Abstract
OBJECTIVE
To systematically review maternal and neonatal outcomes associated with opioid detoxification during pregnancy.
DATA SOURCES
PubMed, PsycINFO, EMBASE, Cochrane, and ClinicalTrials.gov databases were searched from January 1, 1966, to September 1, 2016.
METHODS OF STUDY SELECTION
English-language studies that reported outcomes associated with opioid detoxification among pregnant women with opioid use disorder were included. Nonoriginal research articles (case reports, editorials, reviews) and studies that failed to report outcomes for detoxification participants were excluded. Bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias and quality was assessed using the U.S. Preventive Service Task Force Quality of Evidence scale.
TABULATION, INTEGRATION, AND RESULTS
Of 1,315 unique abstracts identified, 15 met criteria for inclusion and included 1,997 participants, of whom 1,126 underwent detoxification. Study quality ranged from fair to poor as a result of the lack of a randomized control or comparison arm and high risk of bias across all studies. Only nine studies had a comparison arm. Detoxification completion (9–100%) and illicit drug relapse (0–100%) rates varied widely across studies depending on whether data from participants who did not complete detoxification or who were lost to follow-up were included in analyses. The reported rate of fetal loss was similar among women who did (14 [1.2%]) and did not undergo detoxification (17 [2.0%]).
CONCLUSIONS
Evidence does not support detoxification as a recommended treatment intervention as a result of low detoxification completion rates, high rates of relapse, and limited data regarding the effect of detoxification on maternal and neonatal outcomes beyond delivery.
Opioid agonist pharmacotherapy (with either methadone or buprenorphine) is endorsed by the American College of Obstetricians and Gynecologists and other professional societies as the optimal treatment for opioid use disorder during pregnancy.1–3 Initial recommendations for the use of pharmacotherapy during pregnancy were largely based on a case report of stillbirth after detoxification coupled with evidence of increased catecholamine release (measured by serial amniocentesis) indicating fetal stress during maternal withdrawal.4,5 Additional support for the effectiveness of opioid pharmacotherapy emerged from data in the 1970s, which demonstrated that women treated with methadone as part of a comprehensive addiction and prenatal care program had similar birth outcomes compared with women without a substance use disorder.6,7
Over the past 15 years, the escalating use of opioids has led to a crisis of epidemic proportions in the United States. As a result, drug treatment admissions for opioid use disorder during pregnancy have risen markedly as have rates of newborns with neonatal abstinence syndrome and the costs necessary to treat them.8–10 Efforts to respond to the opioid epidemic among pregnant women have led to a reappraisal of detoxification during pregnancy, but its efficacy and role as an effective treatment option during pregnancy is unclear.11 Thus, we systematically reviewed the published literature to evaluate the evidence regarding opioid detoxification during pregnancy with a focus on 1) describing the detoxification process; 2) summarizing adverse maternal and neonatal outcomes associated with detoxification including fetal demise, maternal relapse, and neonatal abstinence syndrome; and 3) identifying gaps in the existing literature to guide future research.
SOURCES
The study protocol was developed and the review performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis and Meta-analysis Of Observational Studies in Epidemiology guidelines.12,13 PubMed, PsycINFO, EMBASE, Cochrane, and clinicaltrials.gov electronic databases were searched between January 1, 1966 (when records became indexed on Medline) and September 1, 2016. A reference librarian performed the database search and removed any duplicate records. The references of review articles were also reviewed to ensure capture of all publications related to opioids, pregnancy, and detoxification (Appendix 1, available online at http://links.lww.com/AOG/B79).
STUDY SELECTION
Six authors (M.T., E.E.K., T.E.W., A.P., D.J.H., C.E.M.) were organized into three author pairs and each author independently screened all titles and abstracts for inclusion. To be included, studies had to focus on pregnant women with opioid use disorder who received opioid detoxification as the primary intervention. Studies that were not original research studies, were not in English, and that did not identify detoxification as the intervention were excluded. Titles and abstracts were included in the full-text review if there was any discrepancy in the decisions of author pairs. For full-text review, author pairs independently reviewed each article to identify studies that met inclusion criteria with any disagreements resolved by another author pair. For data extraction, eight authors (M.T., E.E.K., T.E.W., A.P., D.J.H., C.E.M., M.C.M., H.E.J.) were organized into four author pairs and relevant data from included articles were entered into a data extraction tool developed by the authors. The accuracy of extracted data was reviewed by two authors (M.T., E.E.K.). Each study’s design and findings were qualitatively described. Meta-analysis was not performed as a result of heterogeneity in study designs, detoxification processes, and in how and what adverse maternal and neonatal outcomes were assessed. Authors were not contacted and additional data from included studies were not obtained beyond what was available in the published manuscripts.
Each author pair evaluated risk of bias in individual studies using the Cochrane Collaboration’s tool for assessing risk of bias (Appendix 2, available online at http://links.lww.com/AOG/B79)14 and included six domains: selection, performance, detection, attrition, reporting, and other bias. Summary assessments of risk of bias (high risk, unclear risk, and low risk) were made independently by two authors (M.T., E.E.K.) per Cochrane Collaboration’s guidelines. Study quality was assessed independently by two authors (M.T., E.E.K.) according to the risk of bias and the overall evidence provided for adverse maternal and neonatal outcomes using the 3-point U.S. Preventative Services Task Force grading scale (good, fair, poor).15 A “good”-quality study was well designed with no important limitations, a “fair” study was adequate to determine effects on outcomes, but had limitations as a result of the indirect nature of the evidence, and a “poor” study was insufficient to assess effects on outcomes as a result of limited power, important flaws, or a lack of information regarding outcomes.15
RESULTS
Our systematic review captured 1,315 unique citations, of which 110 were assessed for full-text review. Three of these studies were secondary analyses of data from larger studies that met inclusion criteria and were thus excluded to avoid duplicating data.16–18 Fifteen studies were included in the qualitative synthesis (Fig. 1). The publication periods for the included studies ranged from 1975 to 2016. Among these, 10 studies were published after 2000 during the current opioid crisis. Study location varied with eight studies conducted in the United States, one in Canada, and six conducted in Europe and Australia.
Fig. 1. Flow diagram.
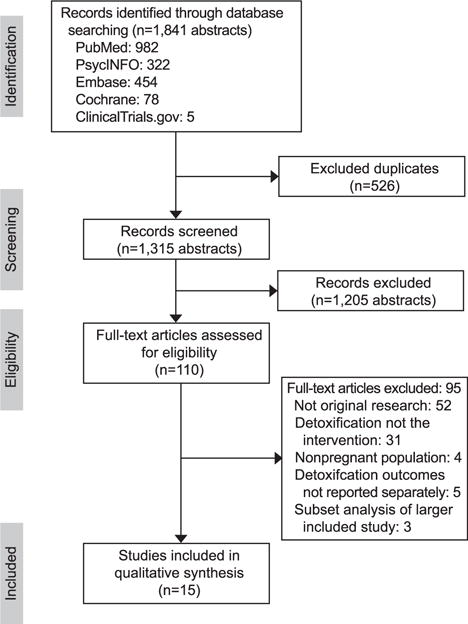
Terplan. Opioid Detoxification During Pregnancy. Obstet Gynecol 2018.
No randomized clinical trials were identified (Table 1). All included studies were observational and only five studies had prospectively collected data.19–23 Before detoxification, most participants had documented opioid use (primarily through urine drug testing), although the distinction between heroin and prescription opioids was not always clear. Other non-opioid illicit substance use was reported in six studies20,23–27 and tobacco use was reported in five.23,25–28 Significant heterogeneity related to the presence of a comparison group and the types of comparison groups used to evaluate differences in maternal and neonatal outcomes existed across the studies. Only nine of the included studies had a comparison group, which ranged from women without opioid use disorder (n=4)20,21,25,29 to women with opioid use disorder on opioid agonist pharmacotherapy (n=4)22,26,28,30 to women with illicit opioid (n=2)20,31 and drug (n=2)22,25 use. The majority of studies also conducted within-group comparisons (eg, successful detoxification compared with in process of methadone taper compared with illicit opioid use after detoxification) rather than prospectively based on their planned treatment regimen.
Table 1.
Summary of Evidence Regarding Detoxification During Pregnancy*
| Author (year; country) |
Study Design |
Total n |
Detox Group(s), n |
Comparison Group(s) |
Summary Outcomes Among Women Undergoing Detoxification |
Quality | ||
|---|---|---|---|---|---|---|---|---|
| Maternal | Birth | Neonatal | ||||||
| Bell et al (2016; United States)19 | Retrospective analysis of prospectively collected data | 301 | Detoxification by 4 methods, total n=301 | None, within-group comparisons made among 4 detoxification groups | Detoxification comp: NR | Demise: n=2 | NAS: 17.2–70.1%† # | Poor |
|
Drug use: 17.4–74%† | Growth: IUGR 5% PTB: 13–19%† |
LOS: NR | |||||
| Dooley et al (2015; Canada)20 | Prospective cohort comparison | 600 | Morphine and bup detoxification in remote First Nations communities, n=86 | Pregnant with occasional opioid use, n=80 | Detoxification comp: 9.3% | Demise: n=2 | NAS: 12.8%↑ | Fair |
| Non-OUD pregnant, n=434 | Drug use: 34.8% | Growth: 3,301 g↓ PTB: 5.8% |
LOS: 2.4 d‖↑ | |||||
| Haabrekke et al (2014; Norway)25 | Retrospective cohort comparison | 187 | Residential detoxification (2004–2008), n=21 | Pregnant with SUD (1991–1996), n=78 | Detoxification comp: 100% | Demise: n=0 | NAS: 0↓ | Poor |
| Non-OUD pregnant (1991–1996), n=58 Non-OUD pregnant (2004–2008), n=30 |
Drug use: 0% | Growth: 3,293 g PTB: 0% |
LOS: NR | |||||
| Stewart et al (2013, United States)27 | Retrospective cohort | 95 | Methadone detoxification, n=95 | None, within-group comparisons between detoxification participants who did (n=42) and did not (n=53) use opioids | Detoxification comp: 80% | Demise: n=3 | NAS: 10–80%† # | Poor |
| Drug use: 44.2% | Growth: 2,788–3,065 g†
# PTB: 10–18%† |
LOS: 3–22 d# | ||||||
| Lund et al (2012, United States)28 | Retrospective cohort comparison | 25 | Methadone detoxification, n=8 | Pregnant with OUD on MM, n=12 | Detoxification comp: 15.7% | Demise: n=0 | NAS: 25% | Poor |
| Pregnant with OUD on BM, n=5 | Drug use: 14.3% | Growth: 3,023 g PTB: 37.5% |
LOS: 7.0 d | |||||
| Jones et al (2008, United States)26 | Retrospective comparison | 175 | Detoxification by 2 methods, total n=123 | Pregnant with OUD on MM, n=52; within-group comparisons between participants who received MM in both detoxification groups (1. n=8; 2. n=20) | Detoxification comp: 58.3–89.3% | Demise: n=3 | NAS: 25–36%† | Poor |
|
Drug use: 15.0–57.1%† # | Growth: 2,824–3,054 g† PTB: 27–36%† |
LOS: 6.0–9.6 d† | |||||
| Kahila et al (2007, Finland)21 | Prospective cohort | 67 | Bup detoxification, n=67 | Finland population data, n=57,759; within-group comparisons between detoxification participants who received BM and were (n=21) and were not compliant (n=37) with treatment visits | Detoxification comp: 13.4% | Demise: n=0 | NAS: 22–100%† # | Fair |
| Drug use: 33.3–45.9%† | Growth: 3,180 g↓ PTB: 0–5.4%† |
LOS: 11.6–28.1 d† # | ||||||
| Luty et al (2003, United Kingdom)29 | Retrospective case series | 101 | Methadone detoxification, n=101 | Published rates of SAB and premature delivery in the general population | Detoxification comp: 41.6% | Demise: n=1 | NAS: NR | Poor |
| Drug use: 95.8% | Growth: 2,564 g PTB: NR |
LOS: NR | ||||||
| Hulse et al (2001; Australia, Portugal, United Kingdom)33 | Retrospective case series | 18 | Rapid detoxification with sedation, n=18** | None | Detoxification comp: NR¶ | Demise: n=0 | NAS: NR | Poor |
| Drug use: 16.7% | Growth: 3,008 g PTB: 0% |
LOS: NR | ||||||
| Sinha et al (2001, United Kingdom)32 | Retrospective case study | 51 | Methadone detoxification, n=51 | None, within-group comparisons between detoxification participants who received MM (n=22) and used opioids (n=19) | Detoxification comp: 19.6% | Demise: n=1 | NAS: 0–81%† # | Poor |
| Drug use: 37.3% | Growth: 2,754–3,800 g† PTB: NR |
LOS: 7.8–33.2 d† # | ||||||
| Dashe et al (1998; United States)24 | Retrospective case series | 34 | Detoxification by 2 methods, total n=34 | None, within-group comparisons among detoxification participants after methadone (n=21) and clonidine (n=13) detoxification and among those who received MM (n=4) and used opioids (n=10) | Detoxification comp: 73.5%‡ | Demise: n=0 | NAS: 15% | Poor |
|
Drug use: 29.4% | Growth: 3,075 g PTB: 18% |
LOS: 8 d§ | |||||
| Kyei-Aboagye et al (1998, United States)22 | Prospective case series | 74 | Drug-free residential detoxification, n=30 | Pregnant with SUD, n=44 (OUD on MM n=15], cocaine use [n=29]) | Detoxification comp: 100% | Demise: n=0 | NAS: NR | Poor |
| Drug use: 0% | Growth: 3,218 g↑ PTB: NR |
LOS: 3 d↓ | ||||||
| LePreau et al (1995, United States)23 | Prospective cohort | 33 | Clonidine detoxification, n=33 | None | Detoxification comp: 69.7% | Demise: n=1 | NAS: NR | Poor |
| Drug use: NR | Growth: 3,008 g PTB: NR |
LOS: NR | ||||||
| Maas et al (1990, Germany)31 | Retrospective case series | 75 | Methadone detoxification, n=58 | Pregnant with OUD, n=17; within-group comparisons among detoxification participants who received MM (n=8) and used opioids (n=32) | Detoxification comp: 29.8% | Demise: n=0 | NAS: 11.8–75%†↓ | Poor |
| Drug use: 56.1% | Growth: 2,625–3,070 g† PTB: 11.8–50%† |
LOS: NR | ||||||
| Wallach et al (1975, United States)30 | Retrospective cohort | 161 | Methadone detoxification, n=100 | Pregnant with OUD on MM, n=61 | Detoxification comp: NR | Demise: n=1 | NAS: NR | Poor |
| Drug use: 100% | Growth: LBW 34% PTB: NR |
LOS: NR | ||||||
NR, not reported; NAS, neonatal abstinence syndrome; LOS, neonatal length of stay; Demise, number of fetal demises; Growth, mean fetal growth; PTB, rate of preterm birth; IUGR, rate of intrauterine growth restriction; SUD, substance use disorder; OUD, opioid use disorder; MM, methadone maintenance; BM, buprenorphine maintenance; SAB, spontaneous abortion; inpt, inpatient; outpt, outpatient; Drug use, rate of illicit drug use or relapse; Detoxification comp, rate of participants completing detoxification process; bup, buprenorphine.
↑ Detoxification outcome significantly greater than nondetoxification comparison outcome; Ydetoxification outcome significantly less than nondetoxification comparison outcome.
Detailed outcome data between detoxification and control and within detoxification comparison groups is reported in Appendix 3 (available online at http://links.lww.com/AOG/B79).
Range across all detoxification groups; 1 participant underwent detoxification twice.
Four patients delivered during their detoxification hospitalization.
NAS treatment duration.
Length of stay does not include 7- to 10-d stay in outpatient facility for follow-up and NAS surveillance.
Two participants on naltrexone maintenance.
Significant within-group differences among detoxification groups.
A total of 1,997 participants, of whom 1,126 underwent detoxification as the primary treatment for opioid use disorder were included (Table 2). Detoxification primarily took place in inpatient settings (n=9) and in two studies, detoxification took place as part of a residential treatment program.22,25 Three studies included patients who were incarcerated at the time of the detoxification.19,25,32 In Bell et al, 108 women with opioid use disorder underwent “involuntary withdrawal” as a result of the absence of opioid pharmacotherapy availability in the penal system.19 In Haabrekke et al, eight women were involuntarily institutionalized and forced into detoxification25; in Sinha et al, of the 10 women who completed detoxification, nine did so without medical supervision (“quit cold turkey”) and one underwent supervised withdrawal in prison.32 Gestational age at the time of withdrawal was reported in all but the oldest study30 and predominantly occurred in the second or third trimester.
Table 2.
Summary of Detoxification Process in Included Studies
| Author | Detoxification, n |
Setting | Trimester or Gestational Age at Detoxification, n (%)* |
Duration | Pharmacotherapy | Fetal Monitoring | Behavioral Health | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Bell et al19 | 301 | Detoxification 1: jail | 1: 1st: 10 (9), 2nd: 65 (60), 3rd: 33 (31) | 1: NR | 1: Clonidine, antiemetic, antidiarrheal | FHT | 1: None | Delivery |
| Detoxification 2: inpatient | 2: 1st: 4 (17), 2nd: 10 (43), 3rd: 9 (39) | 2: 5–8 d | 2: Buprenorphine | 2: Residential or 8 h daily | ||||
| Detoxification 3: inpatient | 3: 1st: 12 (15), 2nd: 36 (47), 3rd: 29 (38) | 3: 5–8 d | 3: Buprenorphine | 3: None | ||||
| Detoxification 4: outpatient | 4: 1st: 2 (2), 2nd: 37 (40), 3rd: 54 (58) | 4: 8–16 wk | 4: Buprenorphine | 4: Ongoing outpatient | ||||
| Dooley et al20 | 86 | Outpatient | 1st: 14 (16), 2nd: 32 (37), 3rd: 32 (37) | 101 (SD±67) d† | Morphine (n=72); buprenorphine (n=8) | NR | Case-by-case basis | 7–10 d pp |
| Haabrekke et al25 | 21 | Residential‡ | 1st: 4 (19), 2nd: 7 (33), 3rd: 10 (48) | NR | Opioid agonists and pain medications for severe withdrawal | NR | During residential stay for up to 1 y after delivery | Delivery |
| Stewart et al27 | 95 | Inpatient | 20§ | 15–25 d‖ | Neonatal: n=12 for 4.5 y§§ | |||
| Lund et al28 | 8# | Inpatient | 22.8 (SD±8.3)† | 7 d | Methadone | >24 wk | Offered admission to outpatient drug rehabilitation facility¶ | Delivery |
| Jones et al26 | 123 | Inpatient | 20.3 (range 5–39)† | Detoxification 1: 3 d | Methadone | NR | Outpatient | Delivery |
| Detoxification 2: 7 d | Methadone | NR | Outpatient | Delivery | ||||
| Kahila et al21 | 67 | Outpatient | 1st: 38 (57), 2nd–3rd: 13 (21) | NR | Buprenorphine | BPP/growth >24 wk, NST >26 wk | NR | Delivery |
| Luty et al29 | 101 | Inpatient | 1st: 5 (5), 2nd: 54 (53), 3rd: 57 (56) | 19.5 (SE=1.1) d | Methadone | NR | NR | Delivery |
| Hulse et al33 | 18** | NR | 1st: 2 (11), 2nd: 11 (61), 3rd: 6 (33) | NR | Oral or IV sedation | US, n=4 | NR | Delivery |
| Sinha et al32 | 51 | NR†† | 2nd: 51 (100) | NR | Methadone‡‡ | NR | Outpatient during prenatal care | Delivery |
| Dashe et al24 | 34 | Inpatient | 24.2 (SD±8.1)† | 12 (range 3–39) d§ | Methadone for severe withdrawal, n=21; clonidine for mild withdrawal, n=13 | FHT | Outpatient during prenatal care | Delivery |
| Kyei-Aboagye et al22 | 30 | Residential | Up to the 2nd | NR | NR | NR | Part of residential program | Delivery |
| LePreau et al23 | 33 | Inpatient | 15.2 (range 4–36)† | 5 d | Clonidine, antiemetic and antidiarrheal medications | NST >32 wk | Daily group and individual counseling during detoxification, referred to outpatient counseling after detoxification¶ | Delivery |
| Maas et al31 | 58 | Initially inpatient then outpatient | 28 (range 9–40)§ | 9 (range 0–28) wk§ | Methadone | FHT | NR | Delivery |
| Wallach et al30 | 100 | Inpatient | NR | 10 d | Methadone | NR | 2–4 wk of inpatient counseling after detoxification | 6 wk pp |
| Neonatal: N=14 for 4 y |
NR, not reported; IV, intravenous; FHT, fetal heart tone auscultation, US, ultrasonography; NST, nonstress test; pp, postpartum; BPP, biophysical profile; SE, standard error.
n (%) unless otherwise specified.
Mean.
n=8 pregnant women were involuntarily institutionalized.
Median.
Median duration of detoxification for women with and without illicit drug use at delivery.
Program attendance was not reported.
n=51 initiated detoxification, but data for women who did not complete detoxification were not reported.
One participant underwent detoxification twice.
n=1 patient had supervised withdrawal in prison.
n=9 women underwent self-directed “cold turkey” withdrawal.
Reported in Walhovd et al. 18
Pharmacotherapy was specified in all but one study.22 In most studies either methadone or buprenorphine was used, with the exception of LePreau et al, in which clonidine was used followed by phenobarbital,23 Hulse et al, in which sedation was used,33 and Haabrekke et al, in which the type of opioid agonist used was not specified.25 In Bell et al, women who were involuntarily detoxified while incarcerated received clonidine and supportive medications.19 The reported duration of withdrawal ranged from 3 days to 16 weeks and was not reported in five studies. Only seven studies reported fetal monitoring, and only one described fetal monitoring as part of a formal detoxification protocol.23 Behavioral counseling that occurred either concurrently or after withdrawal was mentioned in 11 studies, although descriptions of the type and content of the counseling were vague and adherence was not reported. Prenatal care engagement was reported by most studies, although timing and frequency of visits were not reported. The majority of studies limited maternal follow-up to delivery with only two studies following participants postpartum.20,30
Table 1 summarizes select maternal, birth, and neonatal outcomes for women who underwent detoxification. Detoxification completion rates varied widely (9–100%) among included studies, which was largely secondary to whether data included in analyses were from participants who did not complete detoxification or who were lost to follow-up. In Hulse et al, one participant underwent detoxification twice.33 Importantly, the two studies with detoxification completion rates of 100% were inpatient residential treatment programs, one of which included women who were involuntarily institutionalized.25 Similarly, relapse, captured primarily by positive urine toxicology, ranged from 0 to 100%; this variability was also dependent on which groups of participants were included in the analysis. For example, in Luty et al, 101 women entered the detoxification program, but only 42 women completed the process.29 Among these women, obstetric records were available for only 28 women and of these, four records were incomplete. Among the 24 women with adequate obstetric records, 23 (96%) had a positive urine toxicology at delivery. Importantly, maternal death resulting from opioid overdose was reported by one study. In Wallach et al, two maternal deaths resulting from overdose at 2 and 6 weeks postpartum were reported among women who underwent detoxification during pregnancy.30
Fetal demise including miscarriage was reported in most studies. In detoxification groups (n=1,126), there were 14 total demises: three first trimester (less than 14 weeks of gestation), five second trimester (14 weeks or greater but less than 28 weeks of gestation), one third trimester (28 weeks of gestation or greater), and five with gestational ages not reported. In comparison groups (n=871), there were 17 total demises: five first trimester (one at 13 weeks of gestation and four reported as spontaneous abortions without exact gestational ages), two second trimester, five with birth weight reported (970, 531, 2,200, 1,800, 1,200 g) instead of gestational age, and five with gestational ages not reported. Therefore, the rate of loss among the women undergoing detoxification (1.24%; 95% CI 0.70–2.21) and the rate of loss within the comparison groups (1.95%; 95% CI 1.10–3.10) were similar and both rates were less than the reported rate of fetal loss in the general population.34 The majority of the fetal losses were not attributed to the withdrawal process by the authors because most occurred after detoxification.
Birth weight was reported in 14 studies and intrauterine growth restriction was reported in one. The birth weight of neonates for women who were detoxified was found to be greater than those of women with ongoing illicit drug use in two studies22,27 and significantly less than neonates of women without opioid use disorder in two studies.20,21 Rates of preterm birth varied from 0 to 38% and there were no statistically significant differences reported in the rates of preterm birth between women who underwent detoxification and comparison groups. There was a minimal difference in the rates of preterm birth in the two studies that had a comparison group of women without opioid use disorder (5.5% vs 5.8%20 and 0% vs 0%25).
Neonatal abstinence syndrome was reported in 11 studies and was defined by pharmacotherapy treatment. Across studies, neonatal abstinence syndrome treatment rates ranged from 0 to 100%. Only two studies reported no newborn withdrawal among women who underwent detoxification.25,32 Variability in neonatal abstinence syndrome rates may in part be attributable to variability in treatment thresholds within the studies. Except for Sinha et al,32 which used a scoring system described by Rivers (Rivers score greater than 2),35 all of the studies used the Finnegan scoring system to determine treatment for neonatal abstinence syndrome.36 A Finnegan score greater than 7 was used by one study,20 a score 8 or greater was used by four studies,21,24,25,31 a score 9 or greater was used by two studies,26,28 a score 10 or greater was used by one study,19 and the scores used to treat neonates were not recorded in two studies.27,30 In addition to variability in scoring thresholds, many studies required more than two threshold scores to initiate treatment.19,21,24 Among the neonates whose mothers underwent detoxification, neonatal abstinence syndrome rates were significantly higher in Dooley et al (12.8% vs 6.2%; P<.001)20 and significantly lower in Haabrekke et al (0% vs 76.9%; P<.001)25 compared with pregnant women with illicit opioid use. Higher rates in Dooley et al may be in part the result of only “occasional” opioid use and a high spontaneous “quit” rate among pregnant women in the opioid comparison group. Significantly lower within-group differences were also found in neonatal abstinence syndrome rates among women who successfully completed detoxification compared with women who either resumed illicit opioid use27,32 or who resumed opioid pharmacotherapy during the detoxification process.21,32 Neonatal length of stay was reported in only nine of the studies. Pediatric outcomes beyond the neonatal period were reported for a small percentage of children in two studies. In Wallach et al, normal physical development and psychometric testing (“normal” [n=12], “high normal” [n=1], and “low normal results” [n=1]) for 14 children was provided at 4 years of age.30 Neuroanatomic, neurocognitive, and visual acuity outcomes from Haabrekke were reported for 12 children at 4.5 years of age in Walhovd.18 A detailed summary of maternal and neonatal outcomes reported by included studies is described in Appendices 3 and 4, available online at http://links.lww.com/AOG/B79.
The overall quality of the evidence ranged from “fair” to “poor” primarily as a result of study design, the lack of randomized controls, and a high risk of bias. Bias and quality judgments by the authors were informed by the largely retrospective approaches to data collection, minimal information about the detoxification and comparison group populations, insufficient detail about inclusion and exclusion criteria, self-selection of patients into detoxification groups, and failure to account for lost to follow-up and missing data. Together, these limitations prevent the interpretation of pregnancy outcomes after detoxification. A detailed description of bias and quality assessments for each included study are described in Appendix 2 (http://links.lww.com/AOG/B79).
DISCUSSION
Our review supports the recommendations of the American Society of Addiction Medicine, the American College of Obstetricians and Gynecologists, and the World Health Organization, which promote pharmacotherapy over detoxification for opioid use disorder in pregnancy as a result of low detoxification completion rates, high rates of relapse, and limited data regarding the effect of detoxification on maternal and neonatal outcomes beyond delivery.1–3 Although the current opioid crisis has prompted a reappraisal of detoxification, our review demonstrates that interest in detoxification during pregnancy has been present since the introduction of opioid pharmacotherapy. Although the evidence suggests that fetal demise is not increased with detoxification, loss to follow-up was an important limitation of all studies. As such, the strength of this finding should not be taken as support for abandoning opioid pharmacotherapy as the optimal treatment for opioid use disorder in pregnancy.
Interest in detoxification is driven in part from a desire to decrease the number of neonates with neonatal abstinence syndrome and their associated health care costs. However, our review does not support detoxification for the prevention of neonatal abstinence syndrome as a result of the high rate of relapse and, therefore, continued fetal opioid exposure. Furthermore, relapse as reported in the included studies was likely underreported as a result of lack of follow-up beyond the immediate postpartum period as well as high lost-to-follow-up rates across all studies. Relapse also increases the risk of human immunodeficiency virus, hepatitis, and overdose as exemplified by the two overdose deaths reported by Wallach et al.30
Addiction is a chronic neurochemical disease of brain reward, motivation, memory, and related circuitry whose symptoms manifest in behaviors.37 Detoxification is an acute intervention, which can manage the physical symptoms associated with withdrawal but does not address the chronic cycles of relapse and remission that characterize the illness. To wit, neither the Substance Abuse Mental Health Association nor the American Society of Addiction Medicine considers detoxification as standalone treatment and patients should be advised about risk of relapse from detoxification.1,38 The general addiction literature is illustrative here. Since the 1970s, detoxification has been associated with high rates of relapse39 and low treatment retention in contrast to methadone maintenance.40 A recent Cochrane review contrasting detoxification with buprenorphine maintenance similarly demonstrated increased rates of relapse and poor treatment adherence among individuals receiving detoxification alone.41 Although detoxification can be conceptualized as a door to treatment, the failure to provide ongoing behavioral and psychosocial interventions may contribute to the high rates of relapse associated with this process.38 Among the studies included in this review, few described any ongoing behavioral care after detoxification and none reported any supportive services after delivery.
Although some women may benefit from detoxification, future investigations should be aimed at characterizing the subpopulation of pregnant women for whom withdrawal is most beneficial. Guidelines regarding the optimal treatment regimen (ie, pharmacotherapeutic agent, setting, intensity, and duration of supporting psychosocial services) without increasing maternal and neonatal morbidity and mortality are warranted. Furthermore, as a result of the poor quality of the existing literature, rigorous, multicenter, randomized clinical trials with appropriate control groups are necessary to fully understand the short- and long-term consequences of opioid detoxification compared with pharmacotherapy during pregnancy. An intention-to-treat analytic approach including close attention to participants who are lost to follow-up should be used. To properly assess the risk of relapse, overdose, and overdose death, participants should be followed for at least 1 year after delivery with the effects of postpartum substance use on both maternal and pediatric outcomes evaluated.42–44 Finally, all participants should receive robust behavioral health counseling.
Clinical care considerations for pregnant women with opioid use disorder should be focused on the mother–infant dyad.45 Most participants in the included studies voluntarily participated in the detoxification process, which emphasizes the importance of pregnancy as a time of enhanced maternal investment in behavior change. However, taking advantage of the “pregnancy opportunity” to reinforce patient fears related to fetal opioid exposure and withdrawal by ceasing or not initiating pharmacotherapy should not be the primary driving force behind prevention and treatment efforts. Instead, gender-specific public health and treatment approaches highlighting the chronic nature of addiction and targeting women across the life course should be emphasized. Overall, the dialogue regarding opioid use disorder among women should be modified to emphasize that effective treatments are available before, during, and after pregnancy and efforts to expand comprehensive, women-centered treatment availability and accessibility are a more efficient and effective way to improve maternal and neonatal outcomes within and well beyond the perinatal period.
Acknowledgments
Research reported in this publication was supported in part by the National Institute on Drug Abuse (NIDA) under Award Number K23DA038789 (Dr. Krans). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank Carrie Everstine, MLS, for her assistance with the database searches and systematic review process.
Footnotes
Each author has indicated that he or she has met the journal’s requirements for authorship.
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.American Society of Addiction Medicine (ASAM) National practice guideline for the use of medications in the treatment of addiction involving opioid use. doi: 10.1097/ADM.0000000000000166. Available at: https://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. Retrieved January 26, 2018. [DOI] [PMC free article] [PubMed]
- 2.World Health Organization (WHO) Community management of opioid overdose. Available at: http://apps.who.int/iris/bit-stream/10665/137462/1/9789241548816_eng.pdf. Retrieved January 26, 2018.
- 3.Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e81–94. doi: 10.1097/AOG.0000000000002235. [DOI] [PubMed] [Google Scholar]
- 4.Rementeriá JL, Nunag NN. Narcotic withdrawal in pregnancy: stillbirth incidence with a case report. Am J Obstet Gynecol. 1973;116:1152–6. doi: 10.1016/0002-9378(73)90953-8. [DOI] [PubMed] [Google Scholar]
- 5.Zuspan FP, Gumpel JA, Mejia-Zelaya A, Madden J, Davis R. Fetal stress from methadone withdrawal. Am J Obstet Gynecol. 1975;122:43–6. doi: 10.1016/0002-9378(75)90613-4. [DOI] [PubMed] [Google Scholar]
- 6.Strauss ME, Andresko M, Stryker JC, Wardell JN, Dunkel LD. Methadone maintenance during pregnancy: pregnancy, birth, and neonate characteristics. Am J Obstet Gynecol. 1974;120:895–900. doi: 10.1016/0002-9378(74)90335-4. [DOI] [PubMed] [Google Scholar]
- 7.Finnegan LP. Management of pregnant drug-dependent women. Ann NY Acad Sci. 1978;311:135–46. doi: 10.1111/j.1749-6632.1978.tb16770.x. [DOI] [PubMed] [Google Scholar]
- 8.Martin CE, Longinaker N, Terplan M. Recent trends in treatment admissions for prescription opioid abuse during pregnancy. J Subst Abuse Treat. 2015;48:37–42. doi: 10.1016/j.jsat.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307:1934–40. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 10.Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, Smith PB, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372:2118–26. doi: 10.1056/NEJMsa1500439. [DOI] [PubMed] [Google Scholar]
- 11.Campbell WA. Opioid detoxification during pregnancy: the door continues to open. Am J Obstet Gynecol. 2016;215:258–60. doi: 10.1016/j.ajog.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.s. Preventive Services. Task Force. Grade definitions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions. Retrieved January 26, 2018.
- 16.Jones H. Scientific evidence and practical experience with methadone-assisted withdrawal of heroin-dependent pregnant patients. Heroin Addict Rel Clin Prob. 2008;10:33–8. [Google Scholar]
- 17.Blinick G, Wallach RC, Jerez E. Pregnancy in narcotics addicts treated by medical withdrawal. The methadone detoxification program. Am J Obstet Gynecol. 1969;105:997–1003. doi: 10.1016/0002-9378(69)90117-3. [DOI] [PubMed] [Google Scholar]
- 18.Walhovd KB, Bjornebekk A, Haabrekke K, Siqveland T, Slinning K, Nygaard E, et al. Child neuroanatomical, neurocognitive, and visual acuity outcomes with maternal opioid and polysubstance detoxification. Pediatr Neurol. 2015;52:326–32. e1–3. doi: 10.1016/j.pediatrneurol.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Bell J, Towers CV, Hennessy MD, Heitzman C, Smith B, Chattin K. Detoxification from opiate drugs during pregnancy. Am J Obstet Gynecol. 2016;215:374.e1–6. doi: 10.1016/j.ajog.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Dooley R, Dooley J, Antone I, Guilfoyle J, Gerber-Finn L, Kakekagumick K, et al. Narcotic tapering in pregnancy using long-acting morphine an 18-month prospective cohort study in northwestern Ontario. Can Fam Physician. 2015;61:e88–95. [PMC free article] [PubMed] [Google Scholar]
- 21.Kahila H, Saisto T, Kivitie-Kallio S, Haukkamaa M, Halmesmäki E. A prospective study on buprenorphine use during pregnancy: effects on maternal and neonatal outcome. Acta Obstet Gynecol Scand. 2007;86:185–90. doi: 10.1080/00016340601110770. [DOI] [PubMed] [Google Scholar]
- 22.Kyei-Aboagye K, Acker DB, MacBain D. The effect of postdetoxification drug-free residential living on birth outcome in the pregnant drug abuser. Subst Abus. 1998;19:123–8. doi: 10.1080/08897079809511381. [DOI] [PubMed] [Google Scholar]
- 23.LePreau FJ, Garvey B, Paull N, Stein MD. Opiate detoxification of pregnant women using clonidine: an observational cohort study. J Womens Health. 1995;4:381–86. [Google Scholar]
- 24.Dashe JS, Jackson GL, Olscher DA, Zane EH, Wendel GD., Jr Opioid detoxification in pregnancy. Obstet Gynecol. 1998;92:854–8. doi: 10.1016/s0029-7844(98)00312-3. [DOI] [PubMed] [Google Scholar]
- 25.Haabrekke KJ, Slinning K, Walhovd KB, Wentzel-Larsen T, Moe V. The perinatal outcome of children born to women with substance dependence detoxified in residential treatment during pregnancy. J Addict Dis. 2014;33:114–23. doi: 10.1080/10550887.2014.909698. [DOI] [PubMed] [Google Scholar]
- 26.Jones HE, O’Grady KE, Malfi D, Tuten M. Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. Am J Addict. 2008;17:372–86. doi: 10.1080/10550490802266276. [DOI] [PubMed] [Google Scholar]
- 27.Stewart RD, Nelson DB, Adhikari EH, McIntire DD, Roberts SW, Dashe JS, et al. The obstetrical and neonatal impact of maternal opioid detoxification in pregnancy. Am J Obstet Gynecol. 2013;209:267.e1–5. doi: 10.1016/j.ajog.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Lund IO, Fitzsimons H, Tuten M, Chisolm MS, O’Grady KE, Jones HE. Comparing methadone and buprenorphine maintenance with methadone-assisted withdrawal for the treatment of opioid dependence during pregnancy: maternal and neonatal outcomes. Substance Abuse Rehabil. 2012;3(suppl 1):17–25. doi: 10.2147/SAR.S26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luty J, Nikolaou V, Bearn J. Is opiate detoxification unsafe in pregnancy? J Subst Abuse Treat. 2003;24:363–7. doi: 10.1016/s0740-5472(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 30.Wallach RC, Jerez E, Blinick G. Comparison of pregnancies and births during methadone detoxification and maintenance. Pediatr Ann. 1975;4:46–61. doi: 10.3928/0090-4481-19750701-10. [DOI] [PubMed] [Google Scholar]
- 31.Maas U, Kattner E, Weingartjesse B, Schäfer A, Obladen M. Infrequent neonatal opiate withdrawal following maternal methadone detoxification during pregnancy. J Perinat Med. 1990;18:111–8. doi: 10.1515/jpme.1990.18.2.111. [DOI] [PubMed] [Google Scholar]
- 32.Sinha C, Ohadike P, Carrick P, Pairaudeau P, Armstrong D, Lindow SW. Neonatal outcome following maternal opiate use in late pregnancy. Int J Gynecol Obstet. 2001;74:241–6. doi: 10.1016/s0020-7292(01)00446-5. [DOI] [PubMed] [Google Scholar]
- 33.Hulse GK, O’Neill G, Pereira C, Brewer C. Obstetric and neonatal outcomes associated with maternal naltrexone exposure. Aust N Z J Obstet Gynaecol. 2001;41:424–8. doi: 10.1111/j.1479-828x.2001.tb01322.x. [DOI] [PubMed] [Google Scholar]
- 34.MacDorman MF, Gregory EC. Fetal and perinatal mortality: United States, 2013. Natl Vital Stat Rep. 2015;64:1–24. [PubMed] [Google Scholar]
- 35.Rivers RP. Neonatal opiate withdrawal. Arch Dis Child. 1986;61:1236–9. doi: 10.1136/adc.61.12.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finnegan LP, Connaughton JF, Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2:141–58. [PubMed] [Google Scholar]
- 37.American Society of Addiction Medicine. Public policy statement: definition of addiction. Available at: http://www.asam.org/docs/publicy-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=2. Retrieved January 26, 2018.
- 38.Substance Abuse and Mental Health Services Administration. Detoxification and substance abuse treatment. Substance Abuse and Mental Health Services Administration; 2006. Rockville (MD) (Treatment Improvement Protocol (TIP) Series, No. 45. HHS publication No. (SMA) 13-4131). [Google Scholar]
- 39.Stimmel B, Goldberg J, Rotkopf E, Cohen M. Ability to remain abstinent after methadone detoxification. A six-year study. JAMA. 1977;237:1216–20. [PubMed] [Google Scholar]
- 40.Bass UF, 3rd, Brown BS. Methadone maintenance and methadone detoxification: a comparison of retention rates and client characteristics. Int J Addict. 1973;8:889–95. doi: 10.3109/10826087309033095. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. The Cochrane Database of Systematic Reviews 2016. (5):CD011117. doi: 10.1002/14651858.CD011117.pub2. Art. No. [DOI] [PubMed] [Google Scholar]
- 42.Simkiss DE, Stallard N, Thorogood M. A systematic literature review of the risk factors associated with children entering public care. Child Care Health Dev. 2013;39:628–42. doi: 10.1111/cch.12010. [DOI] [PubMed] [Google Scholar]
- 43.O’Donnell M, Maclean MJ, Sims S, Morgan V, Leonard H, Stanley F. Maternal mental health and risk of child protection involvement: mental health diagnoses associated with increased risk. J Epidemiol Community Health. 2015;69:1175–83. doi: 10.1136/jech-2014-205240. [DOI] [PubMed] [Google Scholar]
- 44.Barnet B, Duggan AK, Wilson MD, Joffe A. Association between postpartum substance Use and depressive symptoms, stress, and social support in adolescent mothers. Pediatrics. 1995;96:659–66. [PubMed] [Google Scholar]
- 45.Klaman SL, Isaacs K, Leopold A, Perpich J, Hayashi S, Vender J, et al. Treating women who are pregnant and parenting for opioid use disorder and the concurrent care of their infants and children: literature review to support national guidance. J Addict Med. 2017;11:178–90. doi: 10.1097/ADM.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]


