Abstract
Background
Cardiac reserve is depressed in patients with heart failure and preserved ejection fraction (HFpEF). The mechanisms causing this are poorly understood.
Objectives
We hypothesized that myocardial injury might contribute to the hemodynamic derangements and cardiac reserve limitations that are present in HFpEF. Markers of cardiomyocyte injury, central hemodynamics, ventricular function, and determinants of cardiac oxygen supply-demand balance were measured.
Methods
Subjects with HFpEF (n = 38) and controls without heart failure (n = 20) underwent cardiac catheterization, echocardiography, and expired gas analysis at rest and during exercise. Central venous blood was sampled to measure plasma high sensitivity troponin T levels as an index of cardiomyocyte injury.
Results
Compared to controls, troponins were more than two-fold higher in subjects with HFpEF at rest and during exercise (p <0.0001). Troponin levels were directly correlated with LV filling pressures (r=0.52, p <0.0001) and diastolic dysfunction (r=−0.43, p=0.002). While myocardial oxygen demand was similar, myocardial oxygen supply was depressed in HFpEF, particularly during exercise (coronary perfusion pressure time integral; 44±9 vs. 30±9 mmHg•sec•min−1•L•dL−1, p<0.0001), and reduced indices of supply were correlated with greater myocyte injury during exercise (r = −0.44, p = 0.0008). Elevation in troponin with exercise was directly correlated with an inability to augment LV diastolic (r=−0.4, p=0.02) and systolic reserve (r=−0.57, p=0.0003), greater increases in LV filling pressures (r=0.55, p <0.0001), blunted cardiac output response(r=−0.44, p=0.002), and more severely depressed aerobic capacity in HFpEF.
Conclusions
Limitations in left ventricular functional reserve and the hemodynamic derangements that develop secondary to these limitations during exercise in HFpEF are correlated with the severity of cardiac injury, assessed by plasma levels of troponin T. Further study is warranted to determine the mechanisms causing myocyte injury in HFpEF, the potential role of ischemia, and to identify and test novel interventions targeted to these mechanisms.
Trial registration
NCT01418248 https://clinicaltrials.gov/ct2/results?term=NCT01418248&Search=Search
Keywords: biomarkers, hemodynamics, exercise, heart failure, HFpEF, troponin T
Introduction
Half of patients with heart failure (HF) have a preserved ejection fraction (HFpEF) (1). While left ventricular (LV) function is often relatively preserved at rest in HFpEF, patients characteristically display limitations in LV diastolic and systolic reserve during exercise (2–13) This impaired ability to augment LV contractility and hasten relaxation is evidenced by blunted increases in systolic and diastolic mechanics during exercise when measured by echocardiography. Reserve limitations lead to increased LV filling pressure and impairment in cardiac output reserve, which promotes symptoms of dyspnea and is associated with increased mortality (5–7,14). While these hemodynamic and LV functional derangements are well-described in HFpEF, the underlying causes of myocardial reserve limitation are not well-defined.
Accumulating data suggest that abnormalities in the coronary microcirculation are important in HFpEF.(15–23) According to the microvascular hypothesis, impaired myocardial nitric oxide (NO) availability due to coronary endothelial dysfunction exerts deleterious effects on ventricular structure and function.(1,15,16) Coronary flow reserve is impaired in HFpEF(17–19) and coronary microvascular density is reduced.(20) When coupled with increases in LV filling pressure that classically develop during exercise in HFpEF, these structural and functional alterations could compromise myocardial blood flow, setting the stage for development of subendocardial ischemia and myocyte injury. However, no mechanistic study has directly evaluated the role of myocardial injury or ischemia in HFpEF, or evaluated whether the development of cardiomyocyte injury might relate to the hemodynamic perturbations or myocardial reserve limitations that develop during stress in this syndrome.
Accordingly, we performed a prospective study to examine the potential role of myocardial injury as a contributor to the pathophysiology of HFpEF. We hypothesized that levels of troponin T, a sensitive and specific biomarker reflective of cardiomyocyte injury, would be greater in HFpEF than controls at rest and during exercise, and that the extent of cardiac injury observed would correlate with pathological hemodynamic changes, reduced myocardial oxygen supply, and ventricular functional limitations observed during exercise in HFpEF.
Methods
Subjects referred to the Mayo Clinic catheterization laboratory for invasive exercise right heart catheterization were enrolled in this prospective study. Some data from this cohort have been previously published (7,24–27), but not as it relates to myocardial injury, the troponins, or their relationships with cardiac function or hemodynamics. Written informed consent was provided by all subjects prior to participation in study-related procedures. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written. The study was approved by the Mayo Clinic Institutional Review Board and the study was registered (NCT01418248).
Study Population
The presence or absence of HFpEF was determined using invasive cardiopulmonary exercise testing in all participants. HFpEF was defined by typical clinical symptoms (dyspnea, fatigue), normal LVEF (≥50%), and elevated left heart filling pressures (pulmonary capillary wedge pressure, PCWP) at rest (>15mmHg) and/or with exercise (≥25mmHg).(7,24–26) Exclusion criteria included clinically significant coronary disease requiring revascularization, significant left-sided valvular disease (>mild stenosis, >moderate regurgitation), infiltrative, restrictive or hypertrophic cardiomyopathy, constrictive pericarditis, pulmonary embolism, and right ventricular myopathies.
Control subjects (n=20) undergoing evaluation for exertional dyspnea according to the same invasive exercise evaluation were also enrolled. Control subjects were required to display no evidence of HF after thorough clinical evaluation, imaging and invasive assessment, including normal rest and exercise PCWP (criteria above) and normal pulmonary artery (PA) pressure at rest and with exercise.
After providing consent, subjects underwent clinical history, physical examination, and resting echocardiography to enable the echocardiographic sonographer to identify optimal windows when simultaneous imaging was performed during catheterization. All patients with history of angina or suspected coronary disease had been evaluated using noninvasive stress testing with or without coronary angiography depending upon stress test results to exclude epicardial coronary artery disease as a cause of symptoms. The presence of coronary disease was defined by: (1) angiographic stenosis of 50–70% severity in ≥1 epicardial coronary artery without objective evidence of myocardial ischemia on stress testing, (2) any prior myocardial infarction, or (3) any history of revascularization.
Hemodynamic Assessment and Exercise Protocol
Right heart catheterization was performed in the supine position with simultaneous expired gas analysis, echocardiography and blood sampling at rest and during cycle ergometry exercise as previously described.(5,7,24–26) Patients were studied on their chronic medications in the fasted state after minimal sedation. Right heart catheterization was performed through a 9 Fr sheath via the right internal jugular vein. Right atrial (RA) pressure, PA pressures, and PCWP were measured at end expiration (mean of ≥3 beats) using 2 Fr high fidelity micromanometer-tipped catheters (Millar Instruments, Houston, TX) advanced through the lumen of a 7 Fr fluid-filled catheter (Balloon wedge, Arrow). Pressure tracings were digitized (240 Hz) and stored for offline analysis.
Oxygen consumption (VO2) was measured using expired gas analysis at rest and throughout exercise (MedGraphics, St. Paul, MN). Arterial and mixed venous (PA) O2 contents were determined from blood samples obtained at each stage (=hemoglobin*saturation*1.34). Arterial-venous O2 content difference (AVO2diff) was then calculated from the difference in O2 content in these samples. Cardiac output (QC) was determined by the direct Fick method (= VO2/AVO2diff). Following baseline assessments, all hemodynamic measurements were repeated during the first stage of exercise (20 Watts, W) followed by graded 10W increments in workload (3 minute stages) to subject-reported exhaustion.
Assessment of Myocardial O2 Supply and Demand
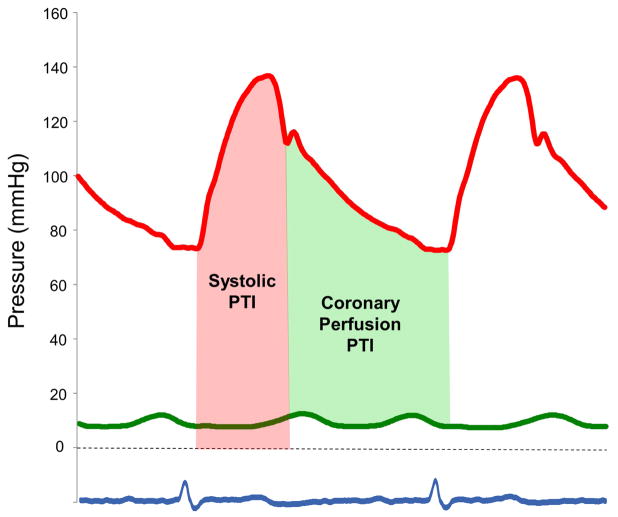
Central aortic pressure waveforms at rest and during exercise were determined from digitized, invasively-measured radial artery pressure tracings using the SphygmoCor software (AtCor, New South Wales, Australia), as previously reported (27). The area under the curve of the ensemble averaged aortic pressure waveform was measured to obtain the systolic pressure time integral (PTI) and diastolic PTI (Figure 1) (28). The systolic PTI (tension time index per beat*heart rate) was taken as an estimate of myocardial O2 demand as described by Sarnoff and colleagues.(29) To adjust for differences in LV wall stress, systolic PTI was also multiplied by LV mass index to derive a corrected systolic PTI (systolic PTIC).(29,30) The rate-pressure product (RPP), another surrogate of myocardial O2 demand, was determined from the product of systolic blood pressure and heart rate (31).
Figure 1. Estimates of Myocardial Oxygen Supply and Demand.
The area under the curve of the aortic pressure waveform was measured to obtain the systolic pressure time integral (PTI, pink area) and diastolic PTI. The systolic PTI provides an estimate of myocardial oxygen demand. Myocardial oxygen supply was assessed by the coronary perfusion PTI (light green area), calculated as the difference between the aortic diastolic PTI and simultaneously-measured pulmonary capillary wedge pressure (PCWP) waveforms (green line).
Myocardial O2 supply was assessed by the coronary perfusion PTI, calculated as the difference between the aortic diastolic PTI and simultaneously-measured PCWP waveforms, as described by Buckberg et al.(28) To adjust for differences in myocardial O2 delivery related to anemia, the raw coronary perfusion PTI was multiplied by arterial O2 content to derive the corrected coronary perfusion PTI (coronary PTIC), as recently suggested.(30) The balance of myocardial O2 supply and demand was assessed by the ratio of coronary perfusion PTIC to systolic PTIC (28,30).
Ventricular Function Assessment
Two-dimensional, Doppler, and tissue Doppler echocardiography was performed according to contemporary guidelines by experienced sonographers as previously described (7,24–26). Echocardiographic data were obtained simultaneously with invasive assessment at rest and during all stages of exercise. Studies were interpreted offline in a blinded fashion. Left ventricular systolic and diastolic function was assessed using mitral annular tissue velocities using the average of values measured at the lateral and septal annulus (32).
Myocardial Injury Assessment
Blood samples were obtained directly from the superior vena cava after 5 minutes of quiet rest, again after 4 minutes of exercise at 20W, and then at peak exercise. High sensitivity cardiac troponin T levels were measured using an electrochemiluminescent immunoassay by Roche Diagnostics (Indianapolis, IN). The interassay coefficient of variation (CV) is 4.2% at 29.1 ng/L and 2.5% at 2171 ng/L. Troponin T levels >14.0 ng/L were defined as abnormal (33).
Plasma NT-proBNP levels were measured at rest and exercise from the same samples, and growth differentiation factor-15 (GDF-15) was also assessed at rest as another biomarker reflective of myocardial injury/stress.(34) Plasma levels of NT-proBNP (CV of 2.6% at 137.0 pg/mL and 2.0% at 4805 pg/mL) and GDF-15 (CV of 2.7% at 1368 pg/mL and 2.2% at 7044 pg/mL) were determined by Electrochemiluminescent immunoassays by Roche Diagnostics.
Statistical Analysis
Data are reported as mean (SD), median (IQR) or number (%) unless otherwise specified. Between-group differences were compared using an unpaired t test, Wilcoxon rank-sum test, or Fisher exact test, as appropriate. Within group differences were assessed by one-way repeated measures ANOVA. Correlations were assessed using Pearson’s test, where non-normally distributed variables were log transformed. Multivariable regression analysis was used to adjust for age, body mass index (BMI), renal function, and the presence of coronary artery disease.
Results
Compared to controls, subjects with HFpEF were older, had higher BMI and were more likely to be treated with β-blockers (Table 1). There were no significant differences in sex or comorbidities, or other medication use. Subjects with HFpEF displayed lower hemoglobin and higher creatinine, NT-proBNP and GDF-15 levels than controls. While left ventricular size, mass and EF were similar in HFpEF and controls, HFpEF subjects displayed more diastolic dysfunction evidenced by higher transmitral E velocity and E/e′ ratio and larger left atrial volumes (Table 1). None of the subjects developed evidence of regional ischemia by electrocardiography or focal wall motion abnormalities during the examination.
Table 1.
Baseline Characteristics
| Control (n=20) | HFpEF (n=38) | P value | |
|---|---|---|---|
| Age (years) | 62±11 | 69±11 | 0.01 |
| Female sex (%) | 50 | 50 | 1.0 |
| Body mass index (kg/m2) | 27.5±4.8 | 34.1±6.8 | 0.0003 |
|
| |||
| Comorbidities | |||
| Hypertension (%) | 90 | 92 | 1.0 |
| Coronary disease* (%) | 20 | 39 | 0.4 |
| Diabetes (%) | 20 | 39 | 0.4 |
| Atrial fibrillation (%) | 10 | 18 | 0.5 |
|
| |||
| Medications | |||
| ACEI or ARB (%) | 50 | 71 | 0.2 |
| β-blocker (%) | 40 | 74 | 0.02 |
| Loop diuretic (%) | 15 | 39 | 0.1 |
| Nitrate (%) | 25 | 32 | 0.6 |
|
| |||
| Laboratories | |||
| Hemoglobin (g/dL) | 13.9±1.3 | 12.5±1.4 | 0.0003 |
| Creatinine | 1.0 (0.8, 1.1) | 1.15 (0.9, 1.5) | 0.02 |
| GDF-15 (pg/mL) | 680 (613, 954) | 1387 (999,2119) | <0.0001 |
|
| |||
| Echocardiography | |||
| LV end-diastolic dimension (mm) | 48±4 | 48±5 | 0.7 |
| LV mass index (g/m2) | 88±20 | 86±22 | 0.8 |
| LV ejection fraction | 62±8 | 61±8 | 0.9 |
| Mitral E (cm/sec) | 66±18 | 92±25 | <0.0001 |
| E/A ratio | 1.0±0.6 | 1.3±0.7 | 0.3 |
| E/e′ ratio | 8±2 | 14±6 | <0.0001 |
| LA volume index (ml/m2) | 29 (20, 41) | 39 (33, 57) | 0.01 |
The presence or absence of coronary disease was determined by angiography in 15 controls (75%) and 26 HFpEF patients (68%), by stress imaging in 6 HFpEF patients and 2 controls, and by clinical history alone in 6 HFpEF patients and 3 controls.
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; E/A, ratio of early to late diastolic transmitral flow velocity; E/e′, ratio of early diastolic mitral inflow velocity to early diastolic mitral annular tissue velocity; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; and NT-proBNP, N-terminal pro B-type natriuretic peptide.
Myocardial Oxygen Supply, Demand, and Myocyte Injury at Rest
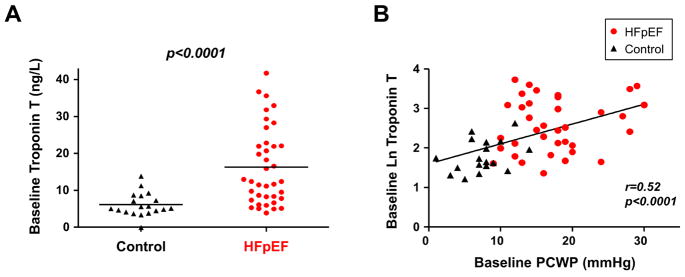
Compared to controls, HFpEF subjects displayed higher biventricular filling pressures, lower arterial O2 content, and higher PA pressures at rest, while blood pressure, heart rate, and QC were similar (Table 2). Troponin T levels were significantly elevated in subjects with HFpEF compared to controls at rest (Figure 2, Table 2). These differences persisted after adjusting for age, BMI, renal function, and history of coronary artery disease (p<0.05). Troponin T levels were directly correlated with invasively measured PCWP at rest (Figure 2) as well as NT-proBNP (r=0.58, p<0.0001) and GDF-15 (r=0.67, p<0.0001).
Table 2.
Hemodynamics, Myocardial O2 Supply and Demand, Function, and Troponins at Rest and during Exercise
| Rest | 20W | Peak | ||||
|---|---|---|---|---|---|---|
| Control | HFpEF | Control | HFpEF | Control | HFpEF | |
| Hemodynamics | ||||||
| Heart rate (min−1) | 69±13 | 66±11 | 92±14 | 88±16 | 120±16 | 98±16‡ |
| Systolic blood pressure (mmHg) | 142±24 | 150±22 | 173±26 | 175±29 | 187±26 | 185±34 |
| Right atrial pressure (mmHg) | 4±2 | 11±4‡ | 9±3 | 21±5‡ | 8±4 | 23±6‡ |
| PA systolic pressure (mmHg) | 28±6 | 43±12‡ | 38±10 | 68±12‡ | 42±9 | 72±12‡ |
| PA mean pressure (mmHg) | 17±4 | 28±8‡ | 25±6 | 49±11‡ | 27±7 | 50±8‡ |
| PCWP (mmHg) | 8±3 | 18±6‡ | 15±5 | 32±5‡ | 14±5 | 34±6‡ |
| Cardiac output (l/min) | 5.3±1.9 | 5.0±1.2 | 8.2±1.9 | 6.6±2.0† | 11.8±3.6 | 7.7±2.4‡ |
|
| ||||||
| Myocardial Oxygen Supply & Demand | ||||||
| Systolic PTI (mmHg•sec•min−1) | 2817±581 | 2845±547 | 3818±661 | 3697±904 | 4311±615 | 3972±1001 |
| Coronary PTI (mmHg•sec•min−1) | 3171±497 | 2762±537† | 2537±360 | 1984±396‡ | 2186±340 | 1800±330‡ |
| Coronary/Systolic PTI | 1.17±0.31 | 1.01±0.28* | 0.69±0.18 | 0.57±0.18* | 0.52±0.12 | 0.48±0.14 |
| Rate pressure product (mmHg•min−1) | 9888±2722 | 10015±2482 | 15955±3817 | 15742±4813 | 22614±4599 | 18304±5597† |
| Systolic PTIC (mmHg•sec•min−1•kg•m−2) | 245±72 | 246±69 | 330±88 | 317±95 | 372±79 | 344±109 |
| Coronary PTIC (mmHg•sec•min−1•L•dL−1) | 54±12 | 41±11‡ | 44±9 | 30±9‡ | 39±8 | 28±7‡ |
| Coronary PTIC/systolic PTIC | 0.24±0.07 | 0.18±0.05† | 0.14±0.04 | 0.10±0.04† | 0.11±0.03 | 0.09±0.03* |
| Arterial O2 content (mL/dL) | 16.9±1.7 | 14.9±2.0‡ | 17.4±1.7 | 15.3±2.2‡ | 17.9±1.6 | 15.8±2.2‡ |
|
| ||||||
| Ventricular function | ||||||
| Mitral annular e′ velocity (cm/s) | 9±2 | 7±2† | 10±3 | 7±2† | 14±4 | 8±3‡ |
| Mitral annular s′ velocity (cm/s) | 8±2 | 6±2† | 9±2 | 7±2† | 11±2 | 8±2‡ |
|
| ||||||
| Cardiomyocyte Injury and Stress | ||||||
| hs troponin T (ng/L) | 5.2 (4.2,8.6) | 12.4 (7.8,22.3)‡ | 6.0 (5.0,9.6) | 13.8(8.2,25.1)‡ | 6.3 (4.8,9.7) | 13.8 (8.9,26.2)‡ |
| NT-proBNP (pg/mL) | 100 (54, 236) | 436 (147, 978)‡ | 123 (49, 265) | 490 (156, 1104)‡ | 122 (47, 279) | 484 (155, 1133)‡ |
p<0.05
p<0.01, and
p<0.001 vs. controls.
Coronary PTIC indicates the product of coronary perfusion pressure time integral (PTI) and arterial oxygen content; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; systolic PTIC, the product of systolic PTI and LV mass index to adjust for differences in LV wall stress; and other abbreviations as in Table 1.
Figure 2. Myocardial Injury at Rest and Correlations with Filling Pressures.
(A) Troponin T levels were significantly higher in patients with heart failure with preserved ejection fraction (HFpEF) (red) as compared to controls (black). (B) Resting plasma troponin T levels were significantly correlated with simultaneously measured PCWP. Abbreviations as in Figure 1.
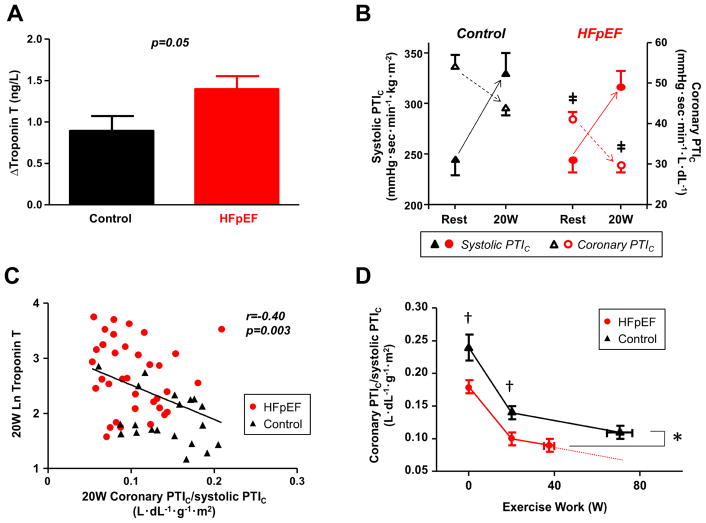
Resting RPP, systolic PTI, and systolic PTIC were similar in HFpEF and controls, indicating no differences in baseline myocardial oxygen demand. However, resting coronary perfusion PTI and PTIC were both significantly reduced in the HFpEF group, resulting in imbalance between myocardial O2 supply and demand (Table 2, Figure 3B and 3D). Resting troponin T levels varied inversely with coronary PTIC (r= −0.44, p=0.001) and coronary PTIC/systolic PTIC ratio (r=−0.38, p=0.006). Troponin levels were unrelated to myocardial oxygen demand assessed by systolic PTI, PTIC or RPP (not shown).
Figure 3. Myocardial Injury and Oxygen Supply-Demand Relationships during Exercise.
(A) Compared to controls, subjects with HFpEF developed greater increase in troponin T levels during low-level exercise. (B) At rest, systolic PTIC was similar in HFpEF and controls but coronary perfusion PTIC was significantly reduced in the HFpEF group, resulting in imbalance between myocardial O2 supply and demand. With low-level (20W) exercise, systolic PTIC increased while coronary perfusion PTIC decreased. As compared to controls, coronary perfusion PTIC remained significantly lower in HFpEF subjects during exercise. ‡p<0.001 vs. controls. (C) Troponin T levels were correlated with lower coronary PTIC/systolic PTIC ratio at 20W exercise. (D) Coronary PTIc/systolic PTIc ratio as a function of workload. Group differences in the ratio were slightly attenuated at peak exercise, likely due to the differing absolute workloads achieved, but remained significant. Solid lines depict measured data, and the dotted line depicts the hypothetical values if HFpEF subjects had been able to reach the same workload at peak as the control subjects based upon the trajectory of change. *p<0.05 vs. controls; †p<0.01 vs. controls. Coronary PTIC indicates the product of coronary perfusion pressure time integral PTI and arterial oxygen content; systolic PTIC, the product of systolic PTI and LV mass index to adjust for differences in LV wall stress; and other abbreviations as in Figures 1 and 2.
Baseline LV mechanics were impaired in HFpEF subjects compared to controls, with lower LV diastolic (e′) and systolic (s′) tissue velocities (Table 2). Troponin T was inversely correlated with LV diastolic relaxation, assessed by mitral annular e′ velocity (r= −0.43, p=0.002).
Myocardial Oxygen Supply, Demand, and Myocyte Injury with Exercise
Cardiac filling pressures and PA pressures were higher and CO and arterial O2 content were lower in HFpEF as compared to controls during 20W exercise (Table 2). At this matched exercise level, troponin T levels increased in both groups (both p<0.0001, Table 2), but the increase was greater in patients with HFpEF as compared to controls (Figure 3A).
At 20W exercise, RPP, systolic PTI, and systolic PTIC all increased, reflecting an increase in myocardial O2 demand, while coronary perfusion PTI and PTIC decreased (both p<0.0001, Figure 3B and 3D). While myocardial O2 demand was similarly increased in cases and controls (increase in RPP, 58±31% in HFpEF vs. 66±45% in controls, p=0.4; increase in systolic PTIC, 29±18% in HFpEF vs. 38±25% in controls, p=0.2), HFpEF subjects displayed greater decline in myocardial oxygen supply as assessed by coronary perfusion PTIC during 20W exercise (−25±9% in HFpEF vs. −17±6% in controls, p<0.0001, Figure 3B and 3D). This led to further deterioration in myocardial O2 supply and demand imbalance in HFpEF at 20W exercise (0.10±0.04 in HFpEF vs. 0.14±0.04 in controls, p=0.001). Similar findings were observed for non-corrected systolic and coronary perfusion PTI (not shown).
Similar to baseline, there were positive correlations between troponin T levels and PCWP pressures during 20W exercise (r=0.50, p<0.0001), as well as NT-proBNP levels during 20W exercise (r=0.62, p<0.0001). Higher troponin levels during 20W exercise were correlated with lower coronary PTIC (r=−0.44, p=0.0008) and poorer oxygen supply-demand (reduced coronary PTIC/systolic PTIC ratio, Figure 3C). Greater reduction in coronary perfusion PTIC during 20W exercise was correlated with greater increase in troponin T levels (r=−0.31, p=0.02).
At peak exercise HFpEF subjects reached a lower exercise workload (37±16 W vs. 73±28 W, p<0.0001) and shorter exercise duration (12±6 min vs. 22±9, p<0.0001). Group differences in O2 supply-demand relationships were slightly attenuated at peak exercise, likely due to the differing absolute workloads achieved (Table 2, Figure 3D), but remained significant. The increase in troponin T during exercise was correlated with peak workload and exercise time in HFpEF subjects (r=0.38, p=0.03 and r=0.53, p=0.002). During peak exercise, troponin T levels were again directly correlated with PCWP (r=0.55, p<0.0001), NT-proBNP (r=0.58, p<0.0001) and lower coronary perfusion PTIC (r=−0.38, p=0.007). Proportional changes in biventricular filling pressures were correlated with proportional changes in troponin T, with the strongest relationship noted for PCWP (Online Table 1).
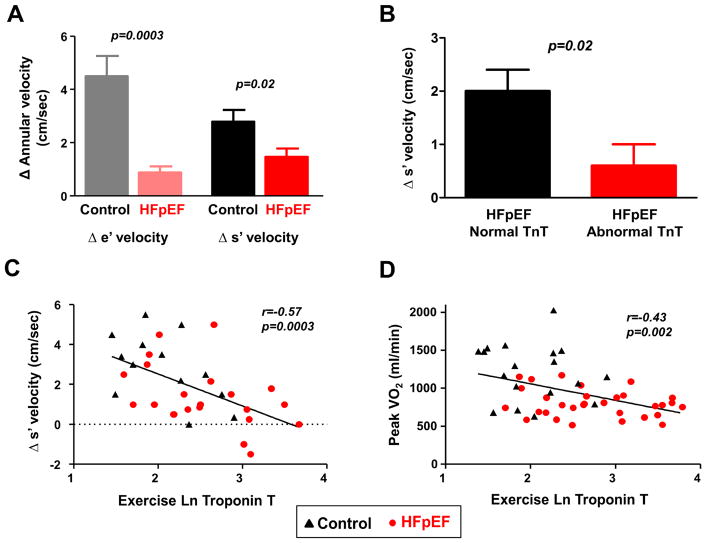
Subjects with HFpEF displayed impaired LV reserve with exercise, manifest by blunted increases in LV diastolic and systolic tissue velocities (Table 2 and Figure 4A). Compared to HFpEF subjects with normal troponin T levels, HFpEF subjects with abnormal troponin T displayed lower LV diastolic tissue velocities during exercise (7.9±1.9 vs 6.3±2.6 cm/sec, p=0.02) and less increase in LV systolic myocardial velocities (Figure 4B). Similar correlations were observed relative to resting troponin T (Online Table 2). Higher troponin T levels during exercise were correlated with poorer LV diastolic reserve (r= −0.4, p=0.02), impaired LV systolic reserve (Figure 4C), lower exercise QC (r= −0.44, p=0.002) and reduced peak aerobic capacity (Figure 4D).
Figure 4. Relationships between Myocardial Injury, Ventricular Reserve, and Exercise Capacity.
(A) Compared to controls, subjects with HFpEF displayed less increase in left ventricular (LV) diastolic (e′) and systolic (s′) mitral annular tissue velocities during peak exercise. (B) Compared to HFpEF subjects with normal troponin T at rest, those with elevated troponin T displayed impaired systolic reserve during peak exercise. (C) Elevated peak exercise troponin T was associated with impaired LV systolic reserve (s′) with exercise. (D) Exercise troponin T levels were inversely correlated with peak oxygen consumption (VO2). Abbreviations as in Figure 2.
Impact of Coronary Artery Disease
Despite similar hemodynamics and myocardial oxygen supply-demand relationships, patients with HFpEF and coronary disease displayed higher troponin T levels at rest and during exercise as compared to HFpEF patients without epicardial coronary disease (Online Table 3). Importantly, the observed correlations between hemodynamics, ventricular function, and troponin T remained significant after excluding HFpEF patients with coronary disease (all p<0.05).
Discussion
This is the first study to directly evaluate relationships between central hemodynamics, ventricular function, myocardial oxygen-supply dynamics, and troponin T, a biomarker reflective of cardiomyocyte injury, both at rest and during exercise. As compared to controls, troponin T was elevated in subjects with HFpEF, and the magnitude of this elevation was directly correlated with the increase in LV filling pressures, measured simultaneously using high fidelity micromanometers. Troponin levels were correlated with lower myocardial oxygen supply and impaired myocardial oxygen supply-demand balance, suggesting that subendocardial ischemia might contribute to myocyte injury. Troponin elevation in HFpEF was associated with limitations in left ventricular systolic and diastolic reserve, as well as the inability to adequately increase cardiac output and oxygen consumption during exercise. These data suggest that myocardial injury may play an important, previously underappreciated role in the pathophysiology of HFpEF, and may provide a novel therapeutic target in this syndrome for which few treatment options exist.
Left ventricular diastolic and systolic reserves during stress are impaired in HFpEF (2–13). Diastolic reserve limitation causes elevation in LV filling pressure, promoting symptoms of dyspnea, development of pulmonary hypertension and worsening mortality (5–7,14). Systolic reserve limitation impairs the ability of the heart to augment stroke volume and cardiac output to meet the metabolic demands of the body during exercise (7–11). The mechanisms causing these myocardial reserve limitations in HFpEF have remained unclear.
Based upon recent studies documenting abnormalities in the coronary microvascular circulation in HFpEF (15–22), we hypothesized that low-grade myocardial injury may develop in HFpEF, possibly in relation to subendocardial ischemia, and that this may partially explain the mechanism of ventricular reserve limitations in HFpEF. Coronary microvascular dysfunction in HFpEF is believed to be related to loss of NO bioavailability due to inflammation (15,16). Coronary microcirculatory deficits in HFpEF might also develop secondary to other common insults, including viral infection (22), radiotherapy exposure (21,35), or microvascular rarefaction (20).
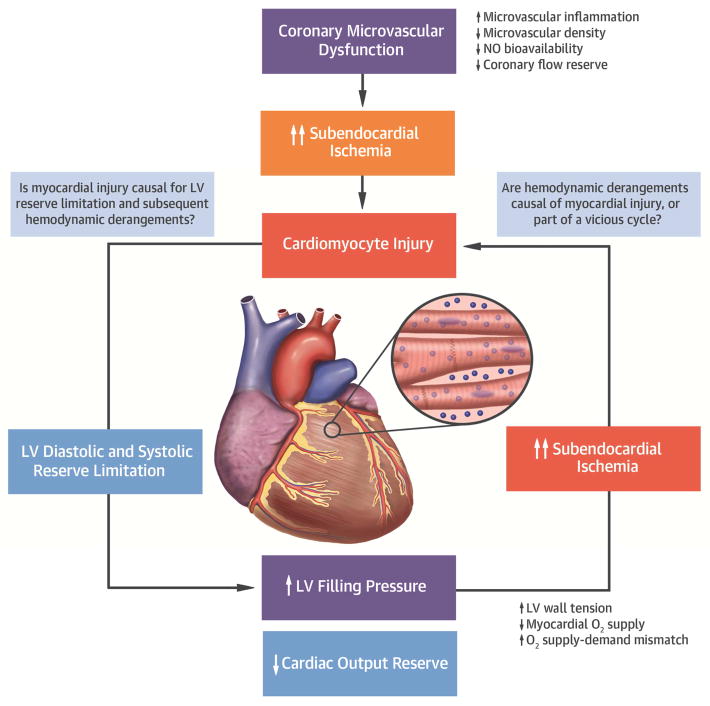
In a case-control study, Hwang et al. reported a surprisingly high rate of “false positive” stress tests (45%) among HFpEF patients without significant epicardial coronary disease by angiography.(36) At the time this was interpreted as a shortcoming of stress imaging in HFpEF, but more recent data suggests that these tests may not have been falsely positive, but rather reflective of microvascular ischemia.(13,17–19,37) In the current study, troponin T levels were elevated in HFpEF subjects compared to controls, even after adjusting for age, BMI, renal function, and the presence of coronary disease. We further demonstrated significantly elevated GDF-15 levels, another biomarker reflecting myocardial injury or stress, in subjects with HFpEF.(34) The elevations in troponin T were associated with more impaired myocardial function and greater increases in filling pressure at rest and during exercise. While causality cannot be assessed from this cross sectional study, the current data provide the framework for a new hypothesis, that myocardial injury is present and worsens during exercise in some patients with HFpEF, and this may contribute to the pathophysiology (Central Illustration).
Central illustration. Conceptual Model Linking Myocardial Injury, LV Diastolic and Systolic Reserve Limitation, and Hemodynamic Derangements during Exercise in HFpEF.
Coronary microvascular dysfunction may contribute to microvascular/subendocardial ischemia and subsequent cardiomyocyte injury, especially in the setting of myocardial O2 supply-demand imbalance. This cardiomyocyte injury may worsen LV diastolic and systolic reserve limitation and cause elevation in LV filling pressure and impairment in cardiac output reserve. Conversely, increase in LV filling pressure that develops secondary to LV reserve dysfunction may lead to cardiomyocyte injury possibly due to myocardial O2 supply-demand mismatch and subendocardial ischemia, or possibly both exacerbating each other. O2 indicates oxygen; NO, nitric oxide; and other abbreviations as in Figures 1 and 4.
The mechanisms of troponin elevation in heart failure remain unclear, but the most prevailing theory is that myocyte injury is mediated by subendocardial ischemia.(38) To explore this further, we measured myocardial oxygen supply at rest and during exercise, defined as the product of the difference between aortic and LV filling pressure during diastole and arterial blood oxygen content.(28,30) Myocardial oxygen supply was reduced in patients with HFpEF at rest, and was decreased to even greater extent in the patients during matched exercise at 20W. Importantly, the reduction in oxygen supply was significantly correlated with increases in troponin levels, which would be consistent with an ischemic mechanism. Oxygen-supply demand ratios decrease as a function of exercise workload intensity, and this may explain why differences in supply-demand ratio between HFpEF and controls at peak exercise were attenuated (Table 2), but still significant (Figure 3D).
We cannot determine from the current data whether increased wall tension caused ischemia, myocyte injury and myocardial dysfunction, or whether impaired myocardial reserve increased wall tension to promote myocyte injury, or possibly some combination of both. Importantly, a number of nonischemic mechanisms may contribute to the cardiac injury, including apoptosis, spontaneous necrosis and inflammation, and further research is warranted to explore whether these mechanisms contribute to injury in HFpEF (39).
Regardless of the mechanism of injury, we observed that subjects with HFpEF displayed higher troponin at rest and greater increases with exercise. This was directly correlated with the observed limitations in LV diastolic and systolic functional reserve, increases in filling pressure, inadequate cardiac output reserve, and impairments in peak aerobic capacity in the HFpEF group. These observations suggest that low-grade myocardial injury may partly explain the inability of the heart to enhance its function during exercise in HFpEF (2–13).
Clinical Implications
Recent studies have reported that elevated troponin levels in HFpEF are associated with adverse outcome.(40,41) This is the first study to directly show that the magnitude of elevation in troponin in HFpEF reflects the severity of increase in LV filling pressures. The current data also provide a mechanism to explain this observation, indicating that these patients have greater ventricular dysfunction and higher filling pressures, and may thus benefit from more aggressive decongestion.
Phenotypic heterogeneity has been recognized as a major limitation in the field of HFpEF (1). While our cross-sectional study cannot determine whether the myocyte injury observed in HFpEF was caused by ischemia or other mechanisms, the current data indicates that there is a group of HFpEF patients where myocardial injury is greater (Figure 2), in whom myocardial reserve is more profoundly depressed (Figure 4), and these patients may respond better to novel therapies targeted to enhance myocardial perfusion or maintain tissue integrity and viability. Importantly, patients with this “injury” phenotype can be readily detected through measurement of high sensitivity troponin levels, a robust, widely available and inexpensive test. This would allow for enrichment in clinical trials of patient most likely to derive benefit from therapies targeting cardiomyocyte injury. Given the relationships with hemodynamics and ventricular function, it may also be that high sensitivity troponins could also serve as a novel surrogate endpoint in clinical trials in HFpEF.
Limitations
This study includes a relatively small sample size, but despite this, we observed highly significant correlations and group differences. By design, subjects were recruited from those referred for cardiac catheterization, introducing selection bias. However, the invasive characterization of patients both at rest and with exercise at the time of troponin sampling is unique in the literature, as is the ability to correlate evidence of myocyte injury with hemodynamics, myocardial O2 supply-demand relationships, and ventricular function. The control group was not truly normal in that they were referred to exercise testing, but this would only be expected to bias our results toward the null. There were baseline differences in age, BMI, and renal function between controls and HFpEF, but the differences in troponin remained significant after adjusting for these confounders, and persisted even among subjects with no history of coronary disease. These comorbidities are also implicated in the pathogenesis of HFpEF. The increase in troponin T levels during exercise was greater in HFpEF patients, but the overall changes observed were rather modest and of unclear clinical significance. This may relate in part to the kinetics of troponin release, which may require a longer period of time to reach peak, particularly if the mechanism of injury is related to ischemia.(33) Indirect indices reflective of myocardial O2 supply-demand were used rather than more direct measures of ischemia such as coronary sinus sampling. Finally, the cross sectional design of our study does not permit assessment of causality and questions regarding the mechanism of injury as well as the directionality of the relationship between injury and hemodynamics require further study in appropriately controlled interventional studies.
Conclusions
Myocardial injury is increased at rest and worsens during low-level exercise in patients with HFpEF, even in the absence of significant epicardial coronary disease. Greater severity of myocyte injury is correlated with impaired myocardial oxygen supply, oxygen supply-demand mismatch, ventricular reserve limitations, and classical hemodynamic derangements that develop during stress and contribute to morbidity and mortality in this cohort. Further study is required to determine the mechanisms underlying cardiomyocyte injury in HFpEF, and to determine whether therapies targeting these mechanisms can improve hemodynamics, restore myocardial reserve, and improve clinical outcomes in patients with HFpEF.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge
Serum troponin T, a marker of cardiomyocyte injury, may be elevated in patients with heart failure and preserved ejection fraction (HFpEF). Patients with HFpEF and more pronounced cardiomyocyte injury display imbalance in myocardial oxygen supply and demand, higher cardiac filling pressures, limited left ventricular functional reserve, and impaired augmentation of cardiac output during exercise.
Translational Outlook
Further studies are needed to clarify the mechanisms underlying cardiomyocyte injury in HFpEF and identify therapeutic strategies to mitigate its consequences.
Acknowledgments
This research was supported by a competitive prospective grants award from the Mayo Clinic and Foundation. Dr. Borlaug is supported by RO1 HL128526, RO1 HL126638, UO1 HL125205 and U10 HL110262. Dr. Melenovsky is supported by the Czech Healthcare Research Grant agency (AZV): 17-28784A. Dr. Olson is supported by RO1 HL126638. Dr. Reddy is supported by T32 HL007111. Dr. Obokata is supported by a research fellowship from the Uehara Memorial Foundation, Japan.
Abbreviations
- QC
cardiac output
- HFpEF
heart failure with preserved ejection fraction
- PA
pulmonary artery
- PCWP
pulmonary capillary wedge pressure
- PTI
pressure time integral
- RAP
right atrial pressure
- RPP
rate-pressure product
- VO2
oxygen consumption
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 3.Tan YT, Wenzelburger F, Lee E, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Phan TT, Abozguia K, Nallur Shivu G, et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–9. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2010;3:588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–9. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abudiab MM, Redfield MM, Melenovsky V, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–85. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosmala W, Rojek A, Przewlocka-Kosmala M, Mysiak A, Karolko B, Marwick TH. Contributions of Nondiastolic Factors to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;67:659–70. doi: 10.1016/j.jacc.2015.10.096. [DOI] [PubMed] [Google Scholar]
- 11.Kosmala W, Przewlocka-Kosmala M, Rojek A, Mysiak A, Dabrowski A, Marwick TH. Association of Abnormal Left Ventricular Functional Reserve with Outcome in Heart Failure with Preserved Ejection Fraction. JACC Cardiovasc Imaging. 2017 doi: 10.1016/j.jcmg.2017.07.028. in press. [DOI] [PubMed] [Google Scholar]
- 12.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–63. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 13.van Empel VP, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2014;3:e001293. doi: 10.1161/JAHA.114.001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorfs S, Zeh W, Hochholzer W, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–12. doi: 10.1093/eurheartj/ehu315. [DOI] [PubMed] [Google Scholar]
- 15.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 16.Franssen C, Chen S, Unger A, et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:312–24. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Kato S, Saito N, Kirigaya H, et al. Impairment of Coronary Flow Reserve Evaluated by Phase Contrast Cine-Magnetic Resonance Imaging in Patients With Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivaratharajah K, Coutinho T, deKemp R, et al. Reduced Myocardial Flow in Heart Failure Patients With Preserved Ejection Fraction. Circ Heart Fail. 2016:9. doi: 10.1161/CIRCHEARTFAILURE.115.002562. [DOI] [PubMed] [Google Scholar]
- 19.Mohammed SF, Majure DT, Redfield MM. Zooming in on the Microvasculature in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016:9. doi: 10.1161/CIRCHEARTFAILURE.116.003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–9. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saiki H, Petersen IA, Scott CG, et al. Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation. 2017;135:1388–1396. doi: 10.1161/CIRCULATIONAHA.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tschope C, Bock CT, Kasner M, et al. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation. 2005;111:879–86. doi: 10.1161/01.CIR.0000155615.68924.B3. [DOI] [PubMed] [Google Scholar]
- 23.Tschope C, Post H. Latent ischaemia as a trigger for a circulus vitiosus of inflammation, fibrosis, and stiffness in HFPEF. Eur J Heart Fail. 2015;17:1210–2. doi: 10.1002/ejhf.439. [DOI] [PubMed] [Google Scholar]
- 24.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8:41–8. doi: 10.1161/CIRCHEARTFAILURE.114.001731. [DOI] [PubMed] [Google Scholar]
- 26.Andersen MJ, Hwang SJ, Kane GC, et al. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:542–50. doi: 10.1161/CIRCHEARTFAILURE.114.002114. [DOI] [PubMed] [Google Scholar]
- 27.Reddy YNV, Andersen MJ, Obokata M, et al. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2017;70:136–148. doi: 10.1016/j.jacc.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckberg GD, Fixler DE, Archie JP, Hoffman JI. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res. 1972;30:67–81. doi: 10.1161/01.res.30.1.67. [DOI] [PubMed] [Google Scholar]
- 29.Sarnoff SJ, Braunwald E, Welch GH, Jr, Case RB, Stainsby WN, Macruz R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol. 1958;192:148–56. doi: 10.1152/ajplegacy.1957.192.1.148. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman JI, Buckberg GD. The myocardial oxygen supply:demand index revisited. J Am Heart Assoc. 2014;3:e000285. doi: 10.1161/JAHA.113.000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–56. doi: 10.1161/01.cir.57.3.549. [DOI] [PubMed] [Google Scholar]
- 32.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol. 2011;57:2398–405. doi: 10.1016/j.jacc.2010.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng JM, Akkerhuis KM, Battes LC, et al. Biomarkers of heart failure with normal ejection fraction: a systematic review. Eur J Heart Fail. 2013;15:1350–62. doi: 10.1093/eurjhf/hft106. [DOI] [PubMed] [Google Scholar]
- 35.Saiki H, Moulay G, Guenzel AJ, et al. Experimental cardiac radiation exposure induces ventricular diastolic dysfunction with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2017;313:H392–H407. doi: 10.1152/ajpheart.00124.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–27. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Konerman MC, Greenberg JC, Kolias TJ, et al. Reduced Myocardial Flow Reserve is Associated with Diastolic Dysfunction and Decreased Left Atrial Strain in Patients with Normal Ejection Fraction and Epicardial Perfusion. J Card Fail. 2017 doi: 10.1016/j.cardfail.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah KS, Maisel AS, Fonarow GC. Troponin in Heart Failure. Heart Failure Clin. 2018;14:57–64. doi: 10.1016/j.hfc.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017;113:1708–1718. doi: 10.1093/cvr/cvx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey A, Golwala H, Sheng S, et al. Factors Associated With and Prognostic Implications of Cardiac Troponin Elevation in Decompensated Heart Failure With Preserved Ejection Fraction: Findings From the American Heart Association Get With The Guidelines-Heart Failure Program. JAMA Cardiol. 2017;2:136–145. doi: 10.1001/jamacardio.2016.4726. [DOI] [PubMed] [Google Scholar]
- 41.Felker GM, Mentz RJ, Teerlink JR, et al. Serial high sensitivity cardiac troponin T measurement in acute heart failure: insights from the RELAX-AHF study. Eur J Heart Fail. 2015;17:1262–70. doi: 10.1002/ejhf.341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.