Abstract
Tendinopathy is a common disease of the musculoskeletal system, particularly in athletes and sports amateurs. In this review, we will present evidence for the critical role of inflammatory mediators and immunocytes in the pathogenesis of tendinopathy and the efficacy of current antiinflammatory therapy and regenerative medicine in the clinic. We hereby propose a hypothesis that in addition to pulling force there may be compressive forces being exerted on the tendon during physical activities, which may initiate the onset of tendinopathy.
We performed literature searches on MEDLINE from the inception of this review to February 2018. No language restrictions were imposed. The search terms were as follows: ("Tendinopathy"[Mesh] OR "Tendon Injuries"[Mesh] OR "Tendinitis"[Mesh] OR "Tendon"[Mesh]) AND (Inflammation OR "Inflammatory mediator*" OR Immunocyte*) OR ("anti inflammatory*" OR "regenerative medicine"). Inclusion criteria included articles that were original and reliable, with the main contents being highly relevant to our review. Exclusion criteria included articles that were not available online or have not been published. We scanned the abstract of these articles first. This was then followed by a careful screening of the articles which might be suitable for our review. Finally, 84 articles were selected as references. This review article is written in the narrative form.
The translational potential of this article: Understanding the mechanisms of inflammation and existing antiinflammatory and regenerative therapies is key to the development of therapeutic strategies in tendinopathy.
Keywords: Compression, Inflammatory mediators, Immunocytes, Pulling force, Tendinopathy
A brief introduction to the tendon
Tendon is a type of dense connective tissue that connects skeletal muscles to bone. From muscle to bone, tendon can be divided into three parts, including muscle tendon junction, tendon and enthesis. Tendons are rich in collagen fibres, mainly type 1 collagen fibrils. The tendon contains a small number of cells, such as tenocytes. Studies have found the presence of a variety of immunocytes, which play important roles in the pathogenesis of tendinopathy [1]. Tendinopathy is a common disease of the musculoskeletal system, characterised by pain, swelling and limited function. Various factors can cause tendon disease, which can be subdivided into two major categories: extrinsic and intrinsic factors. Amongst the extrinsic factors, overuse is considered one of the most important factors, which is associated with sports activities and physical training injuries [2]. Intrinsic factors including age, gender, anatomical abnormalities, systemic diseases (e.g., hyperlipaemia) and genetic diseases (e.g., Marfan syndrome) should also be considered.
Pathology—degeneration or inflammation?
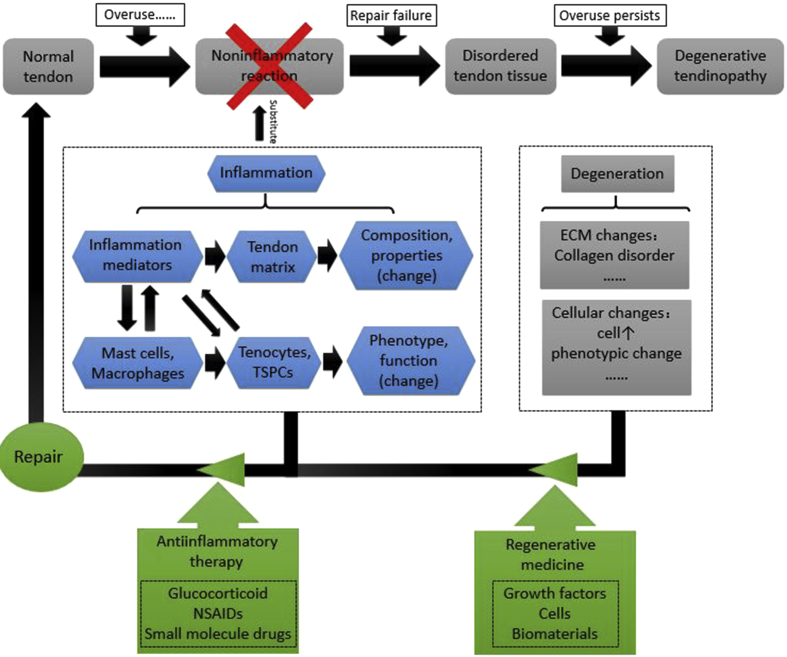
In the 1970s, Puddu et al found that acute inflammatory cells do not play a role in chronic tendinopathy [3]. At the beginning of the 21st century, the theory of degeneration has become the mainstream concept of tendinopathy [4]. Researchers have proposed a continuous process in tendinopathy [5]. Initially, the onset of tendinopathy is caused by many factors, particularly physical overuse [6]. Therefore, in response to the damage caused by overuse, noninflammatory reactions arise in tendon, including tendon thickening and increasing hardness in response to physical stress [6]. If physical overuse persists, this will ultimately lead to tendon self-repair failure, and tendon tissue will become disordered, eventually leading to degenerative tendinopathy [5] (Figure 1). Tendon specimens from symptomatic patients exhibit degenerative changes such as being hypoxic, mucoid, hyaline, myxoid, and showing fatty degeneration [7]. Changes to the paratendon are also very common [8].
Figure 1.
The previous view on pathogenesis of tendinopathy is that it is a series of noninflammatory reactions caused by mechanical loading, which eventually led to degenerative tendinopathy. With the progress of experimental technology, more and more studies have found the presence of inflammatory mediators and immunocytes at the early stage of tendinopathy. Due to inflammation, the composition of tendon matrix was changed, and the phenotype and function of cells in tendon became abnormal. During the early stage of tendinopathy, people need to use some antiinflammatory medications to inhibit the progression of this disease. During the late stages of the disease, inflammation has subsided, which means that antiinflammatory treatments have no value. Because of the poor regenerative capacity of tendon, patients may need to use regenerative medicine strategies to promote the regeneration of tendon. The combination of different treatment modalities at different time points may be one of the future directions in this field. TSPC = tendon stem/progenitor cells.
Inflammation is a series of responses to harmful stimuli, which is considered as an essential physiological process within the human body [9]. Acute and controlled inflammation exerts a protective effect on tissues, but chronic and unregulated inflammation is harmful [9]. There is much opinion that inflammation has no role in tendinopathy, but considering tendinopathy to be purely a degenerative disease may oversimplify the pathogenesis of tendon disease and we may thus overlook the appropriate therapeutic targets [4]. Recently, some experimental models have found evidence of early inflammatory responses in tendinopathy [10]. Human biopsy studies of tendons with smaller tears found significant infiltration of inflammatory mast cells and macrophages [11]. Furthermore, with the advancement of immunohistochemistry and molecular biology techniques, the concept of degenerative tendon disease has become less convincing [4].
It is assumed that there is a continuous transition from physiological to pathological processes in tendon. Factors such as overuse can cause injury to the tendon, as described previously. Under these circumstances, microdamages to tendon fibres occur, and the body will consequently secrete a number of substances to promote healing, which includes inflammatory factors [2]. In the pathogenesis of tendinopathy, inflammation and degeneration may not be two separate processes, as these usually interact with each other. Degenerative process may be triggered by inflammation, and inflammation will also play some roles in later degenerative processes [9] (Figure 1).
Inflammatory mediators and immunocytes in tendinopathy
Inflammatory mediators
Soluble factors such as cytokines and complements that are involved in inflammatory responses are known as inflammatory mediators. Cytokines are signalling molecules that are produced by different cell types. Many different cellular functions can be controlled by cytokines, including cell proliferation, differentiation and apoptosis [12]. Cytokines possess immunomodulatory properties and are also known to play key roles in cell signalling and communication [12].
Interleukin-1β
Interleukin-1β (IL-1β), which may be produced under various pathological conditions such as infection and injury, is an important mediator in the inflammatory response and is implicated in diverse cellular functions [13]. Previously, IL-1β was thought to be produced exclusively by monocytes and macrophages, but it is now known to be produced by some cells in the connective tissue [12]. IL-1β can induce human tenocytes to produce inflammatory mediators such as cyclooxygenase-2 (COX2), prostaglandin E2 (PGE2) and matrix metalloproteinase-1 (MMP-1), and these mediators can accelerate the degradation of tendon extracellular matrix (ECM), which in turn influence the mechanical properties of tendon [14]. A recent study found that IL-1β can cause phenotype loss of tendon stem/progenitor cells (TSPCs) in vitro, which is associated with decreased expression of tendon-related genes, such as scleraxis and tenomodulin. At the same time, the expression of collagen, biglycan and fibromodulin are also downregulated [15]. These results thus suggest that IL-1β will impair TSPCs function and inhibit tendon repair capacity.
Interleukin-6
Cytokines belonging to the interleukin-6 (IL-6) family, include IL-6 itself, as well as IL-11, oncostatin M, ciliary neurotrophic factor, leukaemia inhibitory factor and cardiotrophin-1 [16]. IL-6 is a type of multifunctional Th2 cytokine, which possesses immune regulatory function and which is involved in tendon healing [17], [18]. Expression of some cytokines of the IL-6 family is increased in pathological tendon [16]. When human tendon fibroblasts are subjected to an increased level of cyclical stretching, they can also secrete IL-6 [17]. In addition to the tendon itself, IL-6 expression can also increase in peritendon during tendon exercise [16]. In ruptured human rotator cuff tendon, the expression of IL-6 mRNA is highly increased and immunohistochemical results have shown that IL-6 is mainly concentrated around proliferative blood vessels [19]. IL-6 is associated with collagen synthesis and can lead to increased expression of procollagen markers in the peritendon of human Achilles tendon [20]. When compared to normal or painful Achilles tendon, the expression of oncostatin M and leukaemia inhibitory factor, which belongs to the IL-6 family, was increased in ruptured Achilles tendon [16].
Interleukin-10
Interleukin-10 (IL-10) is a typical antiinflammatory Th2 cytokine which also includes IL-13 and IL-4, with IL-10 being the most potent amongst these [21]. In an IL-10 overexpression mouse model, researchers found some time-dependent effects on healing tendons [22]. But the effects of IL-10 on tendon is still unknown [21]. Ricchetti et al delivered IL-10 into adult murine patellar tendon, and showed that the maximum stress of tendon was increased significantly in the IL-10 group [22]. IL-10 can increase its own expression in tenocytes, and the IL-10-specific receptor IL-10R1 is also known to be expressed on tenocytes and upregulated by proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) [23].
Recently, some research groups found that tenocytes are sensitive to the antiinflammatory cytokines IL-4 and IL-13 [21]. In IL-4 knock-out mice, upregulation of IL-10 and IL-13 can correlate to even better healing properties when compared to the control group, which means that these cytokines might make up for the lack of IL-4 [18].
Interleukin-17A
Interleukin-17A (IL-17A) is a proinflammatory mediator which belongs to the IL-17 family and is associated with tissue destruction and degeneration. An increase of IL-17A expression was found in specimens of early human tendinopathy by Millar et al. Through their studies, they found that IL-17A can influence the synthesis of collagen and can promote the expression of some inflammatory factors in tenocytes, such as TNF-a, IL-6, and IL-8. Additionally, IL-17A is associated with apoptosis of tenocytes. mitogen-activated protein kinase (MAPK) signalling is a major signalling pathway in these biological functions and may become an important target for the treatment of tendinopathy [24].
Interleukin-21
Interleukin-21 (IL-21) is a proinflammatory cytokine belonging to the IL-1 family, which is mainly secreted by natural killer T cells and CD4+ lymphocytes [25]. Recently, researchers found that IL-21 may play an important role in the crosstalk between immunocytes and nonimmune cells, indicating that IL-21 can induce epithelial cells [26] and fibroblasts [27] to secrete more chemokines and matrix metalloproteinases. Campbell found that the IL-21 receptor (IL-21R) is expressed during early tendinopathy, and proinflammatory cytokines can promote the function of IL-21R [28]. In the inflammatory environment of early tendon disease, the increased expression of IL-21R suggests that there is an activated IL-21R tenocyte isotype [28]. This may lead to the weakening of tendon, in a similar manner to joint erosion by activated fibroblasts in rheumatoid arthritis (RA), where it has been shown that the dysregulated production of MMP is associated with the IL-21/IL-21R signalling axis [29]. IL-21 can hardly be detected by polymerase chain reaction or immunohistochemistry, suggesting that IL-21 may bind to IL-21R in tendinopathy [28]. It is possible that the expression of IL-21 take place extremely early during teninopathy and that alternative ligand binding is the more likely scenario in the pathology of tendon diseases [28].
Interleukin-33
Interleukin-33 (IL-33) is a member of the IL-1 superfamily. A study found that compared with normal tendon or torn tendon, the expression level of IL-33 was increased during the early stages of human tendinopathy [30]. IL-33 can induce the expression of type 1 collagen and type 3 collagen in vitro, but type 3 collagen was increased more than type 1 collagen [30]. In addition, recombinant IL-33 can significantly increase the expression of IL-6 and IL-8, which may promote the inflammatory process in tendinopathy [30].
TNF-α
TNF-α appears to be associated with tendinitis and tendon degeneration [31]. TNF-α can strongly activate tenocytes [23], stimulating the tenocytes to produce more proinflammatory and antiinflammatory cytokines, including IL-1β, TNF-α, IL-6 and IL-10, and matrix degradative enzymes [21]. These cytokines further inhibit ECM synthesis such as type I collagen [23], [32]. Recently, Han et al found that the proliferation and tenogenic/osteogenic differentiation of tendon-derived stem cell (TDSC) would be inhibited. However, combining the use of TNF-α and transforming growth factor-β1 (TGFβ1) could promote the proliferation and differentiation of TDSCs in vitro [33]. TNF-α may be involved in apoptosis. In inflamed equine tendons, enhanced expression of TNF-α and caspase-3 activation could indicate a proapoptotic effect [21]. However, Machner et al reported that TNF-α can inhibit the expression of the proapoptotic Fas ligand in human tenocytes, which is in the vicinity of osteoarthritic joints [21].
Substance P
Substance P (SP) is a potent neuropeptide and pain-producing substance, which may be associated with pain in tendon diseases. In recent years, more and more attention has been focused on the role of SP in the pathogenesis of tendinopathy [34]. This type of inflammation involving SP is known as neurogenic inflammation. In tendon, small peripheral sensory neurons and tenocytes themselves can express SP, and noxious stimuli and mechanical loading can trigger the release of SP [35]. Interestingly, the number of mast cells will increase in tendinopathy and mast cells may be a possible target of SP in tendon [36]. In chronic tendon disease, SP can bind to the neurokinin 1 receptors of mast cells, causing them to secrete the preformed substances that are contained within granules and also stimulating the de novo synthesis of leukotriene and prostaglandin [37]. Meanwhile, SP is associated with mediating pain, oedema and fibrosis in tendinopathy [37]. Interestingly, SP can be effectively blocked, which is of clinical interest [34].
Alarmin molecules
Alarmin molecules are endogenous molecules released by dead cells, also known as damage-associated molecular patterns. high-mobility group box-1 (HMGB1), S100 proteins, heat shock protein (HSPs), hypoxia-inducible factor 1α (HIF-1α), and IL-33, are all considered to be alarmin molecules, which may be released by cells in the impaired tendon [38]. The latest research found that in human supraspinatus tendinopathy, HIF-1a and S100A9 were increased in diseased tendons as shown by immunohistochemical analyses, which may have proinflammatory effects [39]. The expression levels of HMGB1 during the early stages of diseased tendons are higher than that in normal or torn tendon [40]. The expression of collagen 3A, tenascin-C and decorin are known to be upregulated by the addition of HMGB1 in vitro, and exogenous HMGB1 will promote tenocytes to secrete inflammatory mediators, such as IL-1β, IL-6, IL-33, C-C Motif Chemokine Ligand 2 (CCL2) and C-X-C Motif Chemokine Ligand 12 (CXCL12) [40]. In summary, alarmin molecules play an important role in tendinopathy, which is likely to be one of the therapeutic targets in future treatments.
Immunocytes and other cells
The role of immunocytes in tendinopathy is still controversial [41]. Many studies have found the negligible presence of immunocytes during the pathogenesis of tendinopathy, but recently, an increasing number of studies have suggested that immunocytes may have a key role in tendinopathy, particularly during the early stages of tendon disease [4], [42]. When compared to healthy control tendons, the number of immunocytes in tendinopathic tendon is higher, but the number of immunocytes is reduced in torn tendons versus intact tendinopathic tissue [41].
Macrophages
Macrophage is a type of white blood cell, which is involved in the injury response and repair of the body. Macrophages have at least two functionally phenotypic states, which differ in the repertoire of their cell surface receptors, effector function and cytokine expression [43]. M1 polarised (classically activated) macrophages are proinflammatory, whereas M2 (alternatively activated) macrophages can restrain inflammation by immunosuppressive cytokines including IL-1RA, IL-10 and IL-4 [43]. Recently, an increasing number of studies have revealed an early onset of inflammation in tendinopathy [10]. When compared to large rotator cuff tears of human tissue biopsy samples, smaller tears exhibit significant inflammatory infiltration, including macrophages [11]. In the study of Dakin et al, tendons at different disease stages were observed to contain different types of macrophages. There are almost no macrophages in normal tendon. However, M1 macrophages predominated in subacute injured tendons, and chronic injured tendons have completely different types of macrophages, namely M2 macrophages [44]. This is similar to our current understanding of tendinopathy. There is inflammation during the early stage of tendon diseases. Subsequently upon further progression of the disease, the inflammation gradually subsides and leaves some degenerative changes.
Mast cells
Mast cells play an important role in the inflammatory process and can rapidly release their characteristic granules together with various mediators [45]. More and more studies have demonstrated increased numbers of mast cells in tendinopathy [10]. Mast cells are more prominent in tendinopathic tissue, particularly around the microvessels, as shown by specific immunolabeling [46]. In tendinopathy, the observed upregulation of SP and prominent mast cells suggest that neurogenic inflammation may be a possibility [37]. Mast cells can be activated by SP, vasoactive intestinal peptide and calcitonin gene related peptide (CGRP), resulting in degranulation and release of histamine, which in turn can lead to axonal stimulation [37]. In a rabbit tendon injury model, the number of mast cells was observed to be significantly increased in repaired tendon, which is accompanied by an increase in the number of sand myofibroblasts and neuropeptide-containing nerve fibres. Thus, Berglund et al hypothesised that there are activated profibrotic neuropeptide–mast cell–myofibroblast pathway during tendon healing [47].
Lymphocytes
Whether or not there are any lymphocytes in tendon and their possible roles in tendinopathy still remain unclear. Some scholars have confirmed that lymphocytes are present in tendons. In human tendinopathic Achilles tendons, Kragsnaes et al found that there are macrophages, T lymphocytes and natural killer (NK) cells [48]. Schubert et al have reported the presence of T and B lymphocytes in chronic Achilles tendinopathy by utilising specific primary monoclonal antibodies [49]. We still need more studies to validate the presence and function of lymphocytes in tendinopathy.
Tenocytes and tendon stem/progenitor cells
Tenocytes are the most abundant cell type in tendons, their major function being the synthesis and secretion of extracellular matrix. Most studies have shown that tenocyte numbers are increased in tendon pathology and that the tenocytes are larger than normal types in healthy tendon [50]. Tenocytes can synthesise various cytokines such as TNF-α, IL-1β, IL-6, IL-10 and vascular endothelial growth factor (VEGF) [51], [52], and mechanical overloading of tenocytes can lead to the release of these cytokines [21]. Tenocyte physiology can be modified by the inflammatory milieu and be transformed into a proinflammatory phenotype [53]. Tenocytes can become more metabolically active under the influence of cytokines, which are part of the inflammatory response [21], and the hyperplasia and hypertrophy of tenocytes may be elicited by inflammatory factors [4]. Healthy and diseased tenocytes treated with interferon-γ (IFNγ) or lipopolysaccharide (LPS), would express the respective target genes unlike untreated cells. It is possible that inflammation can alter the activation status of tenocytes [53].
TSPCs are a unique cell population that has both tendon-specific and stem cell–specific characteristics. TSPCs can maintain the homoeostasis of tendon tissue and can also help to repair the damaged tendon. Multiple factors in the microenvironment of TSPCs can modulate the physiology of these cells, including extracellular matrix, growth factors, oxygen content and mechanics. TSPCs can not only differentiate into normal tendon cells but can also differentiate into nontendon lineages, including fat cells, bone cells and cartilage cells [54]. The differentiation into nontendon lineages may be related to some pathological processes, such as heterotopic ossification and fatty infiltration in the tendon. These suggest that TSPCs may be involved in the process of tendon pathology. Bi et al first proposed a correlation between tendon disease and TSPCs dysfunction [54]. Hu et al found that HIF-2α signalling is markedly activated in human tendons with heterotopic ossification. The abnormal upregulation of HIF-2α may switch TSPCs differentiation into bone rather than tendon [55].
Antiinflammatory therapy—pathway manipulation
Antiinflammatory therapy is still used in the treatment of tendon disease in the clinic, but it is controversial. Most studies have suggested that antiinflammatory treatment has only short-term beneficial effects, but the long-term effects are not good and may even be harmful. Poulsen et al found that glucocorticoids induce senescence in tenocytes, and this may be the cause of the detrimental long-term side-effects of glucocorticoids on tendon [56]. We assume that when the pain is controlled, patients may have the illusion that tendon disease is cured. Such patients then resume their normal physical activities and do not keep up with subsequent treatment, resulting in overuse again. These in turn can aggravate tendon injury, leading to detrimental long-term effects. With a deeper understanding of tendinopathy, the role of inflammation in tendon disease has attracted much research. Maybe it is time to revisit antiinflammatory treatments in tendinopathy. Some antiinflammatory treatments such as small molecule drugs may be a good choice for tendinopathy patients. Many agents can suppress the inflammatory signalling pathway.
In tendinopathy, normal physiological progress are affected by some proinflammatory factors that are implicated in the nuclear factor κB (NF-κB) signalling pathway, and this signalling pathway can be inhibited by small molecule drugs, such as resveratrol or curcumin [57], [58]. Resveratrol, which can be produced by many plants such as peanuts, is thought to be good for human health because of its antioxidant and antiinflammatory properties [57]. Shakibaei et al showed that in human tenocytes, resveratrol can suppress IL-1β–induced activation of the NF-κB pathway, enhancing the production of collagen, tenomodulin and scleraxis expression and inhibiting the expression of genes associated with inflammation and apoptosis [57]. Curcumin, which is also a natural organic compound from some plants, has been confirmed to inhibit IL-1β–induced inflammation and apoptosis in human tenocytes by regulating the NF-κB signalling pathway [58]. In the study of Hu et al, it was shown that the activation of NF-κB signalling pathway can induce the activation of the HIF-2α pathway [55]. Digoxin can inhibit the formation of ectopic ossification in rat tendons and promote the expression of scleraxis (SCX) and tendon repair by regulating the HIF-2α pathway [55].
Some studies have shown that the ability of tenocytes to secrete inflammatory mediators can be affected by regulating the MAPK signalling pathway. Schwartz et al used the p38 MAPK inhibitor SB203580 to treat rats and found that this inhibitor can significantly inhibit the expression of IL-6 in tendon, while had a modest effect on the expression of other ECM and cell proliferation genes [59]. The study by Millar et al found that, in a hypoxic environment, the MAPK and extracellular signal regulated kinases (ERK) signalling pathways will be activated. The production of proinflammatory cytokines and chemokines will be reduced by the MAPK inhibitor SB203580 and ERK inhibitor FR 180204 [60].
Bone morphogenetic proteins (BMPs) are highly expressed in bone and are important regulators of cell differentiation. A study on BMP-7 signalling showed that BMP-7 treatment can upregulate the expression of tendon-related genes, without induction of osteogenic and chondrogenic genes [61]. However, Rui et al found that BMP-2 can promote proteoglycan deposition and induce chondrogenic differentiation of human achilles tendon-derived stem cells (hATDSCs) in vitro, which is associated with chronic tendinopathy [62]. In some animal tendinopathic models, the use of BMP inhibitors such as LDN-193189 can inhibit the formation of heterotopic ossification [63]. The TGF-β signalling pathway can be inhibited by the small molecule SB431542, which can prevent degenerative changes after rotator cuff lesions [64].
Regeneration—augmenting tendon repair
As already mentioned, inflammation exists in diseased tendons and plays an important role in the development of tendinopathy. To a certain extent, antiinflammatory therapy can effectively inhibit the progress of tendon diseases. However, as we all know, there is little inflammation during the late stage of tendon diseases or torn tendon. Most points of views are that inflammation exists during the early stages of tendinopathy. Most patients who seek clinical treatment are often at the late stages of this disease. This is also one of the reasons why we had always considered tendinopathy as a noninflammatory disease. Because the regenerative capacity of tendon tissue is very weak, during the late stage of tendon diseases that have almost no inflammation, the diseased tendon has not yet embarked on the regeneration process. This would imply that antiinflammatory treatment can not completely restore the original structure of healthy tendon and can only be used during the early stages of the disease. We need strategies that can effectively promote tendon regeneration, such as tissue engineering–based therapy. Regenerative medicine is the study of normal developmental mechanisms and the response of tissues and organs to injury, with the ultimate aim of finding effective ways to promote self-repair and regeneration and restore the injured tissue/organ to normal function. In the treatment of tendinopathy, there are a number of different strategies to promote tendon regeneration, which focus mainly on three aspects: growth factors, cells and biomaterials.
Growth factors
During the early repair process following tendon injury, upregulating the expression of some growth factors is beneficial for tendon healing [65], [66]. These factors include basic fibroblast growth factor (bFGF), insulin-like growth factors-1 (IGF-1), platelet-derived growth factor (PDGF), transforming growth factor-β (TGFβ) and VEGF [66], [67], which all play diverse roles during the healing process. However, the role of a single growth factor is always very limited, and the synergy of various growth factors tends to have a better effect. Platelet-rich plasma (PRP) has recently been investigated by many studies [68]. Its composition is complex and includes a large number of growth factors and proteins. A high-quality randomised controlled trial recently demonstrated that autologous PRP injections have better cure rates and pain scores than cortisone injections for up to 2 years after treatment [69]. A study on the effects of PRP with concomitant use of a corticosteroid on tenocytes found that the addition of PRP did not influence the antiinflammatory effects of the corticosteroid but it ameliorated the deleterious side-effects of the corticosteroid [70]. However, because of the variability of PRP components, the use of PRP in clinical therapy still remains controversial. Yan et al studied the influence of leucocytes on PRP-mediated tissue healing, and our study found that leucocyte-poor PRP improved tendon healing with better histological results, which may be a better option for the clinical treatment of tendinopathy when compared to leucocyte-rich PRP [71].
Cells
In the field of regenerative medicine, stem cell therapy can exert beneficial effects on the musculoskeletal system and encompasses a board range of cell types such as bone mesenchymal stem cells (BMSCs), TSPCs, adipose derived mesenchymal stem cells (ADMSCs), embryonic stem cells (ESCs). These different cell types can all exert a positive effect on tendon healing. Two published case studies that evaluated the results of rotator cuff repair with BMSCs injections reported a reduced number of ruptures over time (p < 0.005) [72], [73]. One pilot study, which to our knowledge is the first clinical study to investigate the efficacy of ADMSCs with lateral epicondylitis showed that there were statistically significant positive results [74]. Although there has not yet been any published clinical study to describe the therapeutic effects of TSPCs and ESCs, there is much evidence to validate the self-renewal and differentiation capacity of these cell types. More studies, particularly high-level clinical trials are necessary to validate the efficacy and safety of cell therapy and to elucidate the mechanisms of any observed therapeutic effects.
Biomaterials
With the development of tendon tissue engineering, it is possible to fabricate tissue engineered tendons and select appropriate biomaterials for fabrication of tendon scaffolds. Scaffold materials can provide support and protection for cells, but more importantly, the interaction of cells with scaffold materials can affect cell survival, migration, proliferation and metabolism. Collagen, silk, hyaluronic acid, alginate and decellularised tendon xenografts are all natural biomaterials [75]. Zheng et al had developed a woven silk–collagen sponge scaffold, which was similar to natural tendon with superior mechanical characteristics [76]. Shen et al fabricated a bioactive knitted silk–collagen sponge scaffold which can release stromal cell-derived factor 1α (SDF-1α), enhancing the number of local fibroblast-like cells and inhibiting the accumulation of immunocytes, which enhanced repair of injured tendon [77]. Biomaterials are a key component in the field of tendon tissue engineering.
In summary, antiinflammatory treatments by themselves are far from adequate. For the treatment of tendinopathy, the different stages and different types of this disease should be correctly delineated to achieve effective treatment outcomes. More research studies are needed in this area. Regenerative therapy can greatly improve the healing capacity of damaged tissue. Antiinflammatory therapy combined with regenerative therapy may be able to bring about a new breakthrough and achieve unexpected results in the field of tendinopathy (Figure 1).
Another possible route of pathogenesis—compression with pulling force may cause tendinopathy
Inflammation does play an important role in tendinopathy, but this view may be incomplete. As mentioned earlier, it not appropriate to simplify the pathogenesis of tendinopathy as a type of degenerative diseases. The view that tendinopathy is an inflammatory disease may be incomplete. A research group from Pittsburgh show that low mechanical stimulation can upregulate the expression of tenocyte-related genes in tendon stem cells. However, mechanical overloading can lead to increased expression of both tenocyte-related and nontenocyte-related genes [78]. Overuse is one of the main causative factors of tendinopathy. A study also showed that mechanical stretching can influence the production of inflammatory mediators in tendon [17]. There may exist a complex relationship between inflammation, mechanics and degenerative changes in tendon, which need to be investigated by more studies.
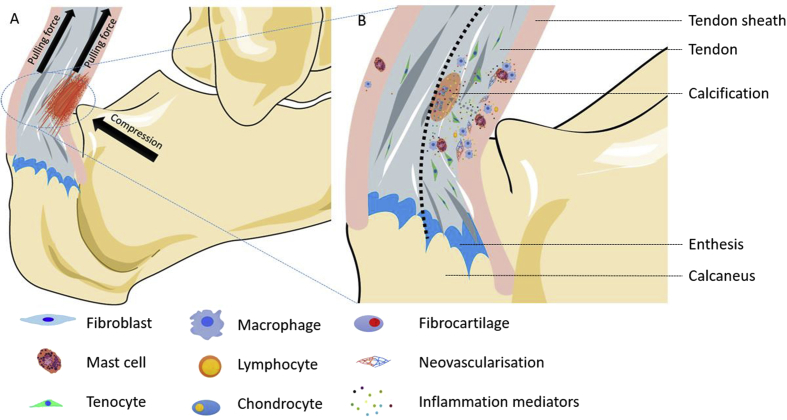
In the treatment of tendon diseases, eccentric exercises maybe a useful method. There are some contradictions between eccentric exercises and the aetiology of tendinopathy—overuse. Pulling force (overuse) can cause tendinopathy, but pulling force (eccentric exercises) can also treat tendinopathy. The possible explanation is that there are many types of forces encountered by the tendon during daily physical activities, including compression and pulling force. There are two types of compression during tendon activity. One is a direct contact with the bone impact, such as Haglund's disease. The Achilles tendon will be subjected to two types of force, compression and pulling force (Figure 2A). The other type of compression mainly exists at the junction between tendon and bone. If the force applied to the tendon is perpendicular to the bone surface, there will be no compression. However, if tendon is subjected to angular tension, the side of the tendon which is near the bone will be under compression.
Figure 2.
(A) Compression is present on the side of the tendon which is near the bone. We assumed that compression and pulling force cause tendinopathy. So tendon tissue that is close to the osteophyma will undergo pathological changes, but the other side will remain normal; (B) Compression and pulling force can initially provoke paratendon inflammation. The upregulation of inflammatory mediators and immunocytes lead to increasing numbers of tenocytes and make cells have abnormal phenotypes. At the same time, it also causes the proliferation of peripheral blood vessels and the onset of calcification.
Combinations of various load types may induce changes in tissue physiology. Cardiac valves are subjected to various types of mechanical loads during physiological activities, such as transvalvular pressures, shear stresses, and bending and axial stress. Abnormal haemodynamics and various types of mechanical loading eventually induce valve pathology. The review of Balachandran et al summarised the relationship between mechanical biology, matrix remodelling, inflammation and valve calcification and pointed out that this complex mechanics can induce tissue inflammation, which lead to valve calcification [79]. Thus, tissue damage caused by multiple types of mechanical forces may also be present in tendon. Soslowsky et al found that the extrinsic compression alone may be insufficient to cause tendinosis, but the injury caused by overuse plus extrinsic compression was more serious [80]. Impingement is a form of mechanical load and adds compressive or shearing load to the normal tensile load on the tendon. The study of Bullock et al showed that, according to the results of magnetic resonance imaging, that there is more Achilles tendinopathy in close proximity to the superior aspect of the calcaneal tuberosity, which is consistent with impingement (67.5%) [81]. It was suggested that the terminology “Achilles impingement tendinopathy” would be more appropriate, as opposed to “Haglund's syndrome,” since this refers to the impingement of the retrocalcaneal bursa and Achilles tendon [81]. During shoulder joint activities, subacromial structures will hit the coracoacromial ligament; therefore, shoulder impingement is one of the most common causes of shoulder tendinopathy [82]. In patellar tendon, Edama et al showed that the impingement may lead to injury between the proximal posterior surface of the patellar tendon and patella during knee activity [83].
Based on these evidences, we support the hypothesis that on the basis of tendon tension, compression may be another contributing factor which can result in tendon disease (Figure 2A). Compression may cause onset of paratendon diseases, such as peritendinitis. Subsequently, paratendon diseases are likely to exert an adverse effect on the tendon itself. Puddu et al proposed a classification system for Achilles tendon disease, which include pure peritendinitis, peritendinitis with tendinosis and tendinosis. In some cases of peritendinitis, they found marked vascular proliferation in the peritendon, which had a tendency to invade the normal tendon. From the newly formed capillary walls, mesenchymal cell types can invade the tendon tissue in a disorganised fashion. Near these mesenchymal cells can be observed newly formed collagen fibres irregularly oriented in comparison with normal tendon bundles [3]. Research has confirmed that the local inflammation does affect tendon. Vieira et al found that induction of localised inflammation in the rat paw can increase MMPs activity in Achilles tendon [84]. Andersson et al showed that paratendinous SP injections could lead to paratendinitis which promoted tendinosis-like changes in Achilles tendon [34].
Conclusions
An increasing number of studies have suggested that there are many inflammatory mediators and immunocytes which are involved in chronic tendinopathy (Table 1). We may be able to put forward a hypothesis (Figure 2B) that compression and pulling force can initially provoke paratendon inflammation. Different inflammatory mediators have different functions and their functions may overlap, which may exert synergistic effects on tendinopathy. First, inflammatory mediators can modulate the composition and properties of the tendon matrix. Second, inflammatory mediators can change the phenotype of some cells in tendon and modify the function of these cells. A series of inflammatory mediators can induce cells in tendon to produce more inflammatory mediators, further aggravating tendon diseases. The cells within tendon also play a key role in tendon disease. Immunocytes and tenocytes can both express inflammatory mediators. But we still cannot draw any conclusion about which type of cells produce inflammatory mediators first.
Table 1.
The roles of inflammatory mediators and immunocytes in tendinopathy.
| Function in tendinopathy | |
|---|---|
| Inflammatory mediators | |
| IL-1β | Inflammatory mediators expression↑ [14]; loss of phenotype [15]; degradation of ECM [14]; ECM synthesis↓ [15] |
| IL-6 | Collagen expression↑ [20] |
| IL-10 | IL-10 expression↑ [23]; maximum stress↓ [22] |
| IL-17A | Inflammatory mediators expression↑ [24]; collagen type III↑ [24]; apoptosis related factors↑ [24] |
| IL-21 | Hardly be detected [28]; proinflammatory cytokines can promote the function of IL-21R [28] |
| IL-33 | Inflammatory mediators expression↑ [30]; collagen expression↑ [30] |
| TNF-α | Inflammatory mediators expression↑ [21]; differentiation of TDSC↓ [33]; apoptosis [21]; ECM synthesis↓ [32] |
| Substance P | Mast cell degranulation [37]; neurogenic inflammation [34] |
| Alarmin molecules | Inflammatory mediators expression↑ [40]; ECM synthesis↑ [40] |
| Immunocytes | |
| Macrophages | Inflammatory infiltration [11] |
| Mast cells | Neurogenic inflammation [37] |
| Lymphocytes | Unclear |
IL = interleukin.
Antiinflammatory treatment such as glucocorticoid and nonsteroidal anti-inflammatory drugs (NSAIDs) does not have a significant effect on tendinopathy and is even harmful to tendon disease prognosis in the long term. Inflammation plays an important role in the development of tendinopathy, so completely denying antiinflammatory therapy may hinder our quest to find an effective treatment modality. Antiinflammatory therapy will be a very important part of future clinical treatment strategies for tendinopathy. New antiinflammatory medications, including small molecule drugs which can manipulate signalling pathways, will attract more attention. In addition to inflammation, there are degenerative changes in tendon diseases, and the regeneration of tendon is often inadequate, which means that antiinflammatory treatment alone is far from enough. The combination of antiinflammatory therapy and regenerative therapy may bring new hope to treatment of tendinopathy but more research studies are needed.
Conflicts of interest
None of the authors had professional or financial affiliations that biased this work.
Acknowledgements
This work of our research group was supported by National key R & D program of China (2017YFA0104900), National key research and development program of China (2016YFC1100204), NSFC grants (81572115, 81572157, 81330041, 81125014, 31271041, 81201396, 81271970, J1103603, 81522029, 31570987, 81401781), Regenerative Medicine in Innovative Medical Subjects of Zhejiang Province and Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents, Zhejiang Province Grants (Z2100086, LY12H06006, LR14H060001, LY14H060003), the Key scientific and technological innovation team of Zhejiang Province (2013TD11), Medical and Health Science and Technology Plan of the Department of Health of Zhejiang Province (2013RCA010, 2014KYB052), Medical Science and Technology Project of Zhejiang Province (201341741), and Zhejiang Provisional Grant (2012C33015), International Science & Technology Cooperation Program of China (2015DFG32130) and Fundamental Research Funds (2017QNA7006) for the Central Universities.
Contributor Information
Weishan Chen, Email: chenweishan@zju.edu.cn.
Weiliang Shen, Email: wlshen@zju.edu.cn.
References
- 1.Millar N.L., Murrell G.A., McInnes I.B. Inflammatory mechanisms in tendinopathy – towards translation. Nat Rev Rheumatol. 2017;13:110–122. doi: 10.1038/nrrheum.2016.213. [DOI] [PubMed] [Google Scholar]
- 2.Abate M., Silbernagel K.G., Siljeholm C., Di I.A., De Amicis D., Salini V. Pathogenesis of tendinopathies: inflammation or degeneration. Arthritis Res Ther. 2009;11:235. doi: 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puddu G., Ippolito E., Postacchini F. A classification of Achilles tendon disease. Am J Sports Med. 1976;4:145–150. doi: 10.1177/036354657600400404. [DOI] [PubMed] [Google Scholar]
- 4.Rees J.D., Stride M., Scott A. Tendons–time to revisit inflammation. Br J Sports Med. 2014;48:1553–1557. doi: 10.1136/bjsports-2012-091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook J.L., Purdam C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 6.Asplund C.A., Best T.M. Achilles tendon disorders. BMJ. 2013;346:f1262. doi: 10.1136/bmj.f1262. [DOI] [PubMed] [Google Scholar]
- 7.Longo U.G., Ronga M., Maffulli N. Achilles Tendinopathy. Sports Med Arthrosc Rev. 2018;26:16–30. doi: 10.1097/JSA.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 8.Maffulli N., Sharma P., Luscombe K.L. Achilles tendinopathy: aetiology and management. J R Soc Med. 2004;97:472–476. doi: 10.1258/jrsm.97.10.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speed C. Inflammation in tendon disorders. Adv Exp Med Biol. 2016;920:209–220. doi: 10.1007/978-3-319-33943-6_20. [DOI] [PubMed] [Google Scholar]
- 10.Millar N.L., Hueber A.J., Reilly J.H., Xu Y., Fazzi U.G., Murrell G.A. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010;38:2085–2091. doi: 10.1177/0363546510372613. [DOI] [PubMed] [Google Scholar]
- 11.Matthews T.J., Hand G.C., Rees J.L., Athanasou N.A., Carr A.J. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489–495. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 12.Mobasheri A., Shakibaei M. Is tendinitis an inflammatory disease initiated and driven by pro-inflammatory cytokines such as interleukin 1β. Histol Histopathol. 2013;28:955–964. doi: 10.14670/HH-28.955. [DOI] [PubMed] [Google Scholar]
- 13.Dinarello C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 14.Yang G., Im H.J., Wang J.H. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K., Asai S., Yu B., Enomoto-Iwamoto M. IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem Biophys Res Commun. 2015;463:667–672. doi: 10.1016/j.bbrc.2015.05.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legerlotz K., Jones E.R., Screen H.R., Riley G.P. Increased expression of IL-6 family members in tendon pathology. Rheumatology (Oxford) 2012;51:1161–1165. doi: 10.1093/rheumatology/kes002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skutek M., van Griensven M., Zeichen J., Brauer N., Bosch U. Cyclic mechanical stretching enhances secretion of Interleukin 6 in human tendon fibroblasts. Knee Surg Sports Traumatol Arthrosc. 2001;9:322–326. doi: 10.1007/s001670100217. [DOI] [PubMed] [Google Scholar]
- 18.Lin T.W., Cardenas L., Glaser D.L., Soslowsky L.J. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39:61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Nakama K., Gotoh M., Yamada T., Mitsui Y., Yasukawa H., Imaizumi T. Interleukin-6-induced activation of signal transducer and activator of transcription-3 in ruptured rotator cuff tendon. J Int Med Res. 2006;34:624–631. doi: 10.1177/147323000603400607. [DOI] [PubMed] [Google Scholar]
- 20.Andersen M.B., Pingel J., Kjær M., Langberg H. Interleukin-6: a growth factor stimulating collagen synthesis in human tendon. J Appl Physiol. 1985;2011(110):1549–1554. doi: 10.1152/japplphysiol.00037.2010. [DOI] [PubMed] [Google Scholar]
- 21.Machner A., Baier A., Wille A., Drynda S., Pap G., Drynda A. Higher susceptibility to Fas ligand induced apoptosis and altered modulation of cell death by tumor necrosis factor-alpha in periarticular tenocytes from patients with knee joint osteoarthritis. Arthritis Res Ther. 2003;5:R253–R261. doi: 10.1186/ar789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricchetti E.T., Reddy S.C., Ansorge H.L., Zgonis M.H., Van Kleunen J.P., Liechty K.W. Effect of interleukin-10 overexpression on the properties of healing tendon in a murine patellar tendon model. J Hand Surg Am. 2008;33:1843–1852. doi: 10.1016/j.jhsa.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.John T., Lodka D., Kohl B., Ertel W., Jammrath J., Conrad C. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. J Orthop Res. 2010;28:1071–1077. doi: 10.1002/jor.21079. [DOI] [PubMed] [Google Scholar]
- 24.Millar N.L., Akbar M., Campbell A.L., Reilly J.H., Kerr S.C., McLean M. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci Rep. 2016;6:27149. doi: 10.1038/srep27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bubier J.A., Sproule T.J., Foreman O., Spolski R., Shaffer D.J., Morse H.C. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci USA. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fina D., Sarra M., Caruso R., Del V.B.G., Pallone F., MacDonald T.T. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut. 2008;57:887–892. doi: 10.1136/gut.2007.129882. [DOI] [PubMed] [Google Scholar]
- 27.Monteleone G., Caruso R., Fina D., Peluso I., Gioia V., Stolfi C. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut. 2006;55:1774–1780. doi: 10.1136/gut.2006.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell A.L., Smith N.C., Reilly J.H., Kerr S.C., Leach W.J., Fazzi U.G. IL-21 receptor expression in human tendinopathy. Mediators Inflamm. 2014;2014:481206. doi: 10.1155/2014/481206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan F.L., Hu W., Lu W.G., Li X., Li J.P., Xu R.S. Targeting interleukin-21 in rheumatoid arthritis. Mol Biol Rep. 2011;38:1717–1721. doi: 10.1007/s11033-010-0285-x. [DOI] [PubMed] [Google Scholar]
- 30.Millar N.L., Gilchrist D.S., Akbar M., Reilly J.H., Kerr S.C., Campbell A.L. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun. 2015;6:6774. doi: 10.1038/ncomms7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosaka Y., Kirisawa R., Ueda H., Yamaguchi M., Takehana K. Differences in tumor necrosis factor (TNF)alpha and TNF receptor-1-mediated intracellular signaling factors in normal, inflamed and scar-formed horse tendons. J Vet Med Sci. 2005;67:985–991. doi: 10.1292/jvms.67.985. [DOI] [PubMed] [Google Scholar]
- 32.Qi J., Chi L., Maloney M., Yang X., Bynum D., Banes A.J. Interleukin-1beta increases elasticity of human bioartificial tendons. Tissue Eng. 2006;12:2913–2925. doi: 10.1089/ten.2006.12.2913. [DOI] [PubMed] [Google Scholar]
- 33.Han P., Cui Q., Yang S., Wang H., Gao P., Li Z. Tumor necrosis factor-α and transforming growth factor-β1 facilitate differentiation and proliferation of tendon-derived stem cells in vitro. Biotechnol Lett. 2017;39:711–719. doi: 10.1007/s10529-017-2296-3. [DOI] [PubMed] [Google Scholar]
- 34.Andersson G., Backman L.J., Scott A., Lorentzon R., Forsgren S., Danielson P. Substance P accelerates hypercellularity and angiogenesis in tendon tissue and enhances paratendinitis in response to Achilles tendon overuse in a tendinopathy model. Br J Sports Med. 2011;45:1017–1022. doi: 10.1136/bjsm.2010.082750. [DOI] [PubMed] [Google Scholar]
- 35.Backman L.J., Fong G., Andersson G., Scott A., Danielson P. Substance P is a mechanoresponsive, autocrine regulator of human tenocyte proliferation. PLoS One. 2011;6:e27209. doi: 10.1371/journal.pone.0027209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pingel J., Wienecke J., Kongsgaard M., Behzad H., Abraham T., Langberg H. Increased mast cell numbers in a calcaneal tendon overuse model. Scand J Med Sci Sports. 2013;23:e353–e360. doi: 10.1111/sms.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott A., Bahr R. Neuropeptides in tendinopathy. Front Biosci (Landmark Ed) 2009;14:2203–2211. doi: 10.2741/3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millar N.L., Murrell G.A., McInnes I.B. Alarmins in tendinopathy: unravelling new mechanisms in a common disease. Rheumatology (Oxford) 2013;52:769–779. doi: 10.1093/rheumatology/kes409. [DOI] [PubMed] [Google Scholar]
- 39.Mosca M.J., Carr A.J., SJB S., Wheway K., Watkins B., Dakin S.G. Differential expression of alarmins-S100A9, IL-33, HMGB1 and HIF-1α in supraspinatus tendinopathy before and after treatment. BMJ Open Sport Exerc Med. 2017;3:e000225. doi: 10.1136/bmjsem-2017-000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akbar M., Gilchrist D.S., Kitson S.M., Nelis B., LAN C., Garcia-Melchor E. Targeting danger molecules in tendinopathy: the HMGB1/TLR4 axis. RMD Open. 2017;3:e000456. doi: 10.1136/rmdopen-2017-000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean B.J., Gettings P., Dakin S.G., Carr A.J. Are inflammatory cells increased in painful human tendinopathy? A systematic review. Br J Sports Med. 2016;50:216–220. doi: 10.1136/bjsports-2015-094754. [DOI] [PubMed] [Google Scholar]
- 42.Fu S.C., Rolf C., Cheuk Y.C., Lui P.P., Chan K.M. Deciphering the pathogenesis of tendinopathy: a three-stages process. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:30. doi: 10.1186/1758-2555-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 44.Dakin S.G., Werling D., Hibbert A., Abayasekara D.R., Young N.J., Smith R.K. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS One. 2012;7:e32333. doi: 10.1371/journal.pone.0032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hakim-Rad K., Metz M., Maurer M. Mast cells: makers and breakers of allergic inflammation. Curr Opin Allergy Clin Immunol. 2009;9:427–430. doi: 10.1097/ACI.0b013e32832e9af1. [DOI] [PubMed] [Google Scholar]
- 46.Scott A., Lian Ø., Bahr R., Hart D.A., Duronio V., Khan K.M. Increased mast cell numbers in human patellar tendinosis: correlation with symptom duration and vascular hyperplasia. Br J Sports Med. 2008;42:753–757. doi: 10.1136/bjsm.2007.040212. [DOI] [PubMed] [Google Scholar]
- 47.Berglund M.E., Hildebrand K.A., Zhang M., Hart D.A., Wiig M.E. Neuropeptide, mast cell, and myofibroblast expression after rabbit deep flexor tendon repair. J Hand Surg Am. 2010;35:1842–1849. doi: 10.1016/j.jhsa.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 48.Kragsnaes M.S., Fredberg U., Stribolt K., Kjaer S.G., Bendix K., Ellingsen T. Stereological quantification of immune-competent cells in baseline biopsy specimens from achilles tendons: results from patients with chronic tendinopathy followed for more than 4 years. Am J Sports Med. 2014;42:2435–2445. doi: 10.1177/0363546514542329. [DOI] [PubMed] [Google Scholar]
- 49.Schubert T.E., Weidler C., Lerch K., Hofstädter F., Straub R.H. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083–1086. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott A., Cook J.L., Hart D.A., Walker D.C., Duronio V., Khan K.M. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56:871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 51.Pufe T., Petersen W.J., Mentlein R., Tillmann B.N. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005;15:211–222. doi: 10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 52.Tohyama H., Yasuda K., Uchida H., Nishihira J. The responses of extrinsic fibroblasts infiltrating the devitalised patellar tendon to IL-1beta are different from those of normal tendon fibroblasts. J Bone Joint Surg Br. 2007;89:1261–1267. doi: 10.1302/0301-620X.89B9.18053. [DOI] [PubMed] [Google Scholar]
- 53.Dakin S.G., Martinez F.O., Yapp C., Wells G., Oppermann U., Dean B.J. Inflammation activation and resolution in human tendon disease. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aac4269. 311ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bi Y., Ehirchiou D., Kilts T.M., Inkson C.A., Embree M.C., Sonoyama W. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 55.Hu J.J., Yin Z., Shen W.L., Xie Y.B., Zhu T., Lu P. Pharmacological regulation of in situ tissue stem cells differentiation for soft tissue calcification treatment. Stem Cells. 2016;34:1083–1096. doi: 10.1002/stem.2306. [DOI] [PubMed] [Google Scholar]
- 56.Poulsen R.C., Watts A.C., Murphy R.J., Snelling S.J., Carr A.J., Hulley P.A. Glucocorticoids induce senescence in primary human tenocytes by inhibition of sirtuin 1 and activation of the p53/p21 pathway: in vivo and in vitro evidence. Ann Rheum Dis. 2014;73:1405–1413. doi: 10.1136/annrheumdis-2012-203146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shakibaei M., Buhrmann C., Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem. 2011;286:11492–11505. doi: 10.1074/jbc.M110.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buhrmann C., Mobasheri A., Busch F., Aldinger C., Stahlmann R., Montaseri A. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2011;286:28556–28566. doi: 10.1074/jbc.M111.256180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz A.J., Sarver D.C., Sugg K.B., Dzierzawski J.T., Gumucio J.P., Mendias C.L. p38 MAPK signaling in postnatal tendon growth and remodeling. PLoS One. 2015;10:e0120044. doi: 10.1371/journal.pone.0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Millar N.L., Reilly J.H., Kerr S.C., Campbell A.L., Little K.J., Leach W.J. Hypoxia: a critical regulator of early human tendinopathy. Ann Rheum Dis. 2012;71:302–310. doi: 10.1136/ard.2011.154229. [DOI] [PubMed] [Google Scholar]
- 61.Klatte-Schulz F., Giese G., Differ C., Minkwitz S., Ruschke K., Puts R. An investigation of BMP-7 mediated alterations to BMP signalling components in human tenocyte-like cells. Sci Rep. 2016;6:29703. doi: 10.1038/srep29703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rui Y., Guo Y., Lin Y., Ma L., Cheng X., Chen H. Experiment of bone morphogenetic protein 2 induced chondrogenic differentiation of human Achilles tendon-derived stem cells in vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013;27:1492–1498. [PubMed] [Google Scholar]
- 63.Yu P.B., Deng D.Y., Lai C.S., Hong C.C., Cuny G.D., Bouxsein M.L. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davies M.R., Liu X., Lee L., Laron D., Ning A.Y., Kim H.T. TGF-β small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One. 2016;11:e0155486. doi: 10.1371/journal.pone.0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heisterbach P.E., Todorov A., Flückiger R., Evans C.H., Majewski M. Effect of BMP-12, TGF-β1 and autologous conditioned serum on growth factor expression in Achilles tendon healing. Knee Surg Sports Traumatol Arthrosc. 2012;20:1907–1914. doi: 10.1007/s00167-011-1772-x. [DOI] [PubMed] [Google Scholar]
- 66.Würgler-Hauri C.C., Dourte L.M., Baradet T.C., Williams G.R., Soslowsky L.J. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg. 2007;16:S198–S203. doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen C.H., Cao Y., Wu Y.F., Bais A.J., Gao J.S., Tang J.B. Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg Am. 2008;33:1834–1842. doi: 10.1016/j.jhsa.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Orchard J., Kountouris A. The management of tennis elbow. BMJ. 2011;342:d2687. doi: 10.1136/bmj.d2687. [DOI] [PubMed] [Google Scholar]
- 69.Gosens T., Peerbooms J.C., van Laar W., den Oudsten B.L. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 70.Jo C.H., Lee S.Y., Yoon K.S., Shin S. Effects of platelet-rich plasma with concomitant use of a corticosteroid on tenocytes from degenerative rotator cuff tears in interleukin 1β-induced tendinopathic conditions. Am J Sports Med. 2017;45:1141–1150. doi: 10.1177/0363546516681294. [DOI] [PubMed] [Google Scholar]
- 71.Yan R., Gu Y., Ran J., Hu Y., Zheng Z., Zeng M. Intratendon delivery of leukocyte-poor platelet-rich plasma improves healing compared with leukocyte-rich platelet-rich plasma in a rabbit achilles tendinopathy model. Am J Sports Med. 2017;45:1909–1920. doi: 10.1177/0363546517694357. [DOI] [PubMed] [Google Scholar]
- 72.Ellera G.J.L., da S.R.C., Silla L.M., Abreu M.R., Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20:373–377. doi: 10.1007/s00167-011-1607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernigou P., Flouzat L.C.H., Delambre J., Zilber S., Duffiet P., Chevallier N. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38:1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 74.Lee S.Y., Kim W., Lim C., Chung S.G. Treatment of lateral epicondylosis by using allogeneic adipose-derived mesenchymal stem cells: a pilot study. Stem Cells. 2015;33:2995–3005. doi: 10.1002/stem.2110. [DOI] [PubMed] [Google Scholar]
- 75.Docheva D., Müller S.A., Majewski M., Evans C.H. Biologics for tendon repair. Adv Drug Deliv Rev. 2015;84:222–239. doi: 10.1016/j.addr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng Z., Ran J., Chen W., Hu Y., Zhu T., Chen X. Alignment of collagen fiber in knitted silk scaffold for functional massive rotator cuff repair. Acta Biomater. 2017;51:317–329. doi: 10.1016/j.actbio.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 77.Shen W., Chen X., Chen J., Yin Z., Heng B.C., Chen W. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–7249. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J., Wang J.H. The effects of mechanical loading on tendons–an in vivo and in vitro model study. PLoS One. 2013;8:e71740. doi: 10.1371/journal.pone.0071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balachandran K., Sucosky P., Yoganathan A.P. Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int J Inflam. 2011;2011:263870. doi: 10.4061/2011/263870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soslowsky L.J., Thomopoulos S., Esmail A., Flanagan C.L., Iannotti J.P., Williamson J.D. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30:1057–1063. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 81.Bullock M.J., Mourelatos J., Mar A. Achilles impingement tendinopathy on magnetic resonance imaging. J Foot Ankle Surg. 2017;56:555–563. doi: 10.1053/j.jfas.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 82.Camargo P.R., Alburquerque-Sendín F., Salvini T.F. Eccentric training as a new approach for rotator cuff tendinopathy: Review and perspectives. World J Orthop. 2014;5:634–644. doi: 10.5312/wjo.v5.i5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edama M., Kageyama I., Nakamura M., Kikumoto T., Nakamura E., Ito W. Anatomical study of the inferior patellar pole and patellar tendon. Scand J Med Sci Sports. 2017;27:1681–1687. doi: 10.1111/sms.12858. [DOI] [PubMed] [Google Scholar]
- 84.Vieira C.P., Guerra F.R., de Oliveira L.P., de Almeida Mdos S., Pimentel E.R. Alterations in the Achilles tendon after inflammation in surrounding tissue. Acta Ortop Bras. 2012;20:266–269. doi: 10.1590/S1413-78522012000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]