Abstract
Objective
Recent studies have suggested a critical role for toll-like receptor 4 (TLR4) in the development of alcoholic liver disease. As TLR4 is widely expressed throughout the body, it is unclear which TLR4-expressing cell types contribute to alcohol-induced liver damage.
Methods
We selectively ablated TLR4 in hepatocytes and myeloid cells. Male mice were fed a liquid diet containing either 5% alcohol or pair-fed a control diet for 4 weeks to examine chronic alcohol intake-induced liver damage and inflammation. In addition, mice were administered a single oral gavage of alcohol to investigate acute alcohol drinking-associated liver injury.
Results
We found that selective hepatocyte TLR4 deletion protected mice from chronic alcohol-induced liver injury and fatty liver. This result was in part due to decreased expression of endogenous lipogenic genes and enhanced expression of genes involved in fatty acid oxidation. In addition, mice lacking hepatocyte TLR4 exhibited reduced mRNA expression of inflammatory genes in white adipose tissue. Furthermore, in an acute alcohol binge model, hepatocyte TLR4 deficient mice had significantly decreased plasma alanine transaminase (ALT) levels and attenuated hepatic triglyceride content compared to their alcohol-gavaged control mice. In contrast, deleting TLR4 in myeloid cells did not affect the development of chronic-alcohol induced fatty liver, despite the finding that mice lacking myeloid cell TLR4 had significantly reduced circulating ALT concentrations.
Conclusions
These findings suggest that hepatocyte TLR4 plays an important role in regulating alcohol-induced liver damage and fatty liver disease.
Keywords: Alcoholic fatty liver disease, Toll-like receptor 4, Hepatocyte, Myeloid cell, Liver injury, Inflammation
Highlights
-
•
Hepatocyte TLR4 ablated mice were protected from both chronic and acute alcohol-induced hepatic triglyceride accumulation.
-
•
Hepatocyte TLR4 ablated mice showed attenuated inflammation in the fat pad and the circulation after chronic alcohol intake.
-
•
Loss of TLR4 in myeloid cells did not affect alcohol-induced development of fatty liver.
1. Introduction
Overconsumption of alcohol is a serious, worldwide health problem. Evidence suggests that inflammation is an essential factor contributing to the initiation and progression of alcoholic liver disease (ALD) [1]. Ethanol metabolites can directly induce inflammation via the activation of NF-κB and production of tumor necrosis factor alpha (Tnfα) [2]. In addition, alcohol impairs gut permeability, leading to translocation of endotoxin/lipopolysaccharide (LPS) to the portal vein and circulation [3], [4], [5], [6]. Circulating LPS interacts with its receptor, toll-like receptor 4 (TLR4), to activate signal transduction and generate inflammatory cytokines, including Tnfα and interleukin 1β (IL-1β) [7]. Previous studies have shown that mutant TLR4 mice [8], or mice deficient in TLR4 [9], [10], display attenuated alcohol-induced fatty liver disease, suggesting a direct role for TLR4 in the development of ALD.
The specific TLR4-expressing cells that contribute to alcohol-induced liver damage remain unclear. TLR4 is widely expressed in several cell types, including hepatocytes [11], [12], [13], [14], [15], [16], [17]. Despite mediating a relatively low inflammatory response [11], [18], [19], several recent studies, including our own, have demonstrated the critical role of hepatocyte TLR4 in disease development. Hepatocyte TLR4 regulates obesity-associated hepatic fat accumulation, inflammation, and insulin resistance [19], [20], [21]. Furthermore, hepatocyte TLR4 mediates the synergistic effects of alcohol and hepatitis C virus-induced liver damage and oncogenesis [12]. These observations led us to hypothesize that hepatocyte TLR4 may regulate alcohol-induced fatty liver disease.
Accumulating evidence also suggests a critical role for macrophage TLR4 in ALD. For instance, Inokuchi et al. have reported that bone marrow-derived TLR4-expressing cells contribute to alcohol-induced steatohepatitis [10]. While bone marrow-derived cells include a variety of TLR4-expressing cell types, we specifically focused on the role of myeloid cell TLR4 in alcohol-induced liver damage and fatty liver development.
In this study, we showed that selective hepatocyte TLR4 deletion protected mice from chronic alcohol-induced liver injury and fatty liver. We found that mice lacking hepatocyte TLR4 had decreased expression of endogenous lipogenic genes, enhanced expression of genes involved in fatty acid oxidation, and reduced expression of inflammatory genes in white adipose tissue. Furthermore, in an acute alcohol binge model, hepatocyte TLR4 deficient mice had significantly decreased plasma alanine transaminase (ALT) levels and attenuated hepatic triglyceride content compared to alcohol-gavaged control mice. In contrast, while mice lacking myeloid cell TLR4 had significantly reduced circulating ALT concentrations, the development of chronic alcohol-induced fatty liver was not affected. Taken together, these findings suggest that hepatocyte TLR4 plays an important role in regulating both chronic and acute alcohol-induced liver damage and fatty liver disease.
2. Materials and methods
2.1. Animal care and alcohol feeding protocol
Tlr4LKO, Tlr4ΔmΦ mice and their corresponding littermate control Tlr4fl/fl mice have been previously described [19]. 10–12 week old male mice on a mixed (B6/129) genetic background were subjected to a modified alcohol feeding protocol to induce early-stage alcoholic liver injury [22]. Mice were acclimated to a control liquid diet (Bio-Serv, F1259SP) for the first day. From days 2–5, a subset of mice was given the liquid diet (Bio-Serv, F1258SP) supplemented with increasing concentrations of ethanol from 1% to 4%. For the subsequent 4 weeks, mice were fed a liquid diet containing either 5% (v/v) ethanol or pair-fed a control diet. Mice were then fasted for 4 h and euthanized for blood and tissue collection. Animals were housed in a temperature-controlled environment on a 12-hour light/12-hour dark cycle. Care of all animals and procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas (UT) Southwestern Medical Center at Dallas.
2.2. Single oral gavage of alcohol
Male mice (10–12 weeks old) were fasted for 2 h in the morning from 8am to 10am. After measuring body weight, mice were given an oral gavage of 5 g/kg BW of alcohol (31.5%, vol/vol) or maltose dextrin (45%, wt/vol). 6 h later, mice were anesthetized for blood and liver collection.
2.3. Determination of hepatic triglyceride contents
Mice were euthanized, and blood was collected from the inferior vena cava. Then 10 mL PBS was slowly delivered to the left ventricle of the heart to remove residual blood. Livers were removed and snap-frozen in liquid nitrogen and stored at −80 °C. Frozen liver tissues were used for lipid extraction by the UT Southwestern Metabolic Phenotyping Core Facility. After extraction, triglyceride contents were measured using Infinity Reagent assays (Thermo Fisher Scientific).
2.4. Plasma parameters
Mice were anesthetized and blood was collected in EDTA-coated tubes. Plasma was separated by centrifugation at 8,000 g for 12 min and stored at −80 °C. Plasma alanine transaminase (ALT) and aspartate aminotransferase (AST) were measured using the Vitros 250 Chemistry Analyzer (Ortho Clinical Diagnostics) by the UT Southwestern Metabolic Phenotyping Core.
2.5. Hepatic oxidative stress
Hepatic oxidative stress was measured by TBARS (TCA Method) Assay Kit (Cayman Chemical Co.) following the manufacturer's instructions.
2.6. Quantitative real-time PCR (qPCR)
Total RNA from liver and white adipose tissue was extracted using RNA Stat60 (Teltest). Complementary DNA was synthesized using the High Capacity cDNA Kit (Applied Biosystems). qPCR was performed using an ABI Prism 7900HT sequence detection system (Applied Biosystems). The relative amounts of all mRNAs were calculated using the ΔΔCT assay. Primers for Tlr4 (ID: Mm0445274_m1), Acc1 (ID: Mm01304277_m1), Fas (ID: Mm00662319_m1), Scd1 (ID: Mm00772290_m1), PPARα (ID: Mm00440939_m1), Cpt1α (ID: Mm01231183_m1), Acox1 (ID: Mm01246834_m1), Tnfα (ID: Mm00443258_m1), IL-6 (ID: Mm00446190_m1), IL-1β (ID: Mm00434228_m1), Mcp1 (ID: Mm00441242_m1), Cd11c (ID: Mm00498698_m1) were purchased from Applied Biosystems. mRNA contents were normalized to 18s (ID: Hs99999901_s1) for liver and GAPDH (ID: Mm99999915_g1) for white adipose tissue, respectively.
2.7. Immunoblot analysis
Liver tissues were homogenized in lysis buffer containing 2% SDS, 50 mM Tris-HCl (pH 6.8) and phosphatase inhibitor (PI78420, Thermo Scientific), followed by centrifugation at 12,000 r.p.m. for 15 min. Supernatants were stored at −20 °C until assays were performed. Tissue protein concentrations were determined by bicinchoninic acid (BCA) Kit (Pierce). 50 μg of protein were loaded onto 4%–12% Bis-Tris gel for electrophoresis (Novex™ NuPAGE™, Invitrogen). Proteins were then transferred onto nitrocellulose membranes and immunoblotted with specific antibodies that recognized ACC1 [23], [24], [25] (508D, rabbit polyclonal anti-mouse antibody), FAS [23], [24] (320D, rabbit polyclonal anti-mouse antibody), SCD1 (Cell Signaling Technology), and β-actin (Cell Signaling Technology). After incubation with ECL (Pierce), chemiluminescence was imaged with a LI-COR Odyssey® Fc imager and quantified using LI-COR Image Studio™ software (LI-COR Biotechnology).
2.8. Circulating cytokines
Plasma concentrations of Tnfα, IL-1β, and Mcp1 were determined using the MILLIPLEX MAP Mouse Cytokine/Chemokine-Premixed 32 Plex assay (Millipore).
2.9. Isolation of peritoneal macrophage, SVF, and adipocytes from control and alcohol-fed mice
After four weeks of diet feeding, mice were anesthetized, and macrophages were collected by flushing the peritoneal cavity with cold PBS. After centrifugation at 1,000 rpm for 10 min at 4 °C, cell pellets were resuspended and plated in RPMI 1640 medium containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Two hours later, floating cells were removed by washing with warm PBS. Adherent macrophages were cultured overnight and used for RNA isolation [19]. Epididymal fat pads were collected from the same mice. Tissues were minced and digested using collagenase (Gibco, #17703-034) at 37 °C for 2 h with shaking. The cell suspension was filtered through a 100 μm cell strainer, washed with DMEM containing 10% FBS, and centrifuged at 800 g for 1 min at room temperature. The floating adipocytes were collected and centrifuged at 500 g for 1min. After removing the lower liquid phase, isolated adipocytes were dissolved in RNA Stat60 (Teltest) for RNA isolation. The remaining cell pellets containing SVF were put on ice immediately. The pellets were passed through a 40 μm cell strainer, washed with cold DMEM buffer, and centrifuged at 500 g for 5 min at 4 °C. Then, SVF were collected as pellets and resuspended in RNA Stat60 (Teltest) for RNA isolation.
2.10. Statistical analyses
All the results are presented as means ± Standard Error of the Mean (SEM). Statistical significance was determined by two-way ANOVA followed by Tukey's Multiple Comparison Test using GraphPad PRISM (version 7, GraphPad, San Diego, CA). Significance was accepted at a value of p < 0.05.
3. Results
3.1. Deletion of TLR4 in hepatocytes protects mice from chronic alcohol-induced hepatic triglyceride accumulation
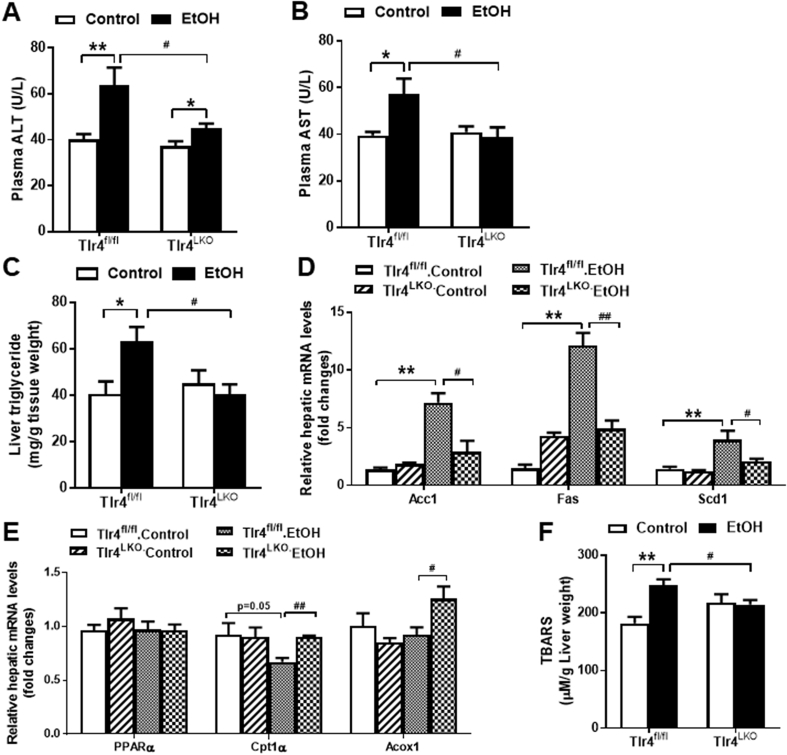
To investigate the role of hepatocyte TLR4 in chronic alcohol-induced fatty liver disease, we selectively ablated TLR4 in hepatocytes as previously described [19]. Hepatocyte TLR4 deficient mice (Tlr4LKO) and their littermate controls (Tlr4fl/fl) were fed either a Lieber-DeCarli liquid diet containing 5% ethanol (v/v) or pair-fed an isocaloric control diet for 4 weeks [22]. As expected, chronic alcohol feeding significantly elevated plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations in Tlr4fl/fl mice (Figure 1A,B). Furthermore, ethanol-fed Tlr4fl/fl mice showed greater hepatic triglyceride content than pair-fed Tlr4fl/fl mice (Figure 1C). In contrast, alcohol-induced elevations of circulating ALT and AST levels, as well as hepatic fat accumulation, were greatly blunted in ethanol-fed Tlr4LKO mice (Figure 1A–C). Over the course of a four-week feeding period, no significant changes in body weight (BW) were observed and similar increases in liver weight to BW ratio were found in both genotypes (data not shown).
Figure 1.
Mice lacking hepatocyte TLR4 exhibit reduced liver injury and decreased hepatic triglyceride accumulation after four weeks of alcohol intake. A, Plasma concentrations of ALT (n = 8–12). B, Plasma concentrations of AST (n = 8–12). C, Liver triglyceride contents (n = 8–10). D, Hepatic mRNA expression of genes involved in endogenous lipogenesis (n = 3–4) and E, fatty acid oxidation (n = 4). F, Hepatic TBARS contents (n = 6–8). *p < 0.05, **p < 0.01, compared between mice of the same genotype fed different diets; #p < 0.05, ##p < 0.01, compared between Tlr4fl/fl and Tlr4LKO mice on the same diet. All data are presented as mean ± SEM.
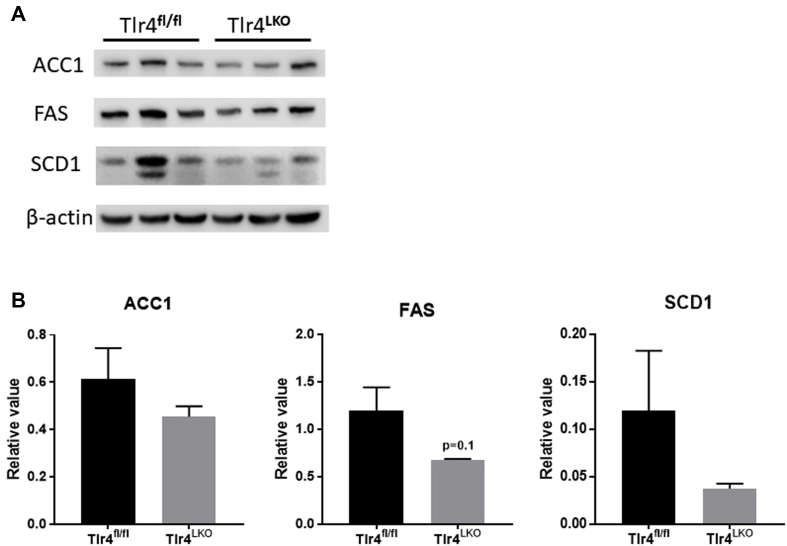
Alcohol exposure enhances lipogenesis and reduces β-oxidation of fatty acids in hepatocytes [26], [27], [28]. Our qPCR analysis showed that the mRNA expression of genes involved in de novo lipogenesis, such as acetyl CoA carboxylase 1 (Acc1), fatty acid synthase (Fas) and stearoyl CoA desaturase 1 (Scd1), were upregulated in Tlr4fl/fl mice after chronic alcohol consumption relative to pair-fed mice (Figure 1D). In contrast, the expression of these lipogenic genes was significantly lower in ethanol-fed Tlr4LKO mice (Figure 1D). Furthermore, the protein expression of ACC1, FAS, and SCD1 tended to be reduced in Tlr4LKO mice after chronic alcohol intake (Supplemental Figs. 1a and b). In addition, we observed a slight, but significant, reduction in hepatic carnitine palmitoyltransferase 1α (Cpt1α) mRNA expression in ethanol-fed Tlr4fl/fl mice (Fig. 1E). In contrast, ethanol-fed Tlr4LKO mice had significantly increased mRNA expression of hepatic acyl-CoA oxidase 1 (Acox1) and were protected from alcohol-induced reductions in Cpt1α (Figure 1E).
Ethanol is mainly metabolized in hepatocytes through the oxidative pathway [29]. One of the harmful effects of ethanol metabolism is the generation of reactive oxygen species, which induces lipid peroxidation and cellular injury. To evaluate whether hepatocyte TLR4 modulates alcohol-induced oxidative stress in mice, we measured the levels of thiobarbituric acid reactive substances (TBARS), a byproduct of lipid peroxidation. We found that ethanol-fed Tlr4fl/fl mice had significantly increased TBARS levels in the liver, indicating elevated hepatic oxidative stress (Figure 1F). However, this elevation was prevented in Tlr4LKO mice (Figure 1F).
Collectively, these data suggest that mice lacking hepatocyte TLR4 are protected from alcohol-induced early-stage liver injury and hepatic triglyceride accumulation.
3.2. Deletion of TLR4 in hepatocytes attenuates chronic alcohol-induced inflammatory response in white adipose tissue
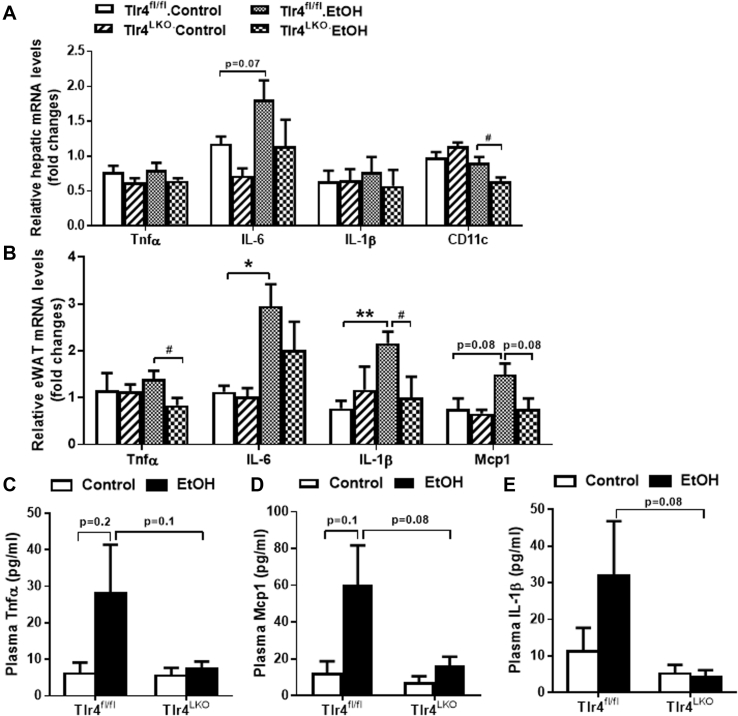
It has been reported that four weeks of Lieber-DeCarli alcohol diet intake induces little or no liver inflammation [22]. Consistent with this, we found that ethanol-fed Tlr4fl/fl mice had increased mRNA expression of hepatic IL-6 compared to control-fed mice, but that hepatic expression of Tnfα and IL-1β was not altered (Figure 2A). Interestingly, ethanol-fed Tlr4LKO mice showed significantly reduced hepatic expression of CD11c compared to that of Tlr4fl/fl mice (Figure 2A).
Figure 2.
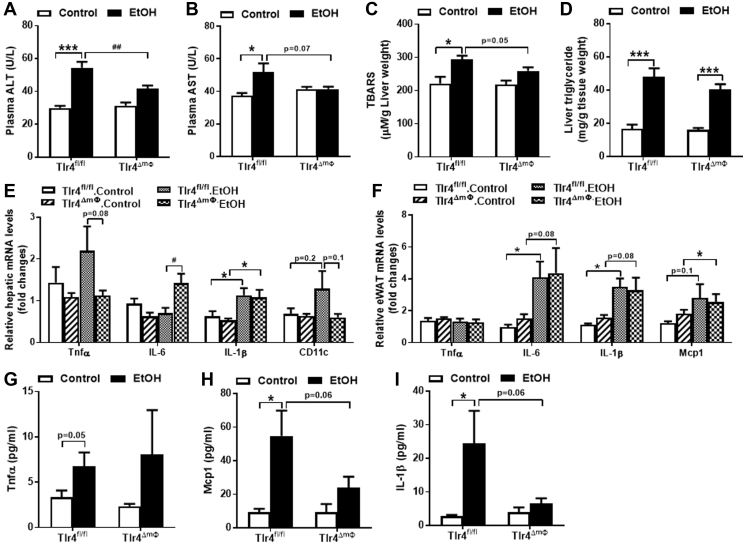
Alcohol-fed hepatocyte TLR4 deficient mice have significantly reduced mRNA expression of inflammatory genes in the epididymal fat pad (eWAT). A, mRNA expression of genes involved in inflammation in the liver (n = 3–5) of mice. B, mRNA expression of genes involved in inflammation in the eWAT (n = 3–4) of mice. Circulating concentrations of C, Tnfα (n = 6–11), D, Mcp1 (n = 5–9), and E, IL-1β (n = 5–9) in Tlr4fl/fl and Tlr4LKO mice after 4 weeks of alcohol-containing diet or pair-fed control diet. *p < 0.05, **p < 0.01, compared between mice of the same genotype fed different diets; #p < 0.05, compared between Tlr4fl/fl and Tlr4LKO mice on the same diet. Data are expressed as mean ± SEM.
Chronic alcohol-fed animals also show increased macrophage infiltration and inflammation in their white adipose tissues (WAT) [30], [31], [32]. We found that epididymal fat (eWAT) expression of IL-6 and IL-1β was significantly increased in ethanol-fed Tlr4fl/fl mice relative to pair-fed mice (Figure 2B). Similarly, circulating levels of Tnfα, IL-1β and monocyte chemoattractant protein-1 (Mcp1) were modestly, although not significantly, increased in Tlr4fl/fl mice after chronic alcohol intake (Figure 2C–E). Notably, these alcohol-induced inflammatory changes were blunted in Tlr4LKO mice (Figure 2B–E), including significantly reduced Tnfα and IL-1β expression in epididymal fat pad, as well as a trend towards decreased circulating Tnfα, Mcp1, and IL-1β levels. These findings were consistent with our previous report that mice lacking hepatocyte TLR4 are protected from chronic high-fat diet induced increases in circulating and adipose tissue-specific inflammatory markers [19].
3.3. Mice lacking hepatocyte TLR4 have reduced triglyceride content in the liver after acute alcohol consumption
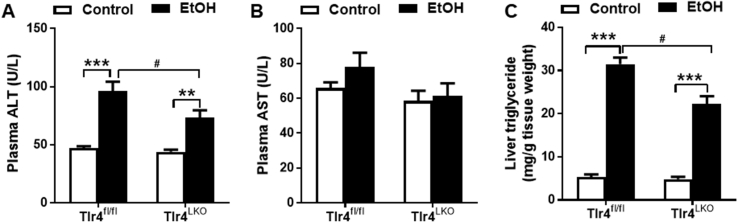
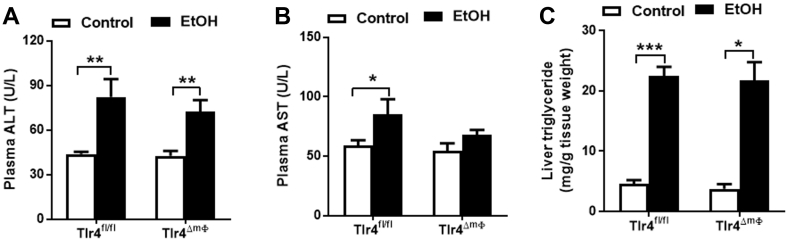
It has been widely reported that acute alcohol consumption leads to triglyceride accumulation in the liver [33], [34], [35]. Consistently, a single oral gavage of alcohol was sufficient to induce liver injury, as measured by elevated plasma ALT levels and dramatically elevated hepatic triglyceride content in Tlr4fl/fl mice (Figure 3A,C). Interestingly, alcohol gavaged-Tlr4LKO mice had attenuated plasma ALT concentrations and accumulated significantly less triglyceride in the liver (Figure 3A,C). There were no changes in plasma AST levels in either genotype after oral alcohol administration (Figure 3B). These findings indicate that mice lacking hepatocyte TLR4 are protected from the acute effects of alcohol on plasma ALT and hepatic lipid.
Figure 3.
TLR4 deletion in hepatocyte attenuates mice from acute alcohol gavage-induced elevations of plasma ALT and hepatic triglyceride content. A, Plasma concentrations of ALT (n = 7–11). B, Plasma concentrations of AST (n = 7–11). C, Liver triglyceride contents (n = 7–11). **p < 0.01, ***p < 0.001, compared between mice of the same genotype fed different diets; #p < 0.05, compared between Tlr4fl/fl and Tlr4LKO mice on the same diet. All data are presented as mean ± SEM.
3.4. Alcohol-fed Tlr4ΔmΦ mice had significantly reduced TLR4 mRNA expression in peritoneal macrophage and SVF
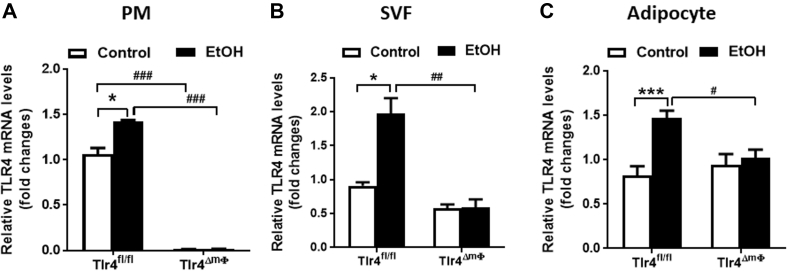
It is largely unknown whether alcohol feeding induces TLR4 expression in macrophages or adipocytes. In this study, we found that alcohol induces TLR4 mRNA expression in multiple cell types, including peritoneal macrophage (PM), stromal vascular fraction (SVF), and adipocytes (Figure 4A–C). These elevations were significantly blunted in alcohol-fed Tlr4ΔmΦ mice (Figure 4B,C). As expected, TLR4 mRNA expression was reduced about 99% in Tlr4ΔmΦ mice because of the Lysozyme M-Cre mediated gene knockdown (Figure 4A).
Figure 4.
Significantly reduced TLR4 mRNA expression in peritoneal macrophages and stromal vascular fraction cells of alcohol-fed Tlr4ΔmΦmice. Tlr4fl/fl and Tlr4ΔmΦ mice were fed either control or alcohol-containing diet for 4 weeks. (A) qPCR analysis of TLR4 mRNA expression in peritoneal macrophages (PM, n = 3–4). (B–C) qPCR analysis of TLR4 mRNA expression in stromal vascular fraction cells (SVF, B) and adipocytes (C) isolated from epididymal white adipose tissue (n = 3–4). *p < 0.05, ***p < 0.001, compared between mice of the same genotype fed different diets; #p < 0.05, ##p < 0.01, ###p < 0.001, compared between Tlr4fl/fl and Tlr4ΔmΦ mice on the same diet. All data are presented as mean ± SEM.
3.5. Deletion of TLR4 in myeloid cells does not attenuate chronic alcohol-induced hepatic triglyceride accumulation
To study the role of myeloid cell TLR4 in alcohol-induced liver damage, we selectively ablated TLR4 in myeloid cells using a Lysozyme M-Cre driver. No significant changes in body weight or liver weight were observed between myeloid cell TLR4 deleted mice (Tlr4ΔmΦ) and their littermate controls (Tlr4fl/fl) during four weeks of control or alcohol-containing liquid diet (data not shown). Chronic alcohol feeding increased circulating ALT and AST levels, and hepatic TBARS content in Tlr4fl/fl mice, but this effect was attenuated in ethanol-fed Tlr4ΔmΦ mice (Figure 5A–C). While mice lacking myeloid cell TLR4 were protected from liver injury, alcohol-induced hepatic triglyceride accumulation was similar between Tlr4ΔmΦ and Tlr4fl/fl mice (Figure 5D).
Figure 5.
Mice lacking myeloid cell TLR4 show significantly reduced circulating ALT levels after chronic alcohol intake. A, Plasma levels of ALT (n = 7–8). B, Plasma levels of AST (n = 7–8). C, Hepatic TBARS contents (n = 6–8). D, Liver triglyceride contents (n = 7–10). E, Hepatic mRNA expression of genes involved in pro-inflammation (n = 4–6). F, eWAT mRNA expression of genes involved in pro-inflammation (n = 5–6). Circulating concentrations of G, Tnfα (n = 6–11), H, Mcp1 (n = 6–11), and I, IL-1β (n = 6–11) in Tlr4fl/fl and Tlr4ΔmΦ mice after four weeks of alcohol-containing diet or pair-fed control diet. *p < 0.05, ***p < 0.001, compared between mice of the same genotype fed different diets; #p < 0.05, ##p < 0.01, compared between Tlr4fl/fl and Tlr4ΔmΦ mice on the same diet. All data are presented as mean ± SEM.
3.6. Deletion of TLR4 in myeloid cells alters circulating inflammatory response after chronic alcohol intake
Considering the potent immune response mediated by macrophage TLR4, we examined the mRNA expression of several inflammatory cytokines in Tlr4ΔmΦ and Tlr4fl/fl mice after four weeks of alcohol consumption. We found that chronic alcohol intake greatly increased mRNA expression of IL-1β in the livers of both Tlr4ΔmΦ and Tlr4fl/fl mice (Figure 5E). Furthermore, qPCR analysis showed that chronic ethanol induced the expression of hepatic IL-6, but blunted the expression of Tnfα and CD11c mRNA in Tlr4ΔmΦ mice as compared to littermate control Tlr4fl/fl mice (Figure 5E).
In epididymal fat pads, both Tlr4ΔmΦ and Tlr4fl/fl mice showed similar increases in IL-6, IL-1β, and Mcp1 mRNA levels in response to ethanol (Figure 5F). Ethanol consumption did not affect Tnfα expression in the fat pad of either genotype (Figure 5F).
Greatly elevated plasma levels of Tnfα, Mcp1, and IL-1β were observed in alcohol-fed Tlr4fl/fl mice compared to control-fed mice (Figure 5G–I). Surprisingly, despite the elevated mRNA expression of several inflammatory cytokines in both liver and adipose tissue, we found a tendency toward reduced circulating concentrations of Mcp1 and IL-1β in Tlr4ΔmΦ mice after chronic alcohol consumption (Figure 5H,I). Deletion of myeloid TLR4 did not affect the levels of circulating Tnfα after ethanol exposure (Figure 5G).
3.7. Mice lacking myeloid cell TLR4 are not protected from acute alcohol-induced hepatic triglyceride accumulation
Next, we examined the effects of acute alcohol intake on liver damage and hepatic triglyceride accumulation in Tlr4ΔmΦ and Tlr4fl/fl mice. We found that a single, oral gavage of alcohol induced comparable increases in plasma ALT and hepatic triglyceride content in both Tlr4ΔmΦ and Tlr4fl/fl mice (Figure 6A,C). Circulating AST concentrations were greatly elevated in Tlr4fl/fl mice after acute alcohol administration, and this effect was slightly but not significantly attenuated in Tlr4ΔmΦ mice (Figure 6B).
Figure 6.
Mice lacking myeloid cell TLR4 were not protected from acute alcohol intake-induced triglyceride accumulation in the liver. A, Plasma concentrations of ALT (n = 6–9). B, Plasma concentrations of AST (n = 6–9). C, Liver triglyceride contents (n = 6–9). *p < 0.05, **p < 0.01, ***p < 0.001, compared between mice of the same genotype fed different diets. All data are presented as mean ± SEM.
4. Discussion
It has been shown that LPS and its cell surface receptor TLR4 play a critical role in the development of ALD [8], [9], [10], [36], yet which TLR4-expressing cell types contribute to the disease are unknown. In the current study, we found that hepatocyte TLR4 deficient mice were protected from both chronic and acute alcohol-induced elevations of plasma ALT and hepatic triglyceride levels. Mechanistically, enhanced de novo lipogenesis [26], [28] and dysregulation of β-oxidation [28], [37] have been shown to contribute to the development of hepatic steatosis following chronic alcohol consumption. Indeed, the mRNA expression of lipogenic genes were elevated in the livers of alcohol-fed wild-type mice but not in mice with global TLR4 mutations [38]. Consistent with this, we found that mice lacking hepatocyte TLR4 had significantly reduced hepatic expression of genes involved in endogenous fatty acid synthesis, including Acc1, Fas, and Scd1. We also observed reduced expression of hepatic Cpt1α in alcohol-fed Tlr4fl/fl mice, indicating decreased fatty acid oxidation. In contrast, ablation of TLR4 in hepatocytes prevented the reduction of Cpt1α and enhanced the expression of Acox1 in mouse livers. Taken together, these findings suggest that suppressed endogenous fatty acid synthesis and improved β-oxidation may partly contribute to the reduction in hepatic triglyceride content in the liver of ethanol-fed Tlr4LKO mice.
The relationship between adipose tissue inflammation and alcoholic liver disease is not well understood. Notably, alcohol-fed animals maintain or even lose their body weight [31], suggesting the activation of different inflammatory mechanisms than those associated with diet-induced obesity. Recently, Parker et al. proposed that alcohol could directly induce adipose tissue damage and contribute to the development of alcoholic liver disease [39]. For instance, chronic alcohol intake promotes macrophage infiltration of epididymal adipose tissue and enhances the expression of pro-inflammatory cytokines [30], [31], [32]. Sebastian et al. has reported that overconsumption of alcohol mediates local inflammation in mouse adipose tissue by activating the cytochrome P4502E1/Bid/C1q-dependent axis [31]. Our data showed that alcohol-fed Tlr4LKO mice had altered expression of both circulating and adipose specific inflammatory markers (Figure 2). It is possible that hepatocyte TLR4 are required for the liver injury induced by alcohol-damaged adipose tissue. Another possibility is that hepatocytes release secreted factor(s) in response to alcohol consumption, which then influence adipose inflammation. Consistent with this, there is recent evidence that altered hepatocyte metabolism can affect the function of other tissues and plays a role in disease development [40], [41], [42], [43]. It remains to be determined how inhibition of hepatocyte TLR4 affects alcohol-induced hepatokines and downstream adipose tissue inflammation.
Kupffer cells, the resident liver macrophages, are a key player in the development of ALD. Deletion of Kupffer cells in rats via gadolinium chloride treatment alleviates alcohol-induced fatty liver disease [44], [45], [46]. In addition, mice lacking TLR4 in hematopoietic cells had significantly reduced hepatic triglyceride content [10]. Surprisingly, we found that myeloid cell-specific deletions of TLR4 did not affect hepatic triglyceride levels after chronic alcohol intake (Figure 5D). The differences between these studies could be due to different animal models (mouse versus rat) or alcohol administration (alcohol in the liquid diet versus intragastric infusion of alcohol). Moreover, gadolinium chloride not only inactivates Kupffer cells [47] but also accumulates in hepatocytes [48], [49] causing hepatotoxicity [49], [50]. Furthermore, it has been reported that Lysozyme M-Cre only mediates a ∼50% gene knockdown in Kupffer cells [51]; therefore, residual TLR4 expression in Kupffer cells could contribute to the alcohol-induced hepatic fat accumulation in Tlr4ΔmΦ mice.
The role of Kupffer cells, as well as Kupffer cell-specific TLR4, in the development of alcohol-induced hepatic inflammation is unclear. Järveläinen et al. showed that Kupffer cell inactivation did not alleviate alcohol-induced inflammatory responses in rat liver [45]. However, other studies have found that loss of Kupffer cells reduced hepatic pathological scores, including inflammation and necrosis, after intragastric alcohol infusions [44], [46]. Using bone marrow transplantation technique, Inokuchi et al. reported that TLR4 expressing-bone marrow cells contribute to alcohol-induced hepatic mRNA expression of IL-6 and IL-1β [10]. In our model, alcohol-fed mice with myeloid cell TLR4 deficiency showed reduced expression of Tnfα and CD11c, as well as significantly elevated IL-6 expression in the liver. Importantly, mice lacking IL-6 are more susceptible to ethanol-induced hepatic steatosis [52], [53], [54]. Furthermore, IL-6 treatment in vivo alleviates liver injury in alcohol-fed mice [55]. Taken together, these hepatic alterations in alcohol-fed Tlr4ΔmΦ mice could play a protective role in reducing liver damage and potentially decreasing circulating inflammatory cytokines.
In the current study, we found that alcohol induced TLR4 mRNA expression in several cell types, including peritoneal macrophages, SVF, and adipocytes. However, alcohol-fed Tlr4ΔmΦ mice showed significantly blunted TLR4 expression in SVF and adipocytes. In contrast, we have previously shown that TLR4 levels are elevated in SVF but unaltered in adipocytes isolated from epididymal white adipose tissue of high-fat diet-fed Tlr4ΔmΦ mice [19]. Importantly, the lower TLR4 expression in these cell types could partly explain the reduced inflammatory conditions in alcohol-fed Tlr4ΔmΦ mice. These findings lend support to the idea that alcohol and high-fat diets activate different inflammatory mechanisms.
In summary, we selectively deleted TLR4 expression in hepatocytes and myeloid cells and assessed the distinct contributions of these two TLR4-expressing cells to alcohol-induced fatty liver disease. Our findings indicate that mice lacking hepatocyte TLR4 had significantly reduced liver damage and hepatic fat content in response to both chronic and acute alcohol administration. In contrast, myeloid cell TLR4 ablated mice showed no differences in hepatic steatosis induced by either acute or chronic alcohol intake. Therefore, targeting hepatocyte TLR4 may be a therapeutic strategy for the treatment of alcoholic fatty liver disease.
Acknowledgments
We thank the Metabolic Phenotyping Core at UTSW for their technical assistance. These studies were supported by the National Institutes of Health grant P01DK088761 to PES, JKE, and JDH, K01AA024809 to LJ, R01DK114036 to CL and by an American Heart Association grant 16SDG27260001 to CL.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.05.015.
Contributor Information
Lin Jia, Email: Lin.Jia@UTSouthwestern.edu.
Joel K. Elmquist, Email: Joel.Elmquist@utsouthwestern.edu.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplemental Figure 1.
Immunoblot analysis of protein expressions in the livers isolated from ethanol-fed Tlr4LKOand Tlr4fl/flmice. A. Immunoblotting of ACC1, FAS, SCD1, and β-actin in livers from Tlr4fl/fl and Tlr4LKO mice after 4-week of alcohol intake (n = 3). B. Quantitation of protein expression using LI-COR Image Studio™ software (n = 3).
References
- 1.Wang H.J., Gao B., Zakhari S., Nagy L.E. Inflammation in alcoholic liver disease. Annual Review of Nutrition. 2012;32:343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen Z., Ajmo J.M., Rogers C.Q., Liang X., Le L., Murr M.M. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-alpha production in cultured macrophage cell lines. American Journal of Physiology Gastrointestinal and Liver Physiology. 2009;296(5):G1047–G1053. doi: 10.1152/ajpgi.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukui H., Brauner B., Bode J.C., Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. Journal of Hepatology. 1991;12(2):162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 4.Fujimoto M., Uemura M., Nakatani Y., Tsujita S., Hoppo K., Tamagawa T. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcoholism Clinical and Experimental Research. 2000;24(4 Suppl):48S–54S. [PubMed] [Google Scholar]
- 5.Nanji A.A., Khettry U., Sadrzadeh S.M., Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. The American Journal of Pathology. 1993;142(2):367–373. [PMC free article] [PubMed] [Google Scholar]
- 6.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50(2):638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Neill L.A., Golenbock D., Bowie A.G. The history of Toll-like receptors - redefining innate immunity. Nature Reviews Immunology. 2013;13(6):453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 8.Uesugi T., Froh M., Arteel G.E., Bradford B.U., Thurman R.G. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 9.Hritz I., Mandrekar P., Velayudham A., Catalano D., Dolganiuc A., Kodys K. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48(4):1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inokuchi S., Tsukamoto H., Park E., Liu Z.X., Brenner D.A., Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcoholism Clinical and Experimental Research. 2011;35(8):1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S., Gallo D.J., Green A.M., Williams D.L., Gong X., Shapiro R.A. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infection and Immunity. 2002;70(7):3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machida K., Tsukamoto H., Mkrtchyan H., Duan L., Dynnyk A., Liu H.M. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(5):1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumura T., Ito A., Takii T., Hayashi H., Onozaki K. Endotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TLR4 gene expression in murine liver and hepatocytes. Journal of Interferon & Cytokine Research – Official Journal of the International Society for Interferon and Cytokine Research. 2000;20(10):915–921. doi: 10.1089/10799900050163299. [DOI] [PubMed] [Google Scholar]
- 14.Migita K., Abiru S., Nakamura M., Komori A., Yoshida Y., Yokoyama T. Lipopolysaccharide signaling induces serum amyloid A (SAA) synthesis in human hepatocytes in vitro. FEBS Letters. 2004;569(1–3):235–239. doi: 10.1016/j.febslet.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 15.Seki E., De Minicis S., Osterreicher C.H., Kluwe J., Osawa Y., Brenner D.A. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nature Medicine. 2007;13(11):1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 16.Wang A.P., Migita K., Ito M., Takii Y., Daikoku M., Yokoyama T. Hepatic expression of toll-like receptor 4 in primary biliary cirrhosis. Journal of Autoimmunity. 2005;25(1):85–91. doi: 10.1016/j.jaut.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Mozer-Lisewska I., Sluzewski W., Kaczmarek M., Jenek R., Szczepanski M., Figlerowicz M. Tissue localization of Toll-like receptors in biopsy specimens of liver from children infected with hepatitis C virus. Scandinavian Journal of Immunology. 2005;62(4):407–412. doi: 10.1111/j.1365-3083.2005.01670.x. [DOI] [PubMed] [Google Scholar]
- 18.Isogawa M., Robek M.D., Furuichi Y., Chisari F.V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. Journal of Virology. 2005;79(11):7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia L., Vianna C.R., Fukuda M., Berglund E.D., Liu C., Tao C. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nature Communications. 2014;5:3878. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchimura K., Hayata M., Mizumoto T., Miyasato Y., Kakizoe Y., Morinaga J. The serine protease prostasin regulates hepatic insulin sensitivity by modulating TLR4 signalling. Nature Communications. 2014;5:3428. doi: 10.1038/ncomms4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao G.N., Zhang P., Gong J., Zhang X.J., Wang P.X., Yin M. Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nature Medicine. 2017;23(6):742–752. doi: 10.1038/nm.4334. [DOI] [PubMed] [Google Scholar]
- 22.Bertola A., Mathews S., Ki S.H., Wang H., Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nature Protocols. 2013;8(3):627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C.W., Moon Y.A., Park S.W., Cheng D., Kwon H.J., Horton J.D. Induced polymerization of mammalian acetyl-CoA carboxylase by MIG12 provides a tertiary level of regulation of fatty acid synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(21):9626–9631. doi: 10.1073/pnas.1001292107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon Y.A., Ochoa C.R., Mitsche M.A., Hammer R.E., Horton J.D. Deletion of ELOVL6 blocks the synthesis of oleic acid but does not prevent the development of fatty liver or insulin resistance. Journal of Lipid Research. 2014;55(12):2597–2605. doi: 10.1194/jlr.M054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metabolism. 2017;26(2):394–406. doi: 10.1016/j.cmet.2017.07.009. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You M., Fischer M., Deeg M.A., Crabb D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) The Journal of Biological Chemistry. 2002;277(32):29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 27.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141(5):1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You M., Matsumoto M., Pacold C.M., Cho W.K., Crabb D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127(6):1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 29.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Research & Health. 2006;29(4):245–254. [PMC free article] [PubMed] [Google Scholar]
- 30.Kang L., Sebastian B.M., Pritchard M.T., Pratt B.T., Previs S.F., Nagy L.E. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcoholism Clinical and Experimental Research. 2007;31(9):1581–1588. doi: 10.1111/j.1530-0277.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- 31.Sebastian B.M., Roychowdhury S., Tang H., Hillian A.D., Feldstein A.E., Stahl G.L. Identification of a cytochrome P4502E1/Bid/C1q-dependent axis mediating inflammation in adipose tissue after chronic ethanol feeding to mice. The Journal of Biological Chemistry. 2011;286(41):35989–35997. doi: 10.1074/jbc.M111.254201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X., Tang Y., Tan X., Li Q., Zhong W., Sun X. Activation of peroxisome proliferator-activated receptor-γ by rosiglitazone improves lipid homeostasis at the adipose tissue-liver axis in ethanol-fed mice. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2012;302(5) doi: 10.1152/ajpgi.00342.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding W.X., Li M., Chen X., Ni H.M., Lin C.W., Gao W. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139(5):1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni H.M., Du K., You M., Ding W.X. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. The American Journal of Pathology. 2013;183(6):1815–1825. doi: 10.1016/j.ajpath.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin C.W., Zhang H., Li M., Xiong X., Chen X., Chen X. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. Journal of Hepatology. 2013;58(5):993–999. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adachi Y., Moore L.E., Bradford B.U., Gao W., Thurman R.G. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108(1):218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Villafranca J., Guillen A., Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie. 2008;90(3):460–466. doi: 10.1016/j.biochi.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z.H., Liu X.Q., Zhang C., He W., Wang H., Chen Y.H. Tlr4-mutant mice are resistant to acute alcohol-induced sterol-regulatory element binding protein activation and hepatic lipid accumulation. Scientific Reports. 2016;6:33513. doi: 10.1038/srep33513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker R., Kim S.J., Gao B. Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations. Nature Reviews Gastroenterology & Hepatology. 2017 doi: 10.1038/nrgastro.2017.116. [DOI] [PubMed] [Google Scholar]
- 40.Wunderlich F.T., Strohle P., Konner A.C., Gruber S., Tovar S., Bronneke H.S. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metabolism. 2010;12(3):237–249. doi: 10.1016/j.cmet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Misu H., Takamura T., Takayama H., Hayashi H., Matsuzawa-Nagata N., Kurita S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metabolism. 2010;12(5):483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 42.El Ouaamari A., Kawamori D., Dirice E., Liew C.W., Shadrach J.L., Hu J. Liver-derived systemic factors drive beta cell hyperplasia in insulin-resistant states. Cell Reports. 2013;3(2):401–410. doi: 10.1016/j.celrep.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghorpade D.S., Ozcan L., Zheng Z., Nicoloro S.M., Shen Y., Chen E. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555(7698):673–677. doi: 10.1038/nature26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adachi Y., Bradford B.U., Gao W., Bojes H.K., Thurman R.G. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20(2):453–460. [PubMed] [Google Scholar]
- 45.Jarvelainen H.A., Fang C., Ingelman-Sundberg M., Lukkari T.A., Sippel H., Lindros K.O. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. Journal of Hepatology. 2000;32(6):900–910. doi: 10.1016/s0168-8278(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 46.Koop D.R., Klopfenstein B., Iimuro Y., Thurman R.G. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Molecular Pharmacology. 1997;51(6):944–950. doi: 10.1124/mol.51.6.944. [DOI] [PubMed] [Google Scholar]
- 47.Hardonk M.J., Dijkhuis F.W., Hulstaert C.E., Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. Journal of Leukocyte Biology. 1992;52(3):296–302. doi: 10.1002/jlb.52.3.296. [DOI] [PubMed] [Google Scholar]
- 48.Wasserman A.J., Monticello T.M., Feldman R.S., Gitlitz P.H., Durham S.K. Utilization of electron probe microanalysis in gadolinium-treated mice. Toxicologic Pathology. 1996;24(5):588–594. doi: 10.1177/019262339602400508. [DOI] [PubMed] [Google Scholar]
- 49.Spencer A.J., Wilson S.A., Batchelor J., Reid A., Pees J., Harpur E. Gadolinium chloride toxicity in the rat. Toxicologic Pathology. 1997;25(3):245–255. doi: 10.1177/019262339702500301. [DOI] [PubMed] [Google Scholar]
- 50.Liu H., Yuan L., Yang X., Wang K. La(3+), Gd(3+) and Yb(3+) induced changes in mitochondrial structure, membrane permeability, cytochrome c release and intracellular ROS level. Chemico Biological Interactions. 2003;146(1):27–37. doi: 10.1016/s0009-2797(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 51.Hevener A.L., Olefsky J.M., Reichart D., Nguyen M.T., Bandyopadyhay G., Leung H.Y. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. The Journal of Clinical Investigation. 2007;117(6):1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong F., Kim W.H., Tian Z., Jaruga B., Ishac E., Shen X. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene. 2002;21(1):32–43. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X., Tachibana S., Wang H., Hisada M., Williams G.M., Gao B. Interleukin-6 is an important mediator for mitochondrial DNA repair after alcoholic liver injury in mice. Hepatology. 2010;52(6):2137–2147. doi: 10.1002/hep.23909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Assal O., Hong F., Kim W.H., Radaeva S., Gao B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cellular and Molecular Immunology. 2004;1(3):205–211. [PubMed] [Google Scholar]
- 55.Hong F., Radaeva S., Pan H.N., Tian Z., Veech R., Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40(4):933–941. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]