repository at www.jacionline.org).
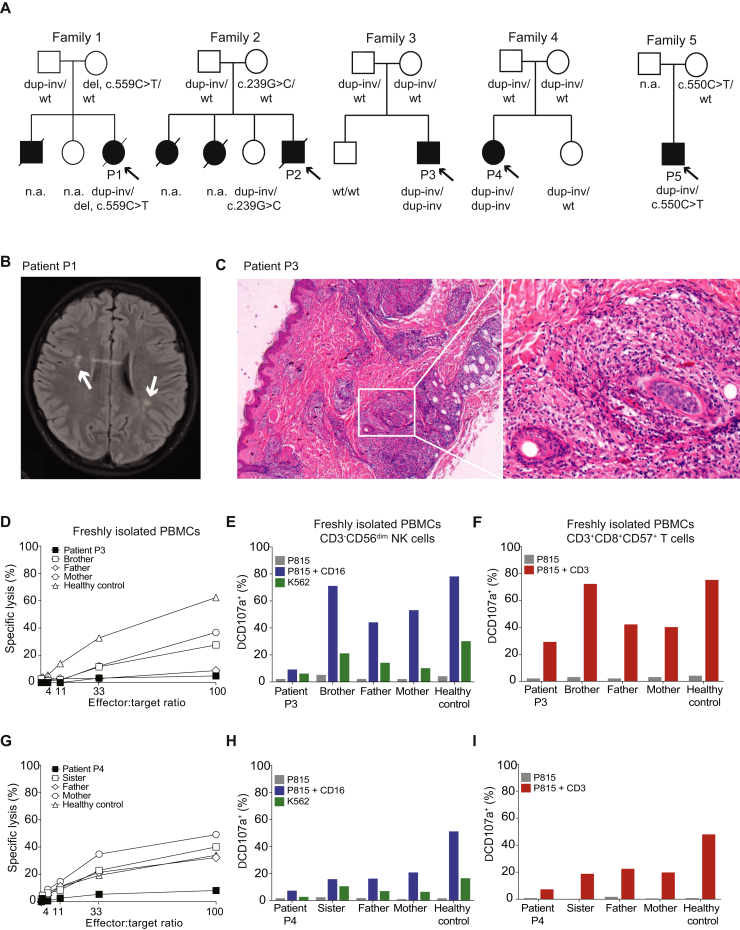
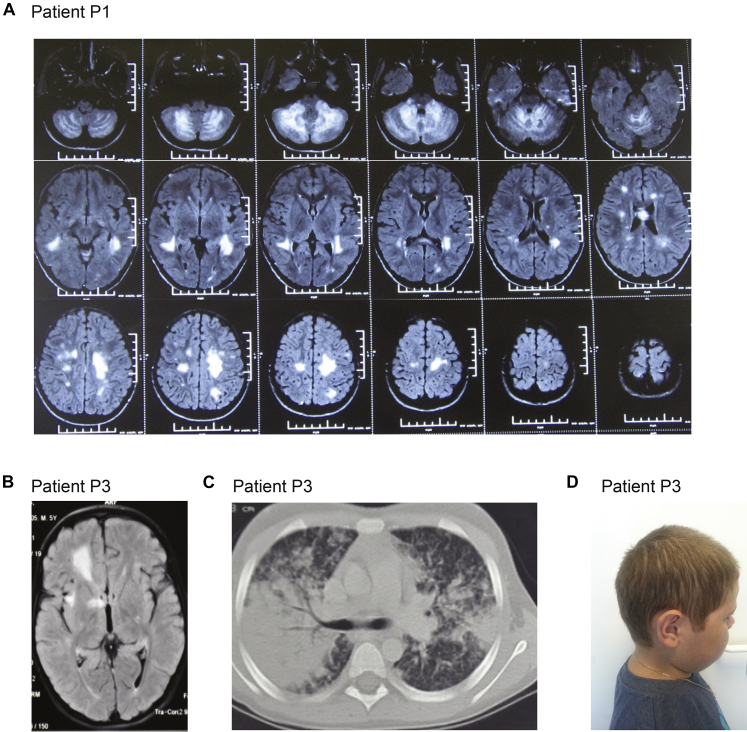
In brief, patient 1 (P1) developed HLH at age 13 years after 8 years of recurrent neuroinflammation (Fig 1, A and B; see Fig E1, A, in this article's Online Repository at www.jacionline.org). Her older brother died in infancy because of severe infectious mononucleosis. Patient 2 (P2) developed fatal HLH at age 15 years. Two of his 3 sisters died at age 13 years, one of an HLH-like syndrome and the other because of brain lymphoma. Patient 3 (P3) developed HLH at age 9 years, after 3 years of recurrent neuroinflammation (Fig E1, B). Patient 4 (P4) suffered from isolated neuroinflammation, without fulfilling HLH criteria. P2, P3, and P4 also had skin granulomas (Fig 1, C). Patient 5 (P5) suffered from recurrent fever flares and EBV viremia, and developed lymphoma at age 13 years. Hypopigmentation was not evident in any of the patients (Fig E1, D). Further clinical information and methods are provided in this article's Online Repository at www.jacionline.org.
Fig 1.
Clinical and laboratory findings. A, Family pedigrees and RAB27A genotype. B, Brain axial magnetic resonance imaging FLAIR images of P1 at diagnosis of HLH showed nonspecific multifocal hyperintense white matter lesions (arrows). C, H&E stain of a skin biopsy from P3 reveals granulomatous infiltrates in the dermis. A nonnecrotizing granuloma (boxed area, left) is presented at a higher magnification (right).D, NK-cell cytotoxic activity of P3 was defective compared with relatives and controls. NK-cell exocytosis (E) and T-cell exocytosis (F) were reduced in P3. NK-cell cytotoxicity (G) and NK (H) and T-cell exocytosis (I) were defective in P4. dup-inv, Duplication/inversion; H&E, hematoxylin and eosin; n.a., not applicable/available; wt, wild type.
Fig E1.
A, Brain MRI of P1 at onset of disease, axial series. B, Brain MRI of P3 at onset of disease. C, Lung computed tomography scan of P3. D, Photo of P3 showing normally pigmented hair.
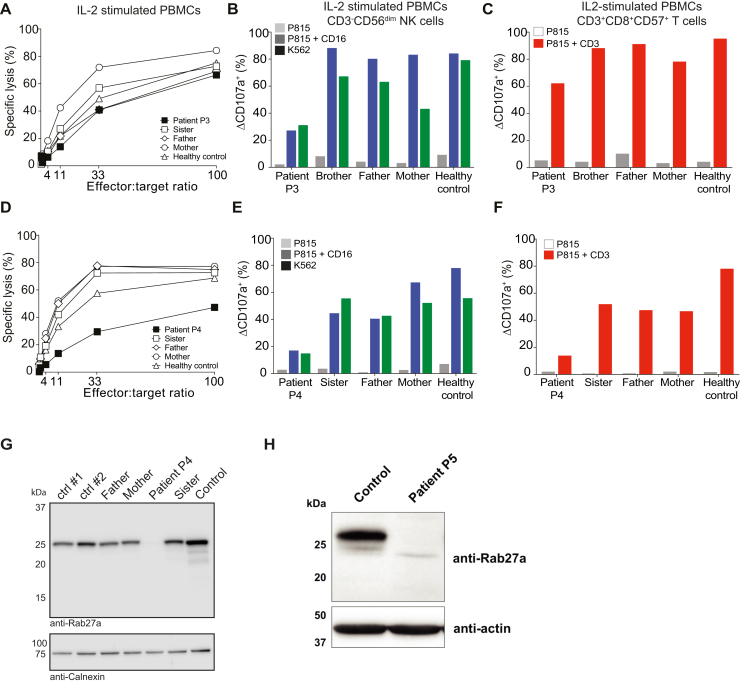
Prompted by a suspicion of HLH, functional investigations of cytotoxic lymphocytes were performed on P1, P3, P4, and P5 (Table E1). Natural killer (NK)-cell cytotoxicity was defective (Fig 1, D and G). Moreover, NK- and T-cell exocytosis was impaired (Fig 1, E and F, H and I), with IL-2 stimulation inducing a partial recovery (see Fig E2, A-F, in this article's Online Repository at www.jacionline.org). Results therefore indicated primary HLH due to defective exocytosis.
Fig E2.
A, NK-cell cytotoxicity assay in IL-2–stimulated PBMCs from P3 and relatives. B, NK-cell degranulation assay in IL-2–stimulated PBMCs from P3 and relatives. C, CD8+CD57+ T-cell degranulation assay in IL-2–stimulated PBMCs from P3 and relatives. D, NK-cell cytotoxicity assay in IL-2–stimulated PBMCs from P4 and relatives. E, NK-cell degranulation assay in IL-2–stimulated PBMCs from P4 and relatives. F, CD8+CD57+ T-cell degranulation assay in IL-2–stimulated PBMCs from P4 and relatives. G, Western blot analysis of Rab27a in P4 and relatives. H, Western blot analysis of Rab27a in P5.
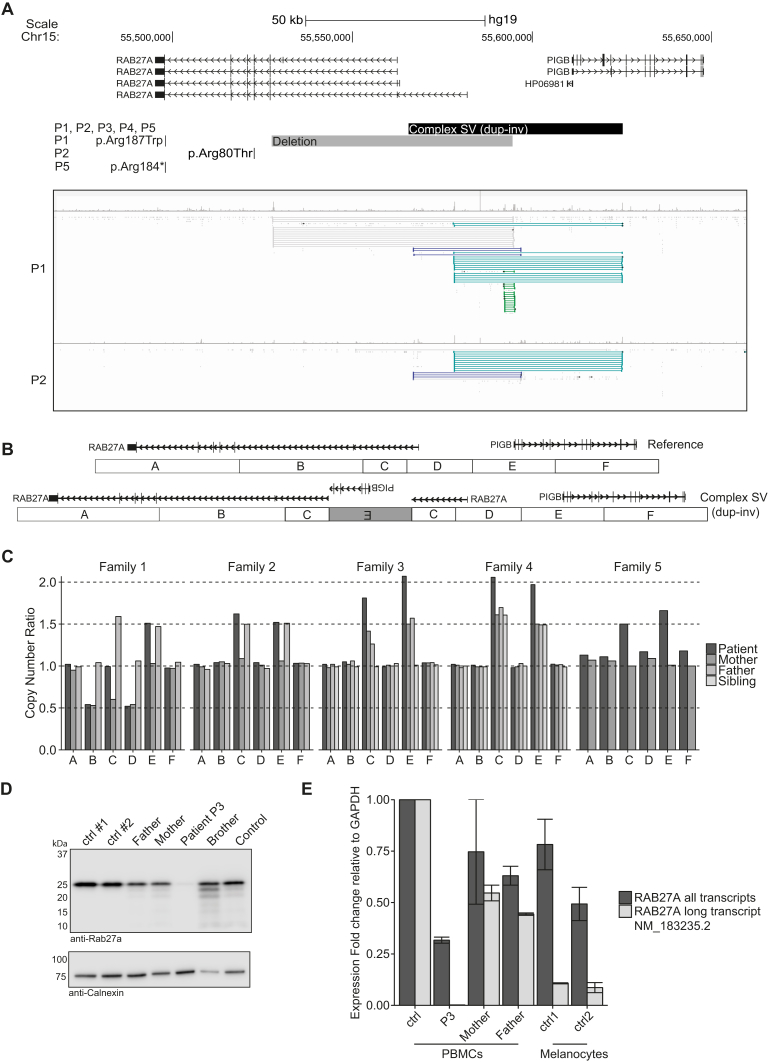
Despite the clinical and immunological findings, a molecular diagnosis of primary HLH was not established by exome sequencing of HLH-associated genes. A heterozygous RAB27A missense variant of unknown significance (p.Arg187Trp) was identified in P1. Moreover, previously reported heterozygous RAB27A mutations were detected in P2 (p.Arg80Thr) and P5 (p.Arg184*) (Fig 2, A).3 To identify possible noncoding mutations, we performed whole-genome sequencing of P1 and P2. Inspection of sequencing data revealed several read pairs aberrant for insert size and orientation at the 5′UTR of RAB27A and over the first 5 exons of the adjacent PIGB, required for glycosylphosphatidylinositol anchor biosynthesis (Fig 2, A). Software-based analysis of structural variants (SVs) confirmed the presence of multiple overlapping SVs in this region, including shared events between P1 and P2 (Fig 2, A).
Fig 2.
Genetic findings. A, Genome browser view over the region chr15:55,467,465-55,656,511 (hg19) including RAB27A and PIGB genes, with screenshots for P1 and P2 showing discordant read pairs. B, Model for the complex SV. C, Segregation analysis by MLPA. D, Rab27a expression in PBMCs evaluated by Western blot in P3, relatives, and healthy controls. E, mRNA expression of RAB27A in PBMCs and melanocytes. The expression of the transcript NM_183235.2 (long) was compared with the total expression of RAB27A. dup-inv, Duplication/inversion; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To confirm the presence of SVs affecting copy number at the 5′UTR of RAB27A, a custom multiplex ligation-dependent probe amplification (MLPA) assay was designed. In P1, a complex SV with a duplication-normal-duplication pattern inherited from the father was confirmed, in addition to a 65-kb deletion inherited from the mother (Fig 2, A and C). P2 and P5, who carried monoallelic coding mutations, were heterozygous for the complex SV (Fig 2, C). Complex SVs are usually associated with cryptic rearrangements.4 Further analysis of split reads and discordant read pairs of the complex SV indicated that one of the duplicated regions was inverted (Fig 2, B and C). In this model, only 1 of several transcriptional start sites (TSSs) of RAB27A, encoding the transcript NM_183235.2, is disrupted by the complex SV, while a copy of PIGB remains intact (Fig 2, B). Validation of one predicted breakpoint supported this model (see Fig E3, A, in this article's Online Repository at www.jacionline.org). Together, identification of biallelic mutations in RAB27A established a diagnosis of atypical GS2 in P1, P2, and P5.
Fig E3.
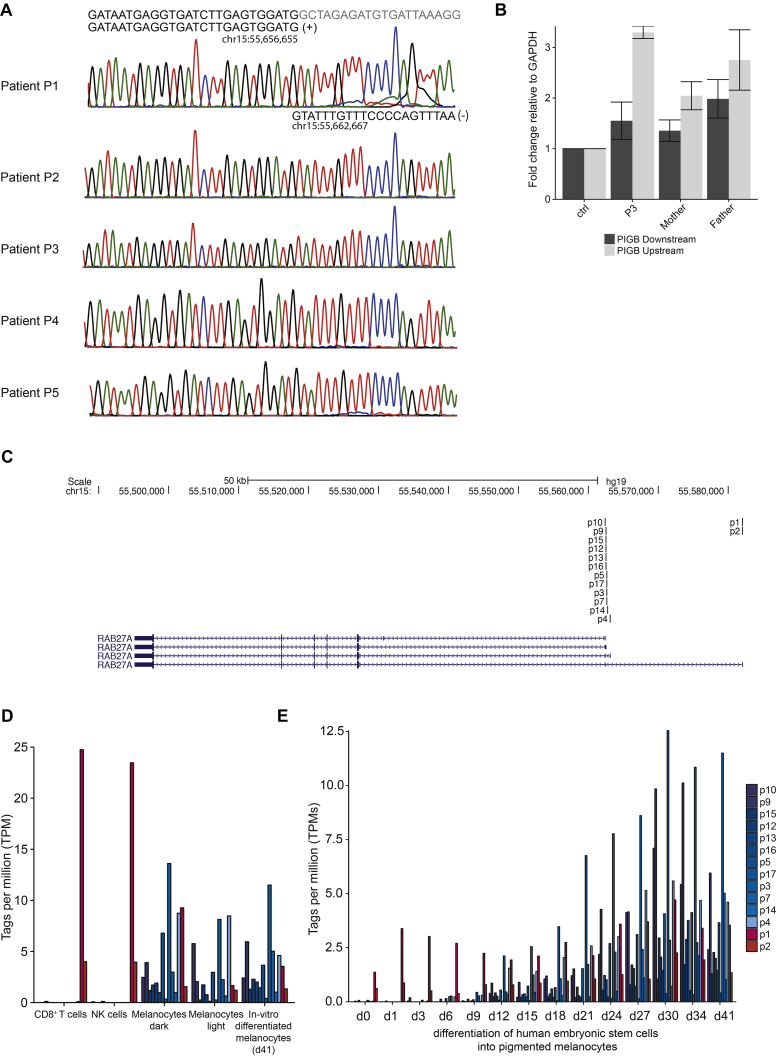
A, Sanger trace for breakpoint C-invE in all patients. B, Quantitative PCR of PIGB to quantify RNA from PBMCs isolated in P3 and relatives. C, UCSC Genome browser screenshot showing localization of the RAB27A TSS according to FANTOM5 CAGE data from human cells. p1 is the TSS for the isoform NM_183235.2. D, Tags per million at the different RAB27A TSS from the FANTOM5 CAGE data. E, Tags per million at the different RAB27A TSS during differentiation of human embryonic stem cells into pigmented melanocytes (d, day). CAGE, Cap analysis gene expression.
The clinical resemblance and geographic proximity prompted analysis of RAB27A by MLPA in P3 and P4, patients who displayed defective lymphocyte exocytosis yet lacked a genetic diagnosis. Indeed, MLPA revealed homozygosity for the complex SV in both patients (Fig 2, C), as confirmed through sequencing of a breakpoint (Fig E3, A). Rab27a protein expression was absent in leukocytes from P3, P4, and P5 (Fig 2, D, and Fig E2, G and H). Thus, all 5 patients carried biallelic RAB27A mutations, and were at least heterozygous for the SV affecting the transcript NM_183235.2.
Eight patients with GS2 and normal pigmentation have been reported to date, all with missense mutations that selectively disrupt binding of Rab27a to Munc13-4 but not to melanophilin, explaining defective lymphocyte yet normal melanocyte function.5, 6 Because the complex SV disrupts only 1 of several TSSs of RAB27A, we hypothesized that lymphocytes and melanocytes might selectively use distinct RAB27A TSSs. Quantitative PCR demonstrated diminished expression of transcript NM_183235.2 in peripheral blood leukocytes from P3 (Fig 2, E), suggesting predominant usage of the TSS for this transcript. This observation is also supported by cap analysis gene expression data from the FANTOM5 project (Fig E3, C-E).7 In contrast, primary as well as embryonic stem cell–derived melanocytes use alternative, downstream TSSs, which were not disrupted by the complex SV (Fig E3, D and E). Quantitative PCR in primary melanocytes confirmed a smaller contribution of transcript NM_183235.2 to total RAB27A expression (Fig 2, E). Transcription of PIGB was maintained by the complex SV (Fig E3, B). Notably, an individual with a homozygous deletion encompassing all RAB27A TSSs displayed classic silvery hair.8 Our data therefore indicate differential TSS usage between leukocytes and melanocytes, explaining the normal pigmentation observed in patients with at least 1 mutation affecting only the upstream RAB27A TSS.
Nonsense mutations in RAB27A are associated with the development of HLH within the first year of life.3 Neurologic involvement affects 55% of patients at diagnosis and 67% during the course of disease.3 Three of the 5 patients reported here displayed severe and recurrent neuroinflammation resembling acute disseminated encephalomyelitis, which preceded onset of full-blown HLH by many years. Functional assays of NK- and T-cell exocytosis should therefore be considered in the diagnostic workup of patients with unexplained neuroinflammatory diseases. Late-onset HLH suggests downstream transcription in activated lymphocytes.
In conclusion, we report 5 patients with atypical GS2 characterized by neuroinflammation, lymphoma, and late-onset HLH. Remarkably, 3 patients manifested skin granulomas.9 Our results elucidate novel structural aberrations affecting the noncoding region of RAB27A, linking the lack of hypopigmentation to differential RAB27A TSS usage between lymphocytes and melanocytes. The identification of a recurrent complex SV in RAB27A suggests a founder effect in the Baltic population. Assessment of this aberration should be included in the genetic workup of patients with defective exocytosis from this area.
Acknowledgments
We thank all family members for their participation. We also thank Stanley Sing Hoi Cheuk for providing melanocytes, the Department of Clinical Genetics of the Karolinska University Hospital for help with MLPA, and the Clinical Genomics unit at SciLifeLab for whole-genome sequencing.
Footnotes
This work was supported by a grant from the European Research Council (ERC) under the European Union's Seventh Framework Programme (grant no. FP/2007-2013; ERC grant agreement no. 311335), the Norwegian Research Council, the Swedish Foundation for Strategic Research, the Wallenberg Foundation, the Karolinska Institutet Center for Innovative Medicine (Y.T.B.), the Swedish Childhood Cancer Foundation (J.-I.H., Y.T.B., M.M.), as well as the Stockholm County Council, the Swedish Cancer Foundation, and the Swedish Research Council (J.-I.H., Y.T.B.), the Cancer and Allergy Foundation of Sweden (J.-I.H.), the German Ministry of Education and Research (grant no. BMBF 01EO1303), and the German Research Foundation (grant no. SFB1160, TP1; S.E.). B.T. is also supported by a doctoral student scholarship from the Board of Postgraduate Studies at Karolinska Institutet. Computations were performed on resources provided by the Swedish National Infrastructure for Computing (SNIC) through the Uppsala Multidisciplinary Center for Advanced Computational Science under Project SNIC b2012204 and b2015280.
Disclosure of potential conflict of interest: S. Ehl reports a grant from Deutsche Forschungsgemeinschaft (grant no. SFB1160 TP4) and a grant from BMBF (grant no. BMBF 01EO1303) during the conduct of the study and grants from Union Chimique Belge outside the submitted work. J.-I. Henter reports grants from the Swedish Childhood Cancer Foundation, the Swedish Cancer Foundation, the Swedish Research Council, the Stockholm County Council, and the Cancer and Allergy Foundation of Sweden during the conduct of the study. M. Meeths reports grants from the Swedish Childhood Cancer Foundation during the conduct of the study. The rest of the authors declare that they have no relevant conflicts of interest.
Contributor Information
Bianca Tesi, Email: Bianca.Tesi@ki.se.
Jelena Rascon, Email: jelena.rascon@vuvl.lt.
Methods
Genome-wide sequencing
Blood samples from the patients and, when available, their parents and siblings were obtained with informed consent according to the Declaration of Helsinki. The study was approved by the Regional Ethics Review Board in Stockholm, Sweden. DNA was isolated according to standard procedure. DNA from P1 and P2 was subjected to whole-genome sequencing with a TruSeq DNA PCR-free protocol followed by sequencing on an Illumina HiSeq X machine with an average coverage of 30×. Sequencing reads were analyzed with the pipeline SpeedSeq. In brief, reads were mapped to the human genome build GRCh37 with burrows-wheeler aligner, while calling of single nucleotide variants and structural variants was performed, respectively, with FreeBayes and Lumpy.E1 The genomic region spanning RAB27A and PIGB was visualized with the Integrative Genomics Viewer.E2 Integrative Genomics Viewer was also used to visualize discordant and split reads at the breakpoints. Split reads were mapped to the reference genome using the BLAT function of the University of California, Santa Cruz (UCSC) genome browser.
Identification of structural variants breakpoints
To confirm the presence of the SV, primers were designed to PCR amplify the breakpoints of the mutant allele based on the information from discordant and split reads. PCR products were sequenced on an ABI3730 Genetic Analyzer. The breakpoint C-invE (Fig 2, B) was captured with the following primers: the forward 5′-CAACAGTTATGCGGCTCTCA-3′ and the reverse 5′-TGCGTGCAGACTGGATAAAG-3′.
Multiplex ligation-dependent probe amplification
To validate the presence of copy number alterations affecting RAB27A and PIGB, we used a custom-designed MLPA assay. MLPA probes were designed to cover the regions involved by the structural variants together with control probes to be used for normalization of the data. Probe sequences are listed in Table E2. The MLPA procedure was carried out according to the manufacturer's instructions (MRC-Holland, Amsterdam, The Netherlands). PCR products were separated on an ABI3500 XL Genetic Analyzer and analyzed with Peak Scanner 2.0 software.
Immunological investigations
PBMCs from the families of P3 and P4, and healthy unrelated controls, were isolated within 24 hours of venipuncture and analyzed by flow cytometry for NK-cell and cytotoxic T-cell exocytosis at resting state and after 36 hours of 500U IL-2 stimulation (R&D Systems, Minneapolis, Minn) as previously described.E3 NK-cell–mediated cytotoxicity was assessed via standard 51Cr 4-hour assay, using labeled K562 cells as target, as previously described.E4 FlowJo (v9.9, Treestar, Ashland, Ore) was used for flow cytometry and Prism (v7, Graphpad, La Jolla, Calif) for statistical analyses and plotting.
Expression studies
Quantitative PCR was used to quantify RAB27A and PIGB transcript in PBMC-derived RNA from P3 and his parents, compared with unrelated healthy controls. Similarly, quantitative PCR was used to quantify transcripts in RNA isolated from melanocytes of healthy donors (n = 2). For RAB27A, one primer pair was designed to capture all isoforms, and another to capture only the isoform NM_183235.2, disrupted by the SV. For PIGB, primer pairs were designed to target the mRNA before (upstream) and after (downstream) the breakpoint.
Publicly available FANTOM5 data were downloaded from http://fantom.gsc.riken.jp/5/data/ (on June 26, 2017). The data set consists of cap analysis gene expression peaks for human samples in the form of relative log expression normalized data.E5 Data for biological replicates (n = 3) were averaged. Further analysis and plotting was done in R version 3.3.1.
Western blot of Rab27a
Cryopreserved PBMCs of patients (P3 and P4) and family members as well as local and transport controls were used to analyze Rab27a protein expression levels. To this end, freshly thawed cells were counted, sedimented, and resuspended in ice-cold lysis buffer (150 mM sodium chloride, 50 mM Tris, 2 mM EDTA, pH 7.6) supplemented with 1% (v/v) Triton X-100, 1% (v/v) Igepal CA-630 (Sigma-Aldrich, St Louis, Mo), and protease inhibitors (ThermoFisher, Waltham, Mass). Postnuclear lysates were separated by SDS-PAGE under reducing conditions (NuPage, ThermoFisher) and transferred to nitrocellulose membranes using the iBlot transfer system (ThermoFisher). Rabbit pAbs against Rab27a and Calnexin were purchased from ProteinTech Group and Enzo Life Science, respectively. For western blot performed on PBMCs from patient 5, the following antibodies were used: anti-RAB27A rabbit polyclonal antibodies #5415 and #5416, diluted 1:5000 (kind gift from Gillian Griffiths) and anti-beta-Actin mouse mAb, diluted 1:10,000 (Clone AC-15, Sigma Aldrich #A1978). Signals were visualized using ECL chemistry (ThermoFisher) and acquired using the Li-Cor Odyssey Fc system or the Fusion Imaging System (Vilber, Collégien, France).
Brief case reports
Patient 1
P1, a girl of Lithuanian origins, presented at the age of 6 years with acute multiple vomiting episodes, headache, weakness, ataxia, and confusion. Cerebrospinal fluid analysis showed minimal pleocytosis and normal protein level. Brain magnetic resonance imaging (MRI) revealed multiple T2 hyperintensive lesions in cerebellum, periventricular regions, corpus callosum, and brain stem, prompting a diagnosis of acute disseminated encephalomyelitis (Fig E1, A). She subsequently experienced 8 relapses within the next 2 years managed with intravenous and oral steroids. During remissions she suffered from mild ataxia and tremor. At the age of 8 years, she was diagnosed with remitting-relapsing multiple sclerosis and started to receive preventive treatment with monthly intravenous immunoglobulin infusions for 18 months and achieved long-lasting remission. During follow-up, she underwent MRI investigations that demonstrated similar patterns, and in addition development of cerebellar atrophy. At age 13 years, following herpes simplex labialis infection, the patient presented with fever, jaundice, hepato- and splenomegaly, pancytopenia, hyperferritinemia, hypofibrinogenemia, and hypoalbuminemia, followed by ascites, pleural effusion, and encephalopathy. Liver enzymes were markedly elevated. Bone marrow biopsy showed no hemophagocytosis. Analysis of cytotoxic lymphocyte function showed defective cytotoxicity and exocytosis (Table E1). Neuroimaging showed nonspecific multifocal hyperintense white matter lesions in both hemispheres in FLAIR MRI (Fig 1, B). Nineteen days later the patient fulfilled the HLH diagnostic criteria (Table E1). Treatment according to HLH-2004 protocol was initiated but the patient's condition deteriorated and she died 48 days later because of massive central nervous system (CNS) lesions provoked by CNS infection (EBV reactivation and Aspergillus fumigatus). Her older brother died at the age of 1 year because of infectious mononucleosis. The patient displayed no signs of oculocutaneous albinism.
Patient 2
P2, a boy of Swedish origin, was diagnosed with benign granuloma of the skin at the age of 2 years. At the age of 2.5 years he suffered from rapidly progressive sensorineuronal hearing loss. At age 3 years, he developed fever, breathing difficulties with interstitial lung infiltrates, hepatosplenomegaly, increased liver enzymes, deteriorating motoric abilities, and low immunoglobulins. A primary EBV infection was detected. Recovery was obtained with antibiotic and antimycotic treatment, and immunoglobulin replacement. He suffered from recurrent episodes regarded as fungal pneumonias, although a fungal infection was never verified. At the age of 14.5 years, he deteriorated and was admitted to the hospital because of fever and coughing. An X-ray revealed interstitial lung disease. He also displayed splenomegaly, pericardial fluid, and elevated liver enzymes. One and a half week later he was again hospitalized with fever and low white blood cells. Spleen biopsy showed infiltration of histiocytic cells and Aspergillus fumigatus. A bone marrow examination did not show signs of malignancy and no or very little hemophagocytosis. He received antibiotics, antimycotics, and corticosteroids. At this point, fulfilling diagnostic criteria (Table E1), a diagnosis of HLH was established, and a secondary form of HLH was suspected. His condition deteriorated, with respiratory difficulties and increased inflammation. He received cyclosporin, antithymocyte globulin, and corticosteroids and later also etoposide. However, he continued to deteriorate and later died in the intensive care unit because of cardiac arrest. Genetic sequencing of PRF1 was performed at the time without findings. One of his 3 sisters died at age 13 years because of an intracerebral tumor, later diagnosed as T-cell lymphoma (immunoblastic lymphoma). Another sister died at age 13 years in a clinical picture of interstitial pneumonia, aplastic anemia, aspergillus, liver disease, and progressive paresis. She also suffered from hearing loss. The third sister is healthy. The patient displayed no signs of oculocutaneous albinism.
Patient 3
P3 is a second child of healthy nonconsanguineous parents of Lithuanian origin. At the age of 5 years, the boy was admitted because of acute ataxia, strabismus, diplopia, sleepiness, and papilledema. Three weeks earlier the patient had an episode of acute respiratory infection. There were multiple focal demyelinating lesions on brain MRI (Fig E1, B). He was diagnosed as having acute demyelinating encephalomyelitis, and intravenous methylprednisolone pulse markedly improved symptoms. Eight months later he was admitted to the intensive care unit because of acute respiratory failure, fever, and reappearance of previously observed neurological symptoms. He also had a maculopapular rash. Skin biopsy showed granulomatous dermatitis, CD3/CD4- and CD3/CD8-positive cells, and CD68-positive histiocytes (Fig 1, C). Lung computed tomography showed focal confluent infiltration and enlarged mediastinal lymph nodes (1-1.3 cm; Fig E1, C). A lung biopsy showed focal lymphocyte and macrophage infiltration (predominance of CD3 T lymphocytes, 20% CD20-positive cells, CD68 macrophages). Slightly decreased immunoglobulin G subtypes were found, but routine test results for cellular immunity were normal. The patient became steroid-dependent for the subsequent 3 years, after which, at the age of 9 years, following tonsillitis, he developed persistent fever with elevated liver enzymes, hepatomegaly, neutropenia, and anemia. Analysis of cytotoxic lymphocyte function showed defective cytotoxicity and exocytosis (Fig 1, D-F). A diagnosis of HLH was established (Table E1) and treatment according to HLH-2004 protocol was initiated. At the age of 10 years, P3 received a hematopoietic stem cell transplantation (HSCT) from his HLA-identical brother. A reduced intensity conditioning based on fludarabine (180 mg/m2) and melphalan (140 mg/m2) was used. For graft-versus-host disease (GvHD) prophylaxis, cyclosporine A and mycophenolate mofetil were used. The patient developed an acute GvHD grade II (skin +++, GI +), which later developed into a chronic form with skin involvement that has resolved. The patient displayed no signs of oculocutaneous albinism (Fig E1, D).
Patient 4
P4 is the second child of healthy nonconsanguineous parents from Lithuania. P4 developed skin granulomas at the age of 16 months. At the age of 1 year 9 months, she developed ataxia, impaired walking, and tremor after varicella infection. The symptoms improved following a course of prednisone, but subsequent similar attacks followed. In 2 exacerbation episodes EBV reactivation was detected. MRI showed numerous demyelinating foci in the cerebellum, and a massive confluent conglomerate in the splenium involving both hemispheres and the spinal cord. At the age of 7 years 8 months, the biopsy of CNS lesions was performed showing nonmalignant reactive lymphoid infiltrates (CD3+, CD4+, CD8+, GranB+, CD30−), nonspecific granulomatous microfoci. No signs characteristic of HLH were detected (Table E1). Analysis of cytotoxic lymphocyte function showed defective cytotoxicity and exocytosis (Fig 1, G-I). At the age of 8 years, P4 received an HSCT from a matched unrelated donor. A reduced-intensity conditioning based on fludarabine (150 mg/m2) and treosulphan (42 g/m2) was used. For GvHD prophylaxis, alemtuzumab (0.6 mg/kg), cyclosporine A, and mycophenolate mofetil were used. At the time of report, the patient is 2 months after HSCT. The patient displayed no signs of oculocutaneous albinism.
Patient 5
P5 is a 14-year-old boy from Russia. He had uncomplicated infectious mononucleosis at age 2 years without signs of HLH and good clinical recovery. At the age of 10 years, he developed recurrent fever flares lasting up to 3 weeks without obvious trigger. The overall condition was not significantly impaired. There were no significant cytopenias and the patient never met diagnostic criteria for HLH. At the age of 11.3 years, the patient rapidly developed splenomegaly with pancytopenia. Splenectomy corrected the cytopenia and diminished the frequency and intensity of fever flares. Enlargement of abdominal lymph nodes was noted and a biopsy showed only follicular hyperplasia. At the age of 12.8 years, he had sudden onset of cervical lymphoadenopathy and was diagnosed with Hodgkin lymphoma, stage IVA. He responded well to treatment according to the GPOH-HD 2002 protocol and is currently in full remission. The combination of recurrent fever flares, splenomegaly, and lymphoma prompted further immunological investigations. NK-cell and cytotoxic T lymphocytes cytotoxicity and exocytosis were defective (Table E1). The patient displayed no signs of oculocutaneous albinism.
Table E1.
Clinical and laboratory findings at diagnosis of HLH
| Family | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Patient | P1 | P2 | P3 | P4 | P5 |
| Ethnical origin | Lithuania | Sweden | Lithuania | Lithuania | Russia |
| Familial disease | Yes | Yes | No | No | No |
| Parental consanguinity | No | No | No | No | No |
| Sex | Female | Male | Male | Female | Male |
| RAB27A allele 1 | Deletion, c.559C>T (p.Arg187Trp) | Dup-Inv | Dup-Inv | Dup-Inv | Dup-Inv |
| RAB27A allele 2 | Dup-Inv | c.239G>C (p.Arg80Thr) | Dup-Inv | Dup-Inv | c.550C>T (p.Arg184*) |
| Age at diagnosis of HLH (y) | 14 | 14.5 | 9 | No HLH | No HLH |
| Fever | Yes | Yes | Yes | No | Yes (intermittent) |
| Splenomegaly | Yes | Yes | No | No | Yes, chronic (splenectomized) |
| Hepatomegaly | Yes | Yes | Yes | No | Transient, self-limiting |
| Hemoglobin (g/L) | 83 | 87 | 90 | Within normal | ND |
| Neutrophils (109/L) | 0.45 | 0.80 | 0.14 | Within normal | ND |
| Platelets (109/L) | 35 | 54 | 194 | Within normal | ND |
| Triglycerides (mmol/L) | 2.49 | 5.8 | 2.71 | Within normal | ND |
| Fibrinogen (g/L) | 0.6 | 0.4 | 0.6 | 1.51 | ND |
| Hemophagocytosis | No | No | Yes | ND | ND |
| Ferritin (μg/L) | 2451 | 49000 | 12000 | Within normal | ND |
| Soluble CD25 (pg/mL) | 20512.4 | ND | 16437 | 1760 | ND |
| NK-cell activity | ND | ND | Defective | Defective | Defective |
| NK-cell degranulation | Defective, 3.1% ΔCD107a | ND | Defective, 6% ΔCD107a | Defective, 3.3% ΔCD107a | Defective, 0.6% ΔCD107a |
| Neurological manifestations | Yes, before HLH onset | Yes | Yes, before HLH onset | Yes | No |
| Pathological CSF | Yes | ND | Yes | Yes | ND |
| Treatment active disease | HLH-2004 | Cortico, CSA, ATG | HLH-2004 | Cortico, MMF | |
| Remission at 2 mo | No | No | Yes | NA | |
| Age at HSCT | Not done | 10 | 7 | ||
| Outcome and follow-up | Deceased 48 d after HLH onset | Alive 19 mo after HSCT | Alive at 7 y | Alive at 16 y | |
| Other manifestations | No | Skin granuloma, lung infiltrates | Skin granuloma, lung infiltrates | Skin granuloma, isolated CNS involvement | Hodgkin lymphoma at 13 y, recurrent fever episodes from 10 y |
ATG, Antithymocyte globulin; Cortico, corticosteroids; CSA, cyclosporine A; CSF, cerebrospinal fluid; Dup-Inv, Duplication-Inversion; MMF, mycophenolate mofetil; NA, not applicable/available; ND, no data.
Table E2.
MLPA probes
| Gene | Size (bp) | Used for Figure 2, C |
|---|---|---|
| GABRA4_fam-pilot | 84 | Control probe |
| RAB27A_probe5 | 87 | Probe C in Figure 2, C |
| RAB27A_probe6 | 90 | |
| RAB27A_probe7 | 93 | |
| RAB27A_probe8 | 96 | Probe D in Figure 2, C |
| RAB27A_probe4 | 99 | Probe B in Figure 2, C |
| RAB27A_probe3 | 102 | |
| Stern PCLN13q | 105 | Control probe |
| RAB27A_probe10 | 108 | Probe E in Figure 2, C |
| RAB27A_probe9 | 111 | |
| RAB27A_probe1 | 114 | Probe A in Figure 2, C |
| PIGB_probe11 | 117 | Probe F in Figure 2, C |
| RB1ex23 | 129 | Control probe |
| MRPL41_e1_132 | 132 | Control probe |
| SRY | 135 | Control probe |
| GABRA4_fam-pilot_A | gggttccctaagggttggaCAGCCTGTTGTCATAACCATCG | |
| GABRA4_fam-pilot_B | /5Phos/AGCAAACTGTCCAGGATGCGtctagattggatcttgctggcac | |
| Stern PCLN13q_A | gggttccctaagggttggaGACACAAGGGTGTAAAATGCACG | |
| Stern PCLN13q_B | /5Phos/TTTCAGGGTGTGTTTGCATATGATTTAATCAATCAGTATGtctagattggatcttgctggcac | |
| RB1ex23_A | gggttccctaagggttggaGTCACCAATACCTCACATTCCTCGAAGCCCTTACAAGTTTCCT | |
| RB1ex23_B | /5Phos/AGTTCACCCTTACGGATTCCTGGAGGGAACATCTATATTTCACCtctagattggatcttgctggcac | |
| MRPL41_e1_132_A | gggttccctaagggttggaGACCCTGACAACCTGGAAAAGTACGGCTTCGAGCCCACACAGGAG | |
| MRPL41_e1_132_B | /5Phos/GGAAAGCTCTTCCAGCTCTACCCCAGGAACTTCCTGCGCTAGCTGtctagattggatcttgctggcac | |
| SRY_A | gggttccctaagggttggaCAGTGCAAAGGAAGGAAGAGCTTCTCCGGAGAGCGGGAATATTCT | |
| SRY_B | /5Phos/CTTGCACAGCTGGACTGTAATCATCGCTGTTGAATACGCTTAACATAGtctagattggatcttgctggcac | |
| RAB27A_p5_A | gggttccctaagggttggaGGACTTCAGGAAGCTGCAATGTTT | |
| RAB27A_p5_B | /5Phos/GCTTTTGTGAATTTCCTTCCCtctagattggatcttgctggcac | |
| RAB27A_p6_A | gggttccctaagggttggaCACAGAACCTCAGAGAAGCCTTGA | |
| RAB27A_p6_B | /5Phos/GGGGCAACTGGTCAACCAATTAGGtctagattggatcttgctggcac | |
| RAB27A_p7_A | gggttccctaagggttggaCAGACCTCGTGGTTCCCACTCAGA | |
| RAB27A_p7_B | /5Phos/GGCAGCCAAGTTCATCCCTCTCAGTGGtctagattggatcttgctggcac | |
| RAB27A_p8_A | gggttccctaagggttggaGCCTTCTAAGCCGTCTCGCTGACTTGT | |
| RAB27A_p8_B | /5Phos/GTCTACCTCCACCGCAAATTCCAGCTGtctagattggatcttgctggcac | |
| RAB27A_p4_A | gggttccctaagggttggaGATCCAACTGCTCCCTTCAAGAAGTT | |
| RAB27A_p4_B | /5Phos/GGTAATTAGGGTGAGGTGGAATGATGTACTCtctagattggatcttgctggcac | |
| RAB27A_p3_A | gggttccctaagggttggaCAACTGGCCAGCTGTCACTCAAATGCTAATT | |
| RAB27A_p3_B | /5Phos/GTGTCTATCATCTGCTTTCTCTAATAGCCtctagattggatcttgctggcac | |
| RAB27A_p10_A | gggttccctaagggttggaGTGGCAAGATGGGTGGTAAGTCCTAAATACTTTA | |
| RAB27A_p10_B | /5Phos/GAAGCTGTATGCCAGTTATTTCGTTCCTATGGtctagattggatcttgctggcac | |
| RAB27A_p9_A | gggttccctaagggttggaGCTTTAAGAATGGTGTGGAGGGACCAGAGGTCACTA | |
| RAB27A_p9_B | /5Phos/CTGTGCCTTACAAGGAGCCAACCAGAGCAGCAGtctagattggatcttgctggcac | |
| RAB27A_p1_A | gggttccctaagggttggaGAGGCATGACCATTTGATCGCACCACTCCTTCAGGAAT | |
| RAB27A_p1_B | /5Phos/CCAGGACTTGTCCACACACCGTTCCATTCGCTTCtctagattggatcttgctggcac | |
| PIGB_p_11_A | gggttccctaagggttggaGAGGACCATTTATGTTTCCGGAACAGAATACCAATGCTA | |
| PIGB_p_11_B | /5Phos/CAGAATGTTGAGTCCCCCTACTGACCTACTTCCCTCtctagattggatcttgctggcac |
References
- 1.Janka G.E., Lehmberg K. Hemophagocytic syndromes—an update. Blood Rev. 2014;28:135–142. doi: 10.1016/j.blre.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 2.de Saint Basile G., Ménasché G., Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol. 2010;10:568–579. doi: 10.1038/nri2803. [DOI] [PubMed] [Google Scholar]
- 3.Meeths M., Bryceson Y.T., Rudd E., Zheng C., Wood S.M., Ramme K. Clinical presentation of Griscelli syndrome type 2 and spectrum of RAB27A mutations. Pediatr Blood Cancer. 2010;54:563–572. doi: 10.1002/pbc.22357. [DOI] [PubMed] [Google Scholar]
- 4.Brand H., Collins R.L., Hanscom C., Rosenfeld J.A., Pillalamarri V., Stone M.R. Paired-duplication signatures mark cryptic inversions and other complex structural variation. Am J Hum Genet. 2015;97:170–176. doi: 10.1016/j.ajhg.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Netter P., Chan S.K., Banerjee P.P., Monaco-Shawver L., Noroski L.M., Hanson I.C. A novel Rab27a mutation binds melanophilin, but not Munc13-4, causing immunodeficiency without albinism. J Allergy Clin Immunol. 2016;138:599–601.e3. doi: 10.1016/j.jaci.2015.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cetica V., Hackmann Y., Grieve S., Sieni E., Ciambotti B., Coniglio M.L. Patients with Griscelli syndrome and normal pigmentation identify RAB27A mutations that selectively disrupt MUNC13-4 binding. J Allergy Clin Immunol. 2015;135:1310–1318.e1. doi: 10.1016/j.jaci.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The FANTOM Consortium and the RIKEN PMI and CLST (dgt) A promoter-level mammalian expression atlas. Nature. 2014;507:462–470. doi: 10.1038/nature13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent L.M., Gilbert F., DiPace J.I., Ciccone C., Markello T.C., Jeong A. Novel 47.5-kb deletion in RAB27A results in severe Griscelli syndrome type 2. Mol Genet Metab. 2010;101:62–65. doi: 10.1016/j.ymgme.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerah M.L., DeWitt C.A. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234–243. doi: 10.1159/000368552. [DOI] [PubMed] [Google Scholar]
References
- Chiang C., Layer R.M., Faust G.G., Lindberg M.R., Rose D.B., Garrison E.P. SpeedSeq: ultra-fast personal genome analysis and interpretation. Nat Methods. 2015;12:966–968. doi: 10.1038/nmeth.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdóttir H., Robinson J.T., Mesirov J.P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S.C.C., Theorell J., Entesarian M., Meeths M., Mastafa M., Al-Herz W. Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production. Blood. 2013;121:1345–1356. doi: 10.1182/blood-2012-07-442558. [DOI] [PubMed] [Google Scholar]
- Meeths M., Chiang S.C.C., Wood S.M., Entesarian M., Schlums H., Bang B. Familial hemophagocytic lymphohistiocytosis type 3 (FHL3) caused by deep intronic mutation and inversion in UNC13D. Blood. 2011;118:5783–5793. doi: 10.1182/blood-2011-07-369090. [DOI] [PubMed] [Google Scholar]
- The FANTOM Consortium and the RIKEN PMI and Clst (dgt) A promoter-level mammalian expression atlas. Nature. 2014;507:462–470. doi: 10.1038/nature13182. [DOI] [PMC free article] [PubMed] [Google Scholar]