Abstract
Background
Mitral annular plane systolic excursion (MAPSE) is an M-mode derived echocardiographic marker of left ventricular longitudinal function, the aim of this study is to evaluate the value of MAPSE in assessment of contractile reserve in patients with ischemic cardiomyopathy before cardiac revascularization.
Methods
The study included 50 patients with ischemic cardiomyopathy with ejection fraction (EF) ≤35%, the patients presented to echocardiography laboratory for dobutamine stress echocardiography (DSE) to assess viability and contractile reserve before revascularization, patients with primary valvular disease, and those with significant mitral annular calcifications were excluded from the study. A low dose DSE was done to all patients using standardized incremental infusions of 5, 10, and 20 μg/kg/min and the following parameters were measured at both baseline and peak dose, (EF, wall motion score index(WMSI) and MAPSE). Contractile reserve was measured as the difference between the low dose and baseline values of the EF and WMSI.
Results
The study included 50 patients aged 55.08 ± 7.15 years, 94% were males, the DSE protocol was complete in all patients without serious side effects. A total of eight hundred segments were analyzed, at baseline 65% were dysfunctional including 31.2% hypokinetic, 28.8% were akinetic, and 5% were dyskinetic. At low dose study 70% of the dysfunctional myocardium showed viability, EF increased significantly from 30.84 ± 4.56 to 42.24 ± 8.15%, p < 0.001, the WMSI reduced significantly from 1.92 ± 0.33 to 1.47 ± 0.39, and MAPSE increased significantly from 1.02 ± 0.23 to 1.30 ± 0.30 mm. MAPSE showed a significant positive correlation with EF at both baseline and low dose study (r = 0.283, p = 0.046 & r = 0.348, p = 0.013) respectively and a significant negative correlation with WMSI at both baseline and low dose study (r = −0.3, p = 0.034 & r = −0.409, p = 0.003), respectively. By ROC curve analysis we found that Δ MAPSE ≥2 mm can predict contractile reserve at Δ EF >10% (AUC = 0.6, sensitivity 67.86, specificity 59.09), and Δ MAPSE ≥1.8 mm can predict contractile reserve at ΔWMSI ≤0.20 (AUC = 0.61, sensitivity 65.5, specificity 75.6).
Conclusions
MAPSE is a rapid simple quantitative echocardiographic method that can asses contractile reserve in patients with ischemic cardiomyopathy before cardiac revascularization.
Abbreviations: A, late transmitral inflow velocity; Aa, late diastolic annular velocity; ACEI, angiotensin converting enzyme inhibitors; BP, blood pressure; CAD, coronary artery disease; DSE, dobutamine stress echocardiography; E, early transmitral inflow velocity; Ea, early diastolic annular velocity; ECG, electrocardiogram; EF, ejection fraction; E/Ea, the ratio of early transmitral velocity to early diastolic mitral annular velocity; HR, heart rate; LV, left ventricle; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; MAPSE, mitral annular plane systolic excursion; Sa, systolic annular velocity; TDI, tissue Doppler imaging; WMSI, wall motion score index
Keywords: Mitral annulus systolic excursion, Contractile reserve, Ischemic cardiomyopathy
1. Introduction
Dobutamine stress echocardiography (DSE) is a widely used technique to asses myocardial viability and contractile reserve in patients with ischemic cardiomyopathy.1 To date, the assessment of myocardial contractile reserve is limited to the improvement in left ventricular ejection fraction (EF) or wall motion score index (WMSI) calculated mainly from 2 dimensional (2D) echocardiography during DSE, both methods are reliable in many studies in assessment of contractile reserve but they need expert echocardiographer estimation, proper endocardial border delineation, and are somewhat subjective and prone to reader discordance.2, 3 Mitral annular plane systolic excursion (MAPSE) is an M-mode derived echocardiographic marker of left ventricle (LV) longitudinal function.4, 5 MAPSE has been suggested as a surrogate measurement for LV systolic function.6, 7 it can be easily performed because it does not rely on optimal endocardial definition and the mitral annulus can often be visualized and tracked even when there is poor LV image quality with inadequate endocardial visualization8 the aim of this study is to evaluate the value of MAPSE in assessment of contractile reserve in patients with ischemic cardiomyopathy undergoing cardiac revascularization.
2. Methods
2.1. Study population
The study included 50 patients with ischemic cardiomyopathy with EF ≤ 35% and an ischemic etiology of cardiomyopathy was defined as the presence of any epicardial coronary vessels with ≥75% stenosis in coronary angiography or any history of myocardial infarction or coronary revascularization (either percutaneous coronary intervention or coronary artery bypass grafting),9 the patients presented to echocardiography laboratory for DSE for assessment of viability and contractile reserve before revascularization, patients with primary valvular disease, congenital heart disease, and those with significant mitral annular calcifications that interfere with MAPSE assessment were excluded from the study. In addition, 20 healthy age and gender matched subjects were included as a control group.
2.2. Methods
An informed consent was obtained from all patients and control group. And the study was approved by the ethical committee. The study was carried between November 2015 to august 2016.
2.2.1. Clinical evaluation
All the patients and control were subjected to full history taking, including age, gender, cardiovascular disease risk factors.
2.2.2. Laboratory investigation
Lipid profile, urea, creatinine were measured in all patients.
2.2.3. Coronary angiography data
All patients were presented to echocardiography laboratory for DSE had their coronary angiographic data.
2.2.4. Dobutamine stress echocardiography protocol10
DSE was performed using a low dose protocol to all patients with incremental stages lasting 3 min each, with an initial dose of 5 mcg/kg/min, which was increased to 10 mcg/kg/min, then to the maximal dose 20. The infusion was stopped before the maximal dose was reached if 85% of the maximal predicted heart rate for the age group was achieved, or symptomatic non sustained or sustained ventricular tachycardia were observed. A three-lead electrocardiogram (ECG) to monitor heart rate (HR) continuously, and a 12-lead ECG was recorded every minute. Cuff blood pressure(BP) was measured at each stage. The nature of the procedure was explained to the patients and beta blockers were stopped 48 h prior to DSE in all patients. Echocardiography was performed in the left lateral position using (HD11 XE, Philips) machine and image acquisition at the end of each stage from standard apical and parasternal views, heart rate, blood pressure, and ECG were monitored along the test and the followings were measured at base line echo and at peak dose study:
-
a.)
EF was calculated by modified Simpson method.11
-
b.)
Left ventricular WMSI was calculated using a 16-segment model of the LV. Wall motion for each segment was graded semiquantitatively as normokinesia = 1, hypokinesia = 2, akinesia = 3 and dyskinesia or aneurysm = 4. WMSI was derived from the sum of all 16 segmental wall motion scores, divided by the total number of segments and the presence of myocardial viability was defined as an improvement in regional function of ≥1 grade at low-dose dobutamine (ie, a hypokinetic segment becoming normal or an akinetic segment becoming hypokinetic).11
-
c.)
Pulsed wave Doppler mitral inflow velocities were obtained from the apical 4-chamber view to measure diastolic early filling velocity (E) wave and late diastolic velocity (A) wave, E/A ratio, and the deceleration time of E wave.11
-
d.)
Pulsed wave tissue Doppler imaging (TDI) was obtained after placement of the sample volume at the level of the septal and lateral mitral annuli. From these recordings, early diastolic (Ea), late diastolic mitral annular velocities (Aa), E/Ea, and myocardial systolic annular velocity (Sa) for assessment of global longitudinal systolic function, were measured and averaged.11
-
e.)
MAPSE measured by the use of M-mode echocardiography in an apical view at the septal and lateral mitral annuli, the M-mode cursor should always be aligned parallel to the LV walls. The systolic excursion of mitral annulus should be measured from the lowest point at end-diastole to end of the T-wave on the ECG, MAPSE should be averaged from the septal and lateral mitral annulus.12, 13
-
f.)
Contractile reserve: Contractile reserve was defined according to a continuous parameter as ΔWMSI, which expresses the difference between peak dose and resting WMSI. And also from Δ EF, which expresses the difference between peak dose and resting EF.14, 15, 16
3. Statistical analysis
Statistical analyses were performed by using SPSS system for Windows (version 20 Chicago, IL, USA), Continuous variables were presented as mean ± SD and were compared by Student’s t-test or Mann-Whitney U test for variables with or without normal distribution, respectively. Categorical variables were expressed as percentages and evaluated with a Chi square test or Fisher’s exact test. Correlations between variables were tested by using the Spearman's correlation test. ROC analyses was performed and best cut off value was determined and at that point sensitivity and specificity were determined. The results were considered significant when the p value was less than 0.05.
4. Results
4.1. Baseline characteristics
The baseline demographic, clinical, and angiographic data of the are shown in Table 1, the majority of studied patients were males (94%), NYHA class III–IV were present in 40% of patients,86% were smokers and 28% were diabetics, the majority of patients were receiving acetyl salicylic acid, Angiotensin converting enzyme inhibitors (ACEI), betablokers, diuretics and by coronary angiography multivessel CAD were present in 54% of cases.
Table 1.
comparison between patients and control regarding demographic data.
| Item | Patients (No = 50) | Control group (No = 20) | p value |
|---|---|---|---|
| Age (years) (Mean ± SD) | 55.08 ± 7.15 | 54.40 ± 7.72 | 0.869 |
| Gender (male) (No)(%) | 47(94%) | 17(85%) | 0.343 |
| HR(bpm) (Mean ± SD) | 74.78 ± 5.90 | 75.55 ± 7.28 | 0.521 |
| Systolic BP (mmHg) (Mean ± SD) | 124.46 ± 8.58 | 122.30 ± 7.74 | 0.943 |
| Diastolic BP (mmHg) (Mean ± SD) | 84.72 ± 6.69 | 82.25 ± 6.29 | 0.160 |
| NYHA class I-II(No)(%) | 30 (60) | – | |
| NYHA class III-IV(No)(%) | 20 (40) | – | |
| Total cholesterol(mg/dl) (Mean ± SD) | 250.5 ± 22.6 | 160.2 ± 38.6 | < 0.001 |
| HDL cholesterol(mg/dl) (Mean ± SD) | 30.63 ± 2.33 | 33.3 ± 5.32 | 0.032 |
| LDL cholesterol(mg/dl) (Mean ± SD) | 182.52 ± 17.89 | 125.05 ± 11.74 | < 0.001 |
| Triglycerides(mg/dl) (Mean ± SD) | 165.26 ± 15.09 | 101.45 ± 14.96 | < 0.001 |
| Urea(mg/dl) (Mean ± SD) | 39.5 ± 5.3 | 38.62 ± 6.32 | 0.921 |
| Creatinine(mg/dl) (Mean ± SD) | 0.95 ± 0.42 | 0.85 ± 0.36 | 0.832 |
| Major risk factors for CAD | |||
| Smoking (No)(%) | 43 (86) | 11(55%) | 0.005 |
| Family history of CAD(No)(%) | 10 (20) | – | – |
| Diabetes mellitus(No)(%) | 14 (28) | – | – |
| Hypertension (No)(%) | 7 (14) | – | – |
| Medications | |||
| Aspirin | 50 (100) | ||
| Clopedogrel | 35 (70%) | – | – |
| ACEI | 45 (90) | – | – |
| Diuretics | 48 (96) | – | – |
| b Blockers | 43 (86) | – | – |
| Statins | 50 (100) | – | – |
| Coronary angiographic data | |||
| Single vessel (LAD) | 11 (22) | – | – |
| 2 vessel disease (No)(%) | 12 (24) | – | – |
| 3 vessel disease(No)(%) | 27 (54) | – | – |
Angiotensin converting enzyme inhibitors (ACEI) BP: blood pressure, CAD: coronary artery disease, HDL: High density lipoprotein cholesterol, HR: heart rate,LAD: left anterior descending artery, LCX: left circumflex artery, LAD: left anterior descending artery, LCX: left circumflex artery, LDL: Low density lipoprotein cholesterol, RCA: right coronary artery.
4.2. Results of dobutamine stress echocardiography
The administration of dobutamine was well tolerated by all patients. The protocol was completed in all patients without serious side effects. A total of 800 segments were analyzed at baseline echocardiography in all patients of which 280 segments (35%) were normokinetic, and 520 segments (65%) were dysfunctional including 250 (31.2%) were hypokinetic, 230 (28.8%) were akinetic, and 40 (5%) were dyskinetic. At low dose study, 364 segment (70%) of the dysfunctional myocardium showed viability. The parameters of global LV systolic function including EF, Sa, MAPSE were significantly increased at peak dose in comparison to baseline study and in comparison to control group, the WMSI and LVESV were significantly reduced at peak dose in comparison to low dose and in comparison to control group. Also the E/Ea significantly reduced at peak dose study in comparison to baseline study (Table 2, Fig. 1).
Table 2.
comparison between patients and control group regarding echocardiographic data.
| Patients at baseline DSE No = 50 | Patients at peak dose DSE No = 50 | Control group No = 20 | ||
|---|---|---|---|---|
| LVEDV(ml) | 202.30 ± 49.59 | 191.52 ± 52.53 | 104.35 ± 24.9 | p1 = 0.040*,p2 < 0.001*,p3 < 0.001* |
| LVESV(ml) | 137.73 ± 40.69 | 112.28 ± 43.07 | 38.95 ± 8.57 | p1 < 0.001*,p2 < 0.001*,p3 < 0.001* |
| EF (%) | 30.84 ± 4.56 | 42.24 ± 8.15 | 62.24 ± 4.82 | p1 < 0.001*,p2 < 0.001*,p3 < 0.001* |
| WMSI | 1.92 ± 0.33 | 1.47 ± 0.39 | 1.0 ± 0.0 | p1 < 0.001*,p2 < 0.001*,p3 < 0.001* |
| MAPSE(cm) | 1.02 ± 0.23 | 1.30 ± 0.30 | 2.11 ± 0.26 | p1 < 0.001*,p2 < 0.001*,p3 < 0.001* |
| E (m/sec) | 0.72 ± 0.26 | 0.77 ± 0.29 | 0.79 ± 0.12 | p1 = 0.051, p2 = 0.125,p3 = 0.435 |
| A(m/sec) | 0.56 ± 0.19 | 0.68 ± 0.25 | 0.59 ± 0.09 | p1 < 0.001*, p2 = 0.73, p3 = 0.070 |
| E/A ratio | 1.55 ± 1.03 | 1.45 ± 1.19 | 1.34 ± 0.31 | p1 = 0.409,p2 = 0.820,p3 = 0.103 |
| DT(msec) | 159.56 ± 44.14 | 189.76 ± 57.06 | 188.0 ± 30.97 | p1 < 0.001*, p2 = 0.011*, p3 = 0.869 |
| Sa(m/sec) | 0.06 ± 0.01 | 0.08 ± 0.02 | 0.10 ± 0.02 | p1 < 0.001*,p2 < 0.001*,p3 < 0.001* |
| Ea(m/sec) | 0.07 ± 0.02 | 0.09 ± 0.03 | 0.14 ± 0.04 | p1 < 0.001*,p2 < 0.001*,p3 < 0.001* |
| Aa(m/sec) | 0.07 ± 0.02 | 0.10 ± 0.11 | 0.10 ± 0.02 | p1 < 0.001*,p2 < 0.001*,p3 = 0.228 |
| E/Ea ratio | 11.06 ± 5.28 | 9.22 ± 4.76 | 6.26 ± 1.88 | p1 < 0.001*,p2 < 0.001*,p3 = 0.002* |
A: late diastolic velocity, Aa: late diastolic annular velocity, DT: deceleration time, E: early diastolic velocity, Ea: early diastolic annular velocity, EF:ejection fraction, LVEDV:left ventricular end diastolic volume, LVESV: left ventricular end systolic volume,MAPSE:mitral annular plane systolic excursion, Sa: systolic annular velocity,WMSI: wall motion score index. p1: p value comparing between at base line and at peak low dose,p2: p value comparing between at base line and control,p3: p value comparing between at peak low dose and control,*Statistically significant.
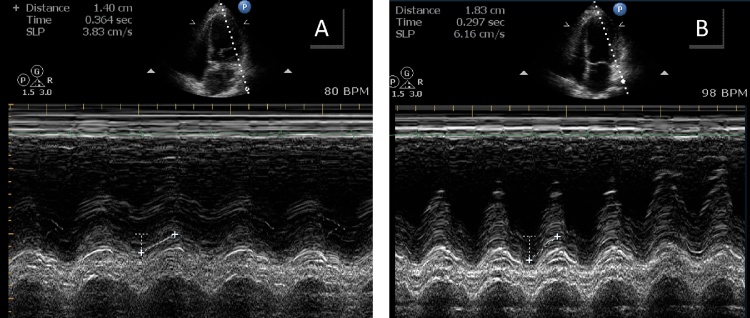
Fig. 1.
Example of mitral annular plane systolic excursion at baseline echocardiography (A) and its improvement at peak dose of dobutamine stress echocardiography(B).
4.3. Correlation analysis
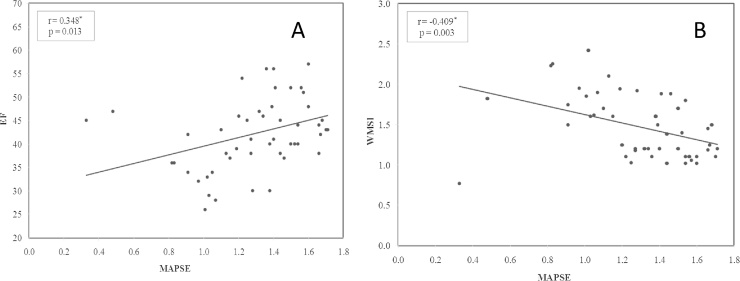
MAPSE showed a significant positive correlation with EF at both baseline and low dose study (r = 0.283, p = 0.046 & r = 0.348, p = 0.013), respectively, also a significant positive correlation with Sa at both baseline and low dose study (r = 0.457, p < 0.001, r = 0.563, p < 0.001), respectively, a significant negative correlation with WMSI at both baseline and low dose study (r = −0.323, p = 0.034 & r = −0.409, p = 0.003) respectively, a significant negative correlation to LVESV at both baseline and low dose study (r = −0.671, p = 0.045, r = −0.408, p = 0.003), respectively, and a non significant correlation with E/Ea at both baseline and low dose study (r = −0.276, p = 0.232, r = −0.133, p = 0.359), respectively (Fig. 2). We found also that ΔMAPSE (the difference between peak dose and baseline study)was significantly positively correlated with ΔEF and ΔSa (r = 0.683, p = 0.032 & r = 0.568, p < 0.001) respectively and significantly negatively correlated to ΔWMSI(r = −0.525, p = 0.002).
Fig. 2.
Correlation between ejection fraction(EF) and mitral annulus plane systolic excursion (MAPSE)(A), wall motion score index (WMSI)and MAPSE in (B) at peak dose of dobutamine stress echocardiography.
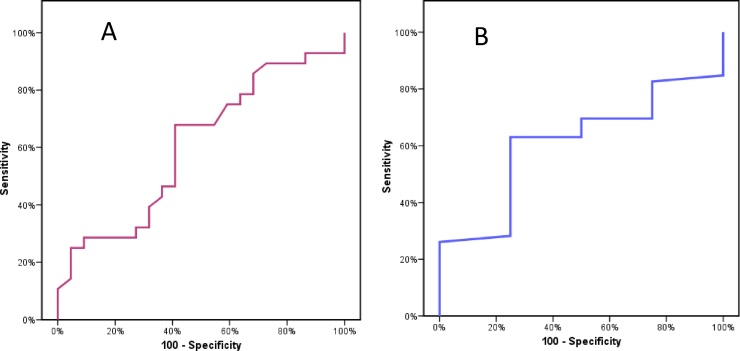
By Roc curve analysis we found that a cutoff Δ MAPSE ≥2 mm can predict contractile reserve at Δ EF >10% (AUC = 0.6, sensitivity 67.86, specificity 59.09, PPV = 67.9,NPV = 59.1), and a cutoff Δ MAPSE ≥1.8 mm can predict contractile reserve at ΔWMSI ≤0.20(AUC = 0.61, sensitivity 65.5, specificity 75.6, PPV = 96.7, NPV = 15.0) (Fig. 3).
Fig. 3.
ROC curve: Δ MAPSE ≥2 mm can predict contractile reserve at Δ EF ≥10%(A), and Δ MAPSE ≥1.8 mm can predict contractile reserve at ΔWMSI ≤0.20 (B).
Using multivariate analysis logistic regression for the different parameters used to asses the global contractile reserve including WMSI,EF, Sa and MAPSE, we found that MAPSE was the most significant parameter used for the assessment of the global contractile reserve at both baseline (B = 4.538, 95% CI LL = 0.814, UL = 8.262, p < 0.001) and low dose DSE (9.068, 95% CI LL = 3.091,UL = 15.045, p < 0.001).
5. Discussion
In patients with CAD and chronic LV dysfunction, it is crucial to distinguish between viable and fibrotic tissue to make adequate clinical decisions, the main clinical issue to search the myocardial viability is that patients with evidence of hibernating myocardium who do not undergo revascularization have poor prognosis with high incidence of cardiac events.17 In contrast, evidence of viable myocardium in patients undergoing successful revascularization is associated with longer survival and improvement of both symptoms and LV function.18 The term hctional myocardium, which was considered to be the result oibernating myocardium was first termed by Rahimtoola to indicate the state of reversible dysfunf a state of persistently impaired myocardial function at rest, caused by reduced coronary blood flow, and which could be partially or completely restored to normal either by improving blood flow or reducing oxygen demand.19 Echocardiography had an important role in selecting those who may benefit from revascularization, echocardiography can detect viable myocardium during infusion of drugs, which have ability to elicit an enhanced contractile response by recruiting contractile proteins, routinely, the dobutamine is the most common stressor used. Sun et al., had demonstrated that the improvement in contractile function during dobutamine infusion was associated with a concomitant increase in myocardial blood flow, the increase in myocardial blood flow occurs because there is persistent albeit reduced coronary flow reserve distal to a stenosis, which dobutamine may exploit. Another mechanism whereby contractile response may be elicited during dobutamine infusion is through its peripheral vasodilator effect, which causes reduction in LV end-systolic wall stress by reducing afterload.20 although DSE is an established method for assessing the myocardial viability however it is subjective as it depends on wall motion analysis, so in our study we had used quantitative parameters in assessment of LV longitudinal systolic performance during DSE using MAPSE and tissue Doppler mitral annular systolic velocities. MAPSE provides a simple and objective method in assessment of LV longitudinal function,21 also TDI has also been introduced in clinical settings as a method of quantifying both segmental and global LV function.22 In our study, MAPSE had a linear correlation to Doppler mitral annular systolic velocities and to the EF at both resting and peak DSE study, and Δ MAPSE was significantly correlated to the parameters of contractile reserve including ΔEF and Δ WMSI meaning that it could be used to asses contractile reserve in patients with ischemic cardiomyopathy, Jason et al.7 studied the predictive value of MAPSE. Using the first 300 studies, an algorithm was developed to predict EF, and concluded that MAPSE measurement was found to be a highly accurate predictor of EF. MAPSE, in addition to being a simple surrogate for EF, may have implications for patient outcomes. Willenheimer et al23 demonstrated that among patients with heart failure, those with decreased MAPSE levels had significantly higher mortality,future work should be done in to assess the correlation between MAPSE measurements and patient outcomes
6. Study limitations
One of the limitations in this study was the limited sample size, also in the assessment of myocardial viability mitral annular motion assessment by M-mode may be influenced by left atrial hemodynamics and left ventricular end-diastolic pressure. Furthermore, since M-mode is performed at the mitral annulus level, measured values might be influenced by the infarct zone or the wall motion of non-infarcted regions. We recommend that this work should be completed with data from post-revascularization phase and with prognostic data from a clinical follow-up in order to assess if MAPSE has a predictive value for recovery of viability or for death or hospitalization. Also another study including comparison of MAPSE with the global longitudinal strain for contractile reserve assessment should be done.
7. Conclusions
MAPSE is a rapid simple quantitative echocardiographic method that can asses contractile reserve in patients with ischemic cardiomyopathy before cardiac revascularization.
Conflict of interest
None declared.
References
- 1.Picano E., Sicari R., Landi P. Prognostic value of myocardial viability in medically treated patients with global left ventricular dysfunction early after an acute uncomplicated myocardial infarction: a dobutamine stress echocardiographic study. Circulation. 1998;98:1078–1084. doi: 10.1161/01.cir.98.11.1078. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez-Chico J.L., Zamorano J.L., Perez de Isla J. Comparison of left ventricular volumes and ejection fractions measured by three-dimensional echocardiography versus by two-dimensional echocardiography and cardiac magnetic resonance in patients with various cardiomyopathies. J Cardiol. 2005;95:809–813. doi: 10.1016/j.amjcard.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Palmieri V., Russo C., Buonomo A. Novel wall motionscore-based method for estimating global left ventricular ejection fraction: validation by real-time 3D echocardiography and global longitudinal strain. Eur J Echocardiogr. 2010;11:125–130. doi: 10.1093/ejechocard/jep177. [DOI] [PubMed] [Google Scholar]
- 4.Simonson J.S., Schiller N.B. Descent of the base of the left ventricle: an echocardiographic index of left ventricular function. J Am Soc Echocardiogr. 1989;2:25–35. doi: 10.1016/s0894-7317(89)80026-4. [DOI] [PubMed] [Google Scholar]
- 5.Henein M.Y., Gibson D.G. Normal long axis function. Heart. 1999;81:111–113. doi: 10.1136/hrt.81.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu K., Liu D., Herrmann S. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging. 2013;14:205–212. doi: 10.1093/ehjci/jes240. [DOI] [PubMed] [Google Scholar]
- 7.Matos J., Kronzon I., Panagopoulos G., Perk G. Mitral annular plane systolic excursion as a surrogate for left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25:969–974. doi: 10.1016/j.echo.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Frielingsdorf J., Schmidt C., Debrunner M. Atrium driven mitral annulus motion velocity reflects global left ventricular function and pulmonary congestion during acute biventricular pacing. J Am Soc Echocardiogr. 2008;21:288–293. doi: 10.1016/j.echo.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Felker G.M., Shaw L.K., O'Connor C.M. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 10.Pellikka P.A., Roger V.L., Oh J.K. Stress echocardiography. Part II. Dobutamine stress echocardiography: implementation, clinical applications and correlations. Mayo Clin Proc. 1995;70:16–27. doi: 10.1016/S0025-6196(11)64660-0. [DOI] [PubMed] [Google Scholar]
- 11.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Carlhäll C., Wigström L., Heiberg E. Contribution of mitral annular excursion and shape dynamics to total left ventricular volume change. Am J Physiol Heart Circ Physiol. 2004;287:H1836–41. doi: 10.1152/ajpheart.00103.2004. [DOI] [PubMed] [Google Scholar]
- 13.Hu K., Liu D., Herrmann S. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging. 2013;14:205–212. doi: 10.1093/ehjci/jes240. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka M., Oki T., Mishiro Y. Early systolic mitral annular motion velocities responses to dobutamine infusion predict myocardial viability in patients with previous myocardial infarction. Am Heart J. 2002;143:552–558. doi: 10.1067/mhj.2002.121266. [DOI] [PubMed] [Google Scholar]
- 15.Karabinos I., Bouki T., Kostopoulos K. Left ventricular long-axis function during dobutamine stress echocardiography for the prediction of post- revascularization recovery comparison with SPECT Thallium scintigraphy. Hosp Chron. 2009;4(4):158–165. [Google Scholar]
- 16.Bouki T., Kranidis A. Left atrioventricular plane response during dobutamine echocardiography. J Am Soc Echocard. 2001;14:1044–1045. doi: 10.1067/mje.2001.117336. [DOI] [PubMed] [Google Scholar]
- 17.Picano E., Sicari R., Landi P. Prognostic value of myocardial viability in medically treated patients with global left ventricular dysfunction early after an acute uncomplicated myocardial infarction: a dobutamine stress echocardiographic study. Circulation. 1998;98:1078–1084. doi: 10.1161/01.cir.98.11.1078. [DOI] [PubMed] [Google Scholar]
- 18.Nesto R.W., Cohn L.H., Collins J.J., Jr. Inotropic contractile reserve: a useful predictor of increased 5 year survival and improved postoperative left ventricular function in patients with coronary artery disease and reduced ejection fraction. Am J Cardiol. 1982;50:39–44. doi: 10.1016/0002-9149(82)90006-6. [DOI] [PubMed] [Google Scholar]
- 19.Rahimtoola S.H. The hibernating myocardium. Am Heart J. 1989;117:211–221. doi: 10.1016/0002-8703(89)90685-6. [DOI] [PubMed] [Google Scholar]
- 20.Sun K.T., Czernin J., Krivokapich J. Effects of dobutamine stimulation on myocardial blood flow, glucose metabolism, and wall motion in normal and dysfunctional myocardium. Circulation. 1996;94:3146–3154. doi: 10.1161/01.cir.94.12.3146. [DOI] [PubMed] [Google Scholar]
- 21.Hoglund C., Alam M., Thorstrand C. Effects of acute myocardial infarction on the displacement of the atrioventricular plane: an echocardiographic study. J Intern Med. 1989;226:251–256. doi: 10.1111/j.1365-2796.1989.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 22.Bolognesi R., Tsialtas D., Barilli A.L. Detection of early abnormalities of left ventricular function by hemodynamic, echo-tissue Doppler imaging and mitral Doppler flow techniques in patients with coronary artery disease and normal ejection fraction. J Am Soc Echocardiogr. 2001;14:764–772. doi: 10.1067/mje.2001.113234. [DOI] [PubMed] [Google Scholar]
- 23.Willenheimer R., Cline C., Erhardt L. Left ventricular atrioventricularplane displacement: an echocardiographic technique for rapid assessment of prognosis in heart failure. Heart. 1997;78:230–236. doi: 10.1136/hrt.78.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]