Abstract
Background
The modification of microbial ecology in human gut by supplementing probiotics may be an alternative strategy to ameliorate or prevent depression.
Objective
The current study was conducted to assess the safety and efficacy of the probiotic strain Bacillus coagulans MTCC 5856 for major depressive disorder (MDD) in IBS patients.
Method
Patients (n = 40) diagnosed for MDD with IBS were randomized (1:1) to receive placebo or B. coagulans MTCC 5856 at a daily dose of 2 × 109 cfu (2 billion spores) and were maintained to the end of double-blind treatment (90 days). Changes from baseline in clinical symptoms of MDD and IBS were evaluated through questionnaires.
Results
Significant change (p = 0.01) in favour of the B. coagulans MTCC 5856 was observed for the primary efficacy measure Hamilton Rating Scale for Depression (HAM-D), Montgomery-Asberg Depression Rating Scale (MADRS), Center for Epidemiological Studies Depression Scale (CES-D) and Irritable bowel syndrome quality of life questionnaire (IBS-QOL). Secondary efficacy measures i.e. Clinical Global Impression-Improvement rating Scale (CGI-I), Clinical Global Impression Severity rating Scale (CGI-S), Gastrointestinal Discomfort Questionnaire (GI-DQ) and Modified Epworth Sleepiness Scale (mESS) also showed significant results (p = 0.01) in B. coagulans MTCC 5856 group compared to placebo group except dementia total reaction scoring. Serum myeloperoxidase, an inflammatory biomarker was also significantly reduced (p < 0.01) when compared with the baseline and end of the study. All the safety parameters remained well within the normal clinical range and had no clinically significant difference between the screening and at the end of the study.
Conclusion
B. coagulans MTCC 5856 showed robust efficacy for the treatment of patients experiencing IBS symptoms with major depressive disorder. The improvement in depression and IBS symptoms was statistically significant and clinically meaningful. These findings support B. coagulans MTCC 5856 as an important new treatment option for major depressive disorder in IBS patients.
Keywords: probiotic, B. coagulans MTCC 5856, LactoSpore®, major depression, irritable bowel syndrome
Major depressive disorder (MDD) is characterised by an increased medical morbidity, mortality, feelings of guilt, low mood, reduced quality of life, disturbed sleep or appetite (1). MDD is one of the most common mental disorders worldwide, with a life time prevalence of 16.2% and a 12-month prevalence of 6.6% in developed countries (2, 3). Furthermore, between 30 and 40% of patients who suffer from MDD never achieve symptom resolution with standard antidepressant treatment (4). Alternative approaches such as cognitive behavioural therapy and lifestyle interventions need highly trained therapists and several weeks to months to achieve effectiveness (5). Therefore, there is a need for new and additional treatment options for depression.
Irritable bowel syndrome (IBS) is characterised by the alterations in bowel function or discomfort, abdominal pain or bloating, and diarrhoea or constipation (6). The prevalence of IBS is estimated between 9 and 23% in the population across the world (6–8) and affects ~21% of the population in South America and ~7% of the population in Southeast Asia (9). Although IBS is classified as a functional gastrointestinal disorder which is a chronic condition, recent developments suggested that IBS may have an impact on extra-intestinal symptoms including genitourinary, musculoskeletal, headaches and fatigue, menstrual, sexual dysfunction, anxiety and mood disorders (7–9). Additionally, clinical symptoms of IBS have been linked with quality of sleep and had co-relation with dementia (10). Thus, patients diagnosed with IBS require specific attention to all psychosocial factors involved including major depression. It has been proposed that gut microbiota is a complex community of over 100 trillion microbial cells, outnumbering the human cells in human bodies by a factor of 10 (11). However, recent findings by Ron Sender et al. (12) suggested that the ratio of bacterial to host cells in humans could be closer to 1:1 which is much lesser compared to earlier reports (12). Regardless of the number of gut microbes reported by various researchers, the role of gut microbiota has been reported to influence human physiology, metabolism, nutrition and immune function, while disruption of gut microbiota has been linked with GI conditions such as IBD, obesity and metabolic disorder (13). It has been postulated that the communication between the gut and brain has multiple routes which include the vagus nerve (VN), the immune system, short-chain fatty acids and tryptophan. However, recent research further suggests that the communication between the gut and the brain may be influenced by the number of the factors, that is, integrity of the intestinal wall, composition/diversity of the gut microbiota and also production of neurotransmitters, hormones, and immune- and neuropeptides by the microbiota in gut (14). Further, several studies have revealed the differences in the composition of the gut microbiota between IBS patients and healthy controls (15). The association between MDD and altered gut microbiota may result in carbohydrate malabsorption and could be associated with mental depression which is defined as a common mental disorder that causes people to experience depressed mood, loss of interest or pleasure, feelings of guilt or low self-worth, disturbed sleep or appetite, low energy and poor concentration (15, 16). Gut microbiota may also be implicated in brain autoimmunity in multiple sclerosis (17). Probiotics are live microorganisms which, when administered in adequate amounts, confer a health benefit on the host (18). Gut microbiota produce immune activating and other signalling molecules (e.g. tryptophan) that may play an important role in regulating the brain and subsequent behaviour (15). On the contrary, bacterial products, such as the Gram-negative endotoxins, can influence mood and cognitive functions (19). Therefore, the modification of microbial ecology by supplementing probiotics may be an alternative option for the management of anxiety and depression (15, 20). LactoSpore®, a commercial proprietary probiotic preparation, is lactose free, non-GMO with GRAS status which contains spores of B. coagulans MTCC 5856 strain (bearing the internal reference number SBC37-01) (21). There are several commercial preparations of B. coagulans reported for the treatment of vaginal infections, adjuvant to antibiotic therapy and lactose intolerance, in human clinical trials (22). Bacillus coagulans MTCC 5856 can withstand high temperature and is found to be stable during processing and storage conditions of functional foods (baked foods, brewed coffee, etc.) (23). Bacillus coagulans MTCC 5856 elicited anti-diarrhoeal activity and inhibited the gastrointestinal motility in an animal model (24). Bacillus coagulans MTCC 5856 was found to be safe and tolerable at a dose of 2 × 109 cfu (spores)/day in humans (25). Further, B. coagulans MTCC 5856 at a dose of 2 × 109 cfu (spores)/day along with standard care of treatment was found to be safe and effective in diarrhoea-predominant IBS patients (22). The effect of B. coagulans MTCC 5856 has not yet been evaluated in patients experiencing IBS symptoms with MDD. Thus, a 90-day randomised, double-blind, placebo-controlled, multi-centre clinical trial was designed to evaluate the safety and efficacy of B. coagulans MTCC 5856 at a dose of 2 × 109 cfu (spores)/day in patients experiencing IBS symptoms with MDD.
Study rationale
The relationships between psychological distress and gastrointestinal symptoms have been reported earlier (26), and most of the patients suffering from IBS identify stress and anxiety as symptom aggravators (27). Microbes are the important link for the communication of the gut–brain axis, and the alteration of gut–brain axis by traditional medicines will be a potential strategy for the management of comorbid central nervous system (CNS) disorders and gastrointestinal problems (15). This suggests that the probiotics or gut microbes may be a useful therapeutic option in stress-related disorders such as depression and anxiety (13, 20). Recent research suggests that the beneficial gut microorganisms may improve the host health both physically and mentally and may be involved in the development of the neural system and behavioural pattern (28). Thus, this placebo-controlled clinical trial was carried out at three sites using B. coagulans MTCC 5856 probiotic in patients with IBS and depression to test the hypothesis that B. coagulans can help in alleviating the symptoms of major depression in patients with IBS.
Experimental procedures
Product description
The investigational product in this study was B. coagulans MTCC 5856 tablets (600 mg) that contained 2 billion spores (333.33 mg), microcrystalline cellulose, starch, sodium starch glycolate and magnesium stearate. Placebo tablets had same ingredients except B. coagulans MTCC 5856. Placebo and investigational products were identical in terms of packaging, taste, colour and texture. B. coagulans MTCC 5856 spore count in the tablet was determined by following pour plate method as described previously (21).
Ethics and informed consent
This trial was conducted in accordance with the clinical research guidelines established by the Drugs and Cosmetics Act, 1940 of India; Drugs and Cosmetics Rules, 1945 of India; Ethical Guidelines for Biomedical Research on Human Participants, 2006 of Indian Council of Medical Research (ICMR) in India; the principles enunciated in the Declaration of Helsinki, Edinburgh (29) (Anonymous, 2000); and the International Conference on Harmonisation (ICH) – harmonised tripartite guideline regarding Good Clinical Practice (GCP). The study was approved by the local ethics committee, a written informed consent was obtained and the study was registered at Clinical Trials Registry − India (www.ctri.nic.in) (identifier: CTRI/2015/05/005754 on 06 May 2015). There were no changes to the methods or planned endpoints after study initiation.
Participants
Subjects were included in the study if indicated ‘Yes’ to all of the inclusion criteria and ‘No’ to all of the exclusion criteria.
Inclusion criteria
Male and/or female subjects ranging in age between 20 and 65 years.
-
Fulfilling Rome III Diagnostic Criteria (30) for Functional IBS. Criterion fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis:
Discomfort or recurrent abdominal pain at least 3 days/month in the last 3 months associated with two or more of the following: improvement with defecation, stool frequency change and change in appearance of stool
Bloating or visible distension at least 3 days/month in the last 3 months
Watery or loose stools without pain occurring in at least 75% of stools
Willingness to follow the protocol requirement as evidenced by written informed consent.
Diagnosed patients with mild to moderate IBS in severity with possible sleep, pain and dementia-associated co-morbidities.
Fulfilling Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (2000) Criteria for MDD.
Willingness to complete subject diaries and study questionnaires.
Agree not to use any medication (prescription and over the counter), including vitamins and minerals, during the course of this study.
Agree not to use any yogurt during the course of this study.
Subjects whose blood chemistries are within a normal range or not considered clinically significant if outside the normal range.
Subject’s assurance that they have not taken antibiotics or other supplements whose primary site of action is in the gastrointestinal tract for a period up to 1 month prior to the start of the study.
Willing to come for regular follow-up visit.
Exclusion Criteria
Any clinically significant medical history, medical finding or an ongoing medical condition exists which in the opinion of the investigator could jeopardise the safety of the subject, impact validity of the study results or interfere with the completion of study according to the protocol.
Significant abnormal findings as determined by baseline history, physical examination, vital signs, haematology, serum chemistry and urinalysis.
History or presence of significant alcoholism or supplement/drug abuse in the past 1 year.
Any medical or surgical conditions which might significantly interfere with the gastrointestinal tract, liver, kidneys and/or blood-forming organs.
History of cardiovascular, renal, hepatic, asthma, glaucoma, pulmonary, neurologic, metabolic or psychiatric disease.
Participation in a clinical study during the preceding 90 days.
History of malignancy or other serious disease.
Any contraindication to blood sampling.
Smoking or consumption of tobacco products.
Blood or blood products donated in past 30 days prior to study supplement administration.
Pregnant female subjects and lactating women.
Prior surgical therapy for obesity.
Patients using yogurt in their daily meal.
Trial Design
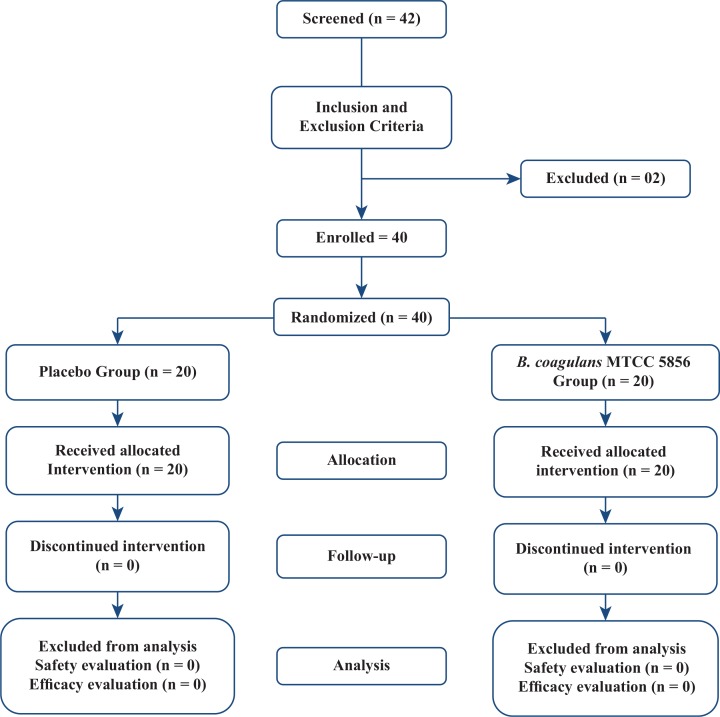
The disposition of the study participants is depicted in Fig. 1. This randomised multi-centre, double-blind, placebo-controlled, parallel-group clinical trial was conducted in India between June 2015 and October 2015 at three different sites: (1) Life Care Hospital, Bangalore, India; (2) Sri Venkateshwara Hospital, Bangalore, India; and (3) Sapthagiri Institute of Medical Sciences and Research Centre, Bangalore, India. The sample size of the study was 40, with 20 subjects randomised to each of the two study arms in a double-blinded manner at a 1:1 ratio. Subjects were blinded and received investigation products dispensed as per randomisation code provided at each site by an authorised person independently. Compliance with study supplement was reviewed at each visit. The daily food intake of the patients was recorded in the patient diaries provided to them at Visit 1 (Day 0). The same was checked and verified at subsequent visits by the investigators. Participants were accompanied by caretakers for their respective visit to the study sites and also were overseen by caretakers during the entire duration of the study. The study consisted of a 90-day intervention period. Subjects visited the study site on screening, baseline/randomisation visit, day 30, day 60, day 90 and day 105. A description of visits 1, 2, 3 and 4 with schedule of events is provided in Table 1.
Fig. 1.
Flow chart of study procedures.
Table 1.
Schedule of events
| Procedure | Screening | Visit 1 (Day 0) Baseline | Visit 2 (Day 30) | Visit 3 (Day 60) | Visit 4 (Day 90) Final visit | Follow-Up visit (At least 15 days from the last visit) |
|---|---|---|---|---|---|---|
| Informed consent | X | |||||
| Medical history | X | |||||
| Physical examination | X | X | X | X | X | |
| Demographicsa | X | X | X | X | X | |
| Vital Signs | X | X | X | X | X | |
| Haematology | X | X | ||||
| Serum chemistry | X | X | ||||
| Myeloperoxidaseb | X | X | ||||
| Urine pregnancy testc | X | |||||
| Randomisation | X | |||||
| Investigational product dispensing | X | X | X | |||
| Gastrointestinal discomfort questionnaire | X | X | X | X | ||
| Irritable bowel syndrome quality of life questionnaire | X | X | X | X | ||
| HAM-D scale | X | X | X | X | ||
| MADRS | X | X | X | X | ||
| CGI-I & CGI-S | X | X | X | X | ||
| CES-D | X | X | X | X | ||
| Dementia checklist | X | X | X | X | ||
| Return of unused IP | X | X | X | |||
| Adverse events | X | X | X | X | X | |
| Concomitant medications | X | X | X | X | X | X |
HAM-D, Hamilton Rating Scale for Depression; MADRS, Montgomery–Asberg Depression Rating Scale; CGI-I & CGI-S, Clinical Global Impression-Improvement Rating Scale and Clinical Global Impression Severity Rating Scale; CES-D, Center for Epidemiological Studies Depression Scale.
Age at screening only.
Only for randomised subjects.
Urine pregnancy test at screening and on early termination, if any.
Intervention
Newly diagnosed patients and patients who were not on any other treatment for major depression with IBS in the past 3 months were enrolled into the study. This criterion was based on physical, medical and medication history of subjects. All enrolled subjects were instructed to self-administer one tablet per day (either Bacillus coagulans MTCC 5856 or placebo) at least 30 min before meal, in the morning as dietary supplement for a period of 90 days.
Efficacy Outcomes
The primary outcome for this study was a mean 90-day change in depression and IBS symptoms as assessed by the Hamilton Rating Scale for Depression (HAM-D) (31), Montgomery–Asberg Depression Rating Scale (MADRS) (32, 33), sleep quality and depressive symptom severity using 11-item Centre for Epidemiological Studies–Depression Scale (CES-D) (34) and Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire (35). Additionally, secondary efficacy assessments included change in Clinical Global Impression-Improvement (CGI-I) Rating Scale, Clinical Global Impression Severity (CGI-S) Rating Scale (36), Dementia – Revised Memory and Behaviour Problem Checklist (RMBPC) (37), Gastrointestinal Discomfort Questionnaire (GI-DQ) (38) and Modified Epworth Sleepiness Scale (mESS) (39) from the baseline till the end of study as subjective test.
Bioassays
Serum myeloperoxidase, an inflammatory biomarker, was also analysed for both the groups (B. coagulans MTCC 5856 and placebo) at baseline and end of the study. The serum myeloperoxidase levels were measured by implementation of enzyme-linked immunosorbent assay (ELISA) techniques according to manufacturer directions (Cayman Chemicals Company, MI, USA).
Safety outcomes
Safety of the study was assessed considering the occurrence of adverse events (AEs), safety blood parameters and change in vital signs (blood pressure and heart rate). Laboratory data were summarised by presenting summary statistics of raw data and change in laboratory values from baseline to end of study relative to normal reference limits. Descriptive physical examinations such as abdomen, extremities, general appearance, head, ear, nose, throat, heart, lungs and neurological were monitored at screening, day 0, day 30, day 60, day 90 and day 120 for safety evaluation. Spontaneously reported or observed AE was assessed at all post-screening study visits. AE was evaluated in terms of intensity (mild, moderate or severe) and possible relationship to the study product. There was only one AE reported which was fever and weakness, which was unrelated and not attributable to the study product because the subject who reported this AE belonged to the placebo group.
Statistical analysis
Statistical Analysis Software (SAS) version 9.2 software was used for data analysis here. Paired ‘t’ test, Analysis of Covariance (ANCOVA) and Wilcoxon signed-rank sum test were used for appropriate data set variables to reach the best possible statistical conclusion between the B. coagulans MTCC 5856-receiving and placebo-receiving groups. Last Observation Carry Forward (LOCF) method was followed for efficacy evaluations of subjects. No formal sample size calculation was performed.
Results
Patient disposition and characteristics
A total of 42 subjects were screened and 40 were enrolled into the study. There were no patient withdrawals or dropouts in this study. Treatment compliance across various visits was checked by the study personnel, and overall treatment compliance for the whole study revealed that 24 (60%) patients met 100% treatment compliance on visit 4 (end of the study) and on an average 66.7% of participants met with 100% treatment compliance during the whole study period (across various visits). At baseline visit (Day 0), no significant difference was observed between the two treatment groups in subject demographics (Table 2). None of the enrolled subjects had abnormal medical history, except for gastrointestinal. Around 3 subjects (7.50%) had earlier GI-related medical history which had no interference with IBS.
Table 2.
Demographics and baseline clinical characteristics
| Placebo (n = 20) | Bacillus coagulans MTCC 5856 (n = 20) | |
|---|---|---|
| Sex, n (%) | ||
| Female | 17 (85) | 17 (85) |
| Male | 03 (15) | 03 (15) |
| Age (years), mean (SD) | ||
| 43.88 ± 9.85 | 40.36 ± 10.28 | |
| Height (cm), mean (SD) | ||
| 157.39 ± 8.49 | 160.1 ± 7.87 | |
| Body mass index (kg/m2) | ||
| 25.9 ± 4.49 | 25.4 ± 4.46 | |
| Smokers, n (%) | ||
| Ex-smoker | 18 (90) | 19 (95) |
| Non-smoker | 01 (5) | 00 |
| Smoker | 01 (5) | 01 (5) |
| Race, n (%) | ||
| Central American | 00 | 00 |
| East Asian | 00 | 00 |
| South Asian | 20 (100) | 20 (100) |
| South American | 00 | 00 |
| South East Asian | 00 | 00 |
| Western European | 00 | 00 |
| White | 00 | 00 |
| Alcohol use | ||
| Non-drinker | 01 (5) | 00 |
| Past drinker | 18 (90) | 19 (95) |
| Occasional drinker | 01 (5) | 01 (5) |
| Current drinker | 00 | 00 |
| Baseline data, mean (SD) | ||
| IBS-QOL | 102.6 ± 21.11 | 106.4 ± 23.44 |
| CGI-I | 3.8 ± 1.01 | 3.7 ± 0.87 |
| CGI-S | 3.7 ± 0.92 | 3.4 ± 0.96 |
| HAM-D | 14.5 ± 3.41 | 13.6 ± 4.41 |
| MADRS | 17.1 ± 4.63 | 16.3 ± 5.40 |
| CES-D | 20.7 ± 4.86 | 19.1 ± 5.25 |
| Dementia – total frequency scoring | 61.3 ± 19.11 | 62.3 ± 17.08 |
| Dementia – total reaction scoring | 61.0 ± 19.83 | 63.8 ± 17.57 |
| mESS | 10.9 ± 2.99 | 10.3 ± 2.43 |
| GI-DQ | 32.5 ± 13.88 | 30.1 ± 15.07 |
CES-D, Center for Epidemiological Studies Depression Scale; CGI-I, Clinical Global Impression-Improvement Rating Scale; CGI-S, Clinical Global Impression Severity Rating Scale; CI, confidence interval; GI-DQ, Gastrointestinal Discomfort Questionnaire; HAM-D, Hamilton Rating Scale for Depression; IBS-QOL, Irritable Bowel Syndrome Quality of Life Questionnaire; MADRS, Montgomery–Asberg Depression Rating Scale; mESS, Modified Epworth Sleepiness Scale.
Values are expressed as mean ± S.D.
Efficacy evaluation
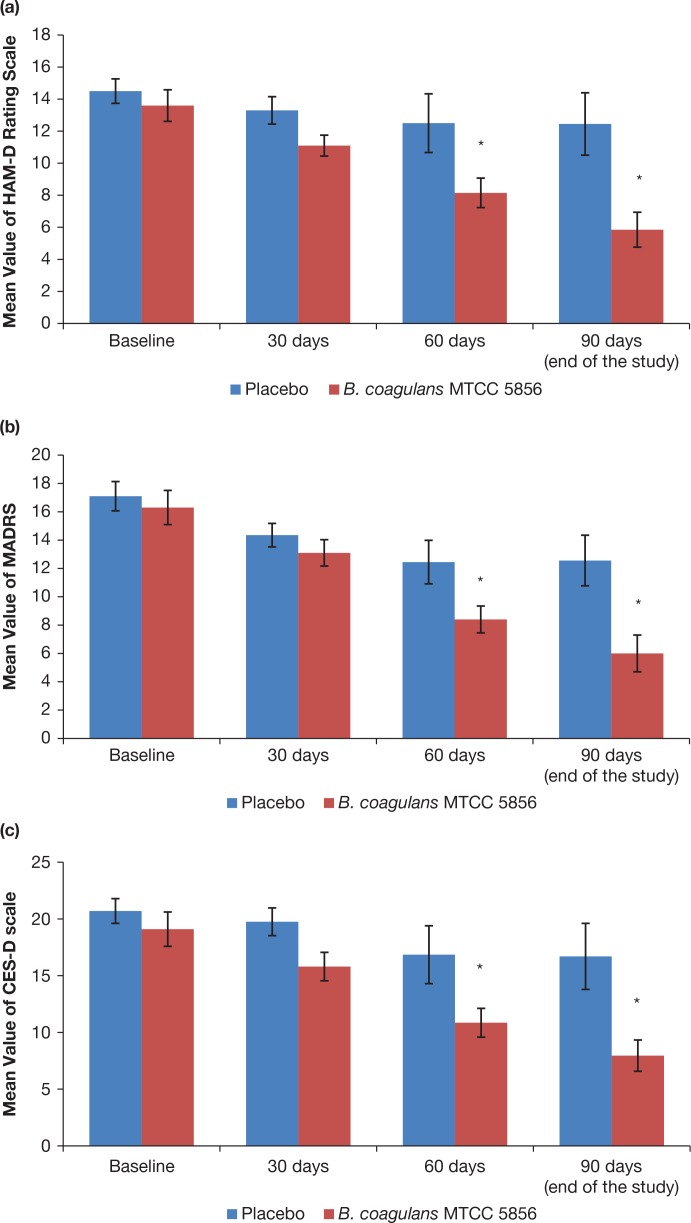
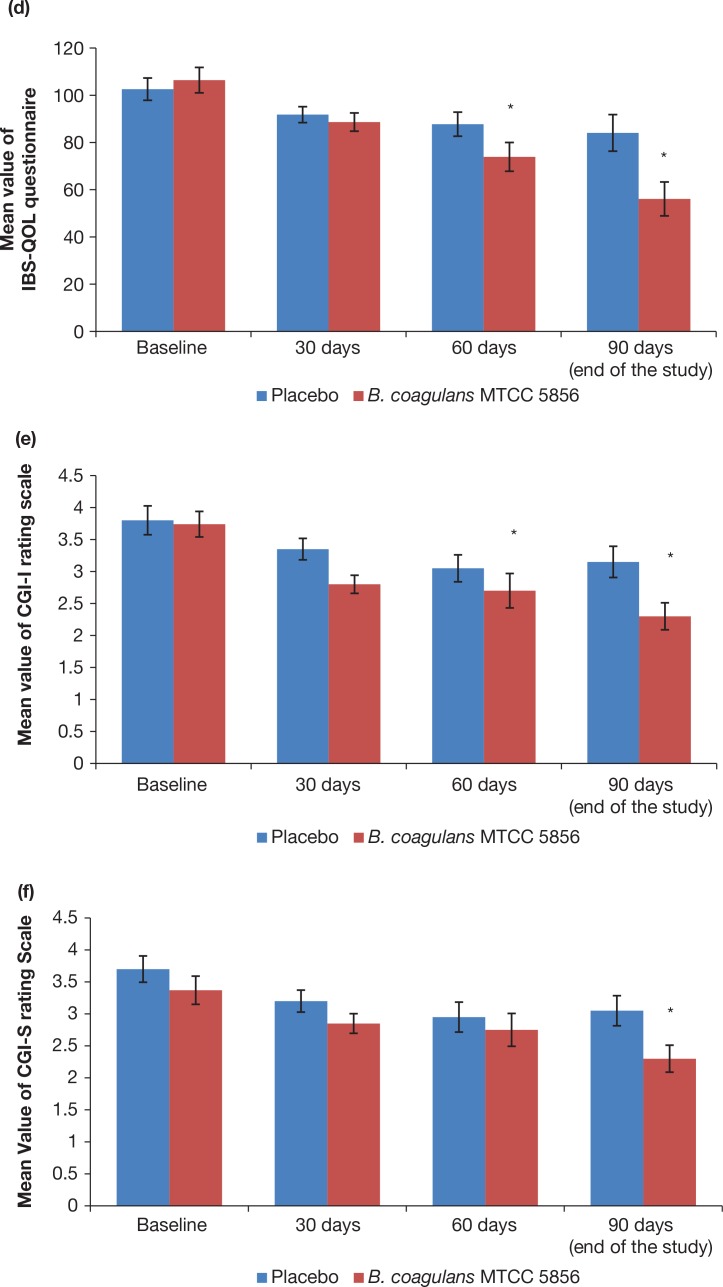
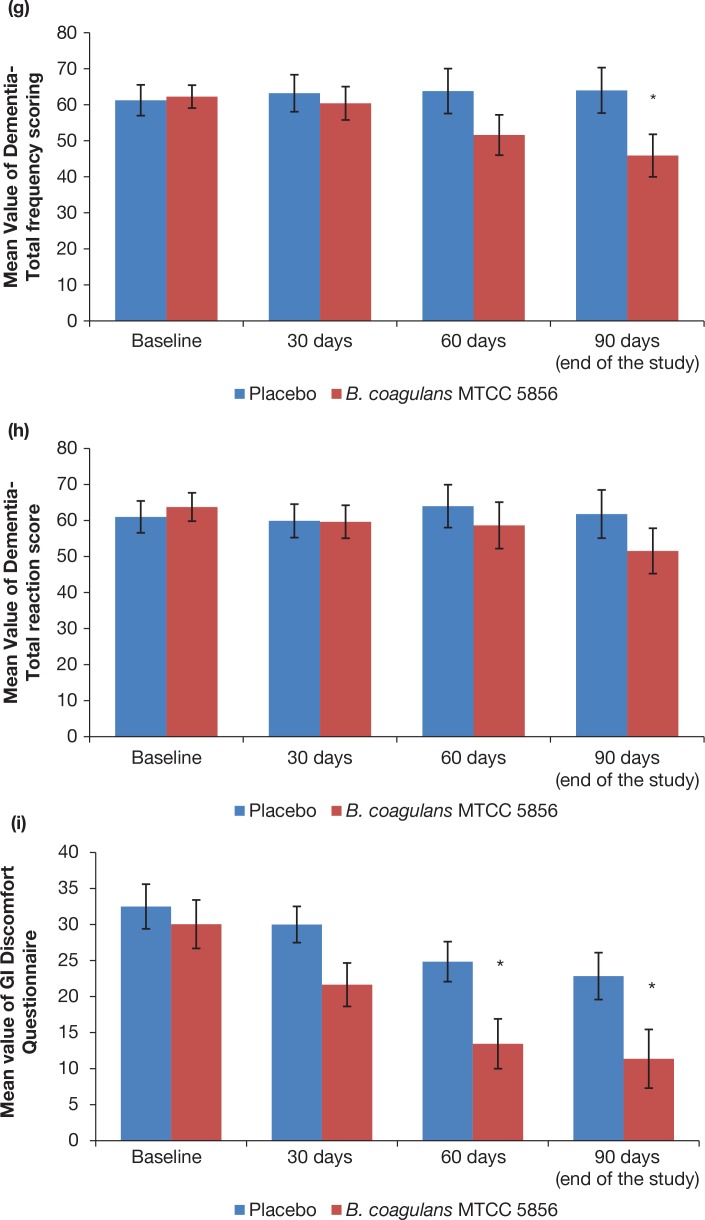
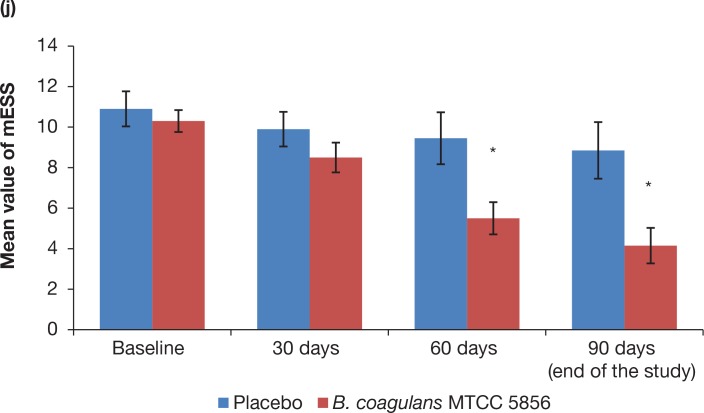
Statistical analysis using Analysis of Co-Variance (ANCOVA) showed that the primary efficacy parameters were found to be statistically significant (p < 0.01) between the B. coagulans MTCC 5856 and placebo groups (Table 3). Similarly, all secondary efficacy parameters were also found to be statistically significant between the B. coagulans MTCC 5856 and placebo groups except for ‘Dementia total reaction scoring’ (Table 3). Furthermore, comparative mean values of efficacy assessments between B. coagulans MTCC 5856 and placebo groups across various visits (baseline, day 30, day 60 and day 90) are as presented for primary efficacy parameters (Fig. 2a–d) and secondary efficacy parameters (Fig. 2e–j). Patients who were on B. coagulans MTCC 5856 had statistically significant difference for the efficacy parameters on day 60 and maintained till the end of the study when compared with placebo (Fig. 2).
Table 3.
Summary of efficacy outcomes at the end of study (day 90): (full analysis set, last observation carried forward, ANCOVA model, 95% CI)
| Efficacy parameters | Bacillus coagulans MTCC 5856 (n = 20) | Placebo (n = 20) | Δ p-value |
|---|---|---|---|
| Primary efficacy outcomes | |||
| HAM-D | |||
| Base line (Day 0) | 13.6 ± 4.41 | 14.5 ± 3.41 | 0.474 |
| End of the study (Day 90) | 5.9 ± 4.88 | 12.5 ± 8.70 | 0.005* |
| Change from baseline to day 90 | p ≤ 0.001* | p = 0.333 | 0.029* |
| MADRS | |||
| Base line (Day 0) | 16.3 ± 5.40 | 17.1 ± 4.63 | 0.618 |
| End of the study (Day 90) | 6.0 ± 5.79 | 12.6 ± 8.00 | 0.007* |
| Change from baseline to day 90 | p ≤ 0.001* | p = 0.056 | 0.031* |
| CES-D | |||
| Base line (Day 0) | 19.1 ± 5.25 | 20.7 ± 4.86 | 0.323 |
| End of the study (Day 90) | 8.0 ± 6.17 | 16.7 ± 13.03 | 0.009* |
| Change from baseline to day 90 | p ≤ 0.001* | p = 0.224 | 0.051* |
| IBS-QOL | |||
| Base line (Day 0) | 106.4 ± 23.44 | 102.6 ± 21.11 | 0.595 |
| End of the study (Day 90) | 56.1 ± 31.26 | 84.1 ± 34.67 | 0.010* |
| Change from baseline to day 90 | p ≤ 0.001* | p = 0.075 | 0.027* |
| Secondary efficacy outcomes | |||
| CGI-I | |||
| Base line (Day 0) | 3.7 ± 0.87 | 3.8 ± 1.01 | 0.835 |
| End of the study (Day 90) | 2.3 ± 0.92 | 3.2 ± 1.09 | 0.011* |
| Change from baseline to day 90 | p ≤ 0.001* | p = 0.096 | 0.141 |
| CGI-S | |||
| Base line (Day 0) | 3.4 ± 0.96 | 3.7 ± 0.92 | 0.277 |
| End of the study (Day 90) | 2.3 ± 0.92 | 3.1 ± 1.05 | 0.022* |
| Change from baseline to day 90 | p = 0.009* | p = 0.058 | 0.396 |
| Dementia – TFS | |||
| Base line (Day 0) | 62.3 ± 17.08 | 61.3 ± 19.11 | 0.862 |
| End of the study (Day 90) | 45.9 ± 26.42 | 64.0 ± 28.26 | 0.043* |
| Change from baseline to day 90 | p = 0.0046* | p = 0.592 | 0.011* |
| Dementia – TRS | |||
| Base line (Day 0) | 63.8 ± 17.57 | 61.0 ± 19.83 | 0.645 |
| End of the study (Day 90) | 51.6 ± 28.19 | 61.8 ± 29.94 | 0.118 |
| Change from baseline to day 90 | p = 0.047* | p = 0.880 | 0.103 |
| GI-DQ | |||
| Base line (Day 0) | 30.1 ± 15.07 | 32.5 ± 13.88 | 0.596 |
| End of the study (Day 90) | 11.4 ± 18.23 | 22.9 ± 14.55 | 0.035* |
| Change from baseline to day 90 | p ≤ 0.001* | p = 0.058 | 0.132 |
| mESS | |||
| Base line (Day 0) | 10.3 ± 2.43 | 10.9 ± 2.99 | 0.490 |
| End of the study (Day 90) | 4.2 ± 3.92 | 8.9 ± 6.24 | 0.007* |
| Change from baseline to day 90 | p ≤ 0.001* | p = 0.171 | 0.018* |
Δ Between-group comparisons were made using the ANCOVA. Within-group comparisons were made using the paired Student’s t-test. *Probability (p) values ≤0.05 are statistically significant.
ANCOVA, analysis of covariance; CES-D, Center for Epidemiological Studies Depression Scale; CGI-I, Clinical Global Impression-Improvement rating Scale; CGI-S, Clinical Global Impression Severity Rating Scale; CI, confidence interval; GI-DQ, Gastrointestinal Discomfort Questionnaire; HAM-D, Hamilton Rating Scale for Depression; IBS-QOL, Irritable Bowel Syndrome Quality of Life Questionnaire; MADRS, Montgomery–Asberg Depression Rating Scale; mESS, Modified Epworth Sleepiness Scale; Dementia – TFS, Dementia – Total frequency scoring; Dementia – TRS, Dementia – Total reaction scoring.
p-value significant (<0.05).
Values are expressed as mean ± SD.
Fig. 2.
Primary and secondary efficacy measures at baseline, 30, 60 and 90 days (end of the study). All the values are expressed as mean ± S.E. (a) Hamilton Depression Rating Scale (HAM-D) on a scale of 0 to 20. (b) Montgomery–Asberg Depression on a scale of 0 to 20. (c) Center for Epidemiologic Studies Depression on a scale of 0 to 30. (d) IBS – Quality of Life score on a scale of 150. High QOL value indicates poor quality of life. (e) Clinical Global Impression-Improvement Rating on a scale of 0 to 5. (f) Clinical Global Impression-Severity Rating on a scale of 0 to 5. (g) Dementia – Total Frequency Scoring on a scale of 0 to 100. (h) Dementia – Total Reaction Scoring on a scale of 0 to 100. (i) Gastrointestinal Discomfort Questionnaire on a scale of 0 to 40. Low value indicates less GI discomfortness. (j) Modified Epworth Sleepiness scoring on a scale of 0 to 15. *p < 0.05 between the treatment groups and also between baseline and end of the study (day 90).
Bioassay
The level of serum myeloperoxidase was significantly reduced (p < 0.01) from the baseline to the end of study (day 90) in patients receiving 2 × 109 spores (cfu)/day of B. coagulans MTCC 5856 (Table 4). However, no significant change in the level of serum myeloperoxidase was observed in the placebo group (p > 0.05) from the baseline to the end of study (day 90) (Table 4).
Table 4.
Effect of Bacillus coagulans MTCC 5856 on the serum myeloperoxidase
| Serum myloperoxidase (ng/mL) | |||
|---|---|---|---|
| Bacillus coagulans MTCC 5856 (n = 20) | Placebo (n = 20) | p-value | |
| Baseline (Day 0) | 12.6 ± 4.31 | 9.81 ± 4.10 | p = 0.0460 |
| End of the study (Day 90) | 7.7 ± 2.57 | 9.8 ± 4.65 | p ≤ 0.01 |
| Change from baseline to day 90 | p ≤ 0.01 | p = 0.9886 | p ≤ 0.01 |
Serum myeloperoxidase was quantified at baseline (Day 0) and end of the study (day 90) of both B. coagulans MTCC 5856 and placebo group. ANCOVA was performed between the group and t-test was performed within the group. Values expressed as mean ± S.D. p < 0.05 considered as significant.
Safety Evaluations
Vital signs such as blood pressure, respiratory rate, pulse rate and any abnormal lab/diagnostic parameters were considered for safety evaluations. No clinically significant changes were recorded for descriptive physical examination in either of the group. Further, no clinically significant abnormal lab values (biochemistry and haematology) were identified (Table 5), and no statistically significant changes in the vitals were observed from the baseline to final visit (Table 6). No serious AEs or significant AEs were noticed in this study. There was only one AE reported, fever and weakness, which resolved without the use of any concomitant medication(s). The unblinding of study product code towards end of the study revealed that the patient with AE belonged to the placebo group.
Table 5.
Biochemistry and haematology values between two treatment groups
| Lab parameter (units) | Visit | Placebo (n = 20) | Bacillus coagulans MTCC 5856 (n = 20) | Normal range |
|---|---|---|---|---|
| Alanine aminotransferase (IU/L) | Screening | 22.6 ± 8.19 | 27.1 ± 15.30 | 0–41 |
| Final visit | 26.9 ± 26.87 | 31.0 ± 25.18 | ||
| Albumin (g/dL) | Screening | 4.5 ± 0.33 | 4.6 ± 0.32 | 3.5–5.2 |
| Final visit | 4.4 ± 0.32 | 4.3 ± 1.01 | ||
| Alkaline Phosphatase (U/L) | Screening | 206.2 ± 84.44 | 210.1 ± 97.16 | 53–128 |
| Final visit | 196.1 ± 72.90 | 214.3 ± 75.42 | ||
| Aspartate aminotransferase (IU/L) | Screening | 25.1 ± 5.95 | 26.4 ± 7.00) | 0–40 |
| Final visit | 27.1 ± 16.69 | 27.3 ± 13.79 | ||
| Blood urea nitrogen (mg/dL) | Screening | 12.9 ± 3.00 | 13.0 ± 5.87 | 5.0–24 |
| Final visit | 11.5 ± 2.75 | 11.4 ± 2.38 | ||
| Fasting blood sugar (mg/dL) | Screening | 104.5 ± 51.03 | 104.1 ± 69.14 | 70–110 |
| Final visit | 105.9 ± 56.70 | 107.2 ± 78.78 | ||
| LDL cholesterol (mg/dL) | Screening | 121.6 ± 24.67 | 127.2 ± 42.56 | Up to 140 |
| Final visit | 123.0 ± 31.43 | 121.1 ± 44.51 | ||
| Potassium (mEq/L) | Screening | 4.8 ± 0.41 | 4.7 ± 0.45 | 3.5–5.2 |
| Final visit | 5.7 ± 1.76 | 7.3 ± 7.44 | ||
| Serum creatinine (mg %) | Screening | 0.8 ± 0.12 | 1.2 ± 1.84 | 0.6–1.4 |
| Final visit | 1.2 ± 2.07 | 0.8 ± 0.12 | ||
| Sodium (mEq/L) | Screening | 140.8 ± 2.63 | 133.2 ± 30.13 | 136–145 |
| Final visit | 138.5 ± 2.89 | 132.6 ± 21.55 | ||
| Total Bilirubin (mg/dL) | Screening | 1.1 ± 0.44 | 1.4 ± 0.72 | 0.1–1.2 |
| Final visit | 1.3 ± 0.57 | 1.6 ± 1.14 | ||
| Total protein (g/dL) | Screening | 7.6 ± 0.53 | 7.6 ± 0.44 | 6.22–8.0 |
| Final visit | 7.4 ± 0.46 | 7.4 ± 0.56 | ||
| Erythrocyte count (×106 cells) | Screening | 4.4 ± 0.61 | 4.6 ± 0.53 | 4.0–6.5 |
| Final visit | 4.6 ± 0.65 | 4.6 ± 0.39 | ||
| Haematocrit (%) | Screening | 37.9 ± 5.37 | 41.5 ± 3.60 | 40–50 |
| Final visit | 37.0 ± 5.63 | 38.1 ± 8.72 | ||
| Haemoglobin (gm %) | Screening | 11.9 ± 2.00 | 13.2 ± 1.52 | 11–16 |
| Final visit | 11.7 ± 2.06 | 12.5 ± 2.15 | ||
| Leukocyte count (cells cu. mm-1) | Screening | 8562.5 ± 1930.07 | 9233.3 ± 2369.35 | 4,000–11,000 |
| Final visit | 8575.0 ± 2879.49 | 8157.5 ± 2413.95 | ||
| Platelet count (×105 per cu. mm) | Screening | 2.7 ± 0.97 | 2.8 ± 0.80 | 1.5–4.5 |
| Final visit | 2.4 ± 0.91 | 2.3 ± 0.70 |
Values are expressed as mean ± S.D.
Table 6.
Change in mean vital signs from baseline to the end of study (90 days)
| Vital parameter | Supplements | Baseline | Day 90 (end of the study) | Change | p |
|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | B. coagulans MTCC 5856 | 122.5 | 121.0 | –1.50 | 0.2674 |
| Placebo | 123.5 | 120.0 | –3.50 | 0.0308 | |
| Diastolic blood pressure (mmHg) | B. coagulans MTCC 5856 | 77.0 | 79.5 | 2.50 | 0.1713 |
| Placebo | 80.5 | 80.0 | –0.50 | 0.7157 | |
| Pulse rate (beats per minute) | B. coagulans MTCC 5856 | 74.6 | 74.6 | 0.00 | 1.0000 |
| Placebo | 76.1 | 74.5 | –1.55 | 0.0841 | |
| Heart rate (beats per minute) | B. coagulans MTCC 5856 | 74.7 | 74.8 | 0.10 | 0.8660 |
| Placebo | 75.8 | 74.8 | –1.00 | 0.1467 | |
| Respiratory rate (breaths per minute) | B. coagulans MTCC 5856 | 21.1 | 21.1 | 0.00 | 1.0000 |
| Placebo | 21.4 | 20.8 | –0.60 | 0.1240 | |
| Weight (kg) | B. coagulans MTCC 5856 | 65.7 | 66.7 | 1.04 | 0.0589* |
| Placebo | 63.1 | 64.0 | 0.91 | 0.0785 | |
| Body mass index (kg/m2) | B. coagulans MTCC 5856 | 25.6 | 25.7 | 0.01 | 0.8956 |
| Placebo | 25.7 | 25.8 | 0.01 | 0.8969 |
p-value is estimated from paired t-test; p < 0.05 is considered as significant.
Discussion
In our previous study, B. coagulans MTCC 5856 treatment exerted a significant change/decrease in the clinical symptoms of IBS such as bloating, vomiting, diarrhoea, abdominal pain and stool frequency towards end of the study (22). Consequently, this study was conducted to evaluate the effect of B. coagulans MTCC 5856 on the clinical symptoms of major depression with IBS. Patients who were not on any other treatment in the past 3 months for major depression with IBS were enrolled. All subjects were asked to self-administer one tablet per day (either Bacillus coagulans MTCC 5856 or placebo) at least 30 min before a meal, in the morning for a period of 90 days. Significant change (p = 0.01) in favour of the probiotic (B. coagulans MTCC 5856) was observed for the primary efficacy measure HAM-D, MADRS, CES-D and IBS-QOL (Table 3). This significant change in the scores of HAM-D, MADRS, CES-D and IBS-QOL is the most relevant parameter to evaluate the clinical significance of depression and IBS (33-34, 38). Additionally, statistical analysis revealed that all primary efficacy measures were statistically significant at day 60 and maintained the same up to end of the study (day 90) (Fig. 2a–d). Similarly, secondary efficacy measures also showed significant results (p = 0.01) in B. coagulans MTCC 5856 group compared to placebo group except dementia total reaction scoring (Fig. 2e–j). Overall, subjects receiving B. coagulans MTCC 5856 (2 × 109 spores) reported a significant change/decrease in their depression clinical symptoms along with decrease in IBS towards end of the study than patients receiving placebo (Table 3). Furthermore, B. coagulans MTCC 5856 was also found to be very beneficial for sleeplessness and, to a lesser extent, for dementia in the current set of patients (Table 3 and Fig. 2g, h). However, these additional benefits may need to be confirmed through extensive further clinical studies on larger population of people with depression and IBS. Recent pre-clinical and clinical research reveals that the human microbiota plays a pivotal role in cognitive and affective functioning (13, 15, 20). This has led to the hypothesis that probiotic supplementation may act as an adjuvant therapy to ameliorate or prevent depression. Gut bacteria have shown to affect depression- and anxiety-like behaviour in animal models (40).
Several human studies suggested that probiotic strains have shown to improve the clinical symptoms such as cognitive performance beneficial effects on anxiety, depression and emotional arousal (13, 15, 40, 41). Moreover, these studies were conducted in healthy subjects and its clinical relevance to disease remains unclear. This is the first study to report the clinical safety and efficacy of B. coagulans MTCC 5856 for the management of MDD in IBS patients. However, a recent placebo-controlled trial concluded that the probiotic Bifidobacterium longum NCC3001 reduces depression but not anxiety scores and increases quality of life in patients with IBS (42). This study provides the additional evidence that probiotics may ameliorate the symptoms of depression in patients with IBS.
Myeloperoxidase (MPO) among other cellular enzymes is responsible for the production of free radicals which leads to the cellular oxidative stress (43). Oxidative stress is linked with the variety of human conditions such as rheumatoid arthritis, Alzheimer’s disease, Parkinson’s disease, cancers, cardiovascular diseases, depression and other neurodegenerative disease (43, 44). MPO has also been studied in various clinical trials as an inflammatory biomarker and used to diagnose the IBD and pro-oxidative processes in patients with depression (44, 45). By considering the clinical importance of MPO and its clinical relation with IBS and depression, we investigated the serum level of MPO in this study. In this study, myeloperoxidase demonstrated a significant decrease in B. coagulans MTCC 5856 (2 × 109 spores) receiving patients from the baseline to the end of study (day 90) unlike the placebo group. This further validates the clinical efficacy of B. coagulans MTCC 5856 in patients experiencing major depression symptoms with IBS.
Despite availability of ample data, the precise mechanism of action of the probiotics in alleviating depression symptoms still remains to be confirmed. However, the basic mechanism of action in disease state (IBS) is mediated by inflammation triggered by loss of the natural eubiotic and its progression towards loss of homeostasis, that is, dysbiosis (9, 13). Further, low-grade, often chronic inflammation and/or immune activation may further increase the risk factor in mood disorders such as depression, anorexia nervosa, obsessive compulsive disorder and autism (46). However, it is also suggested that the production of certain neurotransmitters, hormones, and immune- and neuropeptides and short-chain fatty acids by the probiotics may help in alleviating depression symptoms (46). Likewise, probiotic strain B. coagulans MTCC 5856 has also been reported to produce short-chain fatty acids (acetic, propionic and butyric acid), antimicrobial and anti-inflammatory substances (47, 48). Thus, it may be proposed that production of short-chain fatty acids (acetic, propionic and butyric acid), antimicrobial and anti-inflammatory substances by the B. coagulans MTCC 5856 could be the possible mechanism of action in alleviating depression symptoms. However, further studies are warranted in order to elucidate the precise mechanism of action of probiotic strain B. coagulans MTCC 5856 in managing MDD in IBS patients.
In 2008, European Food Safety Authority granted the Qualified Presumption of Safety (QPS) status to B. coagulans (49), and the Japanese Ministry of Health and Welfare also approved B. coagulans for the improvement in symptoms caused by abnormalities in the intestinal flora or in dysbiosis (22, 25). Additionally, US FDA issued a ‘no questions’ letter to the GRAS notice on the use of B. coagulans MTCC 5856 spore preparations to be used at a maximum level of approximately 2 × 109 cfu/serving in several food categories. Further to the reported safety evidences, we evaluated safety profile of B. coagulans MTCC 5856 at a dose of 2 × 109 cfu per day in patients experiencing major depression with IBS for the 90 days of supplementation. In this trial, there were no serious AEs or significant AEs reported by the patients receiving B. coagulans MTCC 5856 (2 × 109 spores) during the study period suggesting its safety and tolerability in this cohort of patients. There were no clinically abnormal laboratory values and changes in the vital signs between B. coagulans MTCC 5856 and placebo groups (p > 0.05). Thus, B. coagulans MTCC 5856 was found to be safe at a dose of 2 × 109 spores (cfu) per day in patients suffering from MDD with IBS symptoms when supplemented for 90 days.
Conclusions
This is the first investigation of B. coagulans MTCC 5856 as a single probiotic agent at a dose of 2 × 109 spores (cfu) per day which demonstrated efficacy and safety in the patients experiencing MDD symptoms with IBS. The findings of the study indicate that the B. coagulans MTCC 5856 may be a new and alternative approach for the management of MDD in IBS patients. However, further prospective, larger-scale trials with extended follow-up durations are warranted in order to establish underlying mechanism as well as a detailed assessment of therapeutic effects of B. coagulans MTCC 5856 supplementation in managing MDD in IBS patients.
Acknowledgement
We thank clinical trial investigators Dr. Naveen T K, Life Care Hospital; Dr. N. Vijaya Kumar, Sri Venkateshwara Hospital; and Dr. Mohan Kumar R, Sapthagiri Institute of Medical Sciences and Research Centre; and their team members for conducting this clinical trial.
The authors also recognise the following intellectual property rights for ingredients used in the current clinical study.
LACTOSPORE STABLE PROBIOTIC® is a registered logo (U.S Trademark Registration No. 4068336) of Sabinsa Corporation, 20 Lake Drive, East Windsor, NJ, USA 08520.
LACTOSPORE® is a registered brand name (U.S Trademark Registration No. 1701366) of Sabinsa Corporation, 20 Lake Drive, East Windsor, NJ, USA 08520.
The US patent application (15/198158) and PCT application (PCT/US16/40290) of this study have been filed on 30 June 2016.
Conflict of interest and funding
The authors are employees of Sabinsa Corporation/Sami Labs Limited, manufacturer and marketer of LactoSpore®. This work was sponsored and supported by Sabinsa Corporation, NJ 08520, USA.
References
- 1.Uher R, Payne JL, Pavlova B, Perlis RH. Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress Anxiety 2014; 31: 459–71. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Depression. Available from: http://www.who.int/mental_health/management/depression/en/ 2009. Retrieved December 30, 2017. [Google Scholar]
- 3.CBHSQ. Center for Behavioral Health Statistics and Quality Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). 2015; Available from http://www.samhsa.gov/data/. Retrieved December 30, 2017. [Google Scholar]
- 4.Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A. Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry 2016; 3(5): 425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoifodt RS, Strom C, Kolstrup N, Eisemann M, Waterloo K. Effectiveness of cognitive behavioural therapy in primary health care: a review. Fam Pract 2011; 28(5): 489–504. [DOI] [PubMed] [Google Scholar]
- 6.World Gastroenterology Organization (WGO) Irritable bowel syndrome: a global perspective. World Gastroenterology Organisation Global Guideline. Available from: http://www.jupiterpharma.in/journalpdf/IBS WORLD GASTRO.pdf 2009. Retrieved December 30, 2017. [Google Scholar]
- 7.Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. . Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 2014; 109: 1547–61. [DOI] [PubMed] [Google Scholar]
- 8.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712–21. [DOI] [PubMed] [Google Scholar]
- 9.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014; 6: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C-H, Lin C-L, Kao C-H. Irritable bowel syndrome is associated with an increased risk of dementia: a nationwide population-based study. PLoS One 2016; 11(1): e0144589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekirov I, Russell SL, Antunes LC. Gut microbiota in health and disease. Physiol Rev 2010; 90: 859–904. [DOI] [PubMed] [Google Scholar]
- 12.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016; 164: 337–40. [DOI] [PubMed] [Google Scholar]
- 13.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 2015; 26: 26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moschopoulos C, Kratimenos P, Koutroulis I, Shah BV, Mowes A, Bhandari V. The neurodevelopmental perspective of surgical necrotizing enterocolitis: the role of the gut-brain axis. Mediators Inflamm 2018; 7456857: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota–gut–brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol 2014; 592: 2989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledochowski M, Widner B, Sperner-Unterweger B. Carbohydrate malabsorption syndromes and early signs of mental depression in females. Dig Dis Sci 2000; 45: 1255–9. [DOI] [PubMed] [Google Scholar]
- 17.Berer K, Krishnamoorthy G. Commensal gut flora and brain autoimmunity: a love or hate affair? Acta Neuropathol 2012; 123: 639–51. [DOI] [PubMed] [Google Scholar]
- 18.FAO/WHO (2002). Report of a joint FAO/WHO expert consultation on guidelines for the evaluation of probiotics in food. London: World Health Organization and Food and Agriculture Organization of the United Nations. [Google Scholar]
- 19.Krabbe KS, Reichenberg A, Yirmiya R. Low-dose endotoxemia and human neuropsychological functions. Brain Behav Immun 2005; 19: 453–60. [DOI] [PubMed] [Google Scholar]
- 20.Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun 2015; 48: 258–64. [DOI] [PubMed] [Google Scholar]
- 21.Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Eshuis-de Ruiter T, Booij-Veurink J, et al. . Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: a commercial probiotic strain. World J Microbiol Biotechnol 2016; 32: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F, Pande A, et al. . Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant irritable bowel syndrome: a double blind randomized placebo controlled pilot clinical study. Nutr J 2016; 15: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majeed M, Majeed S, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F, et al. . Evaluation of the stability of Bacillus coagulans MTCC 5856 during processing and storage of functional foods. Int J Food Sci Technol 2016; 51(4): 894–901. [Google Scholar]
- 24.Majeed M, Natarajan S, Sivakumar A, Ali F, Pande A, Majeed S, et al. . Evaluation of anti-diarrhoeal activity of Bacillus coagulans MTCC 5856 and its effect on gastrointestinal motility in Wistar rats. Int J Pharma Bio Sci 2016; 7: 311–16. [Google Scholar]
- 25.Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Pande A, Majeed S, et al. . A double-blind, placebo-controlled, parallel study evaluating the safety of Bacillus coagulans MTCC 5856 in healthy individuals. J Clin Toxicol 2016; 6: 283. [Google Scholar]
- 26.Choung RS, Locke GR III, Zinsmeister AR, Schleck CD, Talley NJ. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am J Gastroenterol 2009; 104: 1772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacy BE, Weiser K, Noddin L, Robertson DJ, Crowell MD, Parratt-Engstrom C, et al. . Irritable bowel syndrome: patients’ attitudes, concerns and level of knowledge. Aliment Pharmacol Ther 2007; 25: 1329–41. [DOI] [PubMed] [Google Scholar]
- 28.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015; 17(5): 565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anonymous, 52nd WMA General Assembly, Edinburgh, Scotland 2000. Available from: http://www.wma.net/en/30publications/10policies/b3/. Retrieved December 10, 2017.
- 30.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006; 130: 1377–90. [DOI] [PubMed] [Google Scholar]
- 31.Friedrich M, Grady SE, Wall GC. Effects of antidepressants in patients with irritable bowel syndrome and comorbid depression. Clin Ther 2010; 32, 1221–33. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, et al. . A randomized controlled trial of a probiotic, VSL#3 on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2003; 17: 895–904. [DOI] [PubMed] [Google Scholar]
- 33.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. . Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 2011; 108: 16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, et al. . A randomized controlled trial of a probiotic combination VSL#3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil 2005; 17: 687–96. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchiya J, Barreto R, Okura R, Kawakita S, Fesce E, Marotta F. Single blind follow-up study on the effectiveness of a symbiotic preparation in irritable bowel syndrome. Chin J Dig Dis 2004; 5: 169–74. [DOI] [PubMed] [Google Scholar]
- 36.Baron M. A patented strain of Bacillus coagulans increased immune response to viral challenge. Postgrad Med 2009; 121: 114–18. [DOI] [PubMed] [Google Scholar]
- 37.Jensen GS, Benson KF. Carter SG. GanedenBC30 cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunol 2010; 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. . Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profile. Gastroenterology 2005; 128: 541–51. [DOI] [PubMed] [Google Scholar]
- 39.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–5. [DOI] [PubMed] [Google Scholar]
- 40.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010; 170: 1179–88. [DOI] [PubMed] [Google Scholar]
- 41.Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011; 2: 256–61. [DOI] [PubMed] [Google Scholar]
- 42.Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, et al. . Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 2017; 153(2): 448–59. [DOI] [PubMed] [Google Scholar]
- 43.Ahmadinejad F, Møller SG, Hashemzadeh-Chaleshtori M, Bidkhori G, Jami MS. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017; 6: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talarowska M, Bobin´ska K, Zajączkowska M, Su KP, Maes M, Gałecki P. Impact of oxidative/nitrosative stress and inflammation on cognitive functions in patients with recurrent depressive disorders. Med Sci Monit 2014; 20: 110–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansberry DR, Shah K, Agarwal P, Agarwal N. Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus 2017; 9(1):e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toribio-Mateas M. Harnessing the power of microbiome assessment tools as part of neuroprotective nutrition and lifestyle medicine interventions. Microorganisms 2018; 6(2): 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majeed M, Majeed S, Nagabhushanam K, Arumugam S, Natarajan S, Beede K, et al. . Galactomannan from Trigonella foenum−graecum L. seed: prebiotic application and its fermentation by the probiotic Bacillus coagulans strain MTCC 5856. Food Sci Nutri 2018; 6(3): 666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majeed M, Nagabhushanam K, Arumugam S, Natarajan S, Majeed S, Beede K, et al. . Cranberry seed fiber: a promising prebiotic fiber and its fermentation by the probiotic Bacillus coagulans MTCC 5856. Int J Food Sci Technol 2018. doi: 10.1111/ijfs.13747 Int J Food Sci Technol 2018; 53(7): 1640–1647. [DOI] [Google Scholar]
- 49.EFSA. BIOHAZ Panel (EFSA Panel on Biological Hazards) Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA J 2013; 11(11): 3449, 107 pp. [Google Scholar]