Abstract
Background
Both copper deficiency and overexposure have been associated with adverse health effects. Evidence linking copper to bone mineral density (BMD) and total fracture, however, is limited.
Methods
This nationally representative cross-sectional study enrolled participants from the National Health and Nutrition Examination Survey (2011–2014) in the United States. Using unadjusted and multivariate adjusted logistic regression analyses and a two-piecewise linear regression model with a smoothing function, we evaluated the associations between serum copper levels, bone mineral density and total fracture in 722 participants.
Results
The study sample (n = 722, mean age: 56.47 ± 11.55 y) represented a population of which 47.2% were men; 43.91% were non-Hispanic white, 18.84% non-Hispanic black and 13.71% Mexican American; 25.9% had total fracture. In the multivariate logistic regression analysis, individuals in the lowest category (<98.5 μg/dL) of serum copper concentration had 0.049 g/cm2 lower total femur BMD and 0.045 g/cm2 lower femoral neck BMD than those in the second concentration category (98.5–114 μg/dL). Individuals in the highest category (≥134 μg/dL) of serum copper concentration had an approximately 4-fold increase in the risk of total fracture than those in the second concentration category. There were no significant associations between per 10 μg/dL increases in serum copper levels and total fracture in multivariate logistic regression analysis after multivariate adjustment (all p > 0.05). However, a differential association between serum copper levels and total fractures between men and women was observed (odds ratio = 1.81, 95% confidence interval 1.08–3.03, p = 0.026 for men and odds ratio = 1.07, 95% confidence interval 0.86–1.32, p = 0.552 for women).
Conclusion
Moderate serum copper levels are critically important for bone health. Lower serum copper levels are significantly associated with decreased BMD in the total femur and femoral neck. Higher serum copper levels are significantly associated with increased total fracture, especially in men.
The Translational Potential of this Article
The impact of serum copper concentrations on bone mineral density and total fracture can provide insights into clinical application of copper-containing supplements and biomaterials.
Keywords: Bone mineral density, Fracture, Odds ratio, Serum copper levels
List of abbreviations: Bone mineral density, BMD; Confidence interval, CI; Centers for Disease Control and Prevention, CDC; Diastolic blood pressure, DBP; Dual-energy x-ray absorptiometry, DXA; High-density lipoprotein-cholesterol, HDL-C; National Center for Health Statistics, NCHS; Low-density lipoprotein-cholesterol, LDL-C; National Health and Nutrition Examination Survey, NHANES; Systolic blood pressure, SBP; Standard deviation, SD; Total cholesterol, TC; Triglycerides, TG
Introduction
Copper is an essential trace element involved in many biological processes including enzymatic reactions, nucleic acid synthesis, antioxidant defence, iron metabolism and immune function. Copper deficiency may result in impaired bone and cholesterol metabolism and cardiovascular disorders [1], [2]. Excessive copper has also been also associated with adverse health effects, including damaged lung, renal and liver function [1], [3], [4].
In recent years, owing to increased opportunities for exogenous copper uptake, people have become increasingly concerned about the health effects of copper exposure. For example, increasing copper-containing biomaterials have been developed. Biodegradable magnesium–copper or zinc–copper alloys [5], [6], [7] and copper-containing nanoparticles [8] have been researched and explored in the field of medical implants in recent years, especially for tissue engineering, cardiovascular, orthopaedic and antiinfective applications. Most copper-containing biomaterials were designed to be biodegradable and therefore release copper ions upon degradation. This may lead to increased levels of copper ions in the body.
Many epidemiological studies with mixed findings have investigated the associations between serum copper levels, cardiovascular disease and neurodegenerative diseases [9], [10], [11], [12]. However, the association between copper status and the risk of osteoporosis and related diseases has been examined by only few observational studies and remains controversial because of inconsistent results [13], [14], [15], [16], [17], [18]. Sadeghi et al. investigated the association between plasma copper and bone mineral density (BMD) in 135 Iranian women. They found that plasma copper and lead concentrations were higher in patients with low BMD than in control patients [18]. In another case-control study, the author did not find any statistically significant differences in serum copper concentrations among osteopenic, osteoporotic and control groups [17].
The studies mentioned above included a relatively small number of participants and thus might be underpowered to detect the associations between serum copper levels and BMD, and the impact of copper status on risk of fracture has not been assessed. Therefore, on the basis of the National Health and Nutrition Examination Survey (NHANES, 2011–2014), our study mainly evaluated the associations between serum copper levels, BMD and total fracture in the US population.
Materials and methods
Study population
We analysed data from NHANES 2011–2014, an independent cross-sectional study conducted by the Centers for Disease Control and Prevention and The National Center for Health Statistics designed to assess health and nutritional status in a nationally representative sample of noninstitutionalised, civilian general population in the United States. Survey details regarding plan, operation and design have been previously described [19], [20], [21], [22]. Questionnaire surveys, physical examinations, household interviews including demographic, dietary, health-related questions and examinations and laboratory tests were performed. Our study population included both men and women aged 18 years and older, who were free from diabetes mellitus, rheumatoid arthritis or cancerous diseases and were not exposed to steroids. Three thousand six hundred eight individuals aged 18 years and older in the NHANES 2011–2014 were originally selected as our study sample, and after the exclusion process, 722 individuals were included in our final study sample for further analysis. The NHANES 2011–2014 was approved by the National Center for Health Statistics Ethics Review Board, and informed consent was obtained from all participants.
Measurement of serum copper concentrations
Serum copper concentrations were measured by inductively coupled plasma dynamic reaction cell mass spectrometry, which is a multielement analytical technique capable of trace-level elemental analysis. Detailed instructions on specimen collection and processing can be found on the NHANES websites (https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/CUSEZN_G.htm and https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CUSEZN_H.htm).
Assessments
Demographic data (age, sex and race), physical examination data [standing height (cm), weight (kg), body mass index (BMI, kg/m2), systolic blood pressure (mmHg), diastolic blood pressure (mmHg) and BMD (g/cm2)], laboratory data [serum lipids (mg/dL)] and questionnaire data [smoking status, alcohol consumption, activity status, fractures and major diseases, including cardiovascular disease (congestive heart failure, coronary heart disease and heart attack) and diabetes mellitus] were collected and are described on the NHANES websites at https://wwwn.cdc.gov/Nchs/Nhanes/continuousnhanes/default.aspx?BeginYear=2011 and https://wwwn.cdc.gov/Nchs/Nhanes/continuousnhanes/default.aspx?BeginYear=2013.
For all participants, race (Mexican American, Other Hispanic, Non-Hispanic white, Non-Hispanic black, other race), smoking status (yes or no), alcohol consumption (yes or no) and moderate physical activity (yes or no) were defined. BMD in the total spine, total femur and femoral neck were examined by dual-energy X-ray absorptiometry. Dual-energy X-ray absorptiometry scans were administered to eligible survey participants aged 40 years and older. The scans were acquired on Hologic QDR-4500A fan-beam densitometers (the radiation exposure: less than 20 uSv. Hologic, Inc., Bedford, Massachusetts) using software version Hologic APEX 3.2. All scans were analysed with Hologic APEX, version 4.0, software. Serum lipids, including total cholesterol (TC), triglycerides (TGs) and low-density lipoprotein–cholesterol (LDL-C) were analysed using the Roche Modular P chemistry analyser (enzymatic method). The whole blood glycohaemoglobin level was analysed using the Tosoh G8 glycohaemoglobin analyser.
Self-reported fractures were assessed via questionnaire. Prior low-trauma fractures were defined as self-reported fractures that occurred at an age ≥40 years due to a fall from a standing height or less, a trip/slip or a fall out of bed (hip, wrist and spine) or at an age ≥20 years not due to severe trauma such as a car accident or a hard fall down stairs or from a ladder (fractures other than hip, wrist and spine). Cardiovascular diseases were defined as congestive heart failure, coronary heart disease and heart attack. We defined subjects as having diabetes if they answered yes to the question: “Have you ever been told by a doctor that you have diabetes?” or the subject reported current use of insulin or an oral antihyperglycemic medication. Dyslipidaemia was defined as having high TG (≥200 mg/dL), TC (≥240 mg/dL) and LDL-C (≥160 mg/dL) or low high-density lipoprotein–cholesterol (HDL-C) (≤40 mg/dL).
Statistical analysis
Continuous variables were presented as mean ± standard deviation, while categorical variables were presented as numbers and their proportion. We used the Chi-square tests for categorical variables, one-way analysis of variance for normally continuous variables and the Kruskal–Wallis test for skewed continuous variables. Regression coefficient and corresponding 95% confidence intervals (CI) were calculated using unadjusted and multivariate adjusted logistic regression analyses to determine associations between serum copper quartiles (<98.5 μg/dL, 98.5–114 μg/dL (ref.), 114 to 34 μg/dL and ≥134 μg/dL) or 10 μg/dL increases in serum copper levels and BMD and total fracture. The crude model was adjusted for no variables. The multivariate model was adjusted for age, sex, race, BMI, smoking status, alcohol consumption, moderate physical activity, cardiovascular disease, dyslipidaemia, TG (mg/dL), TC (mg/dL), HDL-C (mg/dL), LDL-C (mg/dL), serum selenium (μg/L) and serum zinc (μg/dL). We selected these confounders on the basis of their associations with the outcomes of interest or change in effect estimate of more than 10%. A two-sided p < 0.05 was considered statistically significant.
To examine the nonlinear association between serum copper levels, BMD and total fracture, we further applied a two-piecewise linear regression model using a smoothing function after adjusting for age, sex, race, BMI, smoking status, alcohol consumption, moderate physical activity, cardiovascular disease, dyslipidaemia, TG, TC, HDL-C, LDL-C, serum selenium and serum zinc. The threshold level was determined using trial and error, including selection of turning points along a predefined interval and then choosing the turning point that gave the maximum model likelihood. In addition, we conducted a log likelihood ratio test comparing the one-line linear regression model with the two-piecewise linear model. Statistical analyses were performed using R packages (http://www.r-project.org).
Results
Descriptive analysis
As shown in Table 1 and Figure 1, 722 subjects aged 18-years or older were included in this study. For exposures of interest, serum copper concentrations (108 vs. 130 μg/dL) were significantly lower in men than in women. For outcomes of interest, BMD in total femur (1.00 vs. 0.91 g/cm2), femoral neck (0.81 vs. 0.76 g/cm2) and total spine (1.02 vs. 0.99 g/cm2) was significantly higher in men than in women, whereas no significant association was found between total fracture and gender. The characteristics of our sample among serum copper quartiles are shown in Table 2. BMI and HDL-C were significantly associated with serum copper quartiles.
Table 1.
Characteristics of study sample: US population aged ≥18 years in the NHANES 2011–2014.
| Characteristic | Total number of particpants (N = 722) |
p | |
|---|---|---|---|
| Men | Women | ||
| Number of participant | 341 | 381 | |
| Age (y) | 56.59 ± 11.37 | 56.36 ± 11.71 | 0.785 |
| Weight (kg) | 84.45 ± 18.40 | 75.20 ± 20.43 | <0.001 |
| Standing height (cm) | 172.93 ± 7.52 | 160.40 ± 6.96 | <0.001 |
| Body mass index (kg/m2) | 28.18 ± 5.53 | 29.19 ± 7.38 | 0.186 |
| Systolic blood pressure (mmHg) | 126.78 ± 17.63 | 125.35 ± 19.41 | 0.318 |
| Diastolic blood pressure (mmHg) | 72.19 ± 13.14 | 70.37 ± 13.08 | 0.026 |
| Serum copper (ug/dL) | 108.42 ± 19.69 | 130.52 ± 25.43 | <0.001 |
| Serum selenium (ug/L) | 133.52 ± 18.46 | 129.33 ± 18.89 | 0.003 |
| Serum zinc (ug/dL) | 81.80 ± 15.87 | 80.44 ± 14.47 | 0.230 |
| Serum lipids | |||
| Total cholesterol | 195.55 ± 42.49 | 200.83 ± 41.12 | 0.091 |
| Triglyceride | 132.26 ± 90.14 | 102.13 ± 52.18 | <0.001 |
| HDL-cholesterol | 50.11 ± 15.91 | 60.29 ± 15.62 | <0.001 |
| LDL-cholesterol | 120.27 ± 39.69 | 120.55 ± 39.21 | 0.946 |
| Bone mineral density (g/cm2) | |||
| Total femur | 1.00 ± 0.14 | 0.91 ± 0.15 | <0.001 |
| Femoral neck | 0.81 ± 0.14 | 0.76 ± 0.15 | <0.001 |
| Total spine | 1.02 ± 0.16 | 0.99 ± 0.16 | 0.048 |
| Race | 0.512 | ||
| Mexican American | 51 (14.96%) | 48 (12.60%) | |
| Other Hispanic | 27 (7.92%) | 43 (11.29%) | |
| Non-Hispanic white | 151 (44.28%) | 166 (43.57%) | |
| Non-Hispanic black | 67 (19.65%) | 69 (18.11%) | |
| Other race | 45 (13.20%) | 55 (14.44%) | |
| Total fracture | 0.775 | ||
| No | 251 (73.61%) | 284 (74.54%) | |
| Yes | 90 (26.39%) | 97 (25.46%) | |
| Cardiovascular diseases | 0.035 | ||
| No | 314 (92.08%) | 365 (95.80%) | |
| Yes | 27 (7.92%) | 16 (4.20%) | |
| Dyslipidaemia | <0.001 | ||
| No | 210 (61.58%) | 302 (79.27%) | |
| Yes | 131 (38.42%) | 79 (20.73%) | |
| Smoking status | <0.001 | ||
| Yes | 189 (55.43%) | 136 (35.70%) | |
| No | 152 (44.57%) | 245 (64.30%) | |
| Alcohol drinking | <0.001 | ||
| Yes | 261 (84.74%) | 219 (62.57%) | |
| No | 47 (15.26%) | 131 (37.43%) | |
| Moderate work activity | 0.070 | ||
| Yes | 106 (31.27%) | 96 (25.20%) | |
| No | 233 (68.73%) | 285 (74.80%) | |
HDL = high-density lipoprotein; LDL = low-density lipoprotein; NHANES = National Health and Nutrition Examination Survey; SD = standard deviation.
Values are mean ± SD or n (%).
Figure 1.
Study sample selection flowchart.
BMD = bone mineral density; NHANES = NHANES = National Health and Nutrition Examination Survey.
Table 2.
Characteristics of study sample among serum copper quartiles.
| Characteristic | Serum copper (ug/dL) |
p | |||
|---|---|---|---|---|---|
| <98.5 | 98.5 to 114 | 114 to 34 | ≥134 | ||
| Number of participants | 142 | 184 | 214 | 182 | |
| Age (y) | 54.95 ± 11.03 | 57.83 ± 11.85 | 56.82 ± 11.26 | 55.86 ± 11.88 | 0.127 |
| Weight (kg) | 79.17 ± 18.25 | 77.55 ± 16.97 | 78.79 ± 21.10 | 82.91 ± 22.52 | 0.065 |
| Standing height (cm) | 170.85 ± 8.67 | 168.31 ± 10.09 | 165.06 ± 9.17 | 162.29 ± 8.09 | <0.001 |
| Body mass index (kg/m2) | 26.99 ± 5.18 | 27.26 ± 4.77 | 28.78 ± 6.75 | 31.44 ± 7.95 | <0.001 |
| Systolic blood pressure (mmHg) | 124.23 ± 16.77 | 124.56 ± 16.90 | 126.80 ± 19.46 | 128.00 ± 20.35 | 0.205 |
| Diastolic blood pressure (mmHg) | 73.31 ± 10.34 | 71.21 ± 13.33 | 70.21 ± 13.38 | 70.85 ± 14.44 | 0.195 |
| Serum selenium (ug/L) | 132.18 ± 18.11 | 132.47 ± 19.08 | 130.27 ± 19.35 | 130.67 ± 18.41 | 0.603 |
| Serum zinc (ug/dL) | 80.14 ± 17.20 | 81.16 ± 13.62 | 80.06 ± 14.93 | 82.95 ± 15.12 | 0.233 |
| Serum lipids (mg/dL) | |||||
| Total cholesterol | 192.24 ± 42.30 | 198.31 ± 41.74 | 198.82 ± 43.14 | 202.56 ± 39.68 | 0.182 |
| Triglyceride | 130.94 ± 96.70 | 116.51 ± 86.87 | 113.06 ± 61.34 | 108.25 ± 50.06 | 0.221 |
| HDL-cholesterol | 52.91 ± 15.83 | 54.84 ± 17.92 | 54.76 ± 13.51 | 59.02 ± 18.38 | 0.006 |
| LDL-cholesterol | 113.57 ± 37.05 | 116.74 ± 37.16 | 127.00 ± 44.29 | 121.83 ± 36.78 | 0.115 |
| Bone mineral density (g/cm2) | |||||
| Total femur | 0.95 ± 0.14 | 0.97 ± 0.16 | 0.93 ± 0.15 | 0.96 ± 0.15 | 0.123 |
| Femoral neck | 0.77 ± 0.13 | 0.80 ± 0.15 | 0.78 ± 0.14 | 0.81 ± 0.15 | 0.086 |
| Total spine | 1.00 ± 0.13 | 0.99 ± 0.17 | 1.01 ± 0.16 | 1.03 ± 0.15 | 0.288 |
| Sex | <0.001 | ||||
| Man | 111 (78.17%) | 116 (63.04%) | 85 (39.72%) | 29 (15.93%) | |
| Woman | 31 (21.83%) | 68 (36.96%) | 129 (60.28%) | 153 (84.07%) | |
| Race | <0.001 | ||||
| Mexican American | 20 (14.08%) | 21 (11.41%) | 28 (13.08%) | 30 (16.48%) | |
| Other Hispanic | 11 (7.75%) | 17 (9.24%) | 22 (10.28%) | 20 (10.99%) | |
| Non-Hispanic white | 68 (47.89%) | 87 (47.28%) | 91 (42.52%) | 71 (39.01%) | |
| Non-Hispanic black | 10 (7.04%) | 25 (13.59%) | 51 (23.83%) | 50 (27.47%) | |
| Other race | 33 (23.24%) | 34 (18.48%) | 22 (10.28%) | 11 (6.04%) | |
| Total fracture | 0.974 | ||||
| No | 104 (73.24%) | 136 (73.91%) | 161 (75.23%) | 134 (73.63%) | |
| Yes | 38 (26.76%) | 48 (26.09%) | 53 (24.77%) | 48 (26.37%) | |
| Cardiovascular diseases | 0.112 | ||||
| No | 138 (97.18%) | 171 (92.93%) | 196 (91.59%) | 174 (95.60%) | |
| Yes | 4 (2.82%) | 13 (7.07%) | 18 (8.41%) | 8 (4.40%) | |
| Dyslipidaemia | 0.257 | ||||
| No | 95 (66.90%) | 124 (67.39%) | 158 (73.83%) | 135 (74.18%) | |
| Yes | 47 (33.10%) | 60 (32.61%) | 56 (26.17%) | 47 (25.82%) | |
| Smoking | 0.693 | ||||
| Yes | 60 (42.25%) | 80 (43.48%) | 103 (48.13%) | 82 (45.05%) | |
| No | 82 (57.75%) | 104 (56.52%) | 111 (51.87%) | 100 (54.95%) | |
| Alcohol drinking | 0.025 | ||||
| Yes | 100 (77.52%) | 128 (78.05%) | 144 (72.73%) | 108 (64.67%) | |
| No | 29 (22.48%) | 36 (21.95%) | 54 (27.27%) | 59 (35.33%) | |
| Moderate physical activity | 0.451 | ||||
| Yes | 38 (26.95%) | 53 (28.96%) | 67 (31.31%) | 44 (24.18%) | |
| No | 103 (73.05%) | 130 (71.04%) | 147 (68.69%) | 138 (75.82%) | |
HDL = high-density lipoprotein; LDL = low-density lipoprotein; SD = standard deviation.
Values are mean ± SD or n (%).
Serum copper levels and bone mineral density
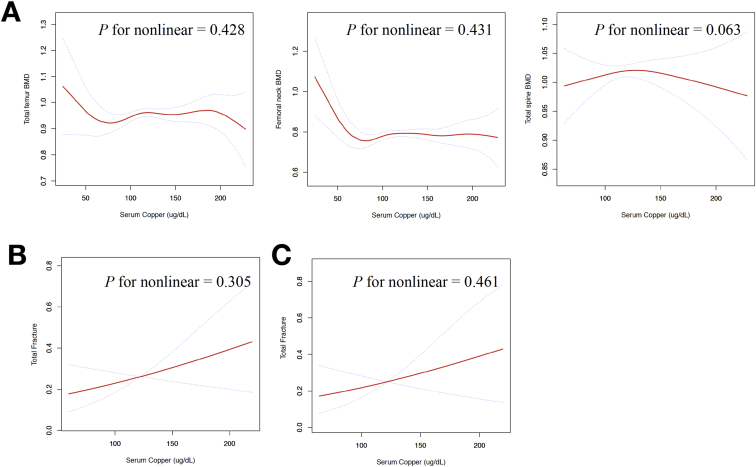
Table 3 and Figure. 2A present the association between serum copper levels and BMD using the logistic regression and two-piecewise linear regression models. In the multivariate logistic regression analysis after multivariate adjustment for age, sex, race, BMI, smoking status, alcohol consumption, moderate physical activity, cardiovascular disease, dyslipidaemia, TG, TC, HDL-C, LDL-C, serum selenium and serum zinc, individuals in the lowest category (<98.5 μg/dL) of serum copper concentration had 0.049 g/cm2 lower total femur BMD and 0.045 g/cm2 lower femoral neck BMD compared to those in the second concentration category (98.5–114 μg/dL). However, there were no significant linear associations between per 10 μg/dL increases in serum copper levels and all site BMD in multivariate logistic regression analysis after multivariate adjustment (all p > 0.05).
Table 3.
Associations between serum copper levels and BMDs.
| Unadjusted model |
Adjusted model |
|||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Total femur BMD (g/cm2) | ||||
| Serum copper (ug/dL) | ||||
| <98.5 | −0.019 (−0.053, 0.015) | 0.27460 | −0.049 (−0.091, −0.007) | 0.02264 |
| 98.5 to 114 | 0 (ref.) | 0 (ref.) | ||
| 114 to 34 | −0.037 (−0.068, −0.005) | 0.02143 | −0.039 (−0.080, 0.003) | 0.06773 |
| ≥134 | −0.010 (−0.043, 0.023) | 0.56254 | −0.028 (−0.074, 0.018) | 0.23504 |
| Per 10 ug/dL increased in serum copper | −0.000 (−0.005, 0.004) | 0.84804 | 0.003 (−0.003, 0.010) | 0.31590 |
| Femoral neck BMD (g/cm2) | ||||
| Serum copper (ug/dL) | ||||
| <98.5 | −0.024 (−0.057, 0.008) | 0.14456 | −0.056 (−0.097, −0.015) | 0.00783 |
| 98.5 to 114 | 0 (ref.) | 0 (ref.) | ||
| 114 to 34 | −0.021 (−0.050, 0.009) | 0.16653 | −0.045 (−0.085, −0.004) | 0.03041 |
| ≥134 | 0.011 (−0.020, 0.043) | 0.47146 | −0.041 (−0.086, 0.005) | 0.07972 |
| Per 10 ug/dL increased in serum copper | 0.003 (−0.002, 0.007) | 0.20775 | 0.001 (−0.005, 0.007) | 0.81142 |
| Total spine BMD (g/cm2) | ||||
| Serum copper (ug/dL) | ||||
| <98.5 | 0.004 (−0.039, 0.047) | 0.85799 | −0.028 (−0.091, 0.035) | 0.38230 |
| 98.5 to 114 | 0 (ref.) | 0 (ref.) | ||
| 114 to 34 | 0.019 (−0.019, 0.057) | 0.33073 | 0.011 (−0.054, 0.077) | 0.73234 |
| ≥134 | 0.036 (−0.003, 0.076) | 0.07440 | −0.018 (−0.089, 0.053) | 0.61736 |
| Per 10 ug/dL increased in serum copper | 0.004 (−0.002, 0.010) | 0.16410 | −0.002 (−0.011, 0.008) | 0.72430 |
BMD = bone mineral density; CI = confidence interval; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
β regression coefficient. Adjust for: age; sex; race; body mass index (kg/m2); smoking statues; alcohol drinking; moderate physical activity; cardiovascular diseases; dyslipidaemia; triglyceride (mg/dL), total cholesterol(mg/dL); HDL-cholesterol (mg/dL); LDL-cholesterol (mg/dL); serum selenium (ug/L); serum zinc (ug/dL).
Figure 2.
(A) Multivariate adjusted smoothing spline plots of serum copper levels and bone mineral density; (B and C) multivariate adjusted smoothing spline plots of serum copper levels and fractures. Model 1 (A and B) is adjusted for age, sex, race, BMI, smoking status, alcohol consumption, moderate physical activity, cardiovascular disease, dyslipidaemia; triglyceride (mg/dL), total cholesterol (mg/dL), HDL-cholesterol (mg/dL), LDL-cholesterol (mg/dL), serum selenium (μg/L) and serum zinc (μg/dL). Model 2 (C) is adjusted for model 1 plus total spine BMD and total femur BMD. The red line represents the line of best-fit, and the blue lines are 95% confidence intervals.
BMD = bone mineral density; HDL = high-density lipoprotein; LDL = low-density lipoprotein
Serum copper levels and total fractures
Table 4 and Figure. 2B and C present the association between serum copper levels and total fractures. In the multivariate logistic regression analysis, after being adjusted for age, sex, race, BMI, smoking status, alcohol consumption, moderate physical activity, cardiovascular disease, dyslipidaemia, TG, TC, HDL-C, LDL-C, serum selenium, serum zinc, total spine BMD and total femur BMD, individuals in the highest category (≥134 μg/dL) of serum copper concentration had an approximately 4-fold increase in risk of total fracture compared to those in the second concentration category (98.5–114 μg/dL). However, there were no significant linear associations between per 10 μg/dL increases in serum copper levels and total fracture in multivariate logistic regression analysis after multivariate adjustment (p = 0.303).
Table 4.
Associations between serum copper levels and total fracture.
| Unadjusted model |
Adjusted model |
Adjusted model plus BMDa |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Serum copper (ug/dL) | ||||||
| <98.5 | 1.035 (0.630, 1.701) | 0.89116 | 1.767 (0.686, 4.550) | 0.23805 | 2.128 (0.610, 7.430) | 0.23641 |
| 98.5 to 114 | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | |||
| 114 to 34 | 0.933 (0.593, 1.466) | 0.76279 | 2.554 (1.061, 6.147) | 0.03645 | 3.555 (1.055, 11.975) | 0.04071 |
| ≥134 | 1.015 (0.637, 1.617) | 0.95029 | 3.358 (1.280, 8.810) | 0.01381 | 4.225 (1.148, 15.558) | 0.03023 |
| Per 10 ug/dL increased in serum copper | 0.977 (0.914, 1.043) | 0.48292 | 1.081 (0.956, 1.223) | 0.21360 | 1.087 (0.927, 1.274) | 0.30336 |
BMD = bone mineral density; CI = confidence interval; HDL = high-density lipoprotein; LDL = low-density lipoprotein; OR = odds ratio.
Adjust for: age; sex; race; body mass index (kg/m2); smoking statues; alcohol drinking; moderate physical activity; cardiovascular diseases; dyslipidaemia; triglyceride (mg/dL), total cholesterol(mg/dL); HDL-cholesterol (mg/dL); LDL-cholesterol (mg/dL); serum selenium (ug/L); serum zinc (ug/dL).
Adjusted model plus total spine BMD; total femur BMD.
Stratified analysis of the association between serum copper levels and BMD and total fractures for gender and age
We examined whether gender and age affected the association between serum copper levels, BMD and total fractures. As shown in Table 5, we observed a differential association between serum copper levels and total fractures between men and women after being adjusted for age, race, BMI, smoking status, alcohol consumption, moderate physical activity, cardiovascular disease, dyslipidaemia, TG, TC, HDL-C, LDL-C, serum selenium, serum zinc, total spine BMD and total femur BMD (odds ratio = 1.81, 95% CI 1.08, 3.03, p = 0.026 for men and odds ratio = 1.07, 95% CI 0.86, 1.32, p = 0.552 for women). Moreover, a significant interactive effect was detected (P for interaction = 0.045).
Table 5.
Effect of sex on the associations between serum copper levels (Per 10 ug/dL increased in serum copper) and BMDs and fracture.
| Men |
Women |
P for interaction | |||
|---|---|---|---|---|---|
| β/OR (95% CI) | p | β/OR (95% CI) | p | ||
| Total femur BMD (g/cm2) | |||||
| Unadjusted model | 0.005 (−0.003, 0.013) | 0.2033 | 0.012 (0.005, 0.018) | 0.0005 | 0.1976 |
| Adjusted model | 0.000 (−0.011, 0.011) | 0.9837 | 0.003 (−0.004, 0.010) | 0.3560 | 0.5962 |
| Femoral neck BMD (g/cm2) | |||||
| Unadjusted model | 0.005 (−0.003, 0.012) | 0.2164 | 0.011 (0.005, 0.018) | 0.0006 | 0.2009 |
| Adjusted model | −0.005 (−0.016, 0.006) | 0.3498 | 0.002 (−0.005, 0.009) | 0.4913 | 0.2021 |
| Total spine BMD (g/cm2) | |||||
| Unadjusted model | 0.011 (−0.000, 0.022) | 0.0571 | 0.008 (−0.000, 0.015) | 0.0630 | 0.6267 |
| Adjusted model | 0.006 (−0.013, 0.025) | 0.5360 | −0.004 (−0.014, 0.007) | 0.4664 | 0.3087 |
| Total fracture | |||||
| Unadjusted model | 0.965 (0.852, 1.092) | 0.5709 | 0.982 (0.897, 1.076) | 0.7045 | 0.8163 |
| Adjusted model | 1.308 (1.025, 1.670) | 0.0312 | 1.016 (0.857, 1.206) | 0.8534 | 0.1592 |
| Adjusted model plus BMDa | 1.805 (1.075, 3.029) | 0.0255 | 1.066 (0.863, 1.317) | 0.5521 | 0.0452 |
BMD = bone mineral density; CI = confidence interval; HDL = high-density lipoprotein; LDL = low-density lipoprotein; OR = odds ratio.
β regression coefficient. Adjust for: age; race; body mass index (kg/m2); smoking status; alcohol drinking; moderate physical activity; cardiovascular diseases; dyslipidaemia; triglyceride (mg/dL), total cholesterol(mg/dL); HDL-cholesterol (mg/dL); LDL-cholesterol (mg/dL); serum selenium (ug/L); serum zinc (ug/dL).
Adjusted model plus total spine BMD; total femur BMD.
As shown in Table 6, there was no differential association between serum copper levels and total femur, femoral neck and total spine BMD among groups aged <50y, 50–65y and >65y after multivariate adjustment. Moreover, there were no significant interactive effects of age on the associations between serum copper levels and BMDs. (all P for interaction >0.05) However, a significant interactive effect of age on the associations between serum copper levels and total fracture was detected (P for interaction = 0.0005).
Table 6.
Effect of age on the associations between serum copper levels (Per 10 ug/dL increased in serum copper) and BMDs and fracture.
| <50 y |
50 to 65 y |
≥65 y |
P for interaction | ||||
|---|---|---|---|---|---|---|---|
| β/OR (95% CI) | p | β/OR (95% CI) | p | β/OR (95% CI) | p | ||
| Total femur BMD (g/cm2) | |||||||
| Unadjusted model | 0.005 (−0.002, 0.012) | 0.1550 | −0.001 (−0.007, 0.006) | 0.8528 | −0.010 (−0.022, 0.001) | 0.0875 | 0.0584 |
| Adjusted model | 0.005 (−0.005, 0.016) | 0.3141 | 0.002 (−0.007, 0.011) | 0.7039 | −0.011 (−0.027, 0.005) | 0.1944 | 0.1139 |
| Femoral neck BMD (g/cm2) | |||||||
| Unadjusted model | 0.008 (0.002, 0.015) | 0.0149 | 0.002 (−0.004, 0.009) | 0.4695 | −0.007 (−0.017, 0.004) | 0.2182 | 0.0426 |
| Adjusted model | −0.001 (−0.012, 0.010) | 0.8804 | −0.001 (−0.010, 0.008) | 0.7660 | −0.013 (−0.029, 0.002) | 0.0997 | 0.2206 |
| Total spine BMD (g/cm2) | |||||||
| Unadjusted model | 0.008 (0.002, 0.015) | 0.0171 | 0.002 (−0.007, 0.011) | 0.6848 | −0.008 (−0.027, 0.011) | 0.4046 | 0.1264 |
| Adjusted model | 0.001 (−0.012, 0.014) | 0.8410 | −0.003 (−0.018, 0.012) | 0.7299 | −0.021 (−0.060, 0.017) | 0.2966 | 0.2312 |
| Total fracture | |||||||
| Unadjusted model | 0.970 (0.865, 1.089) | 0.6107 | 0.890 (0.801, 0.989) | 0.0300 | 1.168 (1.015, 1.344) | 0.0298 | 0.0085 |
| Adjusted model | 1.012 (0.767, 1.334) | 0.9343 | 0.855 (0.679, 1.078) | 0.1851 | 1.35a (0.996, 1.838) | 0.0527 | 0.0005 |
| Adjusted model plus BMDa | 0.989 (0.725, 1.347) | 0.9420 | 0.881 (0.604, 1.284) | 0.5097 | NA | NA | NA |
BMD = bone mineral density; CI = confidence interval; HDL = high-density lipoprotein; LDL = low-density lipoprotein; OR = odds ratio.
β regression coefficient. Adjust for: sex; race; body mass index (kg/m2); smoking status; alcohol drinking; moderate physical activity; cardiovascular diseases; dyslipidaemia; triglyceride (mg/dL), total cholesterol(mg/dL); HDL-cholesterol (mg/dL); LDL-cholesterol (mg/dL); serum selenium (ug/L); serum zinc (ug/dL).
Adjusted model plus total spine BMD; total femur BMD
Discussion
In recent years, biodegradable copper-containing biomaterials have been researched and explored in the field of medical implants. Because of increased opportunities for exogenous copper uptake, people are more concerned about the health effects of exposure to copper. Therefore, investigating the relationship between serum copper levels and bone health can lay a foundation for future design, application, contraindications and postimplantation strategies for copper-containing biomaterials, most notably for orthopaedic biomaterials [23]. This study analysed the data of 722 participants from the NHANES (2011–2014) and found that individuals in the lowest category (<98.5 μg/dL) of serum copper concentration had 0.049 g/cm2 lower total femur BMD and 0.045 g/cm2 lower femoral neck BMD compared to those in the second concentration category (98.5–114 μg/dL). Individuals in the highest category (≥134 μg/dL) of serum copper concentration had an approximately 4-fold increase in risk of total fracture compared to those in the second concentration category. Moreover, there was a significant linear association between per 10 μg/dL increases in serum copper levels and total fracture in men.
Many studies have shown that copper ions are necessary for the health of bone tissue [24], [25]. Our study found that low concentrations of serum copper lead to lower BMD, whereas higher serum copper levels are associated with an increased risk of fracture. Under physiological conditions, copper ions could induce a hypoxic microenvironment such as hypoxia mimicking ions and an increase in BMD by promoting the expression of bone-related genes (ALP, OPN, OPN). This may inhibit active bone resorption and can enhance angiogenesis by promoting the expression of vascular endothelial growth factor [26], [27], [28]. In addition, the activity of lysyl oxidase, which is necessary for covalent cross-linking of collagen, is dependent on copper ions. Collagen is the main component of bone tissue extracellular matrix, and the integrity of bone matrix is very important to the strength and plastic deformation of bone, meaning copper can affect the formation of extracellular matrix of bone tissue [29], [30], [31]. Copper can also promote both osteogenesis and adipogenic differentiation of bone mesenchymal stem cells (BMSC), but gives priority to the differentiation of the osteogenic lineage [30]. Therefore, the lack of copper ions will harm the bone tissue, lead to the enhancement of bone resorption and the disorder of lysyl oxidase [32], decrease cancellous bone, weaken bone formation and mineralisation, impair cartilage integrity and eventually reduce bone density. In animal experiments, copper deficiency has been found to lead to bone deformities, hypoplasia, fragile bones and frequent fractures. Less loss of axial and peripheral bone mass has been found in ovariectomy-induced reduction of bone mass in rats supplemented with copper, and it is suggested that copper has therapeutic potential in osteoporosis [33]. A decrease in bone mass due to copper deficiency has also been observed in goats [34]. In epidemiological studies, serum copper levels in patients with osteoporosis, lumbar osteopenia and femoral neck fracture were lower than those in the normal group, and low serum copper levels were also considered as a risk factor for age-related osteoporosis. Some even suggested that copper could be used as a simple radiation-free screening method for osteoporosis. In terms of diet, a low-copper diet is thought to be associated with osteoporosis and ischaemic heart disease, and adequate dietary copper intake is important for maintaining healthy bone and cartilage [35], [36], [37].
On the other hand, the harm caused by excessive copper levels has also been studied. For example, copper has direct toxicity to cartilage and bone in chicken embryo bone tissue [38], and an abundance of serum copper has been found to cause a decrease in bone size and bone density in C57 mice [39]. It was also found that copper could inhibit in vitro osteogenic differentiation of transcription factors such as Runx2 by inducing a hypoxic microenvironment. This thereby regulates the differentiation of rat BMSC, inhibiting osteogenic differentiation, promoting adipogenic differentiation and inhibiting cytoskeletal changes [40]. The apoptosis of BMSC was induced with an increase in copper concentration [41]. Excessive copper also produces large amounts of free radicals that cause lipid peroxidation and interfere with bone metabolism, leading to a decrease in bone cortex and bone strength. Oxidation can also, in itself, promote ageing and reduce bone strength [42], [43], [44], [45]. In Wilson's disease, characterised by copper deposition, epidemiological studies show that the BMD of cortical and cancellous bone are normal, whereas the risk of fracture is increased. As BMD does not evaluate bone microstructure, there may be a decrease in bone strength after excluding factors such as mental state, low BMI and amenorrhea. In addition, excessive copper and decreased bone strength are also associated with other disease manifestations such as rickets and abnormal osteophytes [43], [46]. These findings are also consistent with the increase in fractures we observed at high serum copper concentrations.
Only few observational studies with mixed findings have investigated the associations between serum copper levels and BMD [13], [14], [15], [16], [17], [18]. However, most of these studies were based on patients in hospital, which suggests that their findings might be biased towards unhealthy individuals and therefore cannot be extrapolated to the general population. Additionally, no previous studies have validated the relationship between copper status and fracture. Our research, using a large nationally representative sample of adults in the United States, is the largest cross-sectional study to evaluate the associations between serum copper levels and BMD and fracture.
There were several limitations in this study. First, because of the cross-sectional nature of the study, causal relationships between serum copper levels, BMD and fracture cannot be detected. Second, as an inherent characteristic of cross-sectional studies, some biases are inherent although we tried to minimise these biases using a variety of procedures. Third, risk of BMD and fractures are associated with many factors, such as lifestyle, disease status, metabolic factors and genetic factors. Several fracture risk prediction tools have been developed using different risk factors. Age, BMI, rheumatoid arthritis, smoking status and alcohol status are the most common risk factors. Although wide variables were included in the adjustment, we could not exclude the possibility of residual confounding variables in the analyses, such as serum calcium, phosphorus and vitamin D. Therefore, further stratified cohort studies that include sufficient controls and account for confounding factors are, therefore, needed to elucidate the link between serum copper level and the risk of BMD and fracture.
In conclusion, our study demonstrates that lower serum copper levels are significantly associated with decreased BMD in the total femur and femoral neck. Higher serum copper levels are significantly associated with increased total fracture, especially in men. Moderate serum copper levels are vital for bone health. Prospective cohort studies are still warranted to confirm these findings.
Authors' contributions
XHQ, ZFY and KRD were responsible for the initial plan, study design, data collection, data interpretation, manuscript drafting, statistical analysis and conducting the study. ZHH was responsible for statistical analysis and critical revisions of the manuscript. HQ, ZJJ and ZYM were responsible for critical revisions of the manuscript. This article's contents are solely the responsibility of the authors. ZFY and KRD are the guarantors for this article and have full responsibility for this study. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements/Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 51631009, 11572197 and 81401852), “Chen Guang” Project of the Shanghai Municipal Education Commission, Shanghai Education Development Foundation (No. 14CG14).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jot.2018.05.001.
Contributor Information
Zhifeng Yu, Email: zfyu@outlook.com.
Kerong Dai, Email: krdai@163.com.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Bost M., Houdart S., Oberli M., Kalonji E., Huneau J.F., Margaritis I. Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Biol. 2016;35:107–115. doi: 10.1016/j.jtemb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Klevay L.M. IHD from copper deficiency: a unified theory. Nutr Res Rev. 2016;29(2):172–179. doi: 10.1017/S0954422416000093. [DOI] [PubMed] [Google Scholar]
- 3.Esfahani S.T., Hamidian M.R., Madani A., Ataei N., Mohseni P., Roudbari M. Serum zinc and copper levels in children with chronic renal failure. Pediatr Nephrol. 2006;21(8):1153–1156. doi: 10.1007/s00467-006-0119-1. [DOI] [PubMed] [Google Scholar]
- 4.Skoczynska A., Gruszczynski L., Wojakowska A., Scieszka M., Turczyn B., Schmidt E. Association between the type of workplace and lung function in copper miners. Biomed Res Int. 2016;2016:5928572. doi: 10.1155/2016/5928572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Z., Niu J., Huang H., Zhang H., Pei J., Ou J. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J Mech Behav Biomed Mater. 2017;72:182–191. doi: 10.1016/j.jmbbm.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Li Y., Liu L., Wan P., Zhai Z., Mao Z., Ouyang Z. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: in vitro and in vivo evaluations. Biomaterials. 2016;106:250–263. doi: 10.1016/j.biomaterials.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Chen X. Magnesium-based implants: beyond fixators. J Orthop Translat. 2017;10:1–4. doi: 10.1016/j.jot.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W., Sun Y., Cai M., Zhao Y., Cao W., Liu Z. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat Commun. 2018;9(1):231. doi: 10.1038/s41467-017-02657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zang X., Huang H., Zhuang Z., Chen R., Xie Z., Xu C. The association between serum copper concentrations and cardiovascular disease risk factors in children and adolescents in NHANES. Environ Sci Pollut Res Int. 2018 Apr 6 doi: 10.1007/s11356-018-1816-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Chen A., Li G., Liu Y. Association between copper levels and myocardial infarction: a meta-analysis. Inhal Toxicol. 2015;27(5):237–246. doi: 10.3109/08958378.2015.1030480. [DOI] [PubMed] [Google Scholar]
- 11.Pal A., Kumar A., Prasad R. Predictive association of copper metabolism proteins with Alzheimer's disease and Parkinson's disease: a preliminary perspective. Biometals An Int J Role Metal Ions Biol Biochem Med. 2014;27(1):25–31. doi: 10.1007/s10534-013-9702-7. [DOI] [PubMed] [Google Scholar]
- 12.Arnal N., Morel G.R., de Alaniz M.J., Castillo O., Marra C.A. Role of copper and cholesterol association in the neurodegenerative process. Int J Alzheimers Dis. 2013;2013:414817. doi: 10.1155/2013/414817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahdavi-Roshan M., Ebrahimi M., Ebrahimi A. Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin Cases Miner Bone Metab. 2015;12(1):18–21. doi: 10.11138/ccmbm/2015.12.1.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razmandeh R., Nasli-Esfahani E., Heydarpour R., Faridbod F., Ganjali M.R., Norouzi P. Association of Zinc, Copper and Magnesium with bone mineral density in Iranian postmenopausal women - a case control study. J Diabetes Metab Disord. 2014;13(1):43. doi: 10.1186/2251-6581-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen F.H., Lukaski H.C., Johnson L.K., Roughead Z.K. Reported zinc, but not copper, intakes influence whole-body bone density, mineral content and T score responses to zinc and copper supplementation in healthy postmenopausal women. Br J Nutr. 2011;106(12):1872–1879. doi: 10.1017/S0007114511002352. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhri M.A., Kemmler W., Harsch I., Watling R.J. Plasma copper and bone mineral density in osteopenia: an indicator of bone mineral density in osteopenic females. Biol Trace Elem Res. 2009;129(1–3):94–98. doi: 10.1007/s12011-008-8299-0. [DOI] [PubMed] [Google Scholar]
- 17.Mutlu M., Argun M., Kilic E., Saraymen R., Yazar S. Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J Int Med Res. 2007;35(5):692–695. doi: 10.1177/147323000703500514. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi N., Oveisi M.R., Jannat B., Hajimahmoodi M., Behzad M., Behfar A. The relationship between bone health and plasma zinc, copper lead and cadmium concentration in osteoporotic women. J Environ Health Sci Eng. 2014;12(1):125. doi: 10.1186/s40201-014-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C., Woo J.G., Zhang N. Association between urinary manganese and blood pressure: results from national health and nutrition examination survey (NHANES), 2011–2014. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188145. e0188145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostchega Y., Zhang G., Kit B.K., Nwankwo T. Factors associated with home blood pressure monitoring among US adults: national health and nutrition examination survey, 2011–2014. Am J Hypertens. 2017;30(11):1126–1132. doi: 10.1093/ajh/hpx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu E.W., Chastain H.M., Shin S.H., Wiegand R.E., Kruszon-Moran D., Handali S. Seroprevalence of antibodies to Toxocara species in the United States and associated risk factors, 2011–2014. Clin Infect Dis. 2018;66(2):206–212. doi: 10.1093/cid/cix784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelman S.V., Polonsky W.H. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425–1432. doi: 10.2337/dc16-1974. [DOI] [PubMed] [Google Scholar]
- 23.Chen C.H., O'Keefe R. Clinical translation and application in orthopaedics. J Orthop Translat. 2018;12:A3–A4. doi: 10.1016/j.jot.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rondanelli M., Opizzi A., Perna S., Faliva M.A. Update on nutrients involved in maintaining healthy bone. Endocrinología y Nutrición. 2013;60(4):197–210. doi: 10.1016/j.endonu.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Aaseth J., Boivin G., Andersen O. Osteoporosis and trace elements – an overview. J Trace Elem Med Biol. 2012;26(2):149–152. doi: 10.1016/j.jtemb.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Mir E., Hosseinnezhad A., Bahrami A., Bekheirnia M.R. Adequate serum copper concentration could improve bone density, postpone bone loss and protect osteoporosis in women. Iran J Public Health. 2007;36(2):24–29. [Google Scholar]
- 27.Bari A., Bloise N., Fiorilli S., Novajra G., Vallet-Regí M., Bruni G. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomaterialia. 2017;55:493–504. doi: 10.1016/j.actbio.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Wilson T., Katz J.M., Gray D.H. Inhibition of active bone resorption by copper. Calcif Tissue Int. 1981;33(1):35–39. doi: 10.1007/BF02409410. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger S. Nutritional pathophysiology of obesity and its comorbidities. Academic Press; 2017. Chapter 9-osteoporosis and fracture risk; pp. 209–234. [Google Scholar]
- 30.Rodríguez J.P., Ríos S., González M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J Cell Biochem. 2002;85(1):92–100. [PubMed] [Google Scholar]
- 31.Opsahl W., Zeronian H., Ellison M., Lewis D., Rucker R.B., Riggins R.S. Role of copper in collagen cross-linking and its influence on selected mechanical properties of chick bone and tendon. J Nutr. 1982;112(4):708–716. doi: 10.1093/jn/112.4.708. [DOI] [PubMed] [Google Scholar]
- 32.Sarazin M., Alexandre C., Thomas T. Influence of trace element, protein, lipid, carbohydrate, and vitamin intakes on bone metabolism. Revue du Rhumatisme (Edition Francaise) 2000;67(7):486–497. [Google Scholar]
- 33.Rico H., Roca-Botran C., Hernandez E.R., Seco C., Paez E., Valencia M.J. The effect of supplemental copper on osteopenia induced by ovariectomy in rats. Menopause (New York, NY) 2000;7(6):413–416. doi: 10.1097/00042192-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Buck B.C., Ulrich R., Taube V., Jacobsen B., Ganter M. Osteopenia as a result of copper deficiency in a dwarf Thuringian forest goat. Tierarztliche Praxis Ausgabe G, Grosstiere/Nutztiere. 2012;40(1):45–52. [PubMed] [Google Scholar]
- 35.Klevay L.M. Lack of a recommended dietary allowance for copper may be hazardous to your health. J Am Coll Nutr. 1998;17(4):322–326. doi: 10.1080/07315724.1998.10718769. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhri M.A., Kemmler W., Harsch I., Watling R.J. Plasma copper and bone mineral density in osteopenia: an indicator of bone mineral density in osteopenic females. Biol Trace Elem Res. 2009;129(1):94–98. doi: 10.1007/s12011-008-8299-0. [DOI] [PubMed] [Google Scholar]
- 37.Zheng J., Mao X., Ling J., He Q., Quan J. Low serum levels of zinc, copper, and iron as risk factors for osteoporosis: a meta-analysis. Biol Trace Elem Res. 2014;160(1):15–23. doi: 10.1007/s12011-014-0031-7. [DOI] [PubMed] [Google Scholar]
- 38.Rest J.R. The histological effects of copper and zinc on chick embryo skeletal tissues in organ culture. Br J Nutr. 1976;36(2):243–254. doi: 10.1079/bjn19760076. [DOI] [PubMed] [Google Scholar]
- 39.Massie H.R., Aiello V.R., Shumway M.E., Armstrong T. Calcium, iron, copper, boron, collagen, and density changes in bone with aging in C57BL/6J male mice. Exp Gerontol. 1990;25(5):469–481. doi: 10.1016/0531-5565(90)90035-z. [DOI] [PubMed] [Google Scholar]
- 40.Li S., Wang M., Chen X., Li S.F., Li-Ling J., Xie H.Q. Inhibition of osteogenic differentiation of mesenchymal stem cells by copper supplementation. Cell Prolif. 2014;47(1):81–90. doi: 10.1111/cpr.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burghardt I., Lüthen F., Prinz C., Kreikemeyer B., Zietz C., Neumann H.-G. A dual function of copper in designing regenerative implants. Biomaterials. 2015;44:36–44. doi: 10.1016/j.biomaterials.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 42.Dermience M., Lognay G., Mathieu F., Goyens P. Effects of thirty elements on bone metabolism. J Trace Elem Med Biol Organ Soc Miner Trace Elem (GMS) 2015;32:86–106. doi: 10.1016/j.jtemb.2015.06.005. [eng] [DOI] [PubMed] [Google Scholar]
- 43.A A. Trace element nutrition and bone metabolism. Nutr Res Rev. 1992;5(1):167–188. doi: 10.1079/NRR19920013. [DOI] [PubMed] [Google Scholar]
- 44.Goodman S.B., Qin L. Inflammation and the musculoskeletal system. J Orthop Translat. 2017;10:A1–A2. doi: 10.1016/j.jot.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallo J., Raska M., Kriegova E., Goodman S.B. Inflammation and its resolution and the musculoskeletal system. J Orthop Translat. 2017;10:52–67. doi: 10.1016/j.jot.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quemeneur A.S., Trocello J.M., Ea H.K., Ostertag A., Leyendecker A., Duclos-Vallee J.C. Bone status and fractures in 85 adults with Wilson's disease. Osteoporosis Int. 2014;25(11):2573–2580. doi: 10.1007/s00198-014-2806-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.