Abstract
Background
We evaluated the clinical presentation and risk factors of pulmonary embolism (PE) in soldiers posted at high altitude areas (HAA).
Methods
We conducted a retrospective analysis of all cases of PE presented to us between March 2011 and Aug 2014. The patients were serving at an altitude between 10,000 and 22,000 ft above sea level and PE was diagnosed using the pulmonary CT angiography. Screening for the deep vein thrombosis (DVT) and procoagulant conditions was done at presentation and after six months of treatment. The patients were managed as per the American College of Cardiology (ACC) guidelines and descriptive statistics were used to present the data.
Results
The patients (53 males) had a mean age of 33 ± 4.2 year and were serving at a mean altitude of 12,176 ± 448 feet (ranged between 10,000 and 20,500) at the onset of symptoms. Dyspnea (79%) and tachycardia (68%) were the commonest symptom and sign, respectively. D dimer was positive in 96.2% of the cases while nonspecific T inversion in the ECG was seen in 54.7% of the patients. Procoagulant work up revealed a hereditary thrombophilic condition in 9 out of 53 patients. A total of 44 cases were idiopathic and DVT of lower limb veins was seen in 2 patients. There was no mortality in our case series.
Conclusion
PE is a common complication of HAA and hereditary thrombophilia contributes in a minority of the patients. Further studies are needed to ascertain the risk factors of PE at HAA.
Keywords: Pulmonary embolism, High altitude, Cold, Soldiers, Thrombophilia
1. Introduction
Pulmonary embolism (PE) is a great masquerader with myriad manifestations resulting in significant morbidity and mortality. Progressive dyspnea, pleuritic chest pain and hemoptysis are the cardinal symptoms of PE.1 The risk factors for PE include advanced age, deep vein thrombosis (DVT), trauma, immobilization, surgery, obesity, underlying malignancy, pregnancy, estrogenic drugs, hereditary thrombophilic disorders like Protein C and S, anti-thrombin-III deficiency, anti-phospholipid antibody syndrome and factor-V Leiden mutation.2 Prolonged stay at a high altitude area (HAA) is a less known risk factor for PE.3 Unusual environmental conditions like extreme cold, hypoxia, prolonged immobility, polycythemia and dehydration contribute to the hypercoagulable state in the HAA.4 Previous researchers have studied the profile of PE with DVT, HAA induced DVT and portal system thrombosis.5, 6, 7 Extensive literature search did not reveal any published paper about the HAA induced PE from our country. Hence, we conducted this study to evaluate the profile of PE and the underlying risk factors in soldiers serving at HAA.
2. Methods
This retrospective review of the case records was done at our hospital, which is the designated center to receive the casualties from the HAA located in various parts of North India. The service personnel are deployed at HAA (10,000 to 22,000 feet altitude) for about four to six months continuously prior to a turnover to a plain location. We included all patients with a diagnosis of PE presented to our hospital between March 2011 and Aug 2014. We excluded patients with a known history of medical illness, procoagulant disorder and the presence of any pre-existing condition that may increase the risk of DVT. Routine hematological and biochemical parameters including lipid panel and homocysteine were evaluated in the patients. The coagulation profile includes platelet count, prothrombin time (PT), and partial thromboplastin time activated with Kaolin (PTTK).
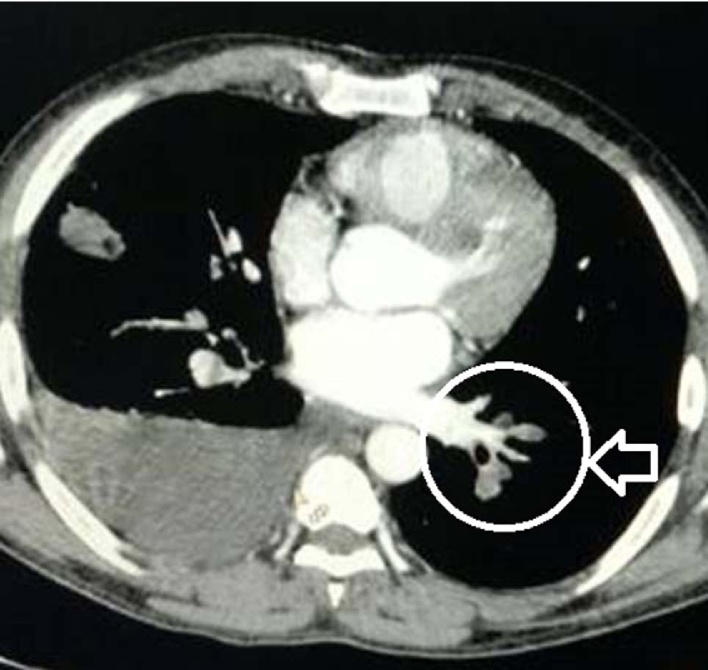
The diagnosis of PE was established using the D-dimer assay, echocardiography and pulmonary CT angiography. A thrombus blocking the main or the branches of the main pulmonary artery is considered diagnostic of the PE as shown in Fig. 1. Doppler examination of the venous system of all four limbs was performed for the presence of DVT. The tests for procoagulant condition were performed at presentation and after six months that include the estimation of proteins C and S, antithrombin-III, anticardiolipin antibody (ACLA), rheumatoid factor (RF), antinuclear antibody (ANA), antibodies to hepatitis B, C and syphilis. The blood samples for the procoagulant condition were collected at our hospital (located at sea level) in container prefilled with citrate as an anticoagulant and analyzed immediately. The first sample was used for the estimation of the antibodies and not used for the estimation of the coagulation factors. All the patients were treated with subcutaneous low molecular weight heparin overlapped with oral anticoagulant (OAC) drugs. Thrombolytic therapy was given using the Alteplase bolus followed by infusion as per the standard protocol.8 The dose of OAC was adjusted periodically to maintain the INR between 2 and 3. After six months of therapy, OAC drugs were stopped for 8 weeks and a second blood sample was collected for the estimation of the coagulation factors. D-dimer estimation was done qualitatively and all other coagulation parameters were estimated quantitatively. The OAC drugs were restarted in the patients diagnosed with a procoagulant condition. The final diagnosis of a procoagulant disorder was based on the combined assessment of all the tests done at the onset and after six months of therapy. The institutional ethics committee approved the study protocol for the data analysis. Individual consent from the patient was not obtained as the study involves only review of the records and the protocol was approved by the institutional ethical committee. We used the descriptive statistics (mean, standard deviation, range and percentage) for the analysis of the study data.
Fig. 1.
CT angiography image showing PE in left pulmonary artery.
3. Results
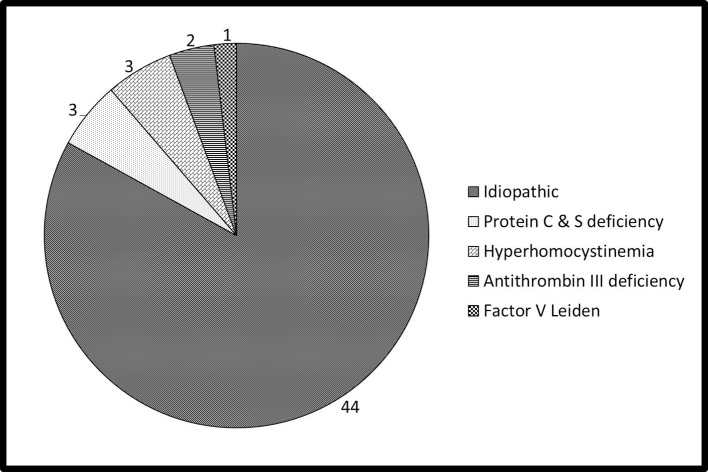
The patients (53 males) of PE had a mean age of 33 ± 4.2 year and were staying at a mean altitude of 12176 ± 448 feet. PE was reported in patients serving at a minimum altitude of 10,000 feet and a maximum altitude of 20,500 feet. At the time of diagnosis, majority of the patients were working at an altitude ranging between 11,000 and 15,000 feet. The majority (49/53) of the patients were non-smokers and low landers (51/53). The clinical features of the patients have been summarized in the Table 1. Briefly, dyspnea and palpitation were the commonest symptoms, whereas tachycardia and tachypnea were the most common findings. D-dimer was positive in 51 (96.2%) cases, whereas non-specific T inversion was seen in 54.7% of the patients. Pleural effusion was present in 18 (33.9%) and prominent pulmonary artery in 14 (26%) patients. DVT of lower limb veins was seen in two patients. Thrombolytic therapy was used in two cases only, who reported in the window period. None of our patients had a fatal outcome during the study observation period. The results of the biochemical and procoagulant work up are shown in Table 2 and Fig. 2 respectively. In brief, majority of the patients had no identifiable cause for the PE and the lipid profile was normal. The tests for ACLA and connective tissue disease profile were negative and polycythemia (hemoglobin >17 gm/dL) was seen in 22 (41.5%) patients at presentation, which was normalized at 3 months.
Table 1.
Clinical features of patients with PE.
| Symptoms | Number | Percentage |
|---|---|---|
| Dyspnea | 42 | 79.2 |
| Palpitations | 36 | 67.9 |
| Chest pain | 14 | 26.4 |
| Cough | 8 | 15 |
| Hemoptysis | 6 | 11.3 |
| Syncope | 1 | 1.8 |
| Signs | ||
| Tachycardia | 36 | 67.9 |
| Tachypnea | 35 | 66 |
| Hypotension | 2 | 3.7 |
| Cyanosis | 2 | 3.7 |
| Hypoxemia | 2 | 3.7 |
| ECG findings | ||
| Sinus tachycardia | 41 | 77.3 |
| Non-specific T inversion | 29 | 54.7 |
| RV overload | 9 | 16.9 |
| S1Q3T3 | 3 | 5.6 |
Table 2.
Coagulation and biochemical profile of patients.
| Parameter | Units | Mean (SD) | Range | Normal Value |
|---|---|---|---|---|
| Hemoglobina | gm/dL | 17.4 (1.9) | 15.4–18.8 | 14–16 |
| Platelet count | per mm3 | 2.3 (2.1) | 1.8–5.4 | 1.5–4 × 106 |
| Bleeding time | sec | 1.3 (0.2) | 1–1.8 | 1–2 |
| Clotting time | sec | 5.1 (0.6) | 4.4–7.7 | 4–8 |
| Prothrombin time | sec | 11.2 (1.1) | 10–13 | 10–14 |
| aPTT | sec | 24.1 (2.6) | 22–36 | 20–35 |
| Protein C | % | 106 (12.2) | 34–144 | 88–150 |
| Protein S | % | 112.4 (18.6) | 45–150 | 89–150 |
| APC resistance ratio | number | 2.4 (0.2) | 0.5–2.9 | >2 |
| Antithrombin III activity | % | 145.7 (12.8) | 55–216 | 120–280 |
| Biochemical parameters | ||||
| Homocysteine | μmol/L | 12.1 (2.7) | 6–32 | 4–15 |
| Total cholesterol | mg/dL | 122.7 (27.9) | 102–246 | 100–200 |
| LDL − cholesterol | mg/dL | 116.4 (21.5) | 78–188 | 100–160 |
| HDL − cholesterol | mg/dL | 45.5 (3.9) | 34–58 | 40–60 |
| Triglycerides | mg/dL | 132.6 (12.4) | 102–196 | <150 |
At the time of presentation.
Fig. 2.
Hereditary thrombophilic conditions in patients with PE.
4. Discussion
Our study has shown that heritable thrombophilic conditions have limited role in the etiology of PE in young soldiers. They contribute to only 17% of the disease burden, whereas idiopathic PE was seen in 83% of the patients as shown in Fig. 1. A study from a neighboring country involving similar patients and climatic conditions also showed the same finding.9 Previous research showed that hereditary thrombophilia is seen in 30% of patients with PE.10 However, our study involved patients at HAA thereby explaining the low prevalence of genetic conditions. The most common genetic risk factor for venous thrombosis has been traditionally associated with factor V Leiden mutation and G20210A mutation in the prothrombin gene. The clinical features of the patients and D-dimer positivity showed similar trends with that of the landmark studies on the subject.11, 12 Pleural effusion was the commonest radiological abnormality in our study, whereas cardiomegaly was the commonest finding in the International Cooperative Pulmonary Embolism Registry (ICOPER) study.12 This could be explained due to the differences in the nativity of the patients and pleural effusion was the 2nd common observation even in the ICOPER study.
Stressful situations lead to elevation of D-dimer and sympathetic activity, which could have contributed towards PE in our patients.13 The soldiers posted at HAA are trained periodically to combat stress though various mechanisms. Decreased fruit intake and vegetables may also contribute to an increased incidence of thrombosis.14 However the ration scales provided for the troops take care of these anomalies, but they may not have access to fresh fruits and vegetables. The tactical situation may demand prolonged immobility and dehydration, which may trigger thrombus formation in HAA.15 The initial hypercoagulable state in HAA is due to the transient increase in platelet count, heightened factor X and XII activity, shortening of prothrombin time, impaired clot retraction and platelet dysfunction.6 The process of acclimatization occurs within few weeks associated with an increase in the hematocrit and decrease in clotting factor levels towards normal. The strengths of our study include the robust diagnostic evaluation of all the patients and the limitations include small sample size, lack of information regarding the dehydration. Another limitation is the study population being entirely of male sex, the findings may not be applicable to the female population.
To conclude, hereditary thrombophilic conditions have a limited contribution in the etiology of the PE at HAA. Further studies at HAA involving large number of patients would confirm the findings observed in our study.
Competing interests
The authors declare that they have no conflict of interest.
References
- 1.Tapson V.F. Acute pulmonary embolism. N Engl J Med. 2008;358:1037–1052. doi: 10.1056/NEJMra072753. [DOI] [PubMed] [Google Scholar]
- 2.Martinelli I., Bucciarelli P., Mannuccio P.M. Thrombotic risk factors: basic pathophysiology. Crit Care Med. 2010;38:S3–S9. doi: 10.1097/CCM.0b013e3181c9cbd9. [DOI] [PubMed] [Google Scholar]
- 3.Khan D.A., Hashim R., Mirza T.M., Matloob-ur-Rehman M. Differentiation of pulmonary embolism from high altitude pulmonary edema. J Coll Physicians Surg Pak. 2003;13:267–270. [PubMed] [Google Scholar]
- 4.West J.B. The physiological basis of high-altitude diseases. Ann Intern Med. 2004;141:789–800. doi: 10.7326/0003-4819-141-10-200411160-00010. [DOI] [PubMed] [Google Scholar]
- 5.Anand A.C., Saha A., Seth A.K., Chopra G.S., Nair V., Sharma V. Symptomatic portal system thrombosis in soldiers due to extended stay at extreme high altitude. J Gastroenterol Hepatol. 2005;20:777–783. doi: 10.1111/j.1440-1746.2005.03723.x. [DOI] [PubMed] [Google Scholar]
- 6.Shishir K. High altitude induced deep venous thrombosis: a study of 28 cases. Indian J Surg. 2006;68:84–88. [Google Scholar]
- 7.Parakh R., Kapadia S.R., Sen I., Agarwal S., Grover T., Yadav A. Pulmonary embolism: a frequent occurrence in Indian patients with symptomatic lower limb venous thrombosis. Asian J Surg. 2006;29:86–91. doi: 10.1016/S1015-9584(09)60113-5. [DOI] [PubMed] [Google Scholar]
- 8.Kearon C., Kahn S.R., Agnelli G., Goldhaber S., Raskob G.E., Comerota A.J. American College of Chest Physicians Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(Suppl. 6):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 9.Khalil K.F., Saeed W. Pulmonary embolism in soldiers serving at high altitude. J Coll Physicians Surg Pak. 2010 Jul;20(7):468–471. [PubMed] [Google Scholar]
- 10.Turan O., Ündar B., Günay T., Akkoçlu A. Investigation of inherited thrombophilias in patients with pulmonary embolism. Blood Coagul Fibrinolysis. 2013;24:140–149. doi: 10.1097/MBC.0b013e328359db0e. [DOI] [PubMed] [Google Scholar]
- 11.Stein P.D., Gottschalk A., Sostman H.D. Methods of prospective investigation of pulmonary embolism diagnosis III (PIOPED III) Semin Nucl Med. 2008;38:462–470. doi: 10.1053/j.semnuclmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldhaber S.Z., Visani L., De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353(9162):1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 13.Wirtz H.P., Bärtschi C., Spillmann M., Ehlert U., Von Käne R. Effect of oral melatonin on the procoagulant response to acute psychosocial stress in healthy men: a randomized placebo controlled study. J Pineal Res. 2008;44(4):358–365. doi: 10.1111/j.1600-079X.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta R., Joshi P., Mohan V., Reddy K.S., Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 15.Girard P., Sanchez O., Leroyer C. Deep venous thrombosis in patients with acute pulmonary embolism: prevalance, risk factors, and clinical significance. Chest. 2005;128:1593–1600. doi: 10.1378/chest.128.3.1593. [DOI] [PubMed] [Google Scholar]