Abstract
Objective
Pancreatic tissue, and islets in particular, are enriched in expression of the interleukin-1 receptor type I (IL-1R). Because of this enrichment, islet β-cells are exquisitely sensitive to the IL-1R ligands IL-1α and IL-1β, suggesting that signaling through this pathway regulates health and function of islet β-cells.
Methods
Herein, we report a targeted deletion of IL-1R in pancreatic tissue (IL-1RPdx1−/−) in C57BL/6J mice and in db/db mice on the C57 genetic background. Islet morphology, β-cell transcription factor abundance, and expression of the de-differentiation marker Aldh1a3 were analyzed by immunofluorescent staining. Glucose and insulin tolerance tests were used to examine metabolic status of these genetic manipulations. Glucose-stimulated insulin secretion was evaluated in vivo and in isolated islets ex vivo by perifusion.
Results
Pancreatic deletion of IL-1R leads to impaired glucose tolerance, a phenotype that is exacerbated by age. Crossing the IL-1RPdx1−/− with db/db mice worsened glucose tolerance without altering body weight. There were no detectable alterations in insulin tolerance between IL-1RPdx1−/− mice and littermate controls. However, glucose-stimulated insulin secretion was reduced in islets isolated from IL-1RPdx1−/− relative to control islets. Insulin output in vivo after a glucose challenge was also markedly reduced in IL-1RPdx1−/− mice when compared with littermate controls. Pancreatic islets from IL-1RPdx1−/− mice displayed elevations in Aldh1a3, a marker of de-differentiation, and reduction in nuclear abundance of the β-cell transcription factor MafA. Nkx6.1 abundance was unaltered.
Conclusions
There is an important physiological role for pancreatic IL-1R to promote glucose homeostasis by suppressing expression of Aldh1a3, sustaining MafA abundance, and supporting glucose-stimulated insulin secretion in vivo.
Keywords: Cytokine, Glucose homeostasis, Inflammation, Insulin, Islet
Highlights
-
•
Pancreatic deletion of IL-1R impairs glucose tolerance in young and old male mice.
-
•
Pancreatic deletion of IL-1R worsens glucose tolerance in obese db/db mice.
-
•
Deletion of IL-1R triggers expression of the de-differentiation marker Aldh1a3.
-
•
IL-1 signaling in pancreatic tissue influences islet health and function.
1. Introduction
Pancreatic islet β-cells secrete insulin in response to fuel stimuli, with glucose regarded as the primary trigger for hormone release [1]. The mature, adult β-cell expresses a multitude of transcription factors (TFs) responsible for maintaining a robust insulin secretory phenotype. Representative examples of such regulatory TFs in β-cells include MafA, Nkx6.1, and Pdx-1. Indeed, Pdx-1 is important for pancreas development and remains expressed in pancreatic β-cells to sustain insulin production [2]; reductions in Pdx-1 impair glucose-stimulated insulin secretion [3]. Nkx6.1 is important for suppression of the glucagon gene and together MafA and Nkx6.1 support glucose-stimulated insulin secretion [4], [5]. Several of these TFs are either reduced in abundance or in nuclear presence in mouse models of obesity and in humans with obesity and T2D [6], [7], [8]. In addition, Aldh1a3 has recently been associated with de-differentiation of β-cells, helping to explain reduced insulin secretion in both rodents and humans [9], [10].
Obesity correlates with increased pancreatic islet size [11], [12], [13], while inflammation is linked with reductions in β-cell function and mass in both type 1 (T1D) and type 2 diabetes (T2D) [14], [15]. A variety of inflammatory stimuli, including free fatty acids and cytokines, have been associated with tissue dysfunction during progression to metabolic disease. For example, elevated levels of interleukin-1β promote key signaling changes within adipose tissue [16], liver [17], and pancreatic islets [18], [19]. The cytokines IL-1α and IL-1β signal through the interleukin-1 receptor type I [20]. These two cytokines are opposed by the interleukin-1 receptor antagonist protein, encoded by the IL-1RN gene [21]. The type II interleukin-1 receptor is proposed to function as a decoy receptor, able to bind ligands, but not communicate an intracellular signal [22]. Collectively, these genes and the proteins they encode are part of the IL-1 signaling system, which is associated with both acute and chronic inflammatory events [23].
Intriguingly, IL-1β has been shown to potentiate glucose-stimulated insulin secretion acutely [24], [25], but impair insulin secretion when β-cells are chronically exposed [26]. Using mouse, rat, and human islets as well as rodent and human derived β-cell lines, we and others have studied the impact of IL-1 signaling on insulin secretion and a variety of other parameters [8], [27], [28], [29], [30], [31], [32]. While a wealth of information on the IL-1 signaling pathway has been gained via these in vitro studies, the pancreas specific role of this pathway in vivo, especially as a homeostatic regulator of metabolism, is not entirely clear.
To our knowledge, we describe herein the first conditional deletion of the IL-1R in pancreatic tissue and the subsequent impact of this deletion on islet function and whole body glucose homeostasis. Several key findings emerged from these studies. 1) In the absence of pancreatic IL-1R signaling, glucose tolerance is impaired, a phenotype that worsens with age and is exacerbated with obesity. 2) Insulin secretion in vivo and ex vivo is diminished in IL-1RPdx1−/− when compared with littermate control mice. 3) MafA abundance is reduced in islets from IL-1RPdx1−/− mice. 4) There is enhanced expression of Aldh1a3, a newly described marker associated with cellular de-differentiation, in the islets of IL-1RPdx1−/− mice.
2. Methods
2.1. Cell Culture, luciferase assays, and reagents
832/13 rat insulinoma cells were cultured as described previously [33]. Mouse and rat IL-1β and TNFα were from Peprotech (Rocky Hill, NJ). NG-monomethyl-l-arginine (L-NMMA) was from Cayman Chemical (Ann Arbor, MI). 832/13 cells were grown in 24-well plates, transduced with the 5x NF-κB luciferase adenovirus and treated as described in the legend to Figure 1. Following treatment, cells were lysed in 1x Passive Lysis Buffer (Promega; Madison, WI) and promoter luciferase activity was measured using the Luciferase Assay System (Promega) on a GloMax luminometer (Promega). Luciferase activity was normalized to total protein content determined via BCA assay.
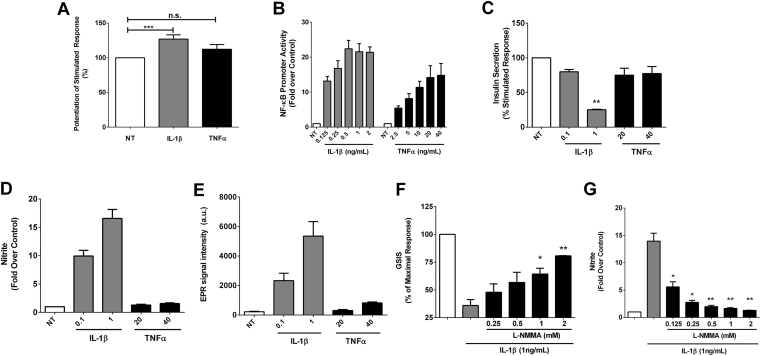
Figure 1.
Interleukin-1 signaling in β-cells potentiates insulin secretion acutely but restricts insulin secretion over time, with nitric oxide functioning as a negative feedback signal. (A) Insulin secretion in cultured 832/13 rat β-cells normalized as percent of the maximal stimulated response after no treatment (NT), or 30 min incubations in either 1 ng/mL IL-1β or 40 ng/mL TNF-α (n = 4, some of which were performed in duplicate or triplicate). (B) Dose response of IL-1β and TNF-α on a 5x NF-κB promoter luciferase reporter gene in cultured 832/13 rat β-cells; luciferase output is normalized to total cellular protein and shown as fold over the unstimulated control cells (n = 4). (C) Insulin secretion in cultured 832/13 rat β-cells normalized as percent of the maximal stimulated response after overnight exposure to either 0.1 and 1 ng/mL IL-1β or 20 and 40 ng/mL TNF-α versus cells left untreated (NT; n = 3, some of which were conducted in duplicate). (D) Total nitrite accumulation in the media in response to the indicated doses of IL-1β or TNF-α (n = 3). (E) Electron paramagnetic resonance spin trapping measurements of nitric oxide radical in response to the indicated doses of IL-1β or TNF-α (n = 3). (F) Insulin secretion in cultured 832/13 rat β-cells normalized as percent of the maximal stimulated response to show the marked reduction in insulin secretion by overnight exposure to 1 ng/mL IL-1β versus the dose-dependent recovery of insulin secretion by L-NMMA (n = 3). (G) Fold increase in total nitrite production in 832/13 cells by exposure to 1 ng/mL IL-1β overnight and the dose-dependent reduction by L-NMMA (n = 3). Error bars represent SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant.
2.2. Nitrite determination, glucose stimulated insulin secretion (GSIS), and electron paramagnetic resonance spectroscopy (EPR)
832/13 cells were grown in 12-well plates. Nitrite in the cell culture media was measured using the Griess assay kit from Promega. GSIS assays were performed as described previously [34], with secreted insulin detected using the Rat Insulin ELISA from Mercodia (Uppsala, Sweden). In addition, cells were lysed with M-PER lysis reagent (Thermo Fisher Scientific, Waltham, MA) to quantify total intracellular protein content by BCA assay (Thermo Fisher Scientific). The spin trap N-methyl-d-glucamine dithiocarbamate (MGD) was purchased from ENZO Life Sciences and used to detect nitric oxide by EPR spectroscopy [8], [35]. The N-methyl-d-glucamine dithiocarbamate–iron complex ((MGD)2-Fe2+) was prepared fresh for each experiment by making stock solutions of 500 mM MGD and 100 mM Fe2+ (from FeSO4.7H2O) in ultrapure water under anaerobic conditions. A final concentration of 25 mM MGD and 5 mM Fe2+ was introduced onto cells in serum free media for 30 min, followed by collection of supernatants for the measurements of the (MGD)2-iron-NO complex. All EPR measurements were conducted using a quartz flat cell at room temperature in a Bruker EMX Plus spectroscope. Typical instrumental conditions were 20 mW microwave power, 5.0 G modulation amplitude, 1 × 105 gain, 0.163 s time constant and 80 G scan range. Quantitation was carried out by measuring and comparing the first peak heights on the spectra.
2.3. Experimental animals
Mice with a pancreas-specific deletion of IL-1R were generated by crossing IL-1R floxed mice [36] with Pdx1-Cre mice (Stock # 014647; The Jackson Laboratory, Bar Harbor, Maine). db/+ mice (Stock # 00697; The Jackson Laboratory) were crossed with IL-1RPdx1−/− mice to generate db/+fl/fl and db/+Pdx1+/− mice. These heterozygous mice were then bred with each other to generate db/dbfl/fl and db/dbPdx1−/− mice. Mice were housed with a 12-hour light/12-hour dark cycle at 22 ± 1 °C and given access to Lab Diet 5001 (Purina) ad libitum throughout the study. For confirmation of IL-1R knockdown, a cohort of 12 week old IL-1Rfl/fl and IL-1RPdx1−/− mice received a single intraperitoneal injection of saline or IL-1β at a concentration of either 1 μg/kg or 3 μg/kg body weight. Animals were sacrificed 30 min post-injection for histological or immunoblot analysis. Non-fasting blood glucose measurements were taken using the Bayer Breeze 2 Glucometer (Bayer HealthCare LLC, Mishawaka, IN). Measurements of body mass and composition (fat, lean, and fluid mass) were assessed by NMR using a Bruker Minispec LF110 Time-Domain NMR system. Upon completion of the study, animals were fasted for 2 h, anesthetized by CO2 asphyxiation then euthanized by decapitation. Liver tissue was snap frozen in liquid nitrogen, and pancreata were fixed in 10% neutral-buffered formalin. Trunk blood was harvested, and the serum fraction was collected. For all experiments, littermates were used as controls with three to four separate cohorts of mice bred to complete the study. All animal procedures were approved by both the Pennington Biomedical Research Center and University of Tennessee Institutional Animal Care and Use Committees.
2.4. GTT, ITT, ex vivo and in vivo GSIS
Glucose tolerance tests were performed in male and female IL-1RPdx1−/− and littermate control mice following a 4 h fast. Mice were intraperitoneally injected with glucose at 2.5 g/kg body weight. Glucose tolerance tests in db/dbfl/fl and db/dbPdx1−/− mice were performed by i.p. injection of glucose at 1.25 g/kg body weight following a 4 h fast. Blood samples were taken from the tail vein at 0, 20, 40, 60, and 120 min after injection and blood glucose was determined with an Alphatrak 2 Glucometer (Zoetis, Kalamazoo, MI). An insulin tolerance test was performed in male and female IL-1RPdx1−/− and littermate control mice after a 2 h fast. Animals were intraperitoneally injected with Humulin R insulin (Lilly, Indianapolis, IN) at 1.0 U/kg body weight. Blood glucose measurements were taken from the tail vein at 0, 15, 30, 45, 60, and 90 min after injection. Blood glucose levels were plotted against time, and the area under the curve was calculated. For in vivo measurements of GSIS, animals were fasted for 4 h, followed by collection of blood via the submandibular vein for baseline measurements of insulin (time = 0 min). Mice were then i.p. injected with glucose at 2.5 g/kg body weight, followed by a second submandibular blood collection 10 min post-injection. Perifusion analyses of insulin secretion using isolated islets of similar size were performed as described previously [37]. Briefly, islet perifusion was conducted by placing islets into a 1-mL glass column, in which each of four distinct channels were operated by high-precision peristaltic pump. Perifusion media and islet chambers were maintained at 37 °C using a large-capacity water bath with fractions collected over time. Islets were sized matched from each animal for the comparisons within and between genotypes.
2.5. Serum ELISA and total acyl glycerol measurements
Serum insulin was measured using the Mouse Insulin ELISA kit from Mercodia. Mouse Proinsulin ELISA and Mouse C-peptide ELISA kits were from Alpco (Salem, NH). For acyl glycerol measurements, 30 mg of isolated liver tissue was homogenized in 300 μL 5% NP-40 solution and heated twice to 95 °C to solubilize all acyl glycerol species. Samples were centrifuged at 4 °C on maximum speed for 2 min, and the supernatant was transferred to a clean, pre-chilled tube. The samples were diluted 10-fold for use with the Triglyceride Determination kit from Sigma Aldrich (St. Louis, MO). Kit protocols were used for all measurements.
2.6. Mouse islet isolation, total RNA extraction, cDNA synthesis, and real-time RT-PCR
Mouse islets were isolated from 8 week old IL-1RPdx1−/− and littermate controls as described previously [38]. Islets were also isolated from 8-week-old male db/+ and db/db mice (B6.BKS(D)-Leprdb/J; stock number 00697) acquired from the Jackson Laboratories. Extraction of RNA, cDNA synthesis, and transcript analysis have been described in detail previously [7], [39].
2.7. Tissue protein isolation and immunoblotting
Isolated liver tissue (50 mg) was homogenized in 500 μL T-PER lysis reagent (Thermo Fisher Scientific) supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). Protein was quantified using a BCA assay. Denaturation of samples and immunoblotting conditions have been described [40]. Antibodies were from Cell Signaling Technology.
2.8. Islet histology and immunohistochemistry
Our methods for sectioning, embedding, and measuring insulin-positive area have been described previously [41]. Antibodies used were as follows: Nkx6.1 (Developmental Studies Hybridoma Bank #F55A12; 1:100 dilution overnight at 4 °C), Pdx-1 (Cell Signaling #5679; 1:300 dilution overnight at 4 °C), MafA (LSBio# LS-C286590; 1:75 dilution overnight at room temperature), Insulin (Dako # A0564), Foxo1 (Cell Signaling #2880; 1:50 dilution overnight at room temperature), p65 (Cell Signaling # 8242; 1:1000 dilution for 2 h at room temperature) and Glucagon (Cell Signaling #2760; 1:300 dilution overnight at 4 °C). All antibodies were detected with either Alexa Fluor secondary conjugation (Alexa 488, Alexa Fluor Plus 555), except for Nkx6.1, which was detected using a biotin/streptavidin exposure method, and, Foxo1, which was detected using a Perkin Elmer TSA Cy3 kit.
2.9. Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). Data were analyzed by either one-way ANOVA using a Tukey post hoc, repeated measures ANOVA (for longitudinal measures of blood glucose, body weight, and body composition), or two-tailed Student's t-test. Data are presented as means ± SEM.
3. Results
3.1. Interleukin-1 signaling in β-cells potentiates insulin secretion acutely but restricts insulin secretion over time, with nitric oxide functioning as a negative feedback signal
IL-1β has both positive and negative effects on insulin secretion, and these outcomes are dependent on duration of β-cell exposure to the cytokine. Using 832/13 rat β-cells, we found that inclusion of IL-1β in the culture media enhanced glucose-stimulated insulin secretion by 27% (Figure. 1A). TNF-α did not recapitulate this effect (Figure 1A), indicating the IL-1 effect is not simply a global response to inflammatory signals, since both cytokines induce robust NF-κB transcriptional activity (Figure 1B). Conversely, overnight exposure to IL-1β, but not TNF-α, reduced insulin secretion in response to glucose (Figure 1C). Nitrite, an index of nitric oxide production, accumulates in response to IL-1β but not to TNF-α (Figure 1D). Using spin trapping coupled with electron paramagnetic resonance spectroscopy, we found that nitric oxide production is selectively driven by IL-1β but not by TNF-α (Figure 1E). Moreover, insulin secretion in the presence of IL-1β could be recovered (Figure 1F) when nitrite production was limited by the broad spectrum NOS inhibitor L-NMMA (Figure 1G). Thus, IL-1β potentiates insulin secretion during acute exposure, but promotes nitric oxide accumulation as a negative feedback regulator to constrain insulin secretion following chronic exposure to the stimulus.
3.2. Deletion of the IL-1R in pancreatic tissue impairs whole body glucose tolerance in young and old male mice, but has only marginal impact in aged female mice
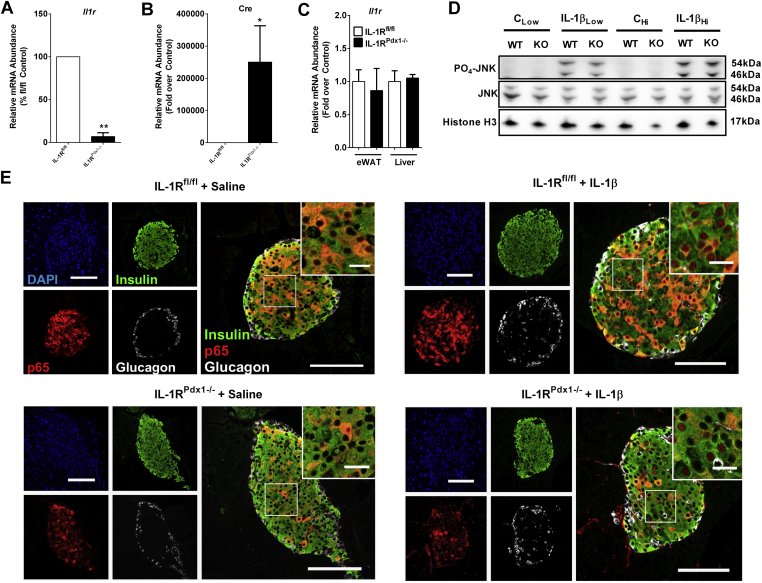
Because IL-1β signaling is capable of both enhancing and impairing insulin secretion (Figure 1 and [24], [25], [42]), we generated a pancreatic specific deletion of the IL-1 receptor (IL-1R) to investigate the in vivo metabolic consequences associated with disrupting this pathway. This was accomplished by crossing IL-1R ‘floxed’ mice with mice expressing cre recombinase driven by the Pdx-1 promoter (IL-1RPdx1−/−). The expression of the IL-1R gene is reduced by 93% in islets isolated from IL-1RPdx1−/− mice relative to floxed control mice (Figure 2A). This reduction is congruent with strong expression of the cre transgene (Figure 2B). Cre expression was not detected in other tissues assessed (not shown), consistent with selectivity of IL-1R deletion (Figure 2C). To assess the functional consequence of this deletion, we injected mice with IL-1β. IL-1R signaling was similar in liver tissue of both control mice and IL-1RPdx1−/− mice (Figure 2D). By contrast, p65 translocation is reduced in pancreatic islets of IL-1RPdx1−/− mice injected with IL-1β, but not in littermate control mice (Figure 2E). Upon quantification of multiple islets, we observed p65 abundance in 78% of nuclei from IL-1β injected floxed control mice (representative image in upper right panel of Figure 2E) versus 23% of nuclei in IL-1β injected IL-1RPdx1−/− mice (image in lower right panel of Figure 2E). In mice receiving saline control injections, only 9% of nuclei from floxed control and 10% of nuclei in IL-1RPdx1−/− mice displayed nuclear p65 localization.
Figure 2.
Deletion of the IL-1R in pancreatic tissue impairs IL-1β signaling in islets in vivo. (A) and (B) Islets were isolated from mice of the indicated genotype and expression of the Il1r and cre genes determined by real-time PCR analyses (n = 4). (C) Mice were injected with either saline (CLow and CHi), 1 (IL-1βLow) or 3 (IL-1βHi) μg/kg body weight of IL-1β. Lysate from homogenized liver was used to blot for phospho- and total JNK. Histone H3 is shown as a loading control. (D) Pancreatic tissue from (C) was stained for insulin (green), NF-κB p65 (red), glucagon (white), and DAPI (blue). Note the vastly reduced p65 nuclear abundance in the IL-1β injected IL-1Rpdx1−/− mice (n = 3). *, p < 0.05; **, p < 0.01.
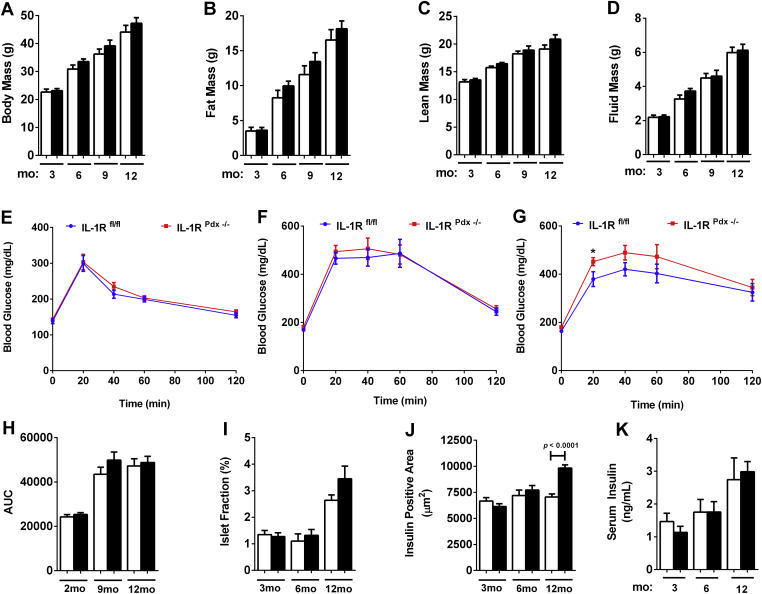
Female IL-1RPdx1−/− and littermate control mice had similar body compositions up to one year of age (Figure 3A – D). Young female mice (12 weeks of age) display no alterations in glucose tolerance due to deletion of the IL-1R in pancreatic tissue (Figure 3E) and even older females (nine months) are still near control values (Figure 3F). However, by one year, there appears to be a modest shift in glucose tolerance in IL-1RPdx1−/− mice relative to floxed control mice (Figure 3G), although the area under the curve measurement is not altered (Figure 3H). The islet fraction is not significantly different between IL-1RPdx1−/− mice and control mice (Figure 3I). Insulin positive area is modestly increased in older IL-1RPdx1−/− mice (12 months; Figure 3J), while serum insulin is not different from control mice (Figure 3K).
Figure 3.
Deletion of the IL-1R in pancreatic tissue impairs reduces glucose tolerance in older female mice. (A) Body mass, (B) fat mass, (C) lean mass, and (D) fluid mass of female mice in three month intervals (n = 8–13). Glucose tolerance tests (GTT) conducted in (E) two month, (F) nine month, and (G) twelve month old female mice (n = 8–13). (H) Area under the curve calculations for each GTT. (I) The percentage of islet fraction, defined as (total islet area/total pancreatic area) x 100. (J) Insulin positive area (square microns) within the islet across the indicated ages. (K) Serum insulin at the indicated ages and genotypes (n = 8–13). White bars indicate littermate controls while black bars represent IL-1Rpdx1−/− mice. *, p < 0.05.
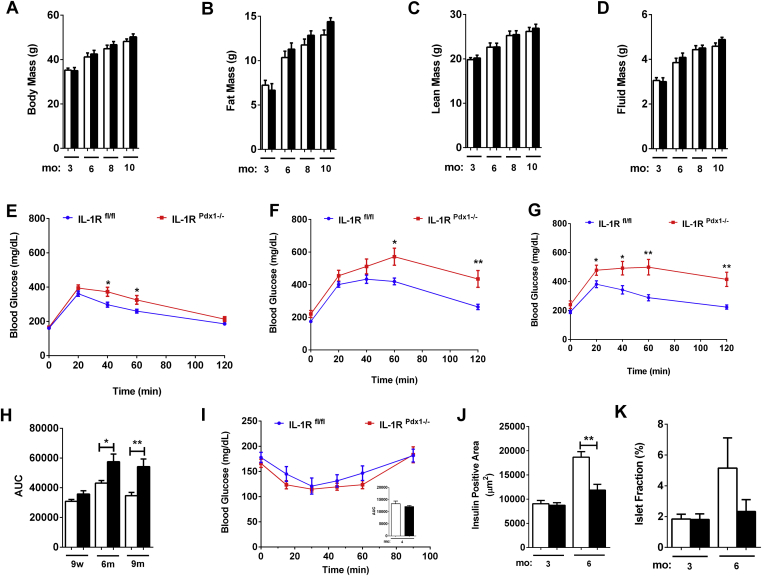
Similar to observations in female mice, there are no growth or body composition differences between male IL-1RPdx1−/− mice when compared with littermate control mice, reflected by similar body, fat, lean, and fluid mass composition (Figure 4A–D). However, in male mice, there is clear glucose intolerance at nine weeks of age (Figure 4E) that is exacerbated at both six (Figure 4F) and nine months of age (Figure 4G). Area under the curve changes at each age are shown in Figure 4H. Insulin tolerance tests were similar between IL-1RPdx1−/− mice and littermate control mice (Figure 4I and AUC inset), indicating that changes insulin sensitivity are not driving reduced glucose tolerance in IL-1RPdx1−/− mice. We note that insulin positive area is reduced in IL-1RPdx1−/− mice by six months of age (Figure 4J). This results in diminished islet area relative to pancreatic area in the IL-1RPdx1−/− mice when compared with littermate controls (Figure 4K).
Figure 4.
Deletion of the IL-1R in pancreatic tissue impairs reduces glucose tolerance in young male mice, a phenotype which is exacerbated with age. (A) Body mass, (B) fat mass, (C) lean mass, and (D) fluid mass of male mice. Glucose tolerance tests (GTT) conducted in (E) nine week, (F) six month, and (G) nine month old male mice (n = 8–15). (H) Area under the curve calculations for each GTT. (I) Insulin tolerance test (ITT) at four months of age with AUC inset (n = 8–10). (J) Insulin positive area (square microns). (K) Islet fraction, defined as (total islet area/total pancreatic area) x 100 (n = 8–10). White bars indicate littermate controls while black bars represent IL-1Rpdx1−/− mice. *, p < 0.05; **, p < 0.01.
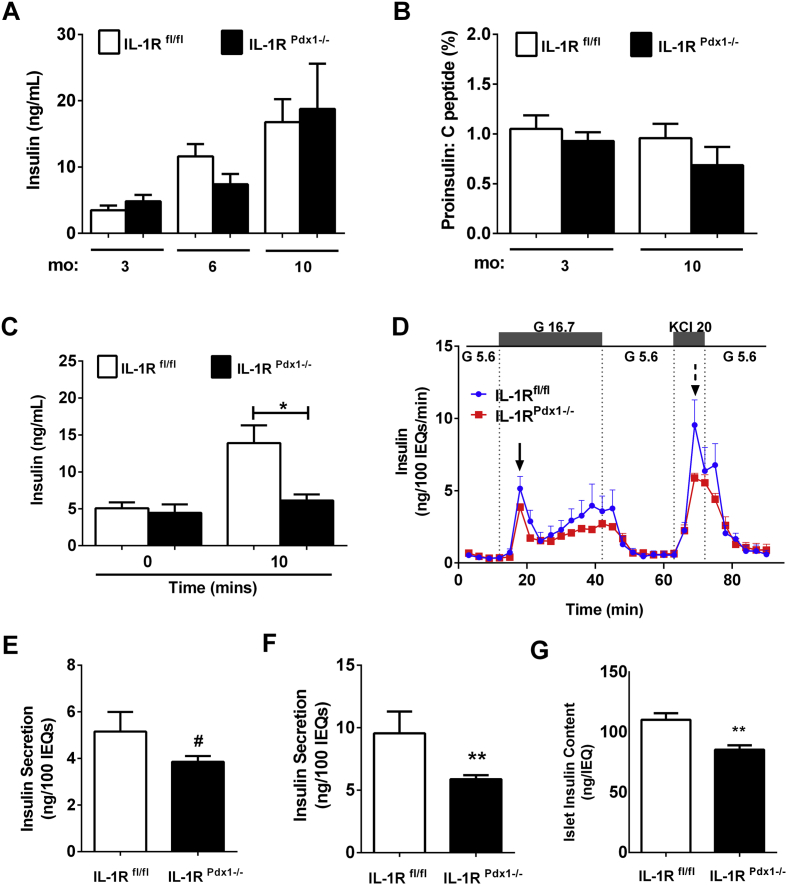
3.3. IL-1RPdx1−/− mice display reduced glucose-stimulated insulin secretion ex vivo and after a glucose challenge in vivo
Serum insulin levels are similar between IL-1RPdx1−/− and littermate control mice (Figure 5A). In addition, the proinsulin/C-peptide ratio, which has been used to indicate ER function [43], is also similar between groups (Figure 5B). Because insulin tolerance was similar between IL-1RPdx1−/− mice and littermate controls (Figure 4I), but glucose tolerance was impaired (Figure 4E–G), we next investigated insulin output after a glucose challenge in vivo. We found that insulin secretion in IL-1RPdx1−/− mice was decreased by 56% when compared with littermate controls 10 min after a glucose bolus (Figure 5C). Consistent with these results, perifusion analyses on isolated islets revealed a decrease in both glucose-stimulated and KCl-induced insulin release (Figure 5D). Quantification of peak glucose-stimulated insulin secretion revealed 25% less output in IL-1RPdx1−/− mice relative to littermate controls (solid arrow in Figure 5D re-plotted in Figure 5E). Similarly, there was a 38% decrease in KCl-induced insulin secretion (dashed arrow in Figure 5D re-plotted in Figure 5F). These results may be due, at least in part, to the 22% reduction in insulin content in islets from the IL-1RPdx1−/− mice (Figure 5G).
Figure 5.
IL-1RPdx1−/−mice display reduced glucose-stimulated insulin secretion in isolated islets and after a glucose challenge in vivo. (A) Serum insulin at each age and genotype (n = 6–10). (B) Serum proinsulin/C-peptide ratio. (C) Serum insulin at baseline (time 0) and 10 min after i.p. injection of glucose (2.5 g/kg body weight; n = 6–8). (D) Perifusion analysis from isolated islets (n = 7–8). (E) Peak glucose-stimulated insulin output from the graph in (D; solid arrow). (F) Peak KCl-induced insulin secretion from the graph in (D; dashed arrow). (G) Insulin content per 50 size-matched islets. #, p < 0.1; *, p < 0.05; **, p < 0.01.
3.4. Increased expression of the de-differentiation marker Aldh1a3 in islets with pancreatic deletion of IL-1R
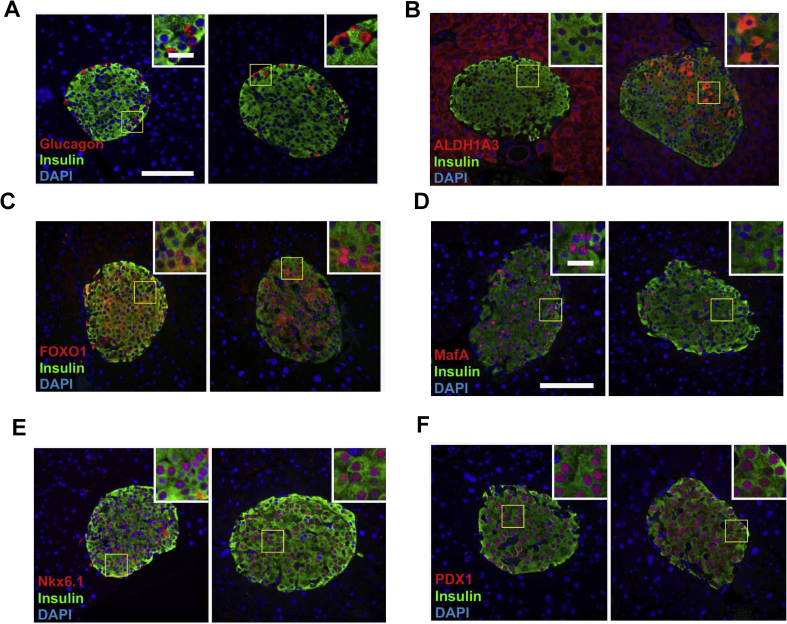
Aldh1a3 expression is elevated in rodent models of obesity and diabetes [7], [9] and in islets from humans with T2D [10]. Because glucose intolerance typically precedes onset of T2D, and IL-1RPdx1−/− mice display impaired glucose tolerance (Figure 5), we investigated islet histology in an effort to understand the underlying phenotype. Glucagon was expressed normally within the islets of both IL-1RPdx1−/− mice and littermate controls (Figure 6A). However, aldh1a3 levels were clearly elevated (Figure 6B). Foxo1 staining trended towards increased nuclear abundance within the islets of IL-1RPdx1−/− mice when compared with littermate controls (images in Figure 6C) although this was heterogeneous; quantification of multiple islets per mouse indicated 43% nuclear localization in IL-1RPdx1−/− mice and 35% Foxo1 nuclear abundance in littermate controls. Much more obvious was that IL-1RPdx1−/− mice had 39% of their nuclei positive for MafA while the littermate control mice displayed 72% nuclear positivity for this transcription factor (images in Figure 6D). The nuclear abundance of Nkx6.1 and Pdx-1 were unaltered between genotypes (Figure 6E,F). Collectively, we interpret this data to indicate that signaling through the IL-1R influences islet health and function.
Figure 6.
Increased expression of the de-differentiation marker Aldh1a3 in islets with pancreatic deletion of IL-1R. Triple-fluorescence staining of fixed pancreatic tissue showing insulin (green) and DAPI (blue). In all panels, littermate controls (n = 6) are on the left while islets from IL-1RPdx1−/− mice (n = 5) are on the right hand side. The red stain indicates (A) glucagon, (B), Aldh1a3, (C), FOXO1, (D) MafA, (E) Nkx6.1, and (F) Pdx-1.Note the orange staining within the islet in panel (B) from IL-1RPdx1−/− mice, indicating increased aldh1a3 in β-cells. Note the reduction in MafA nuclear abundance in panel (D) in IL-1RPdx1−/− mice.
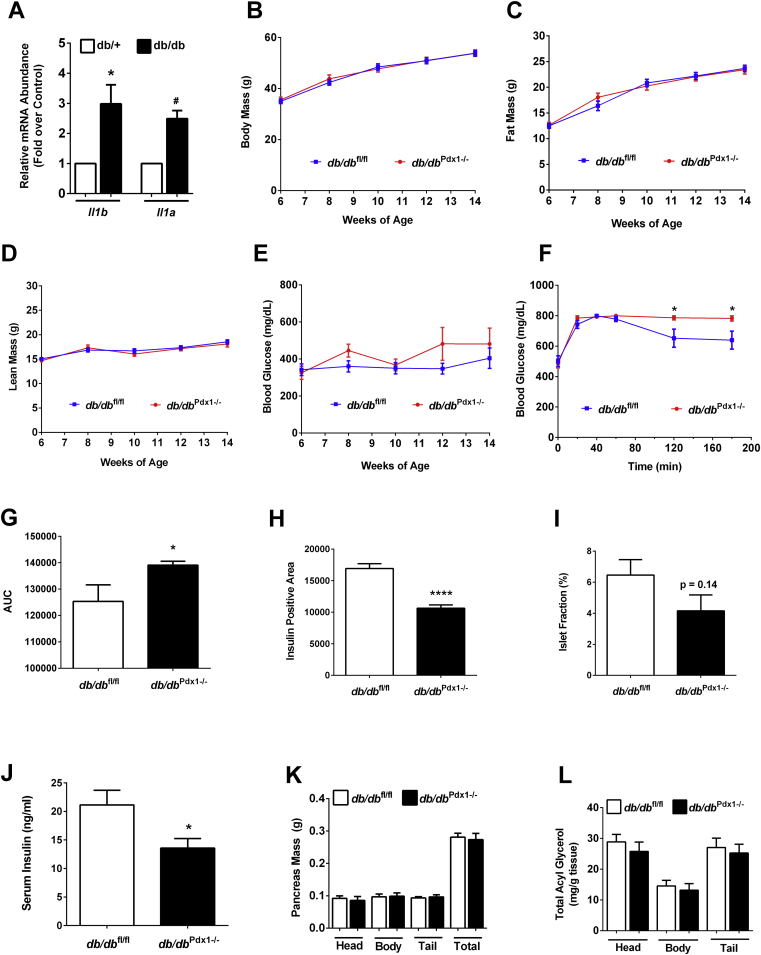
3.5. Deletion of IL-1R in pancreatic tissue of db/db mice worsens glucose tolerance without altering body weight
The db/db mouse model displays markers of pancreatic islet inflammation (see ref. [38] and Figure 7A). To test the hypothesis that reducing IL-1 signaling would alleviate inflammation-induced islet dysfunction and thus improve glucose homeostasis, we generated db/db mice with pancreatic deletion of IL-1R (db/db Pdx1−/−). The db/db Pdx1−/− mice grow normally and have similar total body mass (Fig. 7B), fat mass (Figure 7C), and lean mass (Figure 7D) as their littermate controls. While blood glucose levels over an eight week period trend higher in the db/db Pdx1−/− mice (Figure 7E), glucose intolerance is clearly exacerbated in db/db Pdx1−/− mice (Figure 7F,G). Insulin positive area (Figure 7H) is reduced in the db/db Pdx1−/− mice relative to the littermate control mice, while islet fraction (Figure 7I) trends lower. Accordingly, circulating insulin was also consistently lower in db/db Pdx1−/− mice (Figure 7J). However, there were no differences in either total pancreatic mass or the mass of individual pancreatic sections (Figure 7K). In addition, total lipid content, measured as acyl glycerols, were not different between the genotypes (Figure 7L). Taken together, we interpret this data to indicate that pancreatic IL-1R is necessary to sustain insulin production in states of obesity and insulin resistance and its absence worsens glucose homeostasis in db/db mice.
Figure 7.
Deletion of IL-1R in pancreatic tissue of db/db mice worsens glucose tolerance without changing body weight. (A) Expression of the Il1a and Il1b genes in islets isolated from eight week old db/+ and db/db mice (n = 4–8). (B) Total body mass, (C) fat mass, and (D) lean mass of db/dbf l/fl and db/dbPdx1−/− mice (n = 9). (E) Blood glucose measured over 6–14 weeks of age (n = 9). (F) Glucose tolerance test conducted in 10 week old mice (1.25 g/kg body weight; n = 9). (G) Area under the curve analysis of the GTT shown in (F). (H) Insulin positive area (square microns) and (I) Islet fraction (total islet area/total pancreatic area) x 100. (J) Fasting serum insulin in 14 week old mice (n = 9). (K) Pancreas weight (L) Pancreas TG. #, p < 0.10; *, p < 0.05; ****, p < 0.001.
4. Discussion
We have generated two novel mouse models reported herein, which have revealed an important physiological role for IL-1 receptor signaling in pancreatic tissue. The IL-1R signaling system clearly contributes to whole-body glucose homeostasis by maintaining the health and function of islet β-cells. Indeed, in the absence of pancreatic IL-1R signaling, whole body glucose homeostasis is disrupted in lean mice and worsened in genetically obese db/db mice. Reduced insulin output in response to a glucose challenge was observed in both in vivo and ex vivo analyses. Intriguingly, this observation may be more important in male mice, as female mice were only impacted to a minor degree by this deletion and only at advanced age. With IL-1 signaling typically studied from a pathological viewpoint, our present work offers new insights into the physiological relevance of this pathway and illustrates that it may also include a gender specific effect. The sex differences reported here may have relevance to future design of human clinical trials targeting the IL-1 signaling pathway.
Targeting inflammation to treat metabolic diseases, including T2D, is an emerging theme in both basic and clinical studies. Opinions on this topic range from enthusiastic [44] to more skeptical [45]. In our view, a major impediment to development of targeted clinical therapeutics is a full understanding of both the physiological and pathological roles for cytokine and other inflammation-based signaling pathways. For example, therapeutics targeting the IL-1 system have been underwhelming in terms of clinical outcomes [46] and fail to prevent autoimmune destruction of islets using whole body gene deletion approaches [47]. In addition, mice with global deletion of the IL-1R display reduced sensitivity to leptin and are more susceptible to becoming obese with age [48]. Alternatively, systemic approaches targeting IL-1β in GK rats, a model of T2D, showed reduced inflammation in liver and improved insulin sensitivity [49]. Furthermore, acute increases in IL-1 signaling enhance insulin secretion (Figure 1A) while chronic activation of this pathway reduces insulin output (Figure 1C), consistent with other studies conducted in vitro [24], [25], [50]. Consequently, tissue specific approaches, such as shown herein for pancreatic IL-1R deletion, now provide critical insights into physiological IL-1 signaling in vivo.
Recently, Boni-Schnetzler and colleagues demonstrated that deletion of the IL-1R antagonist protein allows enhanced signaling through the IL-1R pathway, which impairs β-cell proliferation [51]. These results are in agreement with in vitro studies showing decreased incorporation of radiolabeled nucleotides into DNA (an index of proliferation) in the presence of IL-1 pathway activation [8]. From a physiological perspective, postprandial elevations in IL-1 support glucose disposal [52]. Thus, while too much IL-1 signaling is detrimental to β-cell health [8], [29], [51], limiting physiological IL-1 signaling reduces glucose tolerance (Figure 4E–G) without impairing insulin sensitivity (Figures 4I). Accordingly, IL-1R signaling plays an important role in the overall metabolic health of the islet β-cells (Figure 5). Our results are consistent with recent studies using both mouse and human islets ex vivo, where IL-1 signaling enhances docking of insulin granules, supporting a physiological role for IL-1R activation to fine tune β-cell function [25]. From a translational perspective, the ability of IL-1 to potentiate glucose-stimulated insulin secretion increases with BMI, but is lost or markedly reduced in the islets from T2D patients [25].
Insulin production is impaired in the Csf1op/Csf1op mouse model [53], which has a severely diminished macrophage population. Taken together with our results herein, macrophage derived IL-1 is likely to be critical to support islet β-cell health, including sustained MafA abundance, maintenance of the differentiated state, and preservation of secretory function. If true, this would explain why therapies targeting the IL-1 signaling pathway have shown either mixed results or limited efficacy during clinical trials. Indeed, macrophages and β-cells clearly have a symbiotic relationship, with macrophages responding to ATP release from β-cells and β-cells responding to cytokines produced and secreted by macrophages [54], [55].
In our view, β-cells use nitric oxide in a physiological feedback loop to limit insulin secretion, which fits with IL-1 having both potentiating and suppressor activities in insulin secretion assays. The gene encoding the inducible nitric oxide synthase (iNOS) enzyme is robustly responsive to cytokines in pancreatic β-cells [56], [57]. This elevation in iNOS enzyme abundance promotes nitric oxide accumulation, which limits calcium responses from intracellular sources [8] and inhibits mitochondrial aconitase activity [58]. Collectively, these outcomes reduce insulin secretion. We posit that nitric oxide buildup, and subsequent reduction in insulin secretion, prevents hypoglycemic effects that would otherwise occur with exaggerated insulin secretory responses by potentiating agents (in this case IL-1). Nitric oxide is an ideal signal for this purpose due its short lived nature and ability to be quickly synthesized by the iNOS enzyme. Any impact nitric oxide has to damage DNA or become toxic is subject to repair systems present in β-cells [59].
In addition, the ability of IL-1 to enhance chemokine secretion, an event independent of cellular nitric oxide levels [8], is an important communication system with the islet resident macrophages. Interestingly, these macrophages appear to stay in an activated state, which may be distinct from macrophages in other tissues, e.g., lung, where the resting or basal state is the default [60]. The ability of islet macrophages to stay in an ‘activated’ state (relative to other tissue resident macrophages) may thus provide sufficient IL-1 to support physiological health of pancreatic β-cells. Indeed, in the absence of pancreatic IL-1R signaling, insulin content is reduced (Figure 5G), expression of MafA diminishes while Aldh1a3 is elevated (Figure 6), providing an explanation for reduced glucose tolerance (Figure 4E–H). This is significant because reduced insulin content and elevated Aldh1a3 expression are features of failing β-cells in both rodent [7], [9] and human T2D [10].
We suspect that the upregulation of the IL-1R ligands in db/db mice [38], ZDF rats [8], [61], and obese humans [25], [62] is a compensatory response intended to sustain insulin production and secretion. These phenotypes are lost in the absence of pancreatic IL-1R signaling, further deteriorating glucose homeostasis (Figure 7F& 7G). The presence of Aldh1a3 within the islets of lean IL-1RPdx1−/− mice likely indicates extreme stress within the islet (e.g., compensation for age induced insulin resistance) and/or diminished ability to respond to metabolic needs with compensatory insulin output. The deletion of multiple Foxo1 proteins, severe obesity, or deletion of Abcc8, a crucial component of the β-cell KATP –regulated ion channel, all promote increased expression of Aldh1a3 [7], [9], [63]. Thus, Aldh1a3 appears to be a solid indicator of reduced β-cell health, decreased mature β-cell number, or both.
In summary, our present study supports the interpretation that IL-1 receptor signaling has important physiological functions in pancreatic islets. Further studies will address islet β-cell specific deletion as well as include the use of inducible deletion of IL-1R in the adult mouse to account for any potential developmental effects that may have occurred in the present model. Based on these data, the timing and context of any specific therapeutic intervention targeting IL-1 ligands or the IL-1R will need to be carefully evaluated to elicit the desired salutary response without disrupting important physiological mechanisms associated with this pathway. Our observations may also help to explain why anti-inflammatory approaches do not always provide the expected or preferred beneficial outcome in the obese and diabetic populations. These results also provide compelling reasons to further examine gender specific outcomes relevant to IL-1 signaling in pancreatic tissue. Therefore, a more comprehensive understanding of individual signaling pathways, in both physiological and pathological frameworks, is required to provide the next generation of targeted therapeutic approaches.
Acknowledgments
This work was supported by NIH grant P20 GM103528 (J.J.C.), pilot and feasibility funding provided by P30 DK072476 (J.J.C.) and also in part by NARSAD YI Award 25230 (M.J.R.). This research involved use of the Islet Procurement and Analysis Core of the Vanderbilt Diabetes Research and Training Center supported by NIH grant P30 DK020593. This project also used the PBRC Genomics Core as well as the Cell Biology and Bioimaging Core facilities that are supported in part by COBRE (P30GM118430) and NORC (P30 DK072476) center grants from the National Institutes of Health. The authors thank Dr. Robert Noland for useful discussions.
Conflicts of interest
None declared.
References
- 1.Newgard C.B., McGarry J.D. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annual Review of Biochemistry. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- 2.Oliver-Krasinski J.M., Stoffers D.A. On the origin of the beta cell. Genes and Development. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brissova M., Shiota M., Nicholson W.E., Gannon M., Knobel S.M., Piston D.W. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. Journal of Biological Chemistry. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 4.Schisler J.C., Jensen P.B., Taylor D.G., Becker T.C., Knop F.K., Takekawa S. The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet beta cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7297–7302. doi: 10.1073/pnas.0502168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C., Moriguchi T., Kajihara M., Esaki R., Harada A., Shimohata H. MafA is a key regulator of glucose-stimulated insulin secretion. Molecular and Cellular Biology. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo S., Dai C., Guo M., Taylor B., Harmon J.S., Sander M. Inactivation of specific beta cell transcription factors in type 2 diabetes. Journal of Clinical Investigation. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke S.J., Batdorf H.M., Burk D.H., Noland R.C., Eder A.E., Boulos M.S. db/db mice exhibit features of human type 2 diabetes that are not present in weight-matched C57BL/6J mice fed a western Diet. Journal of Diabetes Research. 2017;2017:8503754. doi: 10.1155/2017/8503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke S.J., Stadler K., Lu D., Gleason E., Han A., Donohoe D.R. IL-1beta reciprocally regulates chemokine and insulin secretion in pancreatic beta-cells via NF-kappaB. American Journal of Physiology Endocrinology and Metabolism. 2015;309:E715–E726. doi: 10.1152/ajpendo.00153.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim-Muller J.Y., Fan J., Kim Y.J., Lee S.A., Ishida E., Blaner W.S. Aldehyde dehydrogenase 1a3 defines a subset of failing pancreatic beta cells in diabetic mice. Nature Communications. 2016;7:12631. doi: 10.1038/ncomms12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinti F., Bouchi R., Kim-Muller J.Y., Ohmura Y., Sandoval P.R., Masini M. Evidence of beta-cell dedifferentiation in human type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2016;101:1044–1054. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogilvie R.F. The islands of langerhans in 19 cases of obesity. Journal of Pathology and Bacteriology. 1933;37:473–481. [Google Scholar]
- 12.Mezza T., Muscogiuri G., Sorice G.P., Clemente G., Hu J., Pontecorvi A. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes. 2014;63:994–1007. doi: 10.2337/db13-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke S.J., Karlstad M.D., Collier J.J. Pancreatic islet responses to metabolic trauma. Shock. 2016;46:230–238. doi: 10.1097/SHK.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annual Review of Immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 15.Eizirik D.L., Colli M.L., Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nature Reviews Endocrinology. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 16.Gao D., Madi M., Ding C., Fok M., Steele T., Ford C. Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. American Journal of Physiology Endocrinology and Metabolism. 2014;307:E289–E304. doi: 10.1152/ajpendo.00430.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negrin K.A., Roth Flach R.J., DiStefano M.T., Matevossian A., Friedline R.H., Jung D. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107265. e107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinarello C.A., Donath M.Y., Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Current Opinion in Endocrinology Diabetes and Obesity. 2010;17:314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 19.Padgett L.E., Broniowska K.A., Hansen P.A., Corbett J.A., Tse H.M. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Annals of the New York Academy of Sciences. 2013;1281:16–35. doi: 10.1111/j.1749-6632.2012.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarello C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinkasserer A., Spurr N.K., Cox S., Jeggo P., Sim R.B. The human IL-1 receptor antagonist gene (IL1RN) maps to chromosome 2q14-q21, in the region of the IL-1 alpha and IL-1 beta loci. Genomics. 1992;13:654–657. doi: 10.1016/0888-7543(92)90137-h. [DOI] [PubMed] [Google Scholar]
- 22.Lang D., Knop J., Wesche H., Raffetseder U., Kurrle R., Boraschi D. The type II IL-1 receptor interacts with the IL-1 receptor accessory protein: a novel mechanism of regulation of IL-1 responsiveness. The Journal of Immunology. 1998;161:6871–6877. [PubMed] [Google Scholar]
- 23.Herder C., Dalmas E., Boni-Schnetzler M., Donath M.Y. The IL-1 pathway in type 2 diabetes and cardiovascular complications. Trends in Endocrinology and Metabolism. 2015;26:551–563. doi: 10.1016/j.tem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Yelich M.R. In vivo endotoxin and IL-1 potentiate insulin secretion in pancreatic islets. American Journal of Physiology. 1990;258:R1070–R1077. doi: 10.1152/ajpregu.1990.258.4.R1070. [DOI] [PubMed] [Google Scholar]
- 25.Hajmrle C., Smith N., Spigelman A.F., Dai X., Senior L., Bautista A. Interleukin-1 signaling contributes to acute islet compensation. JCI Insight. 2016;1:e86055. doi: 10.1172/jci.insight.86055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comens P.G., Wolf B.A., Unanue E.R., Lacy P.E., McDaniel M.L. Interleukin 1 is potent modulator of insulin secretion from isolated rat islets of Langerhans. Diabetes. 1987;36:963–970. doi: 10.2337/diab.36.8.963. [DOI] [PubMed] [Google Scholar]
- 27.Burke S.J., Goff M.R., Lu D., Proud D., Karlstad M.D., Collier J.J. Synergistic expression of the CXCL10 gene in response to IL-1beta and IFN-gamma involves NF-kappaB, Phosphorylation of STAT1 at Tyr701, and acetylation of histones H3 and H4. The Journal of Immunology. 2013;191:323–336. doi: 10.4049/jimmunol.1300344. [DOI] [PubMed] [Google Scholar]
- 28.Collier J.J., Burke S.J., Eisenhauer M.E., Lu D., Sapp R.C., Frydman C.J. Pancreatic beta-cell death in response to Pro-inflammatory cytokines is distinct from genuine apoptosis. PLoS One. 2011;6:e22485. doi: 10.1371/journal.pone.0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnush M., Heitmeier M.R., Scarim A.L., Marino M.H., Manning P.T., Corbett J.A. IL-1 produced and released endogenously within human islets inhibits beta cell function. Journal of Clinical Investigation. 1998;102:516–526. doi: 10.1172/JCI844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke S.J., Lu D., Sparer T.E., Masi T., Goff M.R., Karlstad M.D. NF-kappaB and STAT1 control CXCL1 and CXCL2 gene transcription. American Journal of Physiology Endocrinology and Metabolism. 2014;306:E131–E149. doi: 10.1152/ajpendo.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oleson B.J., McGraw J.A., Broniowska K.A., Annamalai M., Chen J., Bushkofsky J.R. Distinct differences in the responses of the human pancreatic beta-cell line EndoC-betaH1 and human islets to proinflammatory cytokines. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2015;309:R525–R534. doi: 10.1152/ajpregu.00544.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collier J.J., Fueger P.T., Hohmeier H.E., Newgard C.B. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes. 2006;55:1398–1406. doi: 10.2337/db05-1000. [DOI] [PubMed] [Google Scholar]
- 33.Hohmeier H.E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C.B. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 34.Collier J.J., White S.M., Dick G.M., Scott D.K. Phosphatidylinositol 3-kinase inhibitors reveal a unique mechanism of enhancing insulin secretion in 832/13 rat insulinoma cells. Biochemical and Biophysical Research Communications. 2004;324:1018–1023. doi: 10.1016/j.bbrc.2004.09.149. [DOI] [PubMed] [Google Scholar]
- 35.Hogg N. Detection of nitric oxide by electron paramagnetic resonance spectroscopy. Free Radical Biology and Medicine. 2010;49:122–129. doi: 10.1016/j.freeradbiomed.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robson M.J., Zhu C.B., Quinlan M.A., Botschner D.A., Baganz N.L., Lindler K.M. Generation and characterization of mice expressing a conditional allele of the Interleukin-1 receptor type 1. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150068. e0150068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang T., Lacik I., Brissova M., Anilkumar A.V., Prokop A., Hunkeler D. An encapsulation system for the immunoisolation of pancreatic islets. Nature Biotechnology. 1997;15:358–362. doi: 10.1038/nbt0497-358. [DOI] [PubMed] [Google Scholar]
- 38.Burke S.J., Karlstad M.D., Regal K.M., Sparer T.E., Lu D., Elks C.M. CCL20 is elevated during obesity and differentially regulated by NF-kappaB subunits in pancreatic beta-cells. Biochimica et Biophysica Acta. 2015;1849:637–652. doi: 10.1016/j.bbagrm.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke S.J., Collier J.J. The gene encoding cyclooxygenase-2 is regulated by IL-1beta and prostaglandins in 832/13 rat insulinoma cells. Cellular Immunology. 2011;271:379–384. doi: 10.1016/j.cellimm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Burke S.J., Batdorf H.M., Eder A.E., Karlstad M.D., Burk D.H., Noland R.C. Oral corticosterone administration reduces insulitis but promotes insulin resistance and hyperglycemia in male nonobese diabetic mice. American Journal Of Pathology. 2017;187:614–626. doi: 10.1016/j.ajpath.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burke S.J., Karlstad M.D., Eder A.E., Regal K.M., Lu D., Burk D.H. Pancreatic beta-Cell production of CXCR3 ligands precedes diabetes onset. BioFactors. 2016;42:703–715. doi: 10.1002/biof.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbett J.A., Sweetland M.A., Wang J.L., Lancaster J.R., Jr., McDaniel M.L. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sims E.K., Chaudhry Z., Watkins R., Syed F., Blum J., Ouyang F. Elevations in the fasting serum proinsulin-to-C-peptide ratio precede the onset of type 1 diabetes. Diabetes Care. 2016;39:1519–1526. doi: 10.2337/dc15-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donath M.Y. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nature Reviews Drug Discovery. 2014;13:465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 45.Maiorino M.I., Bellastella G., Giugliano D., Esposito K. Cooling down inflammation in type 2 diabetes: how strong is the evidence for cardiometabolic benefit? Endocrine. 2017;55:360–365. doi: 10.1007/s12020-016-0993-7. [DOI] [PubMed] [Google Scholar]
- 46.Moran A., Bundy B., Becker D.J., DiMeglio L.A., Gitelman S.E., Goland R. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas H.E., Irawaty W., Darwiche R., Brodnicki T.C., Santamaria P., Allison J. IL-1 receptor deficiency slows progression to diabetes in the NOD mouse. Diabetes. 2004;53:113–121. doi: 10.2337/diabetes.53.1.113. [DOI] [PubMed] [Google Scholar]
- 48.Garcia M.C., Wernstedt I., Berndtsson A., Enge M., Bell M., Hultgren O. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 49.Ehses J.A., Lacraz G., Giroix M.H., Schmidlin F., Coulaud J., Kassis N. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proceedings of the National Academy of Sciences of the USA. 2009;106:13998–14003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Southern C., Schulster D., Green I.C. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an L-arginine-dependent nitric oxide generating mechanism. FEBS Letters. 1990;276:42–44. doi: 10.1016/0014-5793(90)80502-a. [DOI] [PubMed] [Google Scholar]
- 51.Boni-Schnetzler M., Hauselmann S.P., Dalmas E., Meier D.T., Thienel C., Traub S. Beta cell-specific deletion of the IL-1 receptor antagonist impairs beta cell proliferation and insulin secretion. Cell Reports. 2018;22:1774–1786. doi: 10.1016/j.celrep.2018.01.063. [DOI] [PubMed] [Google Scholar]
- 52.Dror E., Dalmas E., Meier D.T., Wueest S., Thevenet J., Thienel C. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nature Immunology. 2017;18:283–292. doi: 10.1038/ni.3659. [DOI] [PubMed] [Google Scholar]
- 53.Banaei-Bouchareb L., Gouon-Evans V., Samara-Boustani D., Castellotti M.C., Czernichow P., Pollard J.W. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. Journal of Leukocyte Biology. 2004;76:359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 54.Weitz J.R., Makhmutova M., Almaca J., Stertmann J., Aamodt K., Brissova M. Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia. 2018;61:182–192. doi: 10.1007/s00125-017-4416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collier J.J., Sparer T.E., Karlstad M.D., Burke S.J. Pancreatic islet inflammation: an emerging role for chemokines. Journal of Molecular Endocrinology. 2017;59:R33–R46. doi: 10.1530/JME-17-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burke S.J., Updegraff B.L., Bellich R.M., Goff M.R., Lu D., Minkin S.C., Jr. regulation of iNOS gene transcription by IL-1beta and IFN-gamma requires a coactivator exchange mechanism. Molecular Endocrinology. 2013;27:1724–1742. doi: 10.1210/me.2013-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heitmeier M.R., Scarim A.L., Corbett J.A. Interferon-gamma increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. Journal of Biological Chemistry. 1997;272:13697–13704. doi: 10.1074/jbc.272.21.13697. [DOI] [PubMed] [Google Scholar]
- 58.Scarim A.L., Heitmeier M.R., Corbett J.A. Irreversible inhibition of metabolic function and islet destruction after a 36-hour exposure to interleukin-1beta. Endocrinology. 1997;138:5301–5307. doi: 10.1210/endo.138.12.5583. [DOI] [PubMed] [Google Scholar]
- 59.Hughes K.J., Meares G.P., Chambers K.T., Corbett J.A. Repair of nitric oxide-damaged DNA in beta-cells requires JNK-dependent GADD45alpha expression. Journal of Biological Chemistry. 2009;284:27402–27408. doi: 10.1074/jbc.M109.046912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferris S.T., Zakharov P.N., Wan X., Calderon B., Artyomov M.N., Unanue E.R. The islet-resident macrophage is in an inflammatory state and senses microbial products in blood. Journal of Experimental Medicine. 2017;214:2369–2385. doi: 10.1084/jem.20170074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jourdan T., Godlewski G., Cinar R., Bertola A., Szanda G., Liu J. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nature Medicine. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H.I., Spinas G.A. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. Journal of Clinical Investigation. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stancill J.S., Cartailler J.P., Clayton H.W., O'Connor J.T., Dickerson M.T., Dadi P.K. Chronic beta-cell depolarization impairs beta-cell identity by disrupting a network of Ca(2+)-Regulated genes. Diabetes. 2017;66:2175–2187. doi: 10.2337/db16-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]