Abstract
Background
To examine the value of speckle tracking echocardiography to detect the presence, extent and severity of coronary artery affection in patients with suspected stable angina pectoris.
Methods
Two hundred candidates with suspected stable angina pectoris and normal resting conventional echocardiography were subjected to speckle tracking echocardiography and coronary angiography. Global and segmental longitudinal peak systolic strain were assessed and were correlated to the results of coronary angiography for each patient.
Results
There was a statistically significant difference in the mean of global longitudinal peak systolic strain between normal coronaries and different degrees of coronary artery disease (CAD) (−20.11 ± 0.8 for normal, −18.34 ± 2.52 for single vessel, −16.14 ± 2.85 for two vessels, −14.81 ± 2.12 for three vessels, −13.01 ± 2.92 for left main disease). GLPSS showed high sensitivity for the diagnosis of single vessel CAD (90%, specificity 95.1%, cutoff value: −18.44, AUC: 0.954); two vessels disease (90%, sensitivity 88.9%, cutoff value −17.35, AUC: 0.906) and for three vessels CAD (cutoff value −15.33, sensitivity 63% and specificity 72.2% AUC 0.681) segmental LPSS also showed statistical significance for localization of the affected vessel for left anterior descending, left circumflex and right coronary artery (ρ = 0.001) and inverse correlation with syntax score that was significant with high and intermediate score (ρ = 0.001) and insignificant for low syntax score (ρ value 0.05).
Conclusion
Two-dimensional speckle tracking echocardiography has good sensitivity and specificity to predict the presence, extent and severity of CAD.
Keywords: Speckle tracking echocardiography, Stable angina pectoris, Coronary angiography
1. Introduction
Noninvasive identification of patients with coronary artery disease (CAD) remains a clinical challenge despite the widespread use of imaging and provocative testing; more than 50% of patients currently referred to coronary angiography show normal or non-obstructive CAD.1
In stable CAD, coronary computed tomography angiography (CTA) is a non-invasive alternative to assess coronary anatomy, but according to expert consensus only selected patients should be considered for CTA.2
Exercise testing is widely used for selecting patients for coronary angiography, but has its clear limitations as emphasized in the European guidelines for stable CAD.3
Measurements of longitudinal motion and deformation are most sensitive markers of CAD, especially in patients with coronary stenosis, where intermittent ischemia may result in subtle forms of stunning that may be detectable with strain measurements.4
Thus we are in need of a simple, non-invasive method to improve the selection of patients who are referred for coronary angiography.
The aim of the study was to evaluate the value of global longitudinal peak systolic strain (GLPSS) performed at rest to predict the presence, extent and severity of CAD in patients with suspected stable angina pectoris.
2. Methods
2.1. Study design
Single center, prospective study enrolled 200 consecutive patients with suspected stable angina pectoris from January 2014 to April 2016. All patients signed an informed consent and the study was approved by the local ethics committee.
Inclusion criteria included: adult patients (age ≥18 years) who presented to outpatient clinic by clinically suspected stable CAD a condition which encompasses several groups of patients: (i) those having symptoms felt to be related to CAD such as dyspnea; (ii) those previously symptomatic with known obstructive or non-obstructive CAD, who have become asymptomatic with treatment and need regular follow-up; (iii) those who report symptoms for the first time and are judged to already be in a chronic stable condition (for instance because history-taking reveals that similar symptoms were already present for several months). All patients were without regional wall motion abnormality on two-dimensional (2D) echocardiography and with preserved systolic function.5
Exclusion criteria included: patients with left ventricular ejection fraction <50% by 2D echocardiography, prior history of percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG), patients presented with acute coronary syndrome (ACS) confirmed by positive cardiac enzymes (serum troponin), congestive heart failure, more than trivial valvular heart disease, intra-ventricular conduction disturbances, pathological Q-waves in the resting electrocardiography (ECG), atrial fibrillation, failure to assess all segments by speckle tracking or patients who refused coronary angiography.
2.2. Baseline evaluation
-
•
On admission all patients had a review of their medical history, included demographic data (age, gender, body mass index [BMI]), presence of risk factors for coronary atherosclerosis (smoking, diabetes, hypertension, dyslipidemia, family history of premature CAD) and associated co-morbidities in addition to general and cardiac examination.
-
•
12 leads surface electrocardiography to exclude any arrhythmia or the presence of Q waves or ST-T wave changes at rest.
-
•
Laboratory investigations beside routine evaluation in the form of complete blood count (CBC), liver function and kidney function, cardiac biomarkers (troponin I and CK-MB) to exclude acute event.
-
•
A complete conventional echocardiographic examination was performed for all patients using Vivid 7 Vingmed-General Electric, Horton, NORWAY apparatus to assess left ventricular wall thickness, internal dimensions, wall motion abnormality, systolic and diastolic function by 2D, M-Mode and Doppler echocardiography.
-
•
Speckle tracking was evaluated by recording three consecutive end-expiratory cardiac cycles using high frame rate (80–100 frames/s) and harmonic imaging was acquired in the apical four-, two-chamber views as well as long axis views for quantification of peak systolic strain by automated function imaging speckle tracking analysis. GLPSS for the complete LV was provided by the software using a 17-segment model in a ‘bull's eye’ plot calculated as the average of a longitudinal peak systolic strain of each view and the mean of the three views the normal value of longitudinal peak systolic strain is −20%.6
-
•
Coronary angiography was performed in less than 1 month from performing echo study by the percutaneous femoral approach. Angiograms were obtained for each coronary vessel in at least 2 projections. A reduction in arterial lumen area of ≥ 50% of any coronary vessel, including left main coronary vessel and >70% for left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA) were considered significant. The analysis of the coronary angiograms was performed visually by an experienced operator who was blinded to the results of the echocardiographic examinations then syntax score was calculated.
All echo reports were read in a blinded manner by three cardiologists; Intra and inter observer agreement were done using (ICC) with values of Intra Class correlation of 0.875 and value of Inter class correlation 0.825.
2.3. Statistical analysis
Statistical analysis was performed using SPSS software (IBM SPSS Statistics for Windows, Version 20.0, IBM Corp., Armonk, NY, USA). Continuous variables were presented as means ± standard deviation and categorical variables as numbers or frequencies. The Chi-square test was used to compare frequencies. One-way analysis of variance was used to compare descriptive parameters after confirming normal distributions. Kappa test was used for categorical data with good agreement; Pearson’s correlation coefficients were used to assess the strength of relationship between continuous variables. Receiver operating characteristic (ROC) curve analysis was used to identify parameters that best predicted the presence of CAD and regional assessment of CAD. The level of evidence was detected in significant (p value <0.05).
3. Results
3.1. Study population
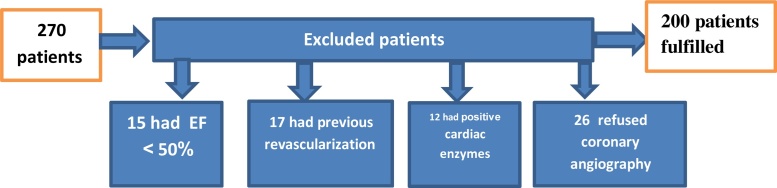
The study included 270 patients only 200 fulfilled the criteria of inclusion and exclusion as shown in the flow chart Fig. 1.
Fig. 1.
Flow chart of included and excluded patients.
Mean age of the studied population was 53.86 ± 8.99 and mean BMI was 28.67 ± 6.28 as shown in Table 1. As regards to risk factors of the studied patients; 125 patients (62.5%) were diabetic, 128 patients (64%) were hypertensive, 114 patients (57%) were dyslipidemic, 79 patients (39.5%) were smoker, 27 patients (13.5%) had a family history as shown in Table 1.
Table 1.
Baseline criteria of studied patients:.
| All patients N = 200 |
||
|---|---|---|
| Mean age (Mean ± SD) | 53.86 ± 8.99 | |
| Gender | male | 110 (55.6%) |
| female | 88 (44.4%) | |
| Diabetes mellitus | 125 (62.5%) | |
| Hypertension | 128 (64%) | |
| Dyslipidemia | 114 (57%) | |
| Smoking | 79 (39.5%) | |
| Family history of CAD | 27 (13.5%) | |
| Results of coronary angiography | Normal coronaries | 50 (25%) |
| Single vessel disease | 70 (35%) | |
| Two vessel disease | 31 (15.5%) | |
| Three vessel disease | 44 (22%) | |
| Left main only | 5 (2.5%) |
BMI: Body mass index.
3.2. Coronary angiography results
According to the results of coronary angiography patients were classified into those with normal coronary angiography, which included 50 patients (25%) and patients with CAD which included 150 patients (75%) Table 1.
-
•
The patients with CAD included 75 patients (37.5%) with a single vessel disease; 31 (15.5%) patients with two vessel disease and 44 (22%) with three vessel disease.
-
•
123 patients (61.7%) had LAD disease as part of single/two or three vessel disease. 86 patients (43%) had LCX disease as part of single/two or three vessel disease. 81 patients (40.5%) had the RCA disease as part of single/two or three vessel disease.
-
•
Five patients (2.5%) had significant left main disease only
-
•
Patients with CAD were classified according to syntax score; 70 patients had low syntax score (<8), 35 had an intermediate syntax score (9–16) and 45 had high syntax score > 16.
3.3. Demographic data of the studied patients as regard to extent of CAD
Demographic data showed statistically significant difference between normal and CAD in term of age (p value 0.027) but there is no statistically significant difference in terms of gender (p value 0.094) and BMI (p value 0.508) and regarding the risk factors, including diabetes, hypertension, dyslipidemia, smoking and family history of coronary artery disease (p value >0.05) as shown in Table 2.
Table 2.
Demographic data and risk factors of the studied patients as regard to extent of CAD.
| Normal | One vessel | Two vessels | Three vessels | One way ANOVA test |
|||
|---|---|---|---|---|---|---|---|
| F/X2 | p-value | ||||||
| Age | Mean ± SD | 51.54 ± 10.09 | 56.08 ± 8.90 | 54.26 ± 7.84 | 52.41 ± 7.85 | 3.136 | 0.027 |
| Range | 45–70 | 43–74 | 42–69 | 41–70 | |||
| Gender | Male | 29 (58%) | 48 (64.0%) | 13 (41.9%) | 20 (45.5%) | 6.394 | 0.094 |
| Female | 21 (42%) | 27 (36.0%) | 18 (58.1%) | 24 (54.5%) | |||
| BMI | Mean ± SD | 28.10 ± 5.97 | 29.38 ± 6.64 | 29.10 ± 6.52 | 27.80 ± 5.84 | 0.777 | 0.508 |
| Range | 20–43 | 20–46 | 20–45 | 21–45 | |||
| Risk factors | DM | 38 (76%) | 39 (52.0%) | 20 (64.5%) | 28 (63.6%) | 7.655 | 0.005 |
| HTN | 35 (70%) | 46 (61.3%) | 15 (48.4%) | 32 (72.7%) | 5.747 | 0.125 | |
| Smoking | 18 (36%) | 16 (12%) | 21 (67%) | 24 (54.6%) | 0.839 | 0.840 | |
| Dyslipidemia | 39 (78%) | 37 (49.3%) | 15 (48.4%) | 23 (52.3%) | 12.134 | 0.007 | |
| Family history | 3 (6%) | 6 (8%) | 8 (25.8%) | 10 (22.7%) | 11.580 | 0.056 | |
BMI: Body mass index, DM: Diabetes mellitus, HTN: Hypertension.
3.4. Echocardiographic data according to extent of CAD
There were no statistically significant differences between the normal, single vessel, two vessels and three vessels disease groups as regard of LV end diastolic diameter (p = 0.932), LV end systolic diameter (p = 0.881), ejection fraction (p = 0.859) or fractional shortening (p = 0.930) as shown in Table 3.
Table 3.
Echocardiographic data according to extent of CAD.
| Normal | One vessel | Two vessels | Three vessels | One way ANOVA test |
|||
|---|---|---|---|---|---|---|---|
| F | P value | ||||||
| LVEDD | Mean ± SD | 49.75 ± 8.56 | 49.95 ± 5.95 | 50.10 ± 5.67 | 50.58 ± 4.36 | 0.146 | 0.932 |
| Range | 41–59 | 37.3–60 | 37–59 | 38–59.2 | |||
| LVESD | Mean ± SD | 33.78 ± 5.03 | 33.27 ± 5.99 | 33.32 ± 4.94 | 32.91 ± 3.95 | 0.222 | 0.881 |
| Range | 23–40 | 20–66 | 22–43 | 22–39 | |||
| FS | Mean ± SD | 32.78 ± 7.61 | 33.12 ± 5.94 | 33.68 ± 5.62 | 33.82 ± 6.58 | 0.253 | 0.859 |
| Range | 26–53 | 23–50 | 24–44 | 23–50 | |||
| EF | Mean ± SD | 61.73 ± 7.23 | 61.70 ± 6.69 | 62.32 ± 6.17 | 62.43 ± 7.31 | 0.149 | 0.930 |
| Range | 50–81 | 50–79 | 51–73 | 50–80 | |||
| SWT | Mean ± SD | 1.0 ± 0.10 | 1.11 ± 1.07 | 0.98 ± 0.05 | 1.02 ± 0.10 | 0.461 | 0.710 |
| Range | 0.9–1.1 | 0.9–1.02 | 0.9–1 | 0.9–1.1 | |||
| PWT | Mean ± SD | 1.11 ± 0.85 | 1.26 ± 0.33 | 0.99 ± 0.05 | 0.97 ± 0.15 | 0.451 | 0.717 |
| Range | 0.9–1.1 | 1–1.1 | 1–1.1 | 1–1.1 | |||
LVEDD = Left ventricular end diastolic diameter, LVESD = Left ventricular end systolic diameter, FS = Fraction shortening, EF = Ejection fraction, SWT = septal wall thickness, PWT = posterior wall thickness.
3.5. Mean of GLPSS in the studied population
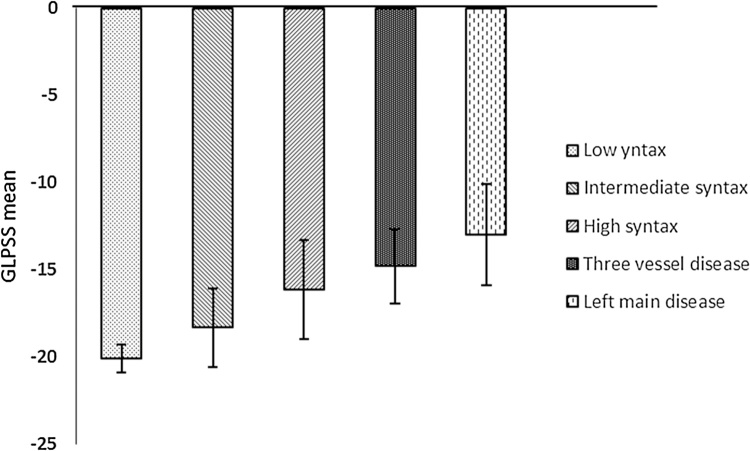
There was a statistically significant difference in mean of GLPSS between those with normal coronaries versus patients with CAD (p value 0.001). Mean GLPSS was −20.11 ± 0.8, −18.34 ± 2.25, −16.14 ± 2.85, −14.81 ± 2.12 and-13.01 ± 2.92 for normal, single vessel, two vessels, three vessels and left main disease, respectively (Table 4) Fig. 2.
Table 4.
Mean of global longitudinal peak systolic strain in the studied population.
| Normal | Single vessel | Two vessels | Three vessels | Left main | One way ANOVA test |
||
|---|---|---|---|---|---|---|---|
| F | P value | ||||||
| Mean of GLPSS | −20.11 ± 0.8 | −18.34 ± 2.52 | −16.14 ± 2.85 | −14.81 ± 2.12 | −13.01 ± 2.92 | 71.296 | 0.001 |
Fig. 2.
Mean of GLPSS in the studied population.
3.6. Post hoc analysis
There was a statistically significant difference in GLPSS between those with normal coronaries versus patients with CAD (p value 0.001) and also when comparing those with normal coronaries for patients with left main disease, single vessel, two vessels or three vessel disease (p value 0.001) as detected by one-way ANOVA test.
There was statistically significant difference in GLPSS When comparing patient with single vessel and three vessel disease (p value 0.002), when comparing patient with two vessels and three vessel disease (p value 0.043), when comparing patients with single vessel to those with left main disease (p value 0.001), when comparing patients with two vessels to those with left main disease (p value 0.005) but when comparing patients with three vessels to those with left main disease or between single and two vessels disease, there was no statistically significant difference detected (p value 0.959, 0.286, respectively) as in Table 5.
Table 5.
Post hoc analysis: Tukey’s test.
| Normal vs Single | Normal vs Two | Normal vs Three | Single vs Two | Single vs Three | Two vs Three | Single vs LM | Two vs LM | Three vs LM | |
|---|---|---|---|---|---|---|---|---|---|
| P value | 0.001 | 0.001 | 0.001 | 0.286 | 0.002 | 0.043 | 0.001 | 0.005 | 0.959 |
3.7. Detection of the number of diseased vessels
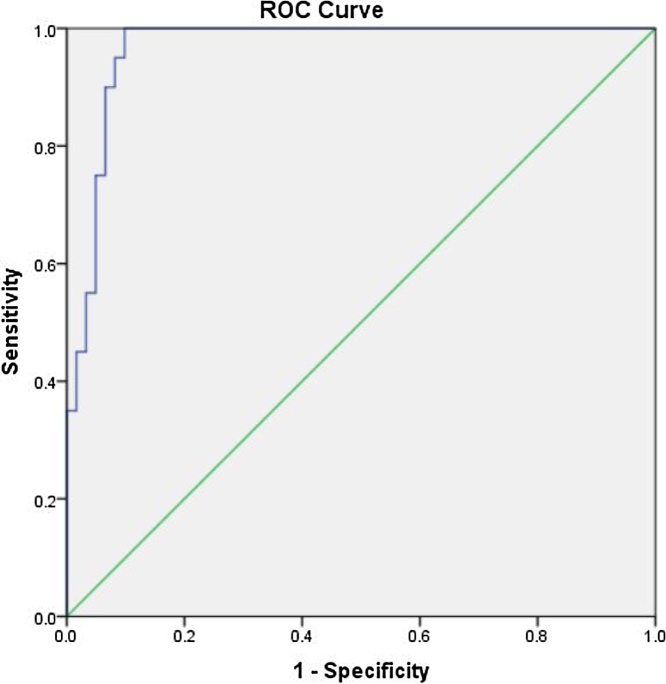
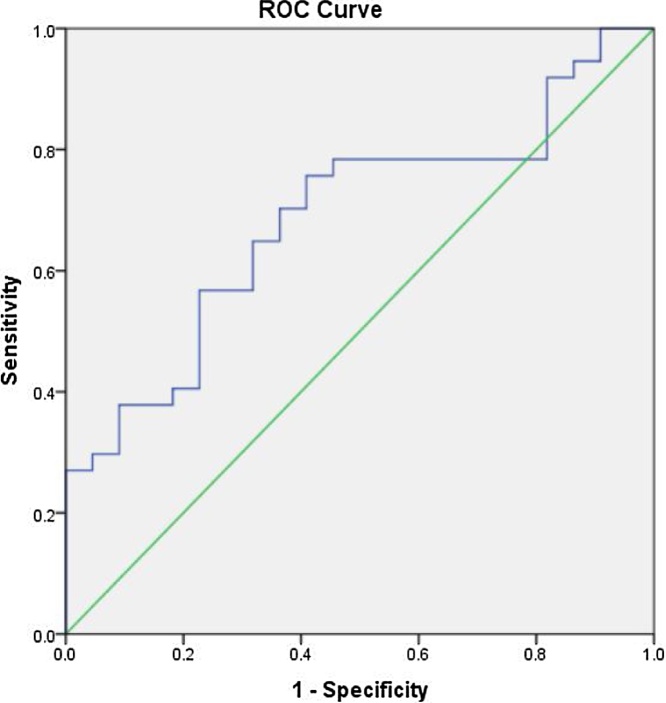
The cutoff value for detection of single vessel disease was −18.4425 with good sensitivity and specificity (90% and 95.1%, respectively) with AUC 0.954 and p value 0.001 (Table 6), Fig. 3.
Table 6.
Detection of number of diseased vessels.
| GLPSS Cut-off value | Sensitivity | Specificity | AUC (95% CI) | p-value | |
|---|---|---|---|---|---|
| Single vessel disease | −18.4425 | 90% | 95.1% | 0.954 (0.907–1.000) | 0.001 |
| Two vessels disease | −17.3597 | 90% | 88.9% | 0.906 (0.807–1.000) | 0.001 |
| Three vessels disease | −15.333 | 63% | 72.2% | 0.681 (0.525–0.837) | 0.041 |
GLPSS: Global longitudinal peak systolic strain.
Fig. 3.
Cut off value for detection of single vessel disease.
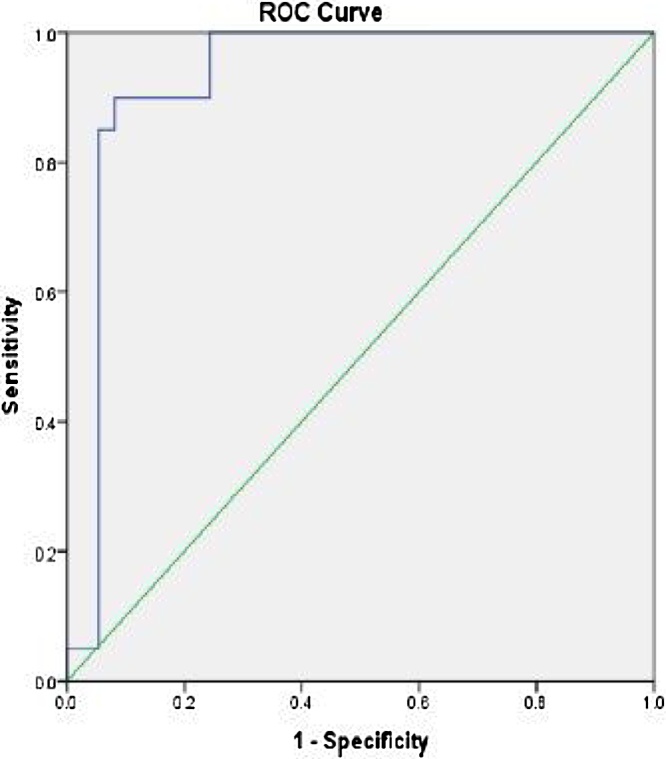
The cutoff value for detection of two vessels disease was −17.3597with good sensitivity and specificity (90% and 88.9%, respectively) with AUC 0.906 and p value 0.001 (Table 6), Fig. 4.
Fig 4.
Cut off value for detection of two vessels disease.
The cutoff value for the detection of three vessels disease was −15.333 with a sensitivity and specificity (63% and 72.2%, respectively) with AUC 0.681 and p value 0.041 (Table 6), Fig. 5.
Fig. 5.
Cutoff value for detection of three vessels disease.
3.8. Localization of the affected vessel
The segmental longitudinal systolic strain had good sensitivity and specificity in localization of the diseased artery:
The cutoff value of segmental LPSS for detection of diseased LAD artery was −18.3 with 90% sensitivity and 91.1% specificity and AUC was 0.93 and p value 0.001 (Table 7).
Table 7.
Localization of the affected vessel.
| Parameters | Segmental LPSS Cut-off value | Sensitivity | Specificity | AUC (95% CI) | p-value |
|---|---|---|---|---|---|
| LAD disease | −18.3000 | 90% | 91.1% | 0.930 (0.863–0.997) | 0.001 |
| LCX disease | −19.3138 | 95% | 80% | 0.912 (0.843–0.982) | 0.001 |
| RCA disease | −18.0850 | 72.9% | 78.8% | 0.798 (0.701–0.895) | 0.001 |
LAD: Left anterior descending, LCX: Left circumflex, RCA: Right coronary artery.
The cutoff value of segmental LPSS for detection of diseased LCX artery was −19.3138 with 95% sensitivity and 80% specificity and AUC was 0.912 and p value 0.001 (Table 7).
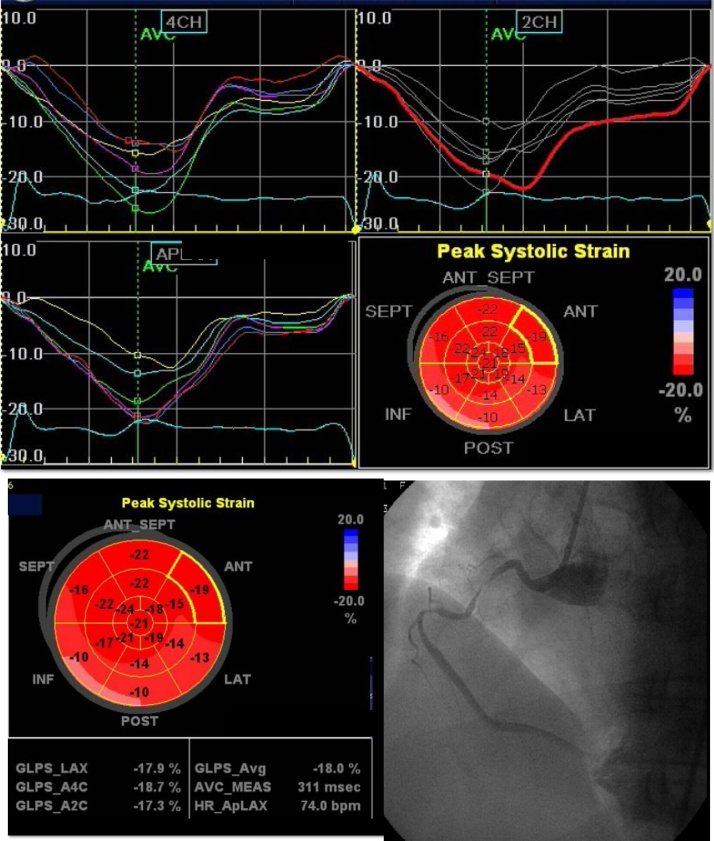
The cutoff value of segmental LPSS for detection of diseased RCA artery was −18.085 with 72.9% sensitivity and 78.8% specificity and AUC was 0.798 and p value 0.001 (Table 7), Fig. 6.
Fig. 6.
Global and segmental longitudinal strain in a patient with RCA lesion there is reduced GLPSS (-18%) and reduced regioal strain in the distribution of the diseased RCA (also there is reduced strain in the distribution of LCX which had insignificant lesion).
3.9. Multivariate regression analysis to assess the effect on GLPSS
Effect of risk factors on global longitudinal peak systolic strain was detected by multivariate logistic regression analysis and there were no statistically significant differences in terms of gender, BMI, DM, HTN, smoking, dyslipidemia and family history as shown in Table 8
Table 8.
Multivariate logistic regression analysis.
| S.E. | Wald | Sig. | Exp (B) | 95% C.I. for EXP(B) |
|||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Gender | −0.162 | 0.330 | 0.242 | 0.623 | 0.850 | 0.445 | 1.623 |
| BMI | −0.791 | 0.331 | 0.271 | 0.542 | 0.276 | 0.432 | 0.671 |
| DM | −0.981 | 0.391 | 0.297 | 0.712 | 0.375 | 0.174 | 0.807 |
| HTN | −0.358 | 0.351 | 1.036 | 0.309 | 0.699 | 0.351 | 1.393 |
| Smoking | 0.383 | 0.487 | 0.618 | 0.432 | 1.467 | 0.564 | 3.811 |
| Dyslipidemia | −1.266 | 0.378 | 0.185 | 0.614 | 0.282 | 0.134 | 0.592 |
| Family history | 1.093 | 0.636 | 2.957 | 0.085 | 2.984 | 0.8585 | 10.37 |
BMI = Body mass index, DM = Diabetes mellitus, HTN = Hypertension.
3.10. Correlation between GLPSS and syntax score
There was an inverse relation between GLPSS and syntax score and it was significant for intermediate and high score (p value 0.001) but insignificant for low score (p value 0.05)
Seventy patients (46.6%) had low syntax score (< 9), 45 patients (30%) had an intermediate syntax score 9, 10, 11, 12, 13, 14, 15, 16 and 35 (23.4%) had high syntax score (>16).
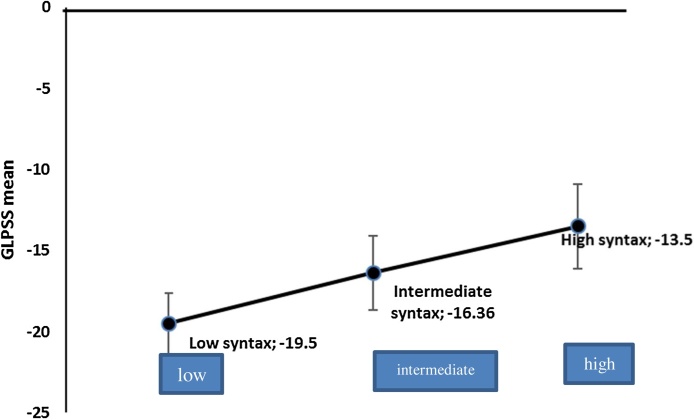
Mean of GLPSS in patients with low, intermediate and high syntax score was −19.5 ± 1.9, −16.35 ± 2.3 and−13.5 ± 2.6, respectively, with cutoff value of −13.753 for the prediction of high syntax score (sensitivity 80%, specificity 91%) and p value 0.001 (Fig. 7)
Fig. 7.
Correlation between GLPSS and syntax score.
4. Discussion
There is considerable interest in the diagnosis of CAD prior to the development of hard endpoints, which are associated with significant morbidity and mortality, but as long as the patient has normal systolic function usually will have a higher threshold for investigation, especially invasive one. However, questions persist regarding the appropriateness and cost effectiveness of screening for CAD along with the optimal approach to screening.
One of the dominant cardiac imaging techniques in patients with suspected cardiac disease is echocardiography. However, conventional echocardiography has a little value in diagnosis and risk stratification of patients with suspected stable angina as most of these patients have a normal wall motion at rest unless there is a history of previous myocardial infarction or myocardial stunning. So, it will be beneficial if another resting module can distinguish severe CAD from less severe CAD.7
Global longitudinal strain measured by 2-D speckle-tracking echocardiography (2-D STE) at rest has been recognized as a sensitive parameter in the detection of significant CAD.8
So in the present study, we evaluated the value of GLPSS at rest to predict the presence, extent and severity of CAD in patients with suspected stable angina pectoris.
Our study included 200 patients with suspected stable angina pectoris without regional wall motion abnormality and with normal systolic function, these patients were subjected to 2D-STE and coronary angiography. Regional and global LPSS were calculated and were correlated to the results of coronary angiography for each patient.
Of the 200 patients included in the present study, 50 patients had normal coronary angiography and 150 had significant CAD. There was statistically insignificant difference between the two groups as regard to the conventional echo parameters (dimensions and ejection fraction) which were concordant with Biering-Sørensen et al.9, Montgomery et al.10 and Nicola et al11 who showed statistically insignificant difference between the two groups with and without CAD in terms of EF and left ventricular internal diameters. But was not in agreement with Hanan Radwan et al. 12 study where there was a lower EF in the group of CAD (59.3 ± 3.2% vs 65.7 ± 4.7% p < 0.000) but in this study the exclusion criteria included patients with severe wall motion abnormality and those with overt heart failure
In the present study, patients with normal coronary artery had small SD of GLPSS (-20.11 ± 0.8) that was in agreement with Hanan Radwan et al12 where GLPSS of normal group was −18.65 ± 0.79, Sameh Bakhoum et al13 where GLPSS of normal group −21.11 ± 0.8 and Vrettos et al14 where GLPSS of normal group −17.39 ± 1.15.
Farsalinos et al15 evaluated the variability of GLPSS measurements among different vendors, They reported mean and SD of GLPSS in 62 volunteers measured by the GE machine (Vivid E9/EchoPac) as −21.0% and 3.9%, respectively and that can be explained by the statistical fact that two data sets − measuring the same variable − may have the same mean but different standard deviations. So variability is responsible for that difference. They recruited 62 individuals on volunteer basis, included both patients with a variety of LV functional states and subjects with normal cardiac function. This fact made the group of 62 individuals more heterogeneous than our group which included only healthy individuals. Other studies found less SD than 3.9 as Biering-Sørensen9 where SD was 2.6 and Nicole et at11 where SD was 2.8 and the last indicated that GLS is more dependent on the 2D-STE software used, rather than ultrasound equipment used to acquire images .
In the present study, there was statistically significant lower global longitudinal peak systolic strain in patients with CAD compared to those with normal coronary artery (p value <0.001) and the effect of risk factors on GLPSS was evaluated by multivariate logistic regression analysis and there were no statistically significant differences in terms of gender, BMI, DM, HTN, smoking, dyslipidemia or family history.
Segmental LPSS also showed statistical significance for localization of the affected vessel for LAD, LCX and RCA (p = 0.001).
Our results are in agreement with Biering-Sørensen 9 study that included 296 consecutive patients with clinically suspected stable angina pectoris without previous cardiac disease, and normal left ventricular ejection fraction (LVEF). GLPSS was significantly lower in patients with CAD compared with patients without CAD (−17.1 ± 2.5% versus −18.8 ± 2.6%; p < 0.001) Furthermore, impaired regional longitudinal systolic strain identifies which coronary artery is stenotic.
Although only patients without a history of heart disease, patients with a normal LVEF, patients with a normal resting ECG were enrolled, and the study population had a relatively low risk of coronary artery disease, but they found that 2-D STE performed at rest was an independent predictor of CAD, and it was even stronger predictor in patients with higher risk of CAD. The main limitation of this study was that radial, transverse, circumferential strain and synchrony analysis were not performed. However, the myocardial fibers most susceptible to ischemia are the longitudinally orientated fibers that are located subendocardially, that is why measurements of longitudinal deformation are thought to be the most sensitive markers of CAD.9
In Choi et al16 study that included 108 patients with chest pain without regional wall motion abnormality at rest echocardiogram, patients were grouped according to the coronary angiography findings as follows; high-risk group with left main or three-vessel CAD (n = 38), low-risk group with one- or two-vessel CAD (n = 28), and a control group without CAD (n = 30). GLPSS was significantly reduced, especially in mid- and basal segments, in the high-risk group. ROC curve analysis demonstrated that mid- and basal GLPSS could effectively detect patients with severe CAD (area under ROC curve = 0.83, 95% CI 0.75-0.91). Although this study could detect CAD but it depended only on number of vessel disease and ignored the degree of obstruction.
Xie et al17, assessed the left ventricular longtidinal, circumfeencial and radial systolic function by STE (global, basal, mid and apical segments) in 45 patients with multivessel CAD compared to 36 subjects with low risk of CAD (control group). The conventional ultrasonic measurement indices were similar between the both groups. Compared with the control group, but global, basal and mid longtidinal, circumfeencial and radial strian was statistically significant lower in multivessel CAD (p value 0.00).
Billehaug Norum et al,18, analyzed 6 studies (778 patients with suspected CAD), which aimed to assess the diagnostic accuracy of GLPSS to predict significant CAD, they concluded that GLPSS measurements at rest only have a modest diagnostic accuracy in predicting CAD among patients presenting with chest pain and it has better diagnostic accuracy with stress test. This can be explained by the selection bias and the presence of history of established CAD that require previous strain images for comparison and also difficult analysis of the regional LPSS to localize which vessel is stenotic.
In the present study, GLPSS showed high sensitivity for detection of the number of diseased vessels with cutoff value −18.44 for single vessel CAD (90%, specificity 95.1%, cutoff value:, AUC: 0.954); −17.35 for two vessels disease (90%, sensitivity 88.9%, cutoff value, AUC: 0.906) and −15.33 for three vessels CAD (sensitivity 63% and specificity 72.2% AUC 0.681), which was in agreement with Biering-Soerensen et al,9, Hanan et al12 and Sameh et al16, that showed GLPSS declined incrementally with increasing severity of CAD defined by increasing number of stenotic coronary vessels.
But the GLPSS diagnostic cutoff value varies significantly among previous studies which can be explained by using different equipment, different design, vendor-dependent 2D-STE software.
In the present study there was an inverse correlation between GLPSS and syntax score that was significant with high and intermediate score (p = 0.001) and insignificant for low syntax score (p value 0.05) with a cutoff value of −13.753 for the prediction of high syntax score (sensitivity 80%, specificity 91%) p value 0.001.
The purpose of the SYNTAX score is to grade lesion complexity for coronary revascularization, so difficult-to-treat anatomies such as trifurcations, bifurcations, tortuosity and calcification are taken into account in the calculation.The score also depends on lesion location and diameter reduction, which usually determines the extent of myocardial ischemia.
Studies have shown that longitudinal strain correlates well with the presence and severity of CAD but limited reports have investigated whether there is correlation between GLPSS and Syntax score.
Tanaka and coworkers19 study showed a modest correlation between SYNTAX scores and the extent of stress-induced myocardial ischemia as assessed on myocardial SPECT (r = 0.647, p < 0.0001) in 158 patients without previous myocardial infarction. These significant correlations were predominantly based on patients with a low SYNTAX score (r = 0.580, p < 0.0001), whereas such a correlation no longer existed in patients with an intermediate-high SYNTAX score (r = –0.033, p = NS).
In patients with an intermediate-high SYNTAX score, however, adding the scoring points related to tortuosity or calcification resulted in a high score, but without an increase in the extent of myocardial ischemia. This may underlie the absence of a significant correlation between SYNTAX score and the extent of myocardial ischemia in such patients.
Vrettos et al14 study was the first to correlate Syntax score and GLPSS in patients with stable angina with normal global and/or regional wall motion. The aim of this study was to investigate the hypothesis-generating idea, which can improve the selection of patients who are referred for coronary angiography. They found that there was a significant inverse correlation between GLPSS and Syntax score values (r2 = 0.3869, p < 0.001). This correlation was weaker in the low-SS group (r2 = 0.1332, p < 0.05), ROC curve analysis identified that the optimal cutoff for the detection of high-syntax score patients was −13.95 (sensitivity = 71%, specificity = 90%, p < 0.001). from the limitation of Vrettos et al, study that some of the baseline characteristics of the population that are known to affect GLPSS were not equally distributed among the participants (age, heart rate, hypertension and some of the medications) and may have contributed to reduced GLPSS values.
The correlation we observed between GLPSS and the syntax score might reflect the underlying relationship between anatomy and function.
4.1. Study implication
Patients with chest pain and inconclusive ECG findings should undergo echocardiographic examinations with strain analysis, even if the conventional echocardiography was within normal as regards to systolic function and wall motion to facilitate the early exclusion of significant coronary disease and thus early discharge and overall cost savings.
Early detection of sub-clinical left ventricular dysfunction prevents the development of complication and facilitate the application of the preventive measure.
4.2. Study limitation
Our study had some limitations. First, we enrolled a relatively small number of patients. Second, coronary angiography was only used to assess the presence, extent and severity of CAD and it is known that this examines only the lumen and doesn't exclude the presence of CAD but we depend on the correlation with significant CAD. Third being single center study.
5. Conclusion
2D STE has good sensitivity and specificity to predict the presence, extent and severity of CAD in patients with suspected stable angina pectoris.
References
- 1.Patel M.R., Peterson E.D., Dai D. Low diagnostic yield of elective coronary angiography. New Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parato V.M.A., Mehta D., Delfino S., Amabili M., Partemi P., Grossi Nardini E. Resting echocardiography for the early detection of acute coronary syndromes in chest pain unit patients. Echocardiography. 2010;27:597. doi: 10.1111/j.1540-8175.2010.01166.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsai W.C., Liu Y.W., Huang Y.Y., Lin C.C., Lee C.H., Tsai L.M. Diagnostic value of segmental longitudinal strain by automated function imaging in coronary artery disease without left ventricular dysfunction. J Am Soc Echocardiogr. 2010;23:1183–1189. doi: 10.1016/j.echo.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Damman P., van Geloven N., Walentin L. Timing of angiography with a routine invasive strategy and long-term outcomes in non-ST segment elevation acute coronary syndrome: a collaborative analysis of individual patient data from the FRISC II (Fragmin and Fast Revascularization During Instability in Coronary Artery Disease), ICTUS (Invasive versus Conservative Treatment in Unstable Coronary Syndromes), and RITA-3 (Intervention versus Conservative Treatment Strategy in Patients With Unstable Angina or Non-ST elevation Myocardial Infarction) Trials. JACC Cardiovasc Interv. 2012;5:191–199. doi: 10.1016/j.jcin.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Montalescot G., Sechtem U., Achenbach S. ESC guidelines on the management of stable coronary artery disease.: the Task Force on the management of stable coronary artery disease of the. European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 6.Ng A.C., Sitges M., Pham P.N. Incremental value of 2-dimensional speckle tracking strain imaging to wall motion analysis for detection of coronary artery disease in patients undergoing dobutamine stress echocardiography. Am Heart J. 2009;158:836–844. doi: 10.1016/j.ahj.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Nesbitt G.C., Mankad S., Oh J.K. Strain imaging in echocardiography: methods and clinical applications. Int J Cardiovascu Imaging. 2009;25(Suppl 1):9–22. doi: 10.1007/s10554-008-9414-1. [DOI] [PubMed] [Google Scholar]
- 8.Zuo H., Yan J., Zeng H. Diagnostic power of longitudinal strain at rest for the detection of obstructive coronary artery disease in patients with type 2 diabetes mellitus. Ultrasound Med Biol. 2015;41:89–98. doi: 10.1016/j.ultrasmedbio.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Biering-Sørensen T., Hoffmann S., Mogelvang R. Myocardial strain analysis by 2-dimensional speckle tracking echocardiography improves diagnostics of coronary artery stenosis in stable angina pectoris. Circ Cardiovasc Imaging. 2014;7:58–65. doi: 10.1161/CIRCIMAGING.113.000989. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery D.E., Puthumana J.J., Fox J.M., Ogunyankin K.O. Global longitudinal strain aids the detection of non-obstructive coronary artery disease in the resting echocardiogram. Eur Heart J Cardiovasc Imaging. 2012;13:579–587. doi: 10.1093/ejechocard/jer282. [DOI] [PubMed] [Google Scholar]
- 11.Gaibazzi Nicola, Pigazzani Filippo, Reverberi Claudio, Porter Thomas R. Rest global longitudinal 2 D strain to detect coronary artery disease in patients undergoing stress echocardiography: a comparison with wall-motion and coronary flow reserve responses. Echo Res Pract. 2014;1:61–70. doi: 10.1530/ERP-14-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radwan Hanan. Ekhlas Hussein Value of global longitudinal strain by two dimensional speckle tracking echocardiography in predicting coronary artery disease severity. Egyp Heart J. 2017 doi: 10.1016/j.ehj.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakhoum Sameh W.G., Taha Hesham S., Abdelmonem Yasser Y., Mirette A.S. Fahim Value of resting myocardial deformation assessment by two dimensional speckle trackingechocardiography to predict the presence, extent and localization of coronary artery affection in patients with suspected stable coronary artery disease. Egypt Heart J. 2016;68:171–179. [Google Scholar]
- 14.Vrettos A., Dawson D., N.Grigoratos C., Nihoyannopoulos P. Correlation between global longitudinal peak systolic strain and coronary artery disease severity as assessed by the angiographically derived SYNTAX score. Echo Res Pract. 2016;3:29–34. doi: 10.1530/ERP-16-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farsalinos K.E., Daraban A.M., Ünlü S., Thomas J.D., Badano L.P., Voigt J.U. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr. 2015;28(10):1171–1181. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Choi J.O., Cho S.W., Song Y.B. Longitudinal. longitudinal 2 D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. Eur J Echocardiogr. 2009;10:695–701. doi: 10.1093/ejechocard/jep041. [DOI] [PubMed] [Google Scholar]
- 17.Xie M.Y., Yin J.B., Lv Q., Wang J. Assessment of the left ventricular systolic function in multi-vessel coronary artery disease with normal wall motion by two dimensional speckle tracking echocardiography. Eur Rev Med Pharmacol Sci. 2015;19:3928–3934. [PubMed] [Google Scholar]
- 18.Billehaug N., Vidar R., Edvardsen T. Diagnostic accuracy of left ventricular longitudinal function by speckle tracking echocardiography to predict significant coronary artery stenosis. BMC Med Ima. 2015;15:25. doi: 10.1186/s12880-015-0067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka H., Chikamori T., Hida S. Relationship of SYNTAX score to myocardial ischemia as assessed on myocardial perfusion imaging. Circ J. 2013;77:2772–2777. doi: 10.1253/circj.cj-13-0099. [DOI] [PubMed] [Google Scholar]