Abstract
Objective
N-acylethanolamines play different roles in energy balance; anandamide (AEA) stimulates energy intake and storage, N-palmitoylethanolamide (PEA) counters inflammation, and N-oleoylethanolamide (OEA) mediates anorectic signals and lipid oxidation. Inconsistencies in the association of plasma N-acylethanolamines with human obesity and cardiometabolic risk have emerged among previous studies, possibly caused by heterogeneous cohorts and designs, and by unstandardized N-acylethanolamine measurements. We aimed to characterize changes in the plasma profile, including N-acylethanolamine levels and ratios associated with obesity, menopause in women, and ageing in men, and to define the significance of such a profile as a biomarker for metabolic imbalance.
Methods
Adult, drug-free women (n = 103 premenopausal and n = 81 menopausal) and men (n = 144) were stratified according to the body mass index (BMI) into normal weight (NW; BMI: 18.5–24.9 kg/m2), overweight (OW; BMI: 25.0–29.9 kg/m2), and obese (OB; BMI ≥30.0 kg/m2). Anthropometric and metabolic parameters were determined. Validated blood processing and analytical procedures for N-acylethanolamine measurements were used. We investigated the effect of BMI and menopause in women, and BMI and age in men, as well as the BMI-independent influence of metabolic parameters on the N-acylethanolamine profile.
Results
BMI and waist circumference directly associated with AEA in women and men, and with PEA in premenopausal women and in men, while BMI directly associated with OEA in premenopausal women and in men. BMI, in both genders, and waist circumference, in women only, inversely associated with PEA/AEA and OEA/AEA. Menopause increased N-acylethanolamine levels, whereas ageing resulted in increasing OEA relative abundance in men. AEA and OEA abundances in premenopausal, and PEA and OEA abundances in lean menopausal women, were directly associated with hypertension. Conversely, PEA and OEA abundances lowered with hypertension in elderly men. Insulin resistance was associated with changes in N-acylethanolamine ratios specific for premenopausal (reduced PEA/AEA and OEA/AEA), menopausal (reduced OEA/AEA) women and men (reduced OEA/AEA and OEA/PEA). PEA and OEA levels increased with total cholesterol, and OEA abundance specifically increased with HDL-cholesterol. Elevated triglyceride levels were associated with increased N-acylethanolamine levels only in menopausal women.
Conclusions
Obesity-related N-acylethanolamine hypertone is characterized by imbalanced N-acylethanolamine ratios. The profile given by a combination of N-acylethanolamine absolute levels and ratios enables imbalances to be identified in relationship with different metabolic parameters, with specific relevance according to gender, menopause and age, representing a useful means for monitoring metabolic health. Finally, N-acylethanolamine system appears a promising target for intervention strategies.
Keywords: Anandamide, N-palmitoylethanolamide, N-oleoylethanolamide, Endocannabinoid system, Obesity, Dysmetabolism
List of abbreviations: 2AG, 2-arachidonoylglycerol; AEA, N-arachidonoylethanolamide; BMI, body mass index; DBP, diastolic blood pressure; ECS, endocannabinoid system; EC, endocannabinoid; HDL, high density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; Int., interaction; NAE, N-acylethanolamine; NW, normal weight; OB, obese; OEA, N-oleoylethanolamide; OW, overweight; PEA, N-palmitoylethanolamide; SBP, systolic blood pressure; SD, standard deviation
Highlights
-
•
Obesity is featured by plasma N-acylethanolamine excess and imbalanced ratios.
-
•
AEA excess is a biomarker of abdominal fat irrespectively of sex and menopause/age.
-
•
PEA and OEA protect from hypertension in gender and menopause/age specific fashion.
-
•
AEA excess in women and OEA deficiency in men are biomarkers of insulin resistance.
-
•
High AEA in men and low OEA in men and menopausal women reflect low HDL-cholesterol.
1. Introduction
N-acylethanolamines (NAEs) are bioactive lipids formed by the fatty acid amidation of membrane phospholipids. They are involved in a wide spectrum of processes, including inflammation, neuroprotection, acute stress, pain perception, anxiety, hypotension, sleep, and energy balance and act by complex synergic and/or anergic interactions depending on the site and environmental context [1].
The role of NAE in energy balance emerged when N-arachidonoylethanolamine (anandamide, AEA) was discovered as the natural ligand of the cannabinoid receptor 1 (CB1) [2], and continued with other NAE members, to be referred to as AEA congeners, N-palmitoylethanolamide (PEA) and N-oleoylethanolamide (OEA). AEA, PEA, and OEA share non-CB targets, such as the transient receptor potential vanilloid 1 (TRPV1), G-protein coupled receptors 55 and 119, and peroxisome proliferator activator receptors (PPARs), and their abundance in tissues is regulated by the availability of diet-derived arachidonic, palmitic and oleic acid, respectively [1], [3], [4]. NAE machinery is ubiquitously expressed and highly redundant. Synthesis begins with N-acyltransferase enzymes generating N-acylphosphatidylethanolamine (NAPE), followed by phospholipase D (NAPE-PLD) removing the phosphatidic acid. Alternative pathways involve phospholipase 2, glycerophosphodiesterase, NAPE-PLC, and a complex pool of lyso-NAPE and glycerophospho-NAPE intermediates. NAEs are inactivated by hydrolysis to fatty acid and ethanolamine, mainly by fatty acid amide hydrolase type 1 (FAAH) but also by FAAH2 and N-acyl-ethanolamine-hydrolyzing acid amidase (NAAA), and exert reciprocal influence by competing for degrading enzymes (entourage effect) [5].
The endocannabinoid system (ECS) consists of specific endocannabinoid (EC) mediators, which along with AEA include 2-arachidonoylglycerol (2AG), CB1 and CB2, and metabolic machinery. ECS is a key orchestrator of energy balance, as systemic CB1-hypersignaling causes obesity, multi-organ inflammation, coronary dysfunction, insulin resistance and dyslipidemia [6]. Although increased circulating ECs are considered markers of ECS hyperactivation, their exact role in determining, maintaining, and reflecting the obese status has not been clarified. AEA levels, in particular, have been reported to be increased, unchanged, or decreased by obesity in different studies [6].
In contrast with AEA, PEA and OEA rouse interest in light of their health promoting function; PEA in countering inflammation [5] and OEA in mediating gut-to-brain anorectic signals and in stimulating lipid β-oxidation and gynoid-vs-android fat distribution [3]. At odds with their individual roles, plasma levels of PEA and OEA were found to correlate with AEA in a direct fashion, and such an association was reported in lean as well as in obese individuals [7], [8], [9]. Whether the relative abundance among NAEs, in addition to their individual levels, can improve the understanding on the regulation of this system, has never been addressed, but it is key to the exploitation of ECS and NAE system as therapeutic targets.
A further challenge to the interpretation of NAE significance in the bloodstream is the multiple levels of interconnection among nutrition, metabolic, and hormonal axes. In these regards, only sparse data are available on the relationship between NAE plasma levels and dysmetabolic features associated with obesity, and no attention has been paid to the influence of menopause and ageing processes on the dynamic of NAE secretion [6].
Finally, a source of inconsistency in this field has been recognized both in the heterogeneity among study populations and designs and in the unstandardized procedural and analytical protocols for NAE measurement [7], [10], [11].
We recently investigated plasma 2AG dependency on factors intrinsically or differentially associated with metabolic worsening, such as gender, body mass index (BMI), menopause in women, and ageing in men and found it to be a valuable biomarker of insulin resistance and dyslipidemia in lean men and menopausal women [12]. Using a similar approach and focusing on the same population, the hypothesis of the present study is that plasma NAE levels as well as their ratios are influenced by BMI and by menopause in women and ageing in men; moreover, we hypothesized that the NAE profile given by levels and ratios could reflect specific dysmetabolic features independently of the above mentioned factors.
2. Materials and methods
2.1. Subjects
As previously described [7], after giving their written informed consent, adult volunteers from the general population were interviewed and examined. Inclusion criterion was a BMI ≥18.5 kg/m2 stable over the previous three months. Exclusion criteria were: shift work, perimenopausal status for females, pharmacological treatment such as estro-progestogens and drugs for type-2-diabetes, hypertension, hypercholesterolemia or hypertriglyceridemia in the previous three months, history of endocrine (except hypothyroidism and obesity), hepatic, renal, tumoral, autoimmune, cardiovascular, hematological, neurological, psychiatric, and allergic diseases. Antipyretic and anti-inflammatory compounds taken before the last month and stable thyroxine replacement were tolerated. Improper blood processing led to exclusion from the study [7], [12].
Two cohorts of 184 females (103 premenopausal: age <53 years, six regular menstrual bleeding in the previous six months; 81 menopausal: age 42–89 years, no menses in the previous 12 months), and 144 males were obtained and further stratified into normal weight (NW; BMI: 18.5–24.9 kg/m2), overweight (OW; BMI: 25.0–29.9 kg/m2), and obese (OB; BMI ≥30.0 kg/m2) (Supplemental Tables 1 and 2) [12].
2.2. Biochemical and NAE measurements
After an overnight fast and following 10 min of saline infusion, blood was withdrawn and immediately centrifuged; the derived plasma and serum were stored at −80 °C and −20 °C, respectively, until analysis. Glucose, triglycerides, total cholesterol, high density lipoprotein (HDL)-cholesterol, and insulin were assayed as previously reported [7]. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as (glucose x insulin)/405 [13]. Plasma NAEs were extracted and measured by liquid chromatography-tandem mass spectrometry in fourteen runs over two months, each including calibrators, 50–70 samples and three replicates of two-level quality controls. Inter-assay imprecision was below 5.2, 4.9, and 7.8%, and functional sensitivity was 0.0195, 0.1953, and 0.1953 pmol/mL for AEA, PEA, and OEA, respectively [7].
2.3. Statistics
Mean, standard deviation (SD), range, and absolute and relative frequencies were used as descriptive statistics. Women and men were analyzed separately. BMI and menopause and BMI and age, were considered as the main factors in women and men, respectively, while metabolic parameters were considered as covariates. In men, age was stratified into six classes (18–29, 30–39, 40–49, 50–59, 60–69, ≥70 years). Anthropometric and metabolic differences among BMI and menopause/age classes in females/males, respectively, were tested by two-way ANOVA, except age, which was tested among male BMI classes by one-way ANOVA.
PEA/AEA, OEA/AEA, and OEA/PEA molar ratios were computed. NAE absolute values and ratios were analyzed as dependent variables by a two-way ANOVA design evaluating the unadjusted effects of BMI and menopause/age in females/males, whereas two-way ANCOVAs were applied to evaluate the same effects adjusted for each metabolic parameter. The linear trend of the polynomial contrast was computed in order to test the BMI effect in both populations and to test the age effect in the male cohort; a simple contrast was applied to test differences between pairs of BMI classes. All the main effects of the explanatory variables (i.e., factors (BMI and menopause/age) and covariates (metabolic parameters)), as well as all possible interactions, were considered in the analyses using a saturated model. Significant interaction between two explanatory variables indicated that they did not act independently on the response variable, i.e., their combined effect was different from the sum of the two separate effects. For example, a significant interaction between BMI and menopause/age indicated that the BMI effect impacted on the NAE variable with a significant difference over classes in different menopausal status/ages. This can also be interpreted as the effect of menopause/age impacting on the NAE variable differently across classes at different BMI levels.
Significantly skewed variables were transformed by the formula log10 (x+k), with k values zeroing the skewness after transformation. All transformed and untransformed variables, except for SBP in females and DBP in females and males, showed a normal distribution at the Kolmogorov–Smirnov test.
Data were managed and analyzed using the SPSS Statistics package (Version 23; IBM Co., Armonk, NY, USA). Two-tailed P values lower than 0.05 were considered statistically significant.
3. Results
3.1. Females
3.1.1. Descriptive features of the female cohorts (Supplemental Table 1)
The anthropometric and metabolic features of premenopausal and menopausal women are detailed elsewhere [12]. In brief, menopausal showed higher SBP (P < 0.001), DBP (P = 0.006), total cholesterol, and triglycerides (both P < 0.001) compared to premenopausal women in the overall cohort. In addition, while showing a similar BMI (P = 0.136), menopausal women in the NW class had a higher waist circumference (P = 0.009) compared to premenopausal women. Both in premenopausal and menopausal cohorts, waist circumference (both P < 0.001), SBP (P < 0.001 and P = 0.047, respectively), DBP (P = 0.014 and P = 0.009, respectively), insulin and HOMA-IR (all P < 0.001) increased, and HDL-cholesterol decreased (P = 0.002 and P = 0.015, respectively) with increasing BMI. In addition, glucose (P = 0.039) and triglycerides (P = 0.003) levels increased with increasing BMI in premenopausal women.
3.1.2. Circulating NAE levels and ratios in the female cohorts
The effect of menopause and BMI as well as their interaction on NAE levels and ratios are reported in Table 1. NAE levels and ratios in premenopausal and menopausal women in each BMI class, along with menopause effect within each BMI class, and with comparisons between pairs of BMI classes in each cohort, are shown in Figure 1.
Table 1.
Results of the two-way ANOVA evaluating the effects of menopause (MP), body mass index (BMI) and their interaction on N-acylethanolamine (NAE) circulating levels and their ratios in the female cohort. Data are shown as P values (sign of the coefficients) of the evaluated effects: non italic data show first order (main) effects; italic data show second order effects (i.e., the interactions between the two main effects). Significant P values are reported in bold.
| Factors | Cohort | AEA | PEA | OEA | PEA/AEA | OEA/AEA | OEA/PEA |
|---|---|---|---|---|---|---|---|
| Effect of MP* | Overall | 0.136 (+) | 0.015 (+) | 0.027 (+) | 0.554 (+) | 0.163 (+) | 0.416 (+) |
| Effect of BMI§ | Premenopausal women | <0.001 (+) | <0.001 (+) | 0.012 (+) | <0.001 (−) | <0.001 (−) | 0.257 (−) |
| Menopausal women | 0.004 (+) | 0.074 (+) | 0.308 (+) | 0.015 (−) | 0.012 (−) | 0.427 (−) | |
| Interaction between MP and BMI$ | 0.142 (−) | 0.367 (−) | 0.428 (−) | 0.199 (+) | 0.056 (+) | 0.924 (+) | |
AEA: N-arachidonoylethanolamide; PEA: N-palmitoylethanolamide; OEA N-oleoylethanolamide.
* Effect of MP on NAE levels and ratios. Positive effect (+): higher NAE values in menopausal than in premenopausal females; negative effect (−): lower NAE values in menopausal than in premenopausal females. § Effect of BMI on NAE levels and ratios. Positive effect (+): NAE values increased with increasing BMI classes. Negative effect (−): NAE values decreased with increasing BMI classes. $ Interaction between BMI and MP effects on NAE levels and ratios. Positive interaction (+): the positive (or negative) effect of BMI on NAE values is higher (or lower) in menopausal than in premenopausal females (i.e.: the positive (or negative) effect of MP increased (or decreased) with increasing BMI). Negative interaction (−): the positive (or negative) effect of BMI on NAE values is lower (or higher) in menopausal than in premenopausal females (i.e.: the positive (or negative) effect of MP decreased (or increased) with increasing BMI).
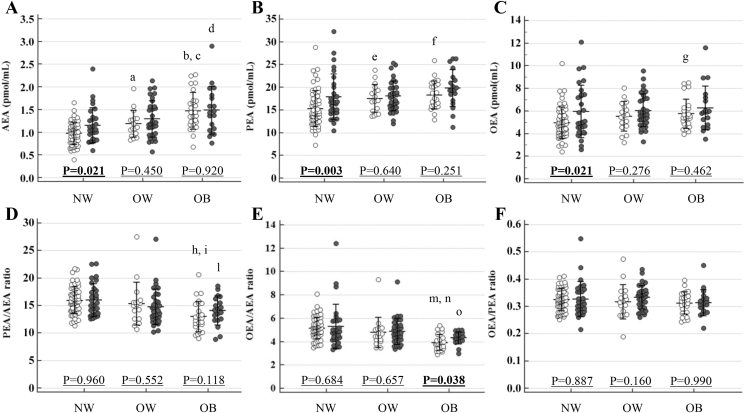
Figure 1.
N-acylethanolamine levels and ratios according to body mass index in premenopausal and menopausal women. Empty dots: premenopausal women. Solid dots: menopausal women. AEA: N-arachidonoylethanolamide; PEA: N-palmitoylethanolamide; OEA N-oleoylethanolamide; NW: normal weight; OW: overweight; OB: obese. AEA (A): aP = 0.011: OW vs. NW premenopausal women; bP < 0.001: OB vs. NW premenopausal women; cP = 0.026: OB vs. OW premenopausal women; dP = 0.004: OB vs. NW menopausal women. No significant differences were found by comparing OW vs. NW (P = 0.114) and OB vs. OW (P = 0.123) menopausal women. PEA (B): eP = 0.013: OW vs. NW premenopausal women; fP < 0.001: OB vs. NW premenopausal women. No significant differences were found by comparing OB vs. OW (P = 0.557) premenopausal women and by comparing pairs of BMI classes within menopausal women (OW vs. NW: P = 0.585; OB vs. NW: P = 0.074 and OB vs. OW: P = 0.176). OEA (C): gP = 0.012: OB vs. NW premenopausal women. No significant differences were found by comparing OW vs. NW (P = 0.118) and OB vs. OW (P = 0.587) premenopausal women and by comparing pairs of BMI classes within menopausal women (OW vs. NW: P = 0.362; OB vs. NW: P = 0.308, and OB vs. OW: P = 0.812). PEA/AEA (D): hP < 0.001: OB vs. NW premenopausal women; iP = 0.004: OB vs. OW premenopausal women; lP = 0.015: OB vs. NW menopausal women. No significant differences were found by comparing OW vs. NW (P = 0.280) premenopausal women and by comparing OW vs. NW (P = 0.070) and OB vs. OW (P = 0.366) menopausal women. OEA/AEA (E): mP < 0.001: OB vs. NW premenopausal women; nP = 0.001: OB vs. OW premenopausal women; oP = 0.012: OB vs. NW menopausal women. No significant differences were found by comparing OW vs. NW (P = 0.104) premenopausal women and by comparing OW vs. NW (P = 0.381) and OB vs. OW (P = 0.069) menopausal women. OEA/PEA (F): No significant differences were found by comparing pairs of BMI classes within premenopausal (OW vs. NW: P = 0.349; OB vs. NW: P = 0.257 and OB vs. OW: P = 0.967) and menopausal (OW vs. NW: P = 0.442; OB vs. NW: P = 0.427 and OB vs. OW: P = 0.137) women.
Menopausal women showed similar AEA (1.29 ± 0.44 vs. 1.13 ± 0.37 pmol/mL, P = 0.136) and higher PEA (18.4 ± 4.2 vs. 16.4 ± 3.8 pmol/mL; P = 0.015) and OEA (6.06 ± 1.89 vs. 5.24 ± 1.39 pmol/mL, P = 0.027) levels compared to premenopausal women (Table 1). Higher AEA (P = 0.021), PEA (P = 0.003) and OEA (P = 0.021) were also found in menopausal vs. premenopausal in NW women (Figure 1A–C). Menopause did not affect PEA/AEA, OEA/AEA and OEA/PEA in the overall cohort (Table 1) but increased OEA/AEA in the OB class (P = 0.038) (Figure 1D–F).
BMI increased AEA, PEA (both P < 0.001), and OEA (P = 0.012) in premenopausal women, increased AEA in menopausal (P = 0.004) women, and reduced PEA/AEA and OEA/AEA in premenopausal (both P < 0.001) and menopausal (P = 0.015 and P = 0.012, respectively) women (Table 1).
No significant interaction between BMI and menopause effect was found to influence the NAE profile. The effects of menopause, BMI, and their interaction on NAE levels and ratios observed after adjusting for each metabolic parameter are reported in Supplemental Table 3.
3.1.3. Association between circulating NAE levels and ratios and metabolic parameters in the female cohorts
The BMI-independent associations of each metabolic parameter with NAE levels (Table 2A) and ratios (Table 2B) were analyzed in the premenopausal and menopausal cohorts along with the effects of menopause and BMI on each association. In addition, correlations between metabolic parameters and the NAE profile were analyzed in each BMI class.
Table 2A.
Correlation of N-acylethanolamine (NAE) circulating levels (A) and their ratios (B) with metabolic parameters evaluated in the overall premenopausal and menopausal cohorts and within body mass index (BMI) classes. Data are shown as P values (sign of the coefficients) of the correlations (non italic data) as well as of the effects of BMI, menopause (MP) and their interaction on correlations (italic data). Significant P values are reported in bold.
| Metabolic parameter | Cohort | Overall |
BMI classes |
Effect of BMI¥ |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal weight (NW) |
Overweight (OW) |
Obese (OB) |
||||||||||||||
| AEA | PEA | OEA | AEA | PEA | OEA | AEA | PEA | OEA | AEA | PEA | OEA | AEA | PEA | OEA | ||
| Waist circumference | Premenopausal | 0.011 (+) | 0.889 (−) | 0.632 (+) | 0.180 (+) | 0.976 (+) | 0.646 (−) | 0.051 (+) | 0.785 (−) | 0.373 (+) | 0.245 (+) | 0.971 (+) | 0.890 (+) | 0.773 (+) | 0.988 (+) | 0.722 (+) |
| Menopausal | 0.001 (+) | 0.040 (+) | 0.080 (+) | 0.054 (+) | 0.389 (+) | 0.259 (+) | 0.020 (+) | 0.050 (+) | 0.286 (+) | 0.146 (+) | 0.531 (+) | 0.356 (+) | 0.909 (+) | 0.974 (+) | 0.899 (+) | |
| MP effectπ | 0.373 (+) | 0.094 (+) | 0.296 (+) | 0.491 (+) | 0.510 (+) | 0.242 (+) | 0.544 (+) | 0.089 (+) | 0.779 (+) | 0.741 (+) | 0.654 (+) | 0.548 (+) | 0.921 (−)& | 0.988 (+)& | 0.893 (−)& | |
| SBP | Premenopausal | 0.102 (+) | 0.371 (+) | 0.036 (+) | 0.549 (+) | 0.958 (−) | 0.475 (+) | 0.276 (+) | 0.730 (+) | 0.217 (+) | 0.277 (+) | 0.238 (+) | 0.105 (+) | 0.625 (+) | 0.336 (+) | 0.399 (+) |
| Menopausal | 0.465 (+) | 0.131 (+) | 0.132 (+) | 0.403 (+) | 0.018 (+) | 0.002 (+) | 0.552 (−) | 0.347 (−) | 0.376 (−) | 0.428 (+) | 0.324 (+) | 0.656 (+) | 0.879 (+) | 0.553 (−) | 0.151 (−) | |
| MP effectπ | 0.488 (−) | 0.698 (+) | 0.619 (−) | 0.836 (+) | 0.076 (+) | 0.078 (+) | 0.216 (−) | 0.425 (−) | 0.130 (−) | 0.916 (−) | 0.986 (−) | 0.471 (−) | 0.834 (−)& | 0.280 (−)& | 0.103 (−)& | |
| DBP | Premenopausal | 0.018 (+) | 0.062 (+) | 0.152 (+) | 0.532 (+) | 0.241 (+) | 0.360 (+) | 0.228 (+) | 0.322 (+) | 0.600 (+) | 0.018 (+) | 0.194 (+) | 0.182 (+) | 0.129 (+) | 0.739 (+) | 0.604 (+) |
| Menopausal | 0.985 (+) | 0.325 (+) | 0.423 (+) | 0.963 (+) | 0.120 (+) | 0.083 (+) | 0.368 (−) | 0.322 (−) | 0.550 (−) | 0.379 (+) | 0.396 (+) | 0.982 (+) | 0.534 (+) | 0.552 (−) | 0.190 (−) | |
| MP effectπ | 0.078 (−) | 0.453 (−) | 0.587 (−) | 0.698 (−) | 0.525 (+) | 0.351 (+) | 0.135 (−) | 0.176 (−) | 0.451 (−) | 0.290 (−) | 0.750 (−) | 0.336 (−) | 0.618 (−)& | 0.503 (−)& | 0.181 (−)& | |
| Glucose | Premenopausal | 0.433 (+) | 0.381 (+) | 0.235 (+) | 0.421 (−) | 0.555 (−) | 0.850 (−) | 0.217 (+) | 0.230 (+) | 0.230 (+) | 0.718 (+) | 0.671 (+) | 0.467 (+) | 0.414 (+) | 0.475 (+) | 0.507 (+) |
| Menopausal | 0.387 (+) | 0.149 (+) | 0.418 (+) | 0.038 (+) | 0.022 (+) | 0.061 (+) | 0.174 (−) | 0.593 (−) | 0.271 (−) | 0.631 (+) | 0.588 (+) | 0.705 (+) | 0.279 (−) | 0.236 (−) | 0.309 (−) | |
| MP effectπ | 0.882 (−) | 0.916 (+) | 0.605 (−) | 0.044 (+) | 0.045 (+) | 0.150 (+) | 0.093 (−) | 0.189 (−) | 0.123 (−) | 0.941 (+) | 0.943 (+) | 0.786 (−) | 0.181 (−)& | 0.182 (−)& | 0.238 (−)& | |
| Insulin | Premenopausal | 0.281 (+) | 0.800 (−) | 0.912 (−) | 0.056 (+) | 0.074 (+) | 0.397 (+) | 0.918 (+) | 0.612 (−) | 0.707 (−) | 0.545 (+) | 0.385 (−) | 0.831 (−) | 0.629 (−) | 0.095 (−) | 0.534 (−) |
| Menopausal | 0.491 (+) | 0.807 (−) | 0.386 (−) | 0.423 (+) | 0.460 (−) | 0.198 (−) | 0.591 (+) | 0.459 (+) | 0.752 (+) | 0.957 (−) | 0.732 (−) | 0.623 (−) | 0.575 (−) | 0.827 (+) | 0.643 (+) | |
| MP effectπ | 0.827 (−) | 0.992 (−) | 0.571 (−) | 0.802 (−) | 0.127 (−) | 0.125 (−) | 0.807 (+) | 0.394 (+) | 0.624 (+) | 0.676 (−) | 0.786 (+) | 0.801 (−) | 0.858 (−)& | 0.252 (+)& | 0.461 (+)& | |
| HOMA-IR | Premenopausal | 0.228 (+) | 0.968 (−) | 0.820 (+) | 0.096 (+) | 0.147 (+) | 0.421 (+) | 0.723 (+) | 0.858 (−) | 0.943 (−) | 0.570 (+) | 0.450 (−) | 0.977 (−) | 0.689 (−) | 0.160 (−) | 0.656 (−) |
| Menopausal | 0.435 (+) | 0.820 (+) | 0.619 (−) | 0.191 (+) | 0.909 (+) | 0.642 (−) | 0.976 (−) | 0.663 (+) | 0.910 (−) | 0.900 (+) | 0.922 (−) | 0.777 (−) | 0.457 (−) | 0.882 (−) | 0.936 (+) | |
| MP effectπ | 0.767 (−) | 0.849 (+) | 0.608 (−) | 0.875 (+) | 0.476 (−) | 0.405 (−) | 0.762 (−) | 0.687 (+) | 0.992 (−) | 0.774 (−) | 0.665 (+) | 0.849 (−) | 0.746 (−)& | 0.439 (+)& | 0.730 (+)& | |
| Total cholesterol | Premenopausal | 0.157 (+) | 0.154 (+) | 0.036 (+) | 0.081 (+) | 0.006 (+) | 0.008 (+) | 0.289 (+) | 0.703 (−) | 0.530 (+) | 0.910 (+) | 0.305 (+) | 0.270 (+) | 0.449 (−) | 0.636 (−) | 0.741 (−) |
| Menopausal | 0.455 (−) | 0.069 (+) | 0.081 (+) | 0.467 (+) | <0.001 (+) | <0.001 (+) | 0.924 (+) | 0.632 (+) | 0.629 (+) | 0.092 (−) | 0.965 (−) | 0.543 (−) | 0.072 (−) | 0.049 (−) | 0.005 (−) | |
| MP effectπ | 0.152 (−) | 0.519 (+) | 0.855 (+) | 0.812 (−) | 0.112 (+) | 0.027 (+) | 0.544 (−) | 0.541 (+) | 0.975 (−) | 0.148 (−) | 0.533 (−) | 0.258 (−) | 0.276 (−)& | 0.166 (−)& | 0.032 (−)& | |
| HDL-cholesterol | Premenopausal | 0.912 (+) | 0.097 (+) | 0.358 (+) | 0.708 (−) | 0.237 (+) | 0.647 (+) | 0.855 (−) | 0.454 (+) | 0.814 (+) | 0.331 (+) | 0.132 (+) | 0.175 (+) | 0.328 (+) | 0.716 (+) | 0.465 (+) |
| Menopausal | 0.596 (+) | 0.053 (+) | 0.043 (+) | 0.293 (+) | 0.010 (+) | 0.022 (+) | 0.364 (+) | 0.410 (+) | 0.281 (+) | 0.402 (−) | 0.896 (−) | 0.883 (+) | 0.182 (−) | 0.053 (−) | 0.124 (−) | |
| MP effectπ | 0.789 (+) | 0.973 (+) | 0.513 (+) | 0.275 (+) | 0.115 (+) | 0.088 (+) | 0.596 (+) | 0.721 (−) | 0.829 (+) | 0.212 (−) | 0.319 (−) | 0.491 (−) | 0.099 (−)& | 0.071 (−)& | 0.093 (−)& | |
| Triglycerides | Premenopausal | 0.693 (+) | 0.795 (−) | 0.916 (+) | 0.110 (+) | 0.181 (+) | 0.148 (+) | 0.409 (+) | 0.734 (−) | 0.913 (−) | 0.096 (−) | 0.258 (−) | 0.409 (−) | 0.022 (−) | 0.086 (−) | 0.122 (−) |
| Menopausal | 0.010 (+) | 0.005 (+) | 0.043 (+) | 0.067 (+) | 0.023 (+) | 0.010 (+) | 0.383 (+) | 0.416 (+) | 0.763 (+) | 0.067 (+) | 0.050 (+) | 0.283 (+) | 0.391 (+) | 0.437 (+) | 0.876 (−) | |
| MP effectπ | 0.076 (+) | 0.017 (+) | 0.130 (+) | 0.762 (+) | 0.417 (+) | 0.337 (+) | 0.860 (−) | 0.457 (+) | 0.793 (+) | 0.017 (+) | 0.024 (+) | 0.181 (+) | 0.054 (+)& | 0.119 (+)& | 0.491 (+)& | |
Table 2B.
| Metabolic parameter | Cohort | Overall |
BMI classes |
Effect of BMI¥ |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal weight (NW) |
Overweight (OW) |
Obese (OB) |
||||||||||||||
| PEA/AEA | OEA/AEA | OEA/PEA | PEA/AEA | OEA/AEA | OEA/PEA | PEA/AEA | OEA/AEA | OEA/PEA | PEA/AEA | OEA/AEA | OEA/PEA | PEA/AEA | OEA/AEA | OEA/PEA | ||
| Waist circumference | Premenopausal | <0.001 (−) | <0.001 (−) | 0.366 (+) | 0.039 (−) | 0.014 (−) | 0.321 (−) | 0.001 (−) | 0.210 (−) | 0.042 (+) | 0.040 (−) | 0.015 (−) | 0.987 (−) | 0.501 (−) | 0.417 (−) | 0.616 (+) |
| Menopausal | 0.009 (−) | 0.012 (−) | 0.817 (+) | 0.049 (−) | 0.194 (−) | 0.431 (+) | 0.222 (−) | 0.055 (−) | 0.384 (−) | 0.115 (−) | 0.258 (−) | 0.433 (+) | 0.846 (−) | 0.840 (−) | 0.838 (+) | |
| MP effectπ | 0.478 (+) | 0.821 (+) | 0.689 (−) | 0.788 (−) | 0.606 (+) | 0.218 (+) | 0.279 (+) | 0.467 (−) | 0.052 (−) | 0.867 (+) | 0.435 (+) | 0.549 (+) | 0.774 (+)& | 0.709 (+)& | 0.865 (−)& | |
| SBP | Premenopausal | 0.130 (−) | 0.835 (+) | 0.013 (+) | 0.354 (−) | 0.980 (+) | 0.210 (+) | 0.203 (−) | 0.970 (+) | 0.071 (+) | 0.685 (−) | 0.767 (+) | 0.221 (+) | 0.801 (+) | 0.827 (+) | 0.845 (+) |
| Menopausal | 0.602 (+) | 0.594 (+) | 0.613 (+) | 0.163 (+) | 0.015 (+) | 0.029 (+) | 0.890 (−) | 0.816 (−) | 0.813 (−) | 0.844 (−) | 0.374 (−) | 0.463 (−) | 0.329 (−) | 0.032 (−) | 0.061 (−) | |
| MP effectπ | 0.144 (+) | 0.831 (+) | 0.140 (−) | 0.099 (+) | 0.075 (+) | 0.448 (+) | 0.318 (+) | 0.875 (−) | 0.099 (−) | 0.909 (+) | 0.385 (−) | 0.176 (−) | 0.368 (−)& | 0.078 (−)& | 0.125 (−)& | |
| DBP | Premenopausal | 0.113 (−) | 0.080 (−) | 0.952 (+) | 0.626 (+) | 0.755 (+) | 0.837 (+) | 0.473 (−) | 0.252 (−) | 0.657 (−) | 0.017 (−) | 0.052 (−) | 0.480 (+) | 0.028 (−) | 0.082 (−) | 0.492 (+) |
| Menopausal | 0.332 (+) | 0.669 (+) | 0.976 (+) | 0.066 (+) | 0.038 (+) | 0.338 (+) | 0.885 (+) | 0.720 (+) | 0.745 (+) | 0.617 (−) | 0.058 (−) | 0.159 (−) | 0.089 (−) | 0.005 (−) | 0.099 (−) | |
| MP effectπ | 0.068 (+) | 0.111 (+) | 0.979 (+) | 0.209 (+) | 0.121 (+) | 0.363 (+) | 0.489 (+) | 0.242 (+) | 0.584 (+) | 0.180 (+) | 0.973 (+) | 0.136 (−) | 0.916 (+)& | 0.295 (−)& | 0.088 (−)& | |
| Glucose | Premenopausal | 0.802 (−) | 0.864 (−) | 0.452 (+) | 0.610 (+) | 0.429 (+) | 0.605 (+) | 0.570 (−) | 0.753 (−) | 0.609 (+) | 0.955 (−) | 0.567 (−) | 0.773 (+) | 0.695 (−) | 0.339 (−) | 0.890 (−) |
| Menopausal | 0.783 (+) | 0.778 (−) | 0.528 (−) | 0.587 (−) | 0.764 (−) | 0.854 (+) | 0.141 (+) | 0.768 (+) | 0.182 (−) | 0.830 (−) | 0.692 (−) | 0.907 (−) | 0.828 (+) | 0.935 (−) | 0.833 (−) | |
| MP effectπ | 0.717 (+) | 0.984 (−) | 0.329 (−) | 0.457 (−) | 0.437 (−) | 0.807 (−) | 0.264 (+) | 0.684 (+) | 0.313 (−) | 0.913 (−) | 0.884 (+) | 0.771 (−) | 0.665 (+)& | 0.524 (+)& | 0.964 (−)& | |
| Insulin | Premenopausal | 0.034 (−) | 0.035 (−) | 0.934 (+) | 0.382 (−) | 0.115 (−) | 0.264 (−) | 0.473 (−) | 0.475 (−) | 0.939 (+) | 0.023 (−) | 0.067 (−) | 0.467 (+) | 0.136 (−) | 0.451 (−) | 0.230 (+) |
| Menopausal | 0.177 (−) | 0.020 (−) | 0.170 (−) | 0.037 (−) | 0.004 (−) | 0.156 (−) | 0.877 (+) | 0.866 (−) | 0.603 (−) | 0.673 (−) | 0.330 (−) | 0.624 (−) | 0.299 (+) | 0.247 (+) | 0.582 (+) | |
| MP effectπ | 0.664 (+) | 0.765 (−) | 0.284 (−) | 0.165 (−) | 0.078 (−) | 0.493 (−) | 0.507 (+) | 0.641 (+) | 0.707 (−) | 0.277 (+) | 0.701 (+) | 0.403 (−) | 0.088 (+)& | 0.168 (+)& | 0.806 (−)& | |
| HOMA-IR | Premenopausal | 0.037 (−) | 0.043 (−) | 0.729 (+) | 0.377 (−) | 0.203 (−) | 0.492 (−) | 0.455 (−) | 0.489 (−) | 0.867 (+) | 0.039 (−) | 0.074 (−) | 0.423 (+) | 0.195 (−) | 0.389 (−) | 0.298 (+) |
| Menopausal | 0.333 (−) | 0.046 (−) | 0.168 (−) | 0.059 (−) | 0.014 (−) | 0.268 (−) | 0.501 (+) | 0.984 (−) | 0.366 (−) | 0.662 (−) | 0.331 (−) | 0.644 (−) | 0.378 (+) | 0.397 (+) | 0.722 (+) | |
| MP effectπ | 0.431 (+) | 0.987 (+) | 0.222 (−) | 0.279 (−) | 0.176 (−) | 0.590 (−) | 0.317 (+) | 0.586 (+) | 0.501 (−) | 0.287 (+) | 0.624 (+) | 0.378 (−) | 0.134 (+)& | 0.230 (+)& | 0.699 (−)& | |
| Total cholesterol | Premenopausal | 0.614 (−) | 0.198 (+) | 0.072 (+) | 0.612 (+) | 0.308 (+) | 0.538 (+) | 0.032 (−) | 0.423 (−) | 0.103 (+) | 0.246 (+) | 0.027 (+) | 0.449 (+) | 0.444 (+) | 0.147 (+) | 0.717 (+) |
| Menopausal | <0.001 (+) | <0.001 (+) | 0.541 (+) | 0.007 (+) | <0.001 (+) | 0.011 (+) | 0.591 (+) | 0.390 (+) | 0.805 (+) | 0.004 (+) | 0.027 (+) | 0.291 (−) | 0.391 (+) | 0.613 (−) | 0.021 (−) | |
| MP effectπ | 0.002 (+) | 0.017 (+) | 0.573 (−) | 0.036 (+) | 0.002 (+) | 0.055 (+) | 0.074 (+) | 0.241 (+) | 0.392 (−) | 0.088 (+) | 0.585 (+) | 0.194 (−) | 0.768 (+)& | 0.221 (−)& | 0.034 (−)& | |
| HDL-cholesterol | Premenopausal | 0.082 (+) | 0.183 (+) | 0.617 (−) | 0.047 (+) | 0.224 (+) | 0.458 (−) | 0.283 (+) | 0.677 (+) | 0.543 (−) | 0.749 (+) | 0.180 (+) | 0.506 (+) | 0.285 (−) | 0.821 (+) | 0.323 (+) |
| Menopausal | 0.153 (+) | 0.014 (+) | 0.253 (+) | 0.203 (+) | 0.064 (+) | 0.474 (+) | 0.594 (−) | 0.934 (+) | 0.392 (+) | 0.195 (+) | 0.054 (+) | 0.613 (+) | 0.997 (−) | 0.980 (+) | 0.874 (−) | |
| MP effectπ | 0.716 (−) | 0.528 (+) | 0.259 (+) | 0.976 (+) | 0.727 (−) | 0.317 (+) | 0.231 (−) | 0.447 (+) | 0.368 (+) | 0.390 (+) | 0.744 (−) | 0.991 (+) | 0.551 (+)& | 0.915 (−)& | 0.491 (−)& | |
| Triglycerides | Premenopausal | 0.413 (−) | 0.400 (−) | 0.779 (+) | 0.365 (−) | 0.764 (−) | 0.509 (+) | 0.128 (−) | 0.236 (−) | 0.796 (+) | 0.216 (+) | 0.817 (+) | 0.728 (−) | 0.126 (+) | 0.713 (+) | 0.495 (−) |
| Menopausal | 0.505 (−) | 0.293 (−) | 0.732 (−) | 0.832 (−) | 0.502 (+) | 0.107 (+) | 0.783 (−) | 0.522 (−) | 0.535 (−) | 0.567 (−) | 0.198 (−) | 0.371 (−) | 0.672 (−) | 0.147 (−) | 0.132 (−) | |
| MP effectπ | 0.993 (−) | 0.772 (−) | 0.658 (−) | 0.655 (+) | 0.484 (+) | 0.444 (+) | 0.274 (+) | 0.544 (+) | 0.571 (−) | 0.279 (−) | 0.214 (−) | 0.535 (−) | 0.246 (−)& | 0.154 (−)& | 0.359 (−)& | |
AEA: N-arachidonoylethanolamide; PEA: N-palmitoylethanolamide; OEA: N-oleoylethanolamide; SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: homeostatic model assessment – insulin resistance; HDL: high density lipoprotein.
¥ Effect of BMI on the correlation between NAE values and metabolic parameters within premenopausal and menopausal cohorts. Positive effect (+): the positive (or negative) correlation found within the premenopausal/menopausal cohort increased (or decreased) with increasing BMI classes. Negative effect (−): the positive (or negative) correlation within the premenopausal/menopausal cohort decreased (or increased) with increasing BMI classes. π Effect of MP on the correlation between NAE values and metabolic parameters in all women and within BMI classes. Positive effect (+): the positive (or negative) correlation is higher (or lower) in menopausal women than in premenopausal women. Negative effect (−): the positive (or negative) correlation is lower (or higher) in menopausal women than in premenopausal women. & Interaction between BMI and MP effects on the correlation between NAE values and metabolic parameters. Positive interaction (+): the positive (or negative) effect of BMI on the correlation is higher (or lower) in menopausal than in premenopausal females (i.e.: the positive (or negative) effect of MP on the correlation increased (or decreased) with increasing BMI classes). Negative interaction (−): the positive (or negative) effect of BMI on the correlation is lower (or higher) in menopausal than in premenopausal females (i.e.: the positive (or negative) effect of MP on the correlation decreased (or increased) with increasing BMI classes).
Waist circumference positively associated with plasma AEA (P = 0.011) in premenopausal, and with AEA and PEA (P = 0.001 and P = 0.040, respectively) in menopausal women, whereas it negatively associated with PEA/AEA and OEA/AEA in premenopausal (both P < 0.001) and menopausal (P = 0.009 and P = 0.012, respectively) cohorts. When analyzed within BMI classes, positive waist circumference association with AEA (P = 0.020) and PEA (P = 0.050) in menopausal OW and with OEA/PEA in premenopausal OW (P = 0.042) were found. In addition, negative waist circumference associations were found with PEA/AEA in NW (P = 0.039), OW (P = 0.001) and OB (P = 0.040) premenopausal and in NW menopausal (P = 0.049) classes, and with OEA/AEA in NW (P = 0.014) and OB (P = 0.015) premenopausal women.
In the premenopausal cohort, positive associations of SBP with OEA (P = 0.036) and OEA/PEA (P = 0.013), and of DBP with AEA (P = 0.018) were found. Furthermore, in OB premenopausal women, AEA directly (P = 0.018) and PEA/AEA inversely (P = 0.017) associated with DBP, and PEA/AEA association with DBP was negatively impacted by BMI (P = 0.028). No associations of BPs with NAE profile were found in the overall menopausal cohort. However, in NW menopausal women, SBP positively associated with PEA (P = 0.018), OEA (0.002), and OEA/PEA (P = 0.029). In addition, in this class, SBP (P = 0.015) and DBP (P = 0.038) were positively associated with OEA/AEA, and both were negatively impacted by BMI (P = 0.032 and P = 0.005, respectively).
No associations were found between NAE levels and the glucose metabolism in the premenopausal and menopausal cohorts. In addition, no association of fasting glucose with NAE profile was detected in premenopausal BMI classes. On the other hand, in menopausal women, direct glucose correlations with AEA (P = 0.038) and PEA (P = 0.022) were found in NW class; these were positively influenced by menopause (P = 0.044 and P = 0.045, respectively). PEA/AEA negatively associated with insulin and HOMA-IR in all (P = 0.034 and P = 0.037, respectively) and in OB (P = 0.023 and P = 0.039, respectively) premenopausal women and with insulin in NW menopausal women (P = 0.037). OEA/AEA negatively associated with insulin and HOMA-IR in premenopausal (P = 0.035 and P = 0.043, respectively) and menopausal (P = 0.020 and P = 0.046, respectively) cohorts and in NW menopausal class (P = 0.004 and P = 0.014, respectively).
When analyzed in overall cohorts, total cholesterol directly associated with OEA in premenopausal women (P = 0.036) and with PEA/AEA and OEA/AEA in menopausal (both P < 0.001) women. Within BMI classes, total cholesterol correlated directly with PEA (P = 0.006) and OEA (P = 0.008) in premenopausal NW; with OEA/AEA in premenopausal OB (P = 0.027); with PEA, OEA (both P < 0.001) and OEA/PEA (P = 0.011) in menopausal NW; with PEA/AEA and OEA/AEA in NW (P = 0.007 and P < 0.001, respectively) and OB (P = 0.004 and P = 0.027, respectively) menopausal women. Total cholesterol also negatively associated with PEA/AEA in OW premenopausal class (P = 0.032). Menopause significantly increased the total cholesterol association with PEA/AEA (P = 0.002 and P = 0.036, respectively) and OEA/AEA (P = 0.017 and P = 0.002, respectively) in all and in NW women, and with OEA in NW women (P = 0.027). In addition, BMI negatively impacted on the total cholesterol association with PEA (P = 0.049), OEA (P = 0.005) and OEA/PEA (P = 0.021). A negative interaction between BMI and menopause effects impacted on the total cholesterol association with OEA (P = 0.032) and OEA/PEA (P = 0.034). In premenopausal women, HDL-cholesterol only positively associated with PEA/AEA in NW class (P = 0.047). At variance, in menopausal women HDL-cholesterol directly associated with OEA (P = 0.043) and OEA/AEA (P = 0.014) in all, and with PEA (P = 0.010) and OEA (P = 0.022) in NW menopausal women. Triglycerides were not associated with the NAE profile in premenopausal women but were directly associated with AEA (P = 0.010), PEA (P = 0.005) and OEA (P = 0.043) in all, with PEA (P = 0.023) and OEA (P = 0.010) in NW, and with PEA in OB (P = 0.050) menopausal women. In addition, menopause had a direct impact on the triglyceride association with PEA in all (P = 0.017) and in OB (P = 0.024) women.
3.2. Males
3.2.1. Descriptive features of the male cohort (Supplemental Table 2)
The anthropometric and metabolic features of the male cohort are detailed elsewhere [12]. In brief, age (P = 0.009), waist circumference, SBP, DBP, insulin, HOMA-IR, and triglycerides increased, while HDL-cholesterol decreased, with BMI (all P < 0.001). SBP also increased with age (P < 0.001). Age and BMI interaction had a negative impact on total cholesterol (P = 0.032), indicating that age correlation with total cholesterol is reduced with increasing BMI.
3.2.2. Circulating NAE levels and ratios in the male cohort
The effects of BMI and age, as well as their interaction, on NAE levels and ratios in the overall cohort as well as within BMI classes, are reported in Table 3. NAE levels and ratios in each BMI class, along with comparisons between pairs of BMI classes, are reported in Figure 2.
Table 3.
Results of the two-way ANOVA evaluating the effects of body mass index (BMI), age and their interaction on N-acylethanolamine (NAE) circulating levels and their ratios in the male cohort. Data are shown as P values (sign of the coefficients) of the evaluated effects: non italic data show first order (main) effects, italic data show second order effects (i.e., the interactions between the two main effects). Significant P values are reported in bold.
| Factors | Cohort | AEA | PEA | OEA | PEA/AEA | OEA/AEA | OEA/PEA |
|---|---|---|---|---|---|---|---|
| Effect of BMI§ | Overall | <0.001 (+) | <0.001 (+) | 0.022 (+) | 0.032 (−) | 0.017 (−) | 0.561 (−) |
| Effect of age* | Overall | 0.474 (−) | 0.657 (−) | 0.340 (+) | 0.589 (+) | 0.014 (+) | 0.039 (+) |
| NW* | 0.987 (+) | 0.372 (+) | 0.969 (+) | 0.244 (+) | 0.920 (−) | 0.288 (−) | |
| OW* | 0.725 (−) | 0.828 (−) | 0.109 (+) | 0.823 (+) | 0.006 (+) | 0.006 (+) | |
| OB* | 0.487 (−) | 0.406 (−) | 0.728 (+) | 0.990 (+) | 0.097 (+) | 0.107 (+) | |
| Interaction BMI x age$ | 0.522 (−) | 0.261 (−) | 0.763 (+) | 0.639 (−) | 0.121 (+) | 0.057 (+) |
AEA: N-arachidonoylethanolamide; PEA: N-palmitoylethanolamide; OEA: N-oleoylethanolamide; NW: normal weight; OW: overweight; OB: obese.
§ Effect of BMI on NAE levels and ratios. Positive effect (+): NAE values increased with increasing BMI classes; negative effect (−): NAE values decreased with increasing BMI classes. * Effect of age on NAE levels and ratios. Positive effect (+): NAE values increased with age; negative effect (−): NAE values decreased with age. $ Interaction between BMI and age effects on NAE levels and ratios. Positive interaction (+): the positive (or negative) effect of age increased (or decreased) with increasing BMI classes (i.e., the positive (or negative) effect of BMI increased (or decreased) with increasing). Negative interaction (−): the positive (or negative) effect of age decreased (or increased) with increasing BMI classes (i.e., the positive (or negative) effect of BMI decreased (or increased) with increasing age).
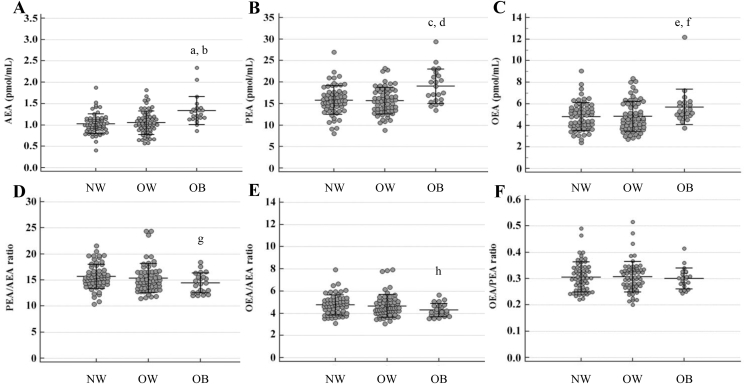
Figure 2.
N-acylethanolamine levels and ratios according to body mass index in men. AEA: N-arachidonoylethanolamide; PEA: N-palmitoylethanolamide; OEA N-oleoylethanolamide; NW: normal weight; OW: overweight; OB: obese. AEA (A): aP < 0.001: OB vs. NW men; bP < 0.001: OB vs. OW men. No significant differences were found by comparing OW vs. NW men (P = 0.690). PEA (B): cP = 0.001: OB vs. NW men; dP < 0.001: OB vs. OW men. No significant differences were found by comparing OW vs. NW men (P = 0.706). OEA (C): eP = 0.022: OB vs. NW men; fP = 0.014: OB vs. OW men. No significant differences were found by comparing OW vs. NW men (P = 0.854). PEA/AEA (D): gP = 0.032: OB vs. NW men. No significant differences were found by comparing OW vs. NW (P = 0.262) and OB vs. OW (P = 0.165) men. OEA/AEA (E): hP = 0.017: OB vs. NW men. No significant differences were found by comparing OW vs. NW (P = 0.407) and OB vs. OW (P = 0.067) men. OEA/PEA (F): no significant differences were found by comparing pairs of BMI classes (OW vs. NW: P = 0.871; OB vs. NW: P = 0.561 and OB vs. OW: P = 0.483) within men.
Mean AEA (1.08 ± 0.29 pmol/mL), PEA (16.2 ± 3.5 pmol/mL) and OEA (4.95 ± 1.44 pmol/mL) values observed in the overall population were positively influenced by BMI (P < 0.001, P = 0.001 and P = 0.022, respectively), while PEA/AEA (P = 0.032) and OEA/AEA (P = 0.017) decreased with BMI (Table 3).
OEA/AEA and OEA/PEA increased with age in all (P = 0.014 and P = 0.039, respectively) and in OW (both P = 0.006) men (Table 3).
No significant interaction between BMI and age effects were found to influence the NAE profile. The effects of BMI, age and their interaction on NAE levels and ratio observed after adjusting for each metabolic parameter are reported in Supplemental Table 4.
3.2.3. Association between circulating NAE levels and ratios and metabolic parameters in the male cohort
The BMI-independent associations of each metabolic parameter with NAE levels (Table 4A) and ratios (Table 4B) were analyzed in men along with the effects of BMI and age on each association. In addition, correlations between metabolic parameters and the NAE profile were analyzed in each BMI class.
Table 4A.
Correlation of N-acylethanolamine (NAE) circulating levels (A) and their ratios (B) with metabolic parameters evaluated in the overall male cohort and within body mass index (BMI) classes. Data are shown as P values (sign of the coefficients) of the correlations (non italic data) as well as of the effects of BMI, age and their interaction on the reported correlations (italic data). Significant P values are reported in bold.
| Metabolic parameter | Overall cohort |
BMI classes |
BMI effect¥ |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal weight (NW) |
Overweight (OW) |
Obese (OB) |
|||||||||||||
| AEA | PEA | OEA | AEA | PEA | OEA | AEA | PEA | OEA | AEA | PEA | OEA | AEA | PEA | OEA | |
| Waist circumference | 0.010 (+) | 0.013 (+) | 0.218 (+) | 0.068 (+) | 0.013 (+) | 0.251 (+) | 0.010 (+) | 0.061 (+) | 0.207 (+) | 0.350 (+) | 0.408 (+) | 0.782 (+) | 0.978 (+) | 0.666 (−) | 0.771 (−) |
| Effect of ageπ | 0.482 (−) | 0.548 (−) | 0.562 (−) | 0.486 (+) | 0.743 (+) | 0.761 (−) | 0.946 (+) | 0.229 (−) | 0.169 (−) | 0.347 (−) | 0.665 (−) | 0.855 (−) | 0.273 (−)& | 0.611 (−)& | 0.928 (−)& |
| SBP | 0.745 (+) | 0.932 (−) | 0.793 (−) | 0.137 (+) | 0.237 (+) | 0.092 (+) | 0.540 (+) | 0.376 (−) | 0.191 (−) | 0.357 (−) | 0.803 (−) | 0.605 (−) | 0.117 (−) | 0.400 (−) | 0.182 (−) |
| Effect of ageπ | 0.061 (+) | 0.109 (+) | 0.101 (+) | 0.342 (+) | 0.217 (+) | 0.159 (+) | 0.265 (+) | 0.613 (−) | 0.909 (+) | 0.212 (+) | 0.093 (+) | 0.196 (+) | 0.489 (+)& | 0.340 (+)& | 0.600 (+)& |
| DBP | 0.069 (−) | 0.026 (−) | 0.020 (−) | 0.136 (−) | 0.025 (−) | 0.215 (−) | 0.480 (−) | 0.129 (−) | 0.023 (−) | 0.264 (−) | 0.447 (−) | 0.331 (−) | 0.784 (−) | 0.687 (+) | 0.789 (−) |
| Effect of ageπ | 0.979 (+) | 0.103 (−) | 0.367 (−) | 0.116 (−) | 0.002 (−) | 0.023 (−) | 0.824 (+) | 0.192 (−) | 0.690 (−) | 0.671 (+) | 0.915 (−) | 0.985 (+) | 0.346 (+)& | 0.335 (+)& | 0.438 (+)& |
| Glucose | 0.395 (−) | 0.045 (−) | 0.111 (−) | 0.150 (+) | 0.264 (+) | 0.582 (+) | 0.706 (−) | 0.143 (−) | 0.057 (−) | 0.134 (−) | 0.020 (−) | 0.145 (−) | 0.051 (−) | 0.010 (−) | 0.120 (−) |
| Effect of ageπ | 0.005 (+) | 0.002 (+) | 0.067 (+) | 0.769 (+) | 0.331 (−) | 0.363 (−) | 0.686 (+) | 0.946 (+) | 0.563 (−) | 0.004 (+) | <0.001 (+) | 0.013 (+) | 0.009 (+)& | <0.001 (+)& | 0.008 (+)& |
| Insulin | 0.837 (−) | 0.975 (−) | 0.069 (−) | 0.380 (−) | 0.552 (−) | 0.003 (−) | 0.614 (+) | 0.911 (+) | 0.442 (−) | 0.922 (−) | 0.838 (+) | 0.735 (−) | 0.752 (+) | 0.650 (+) | 0.290 (+) |
| Effect of ageπ | 0.205 (−) | 0.055 (−) | 0.017 (−) | 0.062 (−) | 0.082 (−) | 0.004 (−) | 0.501 (−) | 0.156 (−) | 0.099 (−) | 0.794 (−) | 0.448 (−) | 0.519 (−) | 0.534 (+)& | 0.904 (+)& | 0.460 (+)& |
| HOMA-IR | 0.992 (+) | 0.936 (+) | 0.085 (−) | 0.576 (−) | 0.746 (−) | 0.008 (−) | 0.742 (+) | 0.808 (−) | 0.236 (−) | 0.904 (+) | 0.686 (+) | 0.903 (−) | 0.715 (+) | 0.611 (+) | 0.261 (+) |
| Effect of ageπ | 0.471 (−) | 0.143 (−) | 0.044 (−) | 0.077 (−) | 0.053 (−) | 0.004 (−) | 0.554 (−) | 0.160 (−) | 0.085 (−) | 0.709 (+) | 0.971 (−) | 0.981 (−) | 0.242 (+)& | 0.373 (+)& | 0.171 (+)& |
| Total cholesterol | 0.460 (+) | 0.049 (+) | 0.330 (+) | 0.029 (+) | 0.002 (+) | 0.005 (+) | 0.112 (−) | 0.240 (−) | 0.070 (−) | 0.592 (+) | 0.185 (+) | 0.554 (+) | 0.603 (−) | 0.781 (−) | 0.439 (−) |
| Effect of ageπ | 0.570 (−) | 0.286 (−) | 0.412 (−) | 0.085 (+) | 0.272 (+) | 0.068 (+) | 0.954 (+) | 0.184 (−) | 0.495 (−) | 0.256 (−) | 0.328 (−) | 0.247 (−) | 0.119 (−)& | 0.216 (−)& | 0.108 (−)& |
| HDL-cholesterol | 0.035 (−) | 0.111 (−) | 0.365 (−) | 0.043 (−) | 0.151 (−) | 0.903 (+) | 0.148 (−) | 0.359 (−) | 0.219 (−) | 0.293 (−) | 0.364 (−) | 0.561 (−) | 0.799 (−) | 0.739 (−) | 0.557 (−) |
| Effect of ageπ | 0.153 (+) | 0.171 (+) | 0.089 (+) | 0.889 (−) | 0.101 (−) | 0.601 (+) | 0.913 (+) | 0.648 (+) | 0.082 (+) | 0.100 (+) | 0.042 (+) | 0.283 (+) | 0.114 (+)& | 0.013 (+)& | 0.420 (+)& |
| Triglycerides | 0.993 (+) | 0.655 (+) | 0.404 (−) | 0.319 (+) | 0.362 (+) | 0.793 (−) | 0.572 (−) | 0.508 (−) | 0.089 (−) | 0.766 (−) | 0.716 (+) | 0.979 (−) | 0.443 (−) | 0.874 (−) | 0.910 (+) |
| Effect of ageπ | 0.305 (+) | 0.621 (−) | 0.686 (−) | 0.899 (+) | 0.454 (−) | 0.091 (−) | 0.212 (+) | 0.757 (−) | 0.860 (−) | 0.509 (+) | 0.894 (−) | 0.746 (+) | 0.577 (+)& | 0.863 (+)& | 0.336 (+)& |
Table 4B.
| Metabolic parameter | Overall cohort |
BMI classes |
BMI effect¥ |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal weight (NW) |
Overweight (OW) |
Obese (OB) |
|||||||||||||
| PEA/AEA | OEA/AEA | OEA/PEA | PEA/AEA | OEA/AEA | OEA/PEA | PEA/AEA | OEA/AEA | OEA/PEA | PEA/AEA | OEA/AEA | OEA/PEA | PEA/AEA | OEA/AEA | OEA/PEA | |
| Waist circumference | 0.458 (−) | 0.099 (−) | 0.299 (−) | 0.694 (+) | 0.472 (−) | 0.222 (−) | 0.151 (−) | 0.166 (−) | 0.775 (−) | 0.701 (−) | 0.336 (−) | 0.617 (−) | 0.601 (−) | 0.605 (−) | 0.898 (+) |
| Effect of ageπ | 0.761 (+) | 0.866 (+) | 0.811 (−) | 0.471 (−) | 0.137 (−) | 0.418 (−) | 0.144 (−) | 0.082 (−) | 0.462 (−) | 0.364 (+) | 0.290 (+) | 0.867 (+) | 0.283 (+)& | 0.152 (+)& | 0.698 (+)& |
| SBP | 0.542 (−) | 0.409 (−) | 0.744 (−) | 0.421 (−) | 0.646 (+) | 0.216 (+) | 0.029 (−) | 0.008 (−) | 0.350 (−) | 0.232 (+) | 0.655 (+) | 0.561 (−) | 0.151 (+) | 0.894 (+) | 0.250 (−) |
| Effect of ageπ | 0.374 (−) | 0.993 (−) | 0.507 (+) | 0.896 (+) | 0.376 (+) | 0.503 (+) | 0.014 (−) | 0.205 (−) | 0.476 (+) | 0.818 (+) | 0.786 (+) | 0.929 (+) | 0.883 (+)& | 0.877 (−)& | 0.826 (−)& |
| DBP | 0.994 (+) | 0.301 (−) | 0.319 (−) | 0.590 (−) | 0.867 (+) | 0.410 (+) | 0.376 (−) | 0.017 (−) | 0.086 (−) | 0.405 (+) | 0.981 (+) | 0.490 (−) | 0.324 (+) | 0.953 (+) | 0.319 (−) |
| Effect of ageπ | 0.032 (−) | 0.177 (−) | 0.556 (+) | 0.130 (−) | 0.163 (−) | 0.886 (+) | 0.034 (−) | 0.447 (−) | 0.314 (+) | 0.393 (−) | 0.490 (−) | 0.923 (+) | 0.781 (−)& | 0.867 (−)& | 0.967 (+)& |
| Glucose | 0.220 (−) | 0.249 (−) | 0.809 (−) | 0.415 (−) | 0.288 (−) | 0.618 (−) | 0.196 (−) | 0.024 (−) | 0.226 (−) | 0.533 (−) | 0.911 (−) | 0.737 (+) | 0.816 (−) | 0.737 (+) | 0.609 (+) |
| Effect of ageπ | 0.590 (−) | 0.332 (−) | 0.565 (−) | 0.090 (−) | 0.104 (−) | 0.737 (−) | 0.588 (−) | 0.227 (−) | 0.323 (−) | 0.845 (+) | 0.897 (−) | 0.807 (−) | 0.432 (+)& | 0.647 (+)& | 0.912 (−)& |
| Insulin | 0.694 (+) | 0.020 (−) | 0.004 (−) | 0.495 (+) | 0.002 (−) | <0.001 (−) | 0.599 (−) | 0.098 (−) | 0.174 (−) | 0.655 (+) | 0.707 (−) | 0.400 (−) | 0.933 (+) | 0.264 (+) | 0.274 (+) |
| Effect of ageπ | 0.560 (−) | 0.089 (−) | 0.156 (−) | 0.523 (+) | 0.135 (−) | 0.017 (−) | 0.421 (−) | 0.214 (−) | 0.422 (−) | 0.530 (−) | 0.483 (−) | 0.915 (−) | 0.396 (−)& | 0.953 (+)& | 0.316 (+)& |
| HOMA-IR | 0.852 (+) | 0.013 (−) | 0.004 (−) | 0.589 (+) | 0.002 (−) | <0.001 (−) | 0.464 (−) | 0.039 (−) | 0.117 (−) | 0.719 (+) | 0.746 (−) | 0.467 (−) | 0.944 (+) | 0.233 (+) | 0.246 (+) |
| Effect of ageπ | 0.395 (−) | 0.063 (−) | 0.175 (−) | 0.821 (+) | 0.086 (−) | 0.025 (−) | 0.357 (−) | 0.149 (−) | 0.344 (−) | 0.490 (−) | 0.523 (−) | 0.983 (+) | 0.475 (−)& | 0.799 (+)& | 0.275 (+)& |
| Total cholesterol | 0.160 (+) | 0.660 (+) | 0.423 (−) | 0.483 (+) | 0.168 (+) | 0.530 (+) | 0.363 (+) | 0.632 (−) | 0.169 (−) | 0.354 (+) | 0.952 (+) | 0.522 (−) | 0.611 (+) | 0.566 (−) | 0.393 (−) |
| Effect of ageπ | 0.564 (−) | 0.710 (−) | 0.975 (−) | 0.154 (−) | 0.661 (+) | 0.100 (+) | 0.044 (−) | 0.339 (−) | 0.538 (+) | 0.587 (+) | 0.869 (−) | 0.461 (−) | 0.364 (+)& | 0.781 (−)& | 0.249 (−)& |
| HDL-cholesterol | 0.243 (+) | 0.158 (+) | 0.628 (+) | 0.231 (+) | 0.004 (+) | 0.035 (+) | 0.326 (+) | 0.822 (−) | 0.367 (−) | 0.633 (+) | 0.561 (+) | 0.910 (+) | 0.987 (+) | 0.606 (−) | 0.514 (−) |
| Effect of ageπ | 0.616 (−) | 0.524 (+) | 0.204 (+) | 0.040 (−) | 0.294 (+) | 0.003 (+) | 0.692 (+) | 0.018 (+) | 0.019 (+) | 0.925 (+) | 0.538 (−) | 0.501 (−) | 0.395 (+)& | 0.338 (−)& | 0.081 (−)& |
| Triglycerides | 0.496 (+) | 0.328 (−) | 0.061 (−) | 0.783 (−) | 0.122 (−) | 0.115 (−) | 0.932 (+) | 0.190 (−) | 0.063 (−) | 0.323 (+) | 0.718 (+) | 0.625 (−) | 0.323 (+) | 0.268 (+) | 0.693 (+) |
| Effect of ageπ | 0.018 (−) | 0.053 (−) | 0.974 (+) | 0.214 (−) | 0.006 (−) | 0.085 (−) | 0.016 (−) | 0.115 (−) | 0.886 (+) | 0.188 (−) | 0.635 (−) | 0.471 (+) | 0.468 (−)& | 0.518 (+)& | 0.181 (+)& |
AEA: N-arachidonoylethanolamide; PEA: N-palmitoylethanolamide; OEA: N-oleoylethanolamide; SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: homeostatic model assessment – insulin resistance; HDL: high density lipoprotein.
¥ Effect of BMI on the correlation between NAE values and metabolic parameters. Positive effect (+): the positive (or negative) correlation found in the overall cohort increased (or decreased) with increasing BMI classes. Negative effect (−): the positive (or negative) correlation found in the overall cohort decreased (or increased) with increasing BMI classes. π Effect of age on the correlation between NAE values and metabolic parameters in the overall cohort and within BMI classes. Positive effect (+): the positive (or negative) correlation increased (or decreased) with age. Negative effect (−): the positive (or negative) correlation decreased (or increased) with age. & Interaction between BMI and age effects on the correlation between NAE values and metabolic parameters. Positive interaction (+): the positive (or negative) effect of age in the overall cohort increased (or decreased) with increasing BMI classes (i.e., the positive (or negative) effect of BMI increased (or decreased) with increasing age). Negative interaction (−): the positive (or negative) effect of age in the overall cohort decreased (or increased) with increasing BMI classes (i.e., the positive (or negative) effect of BMI decreased (or increased) with increasing age).
Waist circumference directly associated with AEA in all and in OW men (both P = 0.010) and with PEA in all and in NW (both P = 0.013) men.
SBP was only found to negatively correlate with PEA/AEA (P = 0.029) and OEA/AEA (P = 0.008) in OW men. DBP negatively associated with PEA (P = 0.026) and OEA (P = 0.020) in all, with PEA in NW (P = 0.025), and with OEA (P = 0.023) and OEA/AEA (P = 0.017) in OW men. Age negatively impacted SBP association with PEA/AEA in OW (P = 0.014) and DBP association with PEA in NW (P = 0.002) classes.
A negative glucose association with PEA was found in all (P = 0.045) and in OB (P = 0.020) men and was positively impacted by age (P = 0.002 and P < 0.001, respectively). The glucose association with PEA was also negatively impacted by BMI (P = 0.010), and positively by the interaction between age and BMI effects (P < 0.001). Glucose also negatively associated with OEA/AEA in OW class (P = 0.024). In the overall cohort, insulin and HOMA-IR were not associated with NAE level, but were negatively associated with OEA/AEA (P = 0.020 and P = 0.013, respectively) and OEA/PEA (both P = 0.004). In NW men, insulin and HOMA-IR negatively associated with OEA (P = 0.003 and P = 0.008, respectively), OEA/AEA (both P = 0.002), and OEA/PEA (both P < 0.001). In addition, in NW men, age negatively impacted the insulin and HOMA-IR association with OEA (both P = 0.004) and with OEA/PEA (P = 0.017 and P = 0.025, respectively). HOMA-IR negatively associated with OEA/AEA also in the OW class (P = 0.039).
Total cholesterol positively associated with PEA (P = 0.049) in all, and with AEA (P = 0.029), PEA (P = 0.002), and OEA (P = 0.005) in NW men. HDL-cholesterol negatively associated with AEA in all (P = 0.035) and in NW (P = 0.043), and directly associated with OEA/AEA (P = 0.004) and OEA/PEA (P = 0.035) in NW men. The latter association was directly impacted by age (P = 0.003). Triglycerides were not associated with the NAE profile.
4. Discussion
There is a non-negligible inconsistency in studies reporting on blood ECs and NAEs in humans, both in terms of concentrations and their association with physiopathology states. The lack of standardization in sample handling and processing is recognized as a major cause of variability in NAE measurement [7], [10], [11]. Heterogeneity may also derive from the possibly unwise approach to investigating human cohorts indistinctly, pooling both genders and various ages, thereby neglecting the impact of such major determinants on metabolic health. Moreover, in spite of the growing knowledge on the interdependency among the specific functions of different NAE compounds, previous studies on human obesity have only focused on the concentration levels of individual NAEs. AEA levels have been found to be unchanged [14], [15], [16] or increased [8], [17], [18], [19], [20], [21], [22], [23] in obesity. Similarly, PEA and OEA have been found to increase with obesity and sometimes not; likewise, it is not clear whether they reflect AEA changes with adiposity measures [8], [16], [20], [21], [23].
By separately addressing the two sexes, and by accounting for menopause in women and ageing in men, we found that AEA hypertone is a primary obesity feature, irrespective of gender and age. We also found that plasma PEA and OEA heightening is associated with BMI in men, independent of age, and in premenopausal women. Moreover, to the best of our knowledge, this is the first study demonstrating that circulating NAEs are regulated not only in terms of individual concentration but also in terms of their relative balance, as represented by the ratios proposed in the present study, in association with the investigated conditions. Previous observations by our group and others reported correlations among fasting AEA, PEA and OEA levels in healthy and in dysmetabolic individuals [7], [8], [9]. In the present study, we clearly show that BMI-related NAE hypertone is characterized by reduced PEA/AEA and OEA/AEA in both genders irrespectively of menopause/ageing states. These data highlight the importance of the NAE balance, rather than their absolute values, in determining or characterizing the obese status.
ECS functions in promoting adipose tissue expansion have been described extensively [6]. Studies using direct measurements of abdominal fat, by dual-energy X-ray absorptiometry or by computed tomography, in sex-mixed populations, reported an association of visceral fat with circulating 2AG [15], [16] and of both visceral and subcutaneous abdominal depots with AEA [18]. However, studies specifically on men, found that plasma AEA was not [24] or was inversely [15] correlated with visceral fat. Although waist circumference is frequently used as an indirect measure of visceral fat, it is essentially related to both visceral and subcutaneous abdominal depots. Studies on sex-mixed populations found a direct association of waist circumference with both circulating AEA and 2AG [19], or with AEA but not 2AG [8], [18]. Conversely, waist circumference was associated with increased 2AG, but not AEA, levels in postmenopausal women [17]. In our study, plasma AEA behaves like a biomarker of waist circumference independently of BMI, gender, fertility status, and age. Such a role was not revealed for 2AG in our previous study in the same cohort [12], which would seem to indicate that each EC is specifically involved in different correlates of a general metabolic impairment.
PEA and OEA were found to increase with waist circumference in a cohort composed of men and women of various ages according to a univariate model [8]. In contrast, recent findings highlighted the PPARα-mediated role of OEA in reducing the accumulation of visceral fat [3]. When absolute levels were considered, our BMI-adjusted approach excluded a direct association of waist circumference with OEA levels, while revealing its direct trend with PEA in menopausal women and in men, possibly reflecting an anti-inflammatory adaptive response to visceral fat inflammation [5]. The scenario changed when ratios were considered, as a strong inverse relationship was found between PEA/AEA and OEA/AEA and waist circumference in women, highlighting the role of OEA and PEA, both PPARα agonists, in female fat distribution.
The effect of menopause on AEA congeners, as well as the impact of ageing on plasma NAEs, have not been investigated to date. Our data showed that menopause elevates PEA, OEA and, in lean women, AEA levels, while not altering their ratios; whereas, in men, age does not influence NAE levels but increases OEA/AEA and OEA/PEA. Previous investigations reported the physiological modulation of AEA levels across the menstrual cycle, revealing higher levels in the follicular than in the luteal phase, and an ovulatory peak [25], [26], as a result of the combined action of estradiol, stimulating AEA synthesis by endothelial cells [27], and progesterone, enhancing AEA degradation by lymphocytes [28]. However, AEA level in menopause has been oppositely reported as similar to the luteal [25] or to the follicular AEA levels [26]. In view of our data, this inconsistency may derive from differences in the metabolic features of the investigated menopausal women. Examining the effect of the menstrual cycle on NAE levels was beyond the scope of the present study. Nonetheless, to avoid potential bias, we verified that, in our premenopausal cohort, the frequency of women in different menstrual phases occurred was similar in the three BMI classes, as determined according to the cycle day and to sex hormone levels (data not shown) [29], [30]. NAE levels we reported for the premenopausal cohort, therefore, should be interpreted as the overall average of the fertile age, when compared with menopausal levels.
AEA mediates vasorelaxing and hypotensive functions by acting on several endothelial and perivascular receptors [31], [32], [33]. The increase in AEA with DBP in our premenopausal women may represent an estrogen-mediated adaptive response to obesity-related hypertension [34]. According to the estrogen depletion, this adaptive mechanism was apparently lost in our menopausal cohort. Besides potentiating AEA action by the entourage effect [31], OEA and PEA are known to directly induce vasodilatory and cardioprotective responses, respectively [32]. In line with this, in our study the increase in OEA absolute and relative abundance may indicate an adaptive response to hypertension both in premenopausal women and in lean menopausal women. The fact that the OEA relationship with hypertension is abolished in overweight/obese menopausal women, may underline the protective contribution of OEA in maintaining a healthy profile. Conversely, in men, lowering the PEA and OEA abundance that accompanies increasing BPs in the elderly, may reflect a loss of protection contributing to ageing-related hypertension.
Physiological insulin secretion has been found to acutely suppress plasma NAE levels, whereas worsening insulin sensitivity results in glucose-mediated NAE elevation in the bloodstream [18], [19], [35], [36]. In line with insulin resistance inherent in the menopausal state, we found a direct association of AEA and PEA levels with fasting glycemia, specifically differentiating between lean menopausal and premenopausal women. Interestingly, OEA is not modulated by the same mechanisms. In men, a PEA inverse relationship with glycemia specifically characterizes young and obese individuals, possibly reflecting the loss of PEA anti-inflammatory function against glucotoxicity [37]. A positive association of AEA with fasting insulin and insulin resistance has been frequently reported [8], [9], [18], [38], [39]. Conversely, contrasting data have been reported for AEA congeners, as they were found to be not [8] or differentially [21] associated with insulin resistance. High AEA and congeners have been reported in type-2 diabetes vs. BMI-matched patients by some authors [35], [36], [40] but not by others [18]. Studies in women have reported increasing plasma NAE with elevated fasting insulin and insulin resistance [9], whereas other studies have not reported such associations in women [14] or in men [14], [15], [41]. When we considered absolute NAE levels, the lack of an association with insulin or HOMA-IR was confirmed. Conversely, PEA/AEA and OEA/AEA revealed strong biomarkers of insulin sensitivity in premenopausal women, highlighting the centrality of AEA function in worsening glucose control. The evaluation of NAE ratios also highlighted the beneficial importance of OEA in menopausal women and in ageing men, since both hyperinsulinemia and insulin resistance were accompanied by reduced OEA relative abundance, particularly in lean individuals.
Our results on BMI-independent NAE relationships with the blood lipid profile represent a further novelty of our findings. Indeed, data in the literature are limited to the direct association of PEA with LDL found in morbidly obese women [9] and to the AEA association with triglycerides found in a small gender-mixed population [18]. No NAE associations with the lipid profile have been found in other studies [15], [24], [38], [41]. In our hands, AEA was a biomarker of low HDL-cholesterol in men, and also of high total cholesterol levels in lean men. Such an AEA role was not detectable in women in any BMI class or fertility status. The primary association between PEA and OEA levels and total cholesterol availability was found in lean individuals of both genders. Menopausal hypercholesterolemia also showed a specific NAE profile, as characterized by increasing PEA and OEA relative abundance over AEA, specifically in lean women. Such a scenario could be explained by focusing on the NAE distribution in different circulating lipid fractions. In fact, while NAEs were believed to prevalently circulate in the albumin-bound form [42], [43], a recent paper by Bilgin et al. [44] showed that compounds in NAE 16:0 class, including PEA, and in 18:1 class, including OEA, largely circulate in lipoprotein fractions, whereas NAEs in the 20:4 class, including AEA, mainly circulate in the lipoprotein-free fraction. These findings could explain the strong association we found between PEA and OEA and total cholesterol, as both these compounds co-circulate in lipoproteins. In addition, we highlighted an OEA role in promoting a healthy cholesterol profile, as shown by associations of its absolute and relative abundance with HDL-cholesterol in menopausal, especially lean, women and in men, especially those who are lean and elderly. These evidences are in line with the beneficial effects of OEA on lipid oxidation observed in previous human studies [3], [4], [20], [45].
Menopausal hypertriglyceridemia is characterized by an NAE hypertone specifically involving a PEA increase but does not alter the NAE ratios. This association could be explained by the biochemical link between NAEs and triglycerides represented by fatty acids serving as precursors and constituents, respectively [4], [5], [20].
Various reasons could explain the depicted derangement in the NAE profile. According to the literature, the most probable causes could be the dysregulation of diverse NAE enzymatic pathways, as well as nutritional habits and their interplay. Recent studies in mice have highlighted that perturbations in the main NAE metabolic pathways, such as the genetic ablation of NAPE-PLD in adipose tissue [46], and the inhibition of FAAH and NAAA expression by inflammatory stimuli in blood cells [47], [48], enhance AEA over PEA and OEA levels, and induce a dysmetabolic and pro-inflammatory profile. However, whether plasma NAEs reflect the dynamics occurring in tissues is not clear. A recent study reported no association of plasma AEA, OEA and 2AG with respective concentrations in abdominal subcutaneous fat and skeletal muscle [49]. In addition, a human study reported that variations in NAE abundance in subcutaneous adipose tissue in response to weight loss or inflammation were not reflected in plasma from the same subjects [22]. In line with their ability to release NAEs in the bloodstream, and with NAE involvement in inflammatory processes [5], [7], [10], [11], blood cells may represent the link between plasma NAE derangement and dysmetabolism. The role of specific dietary fatty acids in modulating the blood level of corresponding NAEs, both in fed and fasting states, and in modifying the metabolic status, has recently emerged in literature [3], [20], [45]. These findings highlight nutritional habits as the possible major determinants of our results. Unfortunately, the main limitation of our study is the lack of diet information in the examined subjects.
Previous studies have rarely addressed the relationships of circulating NAE with metabolic parameters in a BMI-independent fashion. Moreover, menopause and ageing are usually not taken into account. The inconclusiveness of previous data likely also depend on the poor standardization of study populations, as to their physio-pathologic status, and of sampling procedures. Our strength lies in the large population size and in the exclusion of conditions affecting the NAE system, such as medications, concurring diseases, including psychiatric or allergic disturbances, shift-working, venipuncture stress and delayed blood processing. Our statistical design also highlights how most of the associations observed in overall cohorts, covering a wide BMI range, can be missed when focusing on overweight/obese sub-cohorts, possibly clouded by multiple dysmetabolic effects converging on the NAE balance.
5. Conclusions
In line with the current literature on the complex interdependency among lipid bioactive compounds, this study shows that our NAE profile, which is made up of absolute and relative abundances, mirrors metabolic states more effectively than individual AEA, PEA and OEA levels. It is thus an effective biomarker with gender, menopause and ageing specificities.
Among the reported data, two major relationships could be highlighted that define useful and unambiguous biomarkers of the metabolic status of one individual. First, we confirmed the role of AEA excess in featuring obesity and central fat accumulation both in women and men, adding the novel importance of AEA relative abundance in the NAE pool, as expressed by PEA/AEA and OEA/AEA, in reflecting central fat distribution and insulin resistance in women, but not in men. Other minor but context-specific roles for AEA excess were found in featuring hyperglycaemia in lean menopausal women and lowering HDL in men. Second, a clear beneficial role of OEA was demonstrated in men and in menopausal women, as its absolute and relative abundance, as expressed both by OEA/AEA and OEA/PEA, strongly reflect a healthy cholesterol profile and insulin sensitivity.
In addition, NAE associations with BMI, hypertension, glycemia and triglycerides were found to vary, in their extent as well as in their direction, in function of gender and menopause/ageing context. These findings strongly underline the importance of cohort definition when designing studies, and of circumstantiating the result interpretation accordingly.
Our associative evidences do not allow us to define whether circulating NAEs exert a function in themselves or merely reflect the average systemic status. Nonetheless, similarly with what previously highlighted for 2AG, our results in lean individuals suggest that NAE derangement could represent an event preceding or irrespective of obesity [12].
In conclusion, our results support the relevance of the NAE system as a target for interventions aiming at restoring metabolic health in humans. In view of the emerging literature on the effectiveness of diet composition in modifying the NAE circulating profile, our study represents the ground for the utilization of the NAE profiling as a tool for monitoring both patients compliance and responsiveness to possible therapeutic strategies based on nutritional approaches.
Acknowledgements
Financial supports
The study was supported by the European Union (REPROBESITY, FPVII-223713 and NEUROFAST, FPVII-KBBE-2009-3-245009) and by the Emilia-Romagna Region – University Program 2007–2009 (“The unifying inflammatory background of the metabolic syndrome: identification of genetic and metabolic biomarkers profiling tool for patient classification and clinical assessment”, PRUa1a-2007-006).
Author contributions
FF designed the study, performed NAE measurements, data collection and management, statistical analyses and wrote the manuscript. MM1 and IB performed NAE measurements. DIG, AR and GD recruited and examined participants. MM2 contributed to the statistical design and analyses. AMML performed the statistical design. VV, AG and RP contributed to the study concept and design. UP designed the population study and wrote the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.06.002.
Contributor Information
Flaminia Fanelli, Email: flaminia.fanelli@gmail.com.
Marco Mezzullo, Email: marco.mezzullo@gmail.com.
Andrea Repaci, Email: rep.rep@libero.it.
Ilaria Belluomo, Email: ilaria.belluomo@gmail.com.
Daniela Ibarra Gasparini, Email: danielita.ig@gmail.com.
Guido Di Dalmazi, Email: guido.didalmazi@unibo.it.
Marianna Mastroroberto, Email: marianna.mastroroberto@gmail.com.
Valentina Vicennati, Email: vicennati@aosp.bo.it.
Alessandra Gambineri, Email: alessandra.gambineri@aosp.bo.it.
Antonio Maria Morselli-Labate, Email: antoniomaria.morsellilabate@gmail.com.
Renato Pasquali, Email: renato.pasquali@unibo.it.
Uberto Pagotto, Email: uberto.pagotto@unibo.it.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Hussain Z., Uyama T., Tsuboi K., Ueda N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochimica et Biophysica Acta. 2017;1862:1546–1561. doi: 10.1016/j.bbalip.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Devane W.A., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 3.Bowen K.J., Kris-Etherton P.M., Shearer G.C., West S.G., Reddivari L., Jones P.J.H. Oleic acid-derived oleoylethanolamide: a nutritional science perspective. Progress in Lipid Research. 2017;67:1–15. doi: 10.1016/j.plipres.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Naughton S.S., Mathai M.L., Hryciw D.H., McAinch A.J. Fatty Acid modulation of the endocannabinoid system and the effect on food intake and metabolism. The Internet Journal of Endocrinology. 2013;2013:361895. doi: 10.1155/2013/361895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balvers M.G., Verhoeckx K.C., Meijerink J., Wortelboer H.M., Witkamp R.F. Measurement of palmitoylethanolamide and other N-acylethanolamines during physiological and pathological conditions. CNS and Neurological Disorders Drug Targets. 2013;12:23–33. doi: 10.2174/1871527311312010007. [DOI] [PubMed] [Google Scholar]
- 6.Gatta-Cherifi B., Cota D. New insights on the role of the endocannabinoid system in the regulation of energy balance. International Journal of Obesity (London) 2016;40:210–219. doi: 10.1038/ijo.2015.179. [DOI] [PubMed] [Google Scholar]
- 7.Fanelli F., Di Lallo V.D., Belluomo I., De Iasio R., Baccini M., Casadio E. Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional-LC/MS/MS. The Journal of Lipid Research. 2012;53:481–493. doi: 10.1194/jlr.M021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jumpertz R., Guijarro A., Pratley R.E., Piomelli D., Krakoff J. Central and peripheral endocannabinoids and cognate acylethanolamides in humans: association with race, adiposity, and energy expenditure. The Journal of Cinical Endocrinology and Metabolism. 2011;96:787–791. doi: 10.1210/jc.2010-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallipedhi A., Prior S.L., Dunseath G., Bracken R.M., Barry J., Caplin S. Changes in plasma levels of N-arachidonoyl ethanolamine and N-palmitoylethanolamine following bariatric surgery in morbidly obese females with impaired glucose homeostasis. Journal of Diabetes Research. 2015;2015:680867. doi: 10.1155/2015/680867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogeser M., Hauer D., Christina Azad S., Huber E., Storr M., Schelling G. Release of anandamide from blood cells. Clinical Chemistry and Laboratory Medicine. 2006;44:488–491. doi: 10.1515/CCLM.2006.065. [DOI] [PubMed] [Google Scholar]
- 11.Wood J.T., Williams J.S., Pandarinathan L., Courville A., Keplinger M.R., Janero D.R. Comprehensive profiling of the human circulating endocannabinoid metabolome: clinical sampling and sample storage parameters. Clinical Chemistry and Laboratory Medicine. 2008;46:1289–1295. doi: 10.1515/CCLM.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanelli F., Mezzullo M., Belluomo I., Di Lallo V.D., Baccini M., Ibarra Gasparini D. Plasma 2-arachidonoylglycerol is a biomarker of age and menopause related insulin resistance and dyslipidemia in lean but not in obese men and women. Molecular Metabolism. 2017;6:406–415. doi: 10.1016/j.molmet.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Blüher M., Engeli S., Klöting N., Berndt J., Fasshauer M., Bátkai S. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Côté M., Matias I., Lemieux I., Petrosino S., Alméras N., Després J.P. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. International Journal of Obesity (London) 2007;31:692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- 16.Sipe J.C., Scott T.M., Murray S., Harismendy O., Simon G.M., Cravatt B.F. Biomarkers of endocannabinoid system activation in severe obesity. PLoS One. 2010;5:e8792. doi: 10.1371/journal.pone.0008792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engeli S., Böhnke J., Feldpausch M., Gorzelniak K., Janke J., Bátkai S. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Marzo V., Verrijken A., Hakkarainen A., Petrosino S., Mertens I., Lundbom N. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. European Journal of Endocrinology. 2009;161:715–722. doi: 10.1530/EJE-09-0643. [DOI] [PubMed] [Google Scholar]
- 19.Gatta-Cherifi B., Matias I., Vallée M., Tabarin A., Marsicano G., Piazza P.V. Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. International Journal of Obesity (London) 2012;36:880–885. doi: 10.1038/ijo.2011.165. [DOI] [PubMed] [Google Scholar]
- 20.Joosten M.M., Balvers M.G., Verhoeckx K.C., Hendriks H.F., Witkamp R.F. Plasma anandamide and other N-acylethanolamines are correlated with their corresponding free fatty acid levels under both fasting and non-fasting conditions in women. Nutrition and Metabolism (London) 2010;7:49. doi: 10.1186/1743-7075-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martins C.J., Genelhu V., Pimentel M.M., Celoria B.M., Mangia R.F., Aveta T. Circulating endocannabinoids and the polymorphism 385C>A in fatty acid amide hydrolase (FAAH) gene may identify the obesity phenotype related to cardiometabolic risk: a study conducted in a brazilian population of complex interethnic admixture. PLoS One. 2015;10:e0142728. doi: 10.1371/journal.pone.0142728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montecucco F., Lenglet S., Quercioli A., Burger F., Thomas A., Lauer E. Gastric bypass in morbid obese patients is associated with reduction in adipose tissue inflammation via N-oleoylethanolamide (OEA)-mediated pathways. Thrombosis and Haemostasis. 2015;113:838–850. doi: 10.1160/TH14-06-0506. [DOI] [PubMed] [Google Scholar]
- 23.Quercioli A., Carbone F., Bonaventura A., Liberale L., Pataky Z., Thomas A. Plasma palmitoylethanolamide (PEA) as a potential biomarker for impaired coronary function. International Journal of Cardiology. 2017;231:1–5. doi: 10.1016/j.ijcard.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Kantae V., Nahon K.J., Straat M.E., Bakker L.E.H., Harms A.C., van der Stelt M. Endocannabinoid tone is higher in healthy lean South Asian than white Caucasian men. Scientific Reports. 2017;7:7558. doi: 10.1038/s41598-017-07980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam P.M., Marczylo T.H., El-Talatini M., Finney M., Nallendran V., Taylor A.H. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma. Analytical Biochemistry. 2008;380:195–201. doi: 10.1016/j.ab.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 26.El-Talatini M.R., Taylor A.H., Konje J.C. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertility and Sterility. 2010;93:1989–1996. doi: 10.1016/j.fertnstert.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 27.Maccarrone M., Bari M., Battista N., Finazzi-Agrò A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood. 2002;100:4040–4048. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- 28.Maccarrone M., Bari M., Di Rienzo M., Finazzi-Agrò A., Rossi A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. Journal of Biological Chemistry. 2003;278:32726–32732. doi: 10.1074/jbc.M302123200. [DOI] [PubMed] [Google Scholar]
- 29.Fanelli F., Belluomo I., Di Lallo V.D., Cuomo G., De Iasio R., Baccini M. Serum steroid profiling by isotopic dilution-liquid chromatography-mass spectrometry: comparison with current immunoassays and reference intervals in healthy adults. Steroids. 2011;76:244–253. doi: 10.1016/j.steroids.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Fanelli F., Fazzini A., Mezzullo M., Baccini M., Gambineri A., Vicennati V. 18th european congress of endocrinology, Munich, endocrine abstract EP 658. 2016. Sex hormone and steroid precursor measurement by simple and rapid lc-ms/ms: comparison with current routine immunoassays. [Google Scholar]
- 31.Ho W.S., Barrett D.A., Randall M.D. 'Entourage' effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. British Journal of Pharmacology. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montecucco F., Di Marzo V. At the heart of the matter: the endocannabinoid system in cardiovascular function and dysfunction. Trends in Pharmacological Sciences. 2012;33:331–340. doi: 10.1016/j.tips.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Stanley C.P., Hind W.H., Tufarelli C., O'Sullivan S.E. The endocannabinoid anandamide causes endothelium-dependent vasorelaxation in human mesenteric arteries. Pharmacological Research. 2016;113:356–363. doi: 10.1016/j.phrs.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zubrzycki M., Liebold A., Janecka A., Zubrzycka M. A new face of endocannabinoids in pharmacotherapy. Part I: protective role of endocannabinoids in hypertension and myocardial infarction. Journal of Physiology and Pharmacology. 2014;65:171–181. [PubMed] [Google Scholar]
- 35.Matias I., Gonthier M.P., Orlando P., Martiadis V., De Petrocellis L., Cervino C. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. The Journal of Cinical Endocrinology and Metabolism. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 36.Matias I., Gonthier M.P., Petrosino S., Docimo L., Capasso R., Hoareau L. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells. British Journal of Pharmacology. 2007;152:676–690. doi: 10.1038/sj.bjp.0707424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoareau L., Buyse M., Festy F., Ravanan P., Gonthier M.P., Matias I. Anti-inflammatory effect of palmitoylethanolamide on human adipocytes. Obesity (Silver Spring) 2009;17:431–438. doi: 10.1038/oby.2008.591. [DOI] [PubMed] [Google Scholar]
- 38.Quercioli A., Pataky Z., Vincenti G., Makoundou V., Di Marzo V., Montecucco F. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. European Heart Journal. 2011;32:1369–1378. doi: 10.1093/eurheartj/ehr029. [DOI] [PubMed] [Google Scholar]
- 39.Wang X., Yu Q., Yue H., Zhang J., Zeng S., Cui F. Circulating endocannabinoids and insulin resistance in patients with obstructive sleep apnea. BioMed Research International. 2016;2016 doi: 10.1155/2016/9782031. 9782031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grapov D., Adams S.H., Pedersen T.L., Garvey W.T., Newman J.W. Type 2 diabetes associated changes in the plasma non-esterified fatty acids, oxylipins and endocannabinoids. PLoS One. 2012;7:e48852. doi: 10.1371/journal.pone.0048852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Marzo V., Côté M., Matias I., Lemieux I., Arsenault B.J., Cartier A. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009;52:213–217. doi: 10.1007/s00125-008-1178-6. [DOI] [PubMed] [Google Scholar]
- 42.Giuffrida A., Rodriguez de Fonseca F., Nava F., Loubet-Lescoulié P., Piomelli D. Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. European Journal of Pharmacology. 2000;408:161–168. doi: 10.1016/s0014-2999(00)00786-x. [DOI] [PubMed] [Google Scholar]
- 43.Zolese G., Falcioni G., Bertoli E., Galeazzi R., Wozniak M., Wypych Z. Steady-state and time resolved fluorescence of albumins interacting with N-oleylethanolamine, a component of the endogenous N-acylethanolamines. Proteins. 2000;40:39–48. doi: 10.1002/(sici)1097-0134(20000701)40:1<39::aid-prot60>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 44.Bilgin M., Bindila L., Graessler J., Shevchenko A. Quantitative profiling of endocannabinoids in lipoproteins by LC-MS/MS. Analytical and Bioanalytical Chemistry. 2015;407:5125–5131. doi: 10.1007/s00216-015-8559-8. [DOI] [PubMed] [Google Scholar]
- 45.Pu S., Eck P., Jenkins D.J., Connelly P.W., Lamarche B., Kris-Etherton P.M. Interactions between dietary oil treatments and genetic variants modulate fatty acid ethanolamides in plasma and body weight composition. British Journal of Nutrition. 2016;115:1012–1023. doi: 10.1017/S0007114515005425. [DOI] [PubMed] [Google Scholar]
- 46.Geurts L., Everard A., Van Hul M., Essaghir A., Duparc T., Matamoros S. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nature Communications. 2015;6:6495. doi: 10.1038/ncomms7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alhouayek M., Bottemanne P., Makriyannis A., Muccioli G.G. N-acylethanolamine-hydrolyzing acid amidase and fatty acid amide hydrolase inhibition differentially affect N-acylethanolamine levels and macrophage activation. Biochimica et Biophysica Acta. 2017;1862:474–484. doi: 10.1016/j.bbalip.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Alhouayek M., Bottemanne P., Subramanian K.V., Lambert D.M., Makriyannis A., Cani P.D. N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. The FASEB Journal. 2015;29:650–661. doi: 10.1096/fj.14-255208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinitz S., Basolo A., Piaggi P., Piomelli D., Jumpertz von Schwartzenberg R., Krakoff J. Peripheral endocannabinoids associated with energy expenditure in native Americans of Southwestern Heritage. The Journal of Cinical Endocrinology and Metabolism. 2017;103:1077–1087. doi: 10.1210/jc.2017-02257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.