Abstract
Rationale: E-cigarettes vaporize propylene glycol/vegetable glycerin (PG/VG), nicotine, and flavorings. However, the long-term health effects of exposing lungs to vaped e-liquids are unknown.
Objectives: To determine the effects of chronic vaping on pulmonary epithelia.
Methods: We performed research bronchoscopies on healthy nonsmokers, cigarette smokers, and e-cigarette users (vapers) and obtained bronchial brush biopsies and lavage samples from these subjects for proteomic investigation. We further employed in vitro and murine exposure models to support our human findings.
Measurements and Main Results: Visual inspection by bronchoscopy revealed that vaper airways appeared friable and erythematous. Epithelial cells from biopsy samples revealed approximately 300 proteins that were differentially expressed in smoker and vaper airways, with only 78 proteins being commonly altered in both groups and 113 uniquely altered in vapers. For example, CYP1B1 (cytochrome P450 family 1 subfamily B member 1), MUC5AC (mucin 5 AC), and MUC4 levels were increased in vapers. Aerosolized PG/VG alone significantly increased MUC5AC protein in human airway epithelial cultures and in murine nasal epithelia in vivo. We also found that e-liquids rapidly entered cells and that PG/VG reduced membrane fluidity and impaired protein diffusion.
Conclusions: We conclude that chronic vaping exerts marked biological effects on the lung and that these effects may in part be mediated by the PG/VG base. These changes are likely not harmless and may have clinical implications for the development of chronic lung disease. Further studies will be required to determine the full extent of vaping on the lung.
Keywords: vaping, tobacco, mucin, chronic obstructive pulmonary disease
At a Glance Commentary
Scientific Knowledge on the Subject
Limited information is available on the effects of chronic e-cigarette use on the lung.
What This Study Adds to the Field
We show that chronic e-cigarette use alters ∼200 proteins in airway epithelia. Further confirmation of these changes is provided by in vitro and in vivo studies.
E-cigarettes represent a relatively new alternative to tobacco smoking that use a propylene glycol/vegetable glycerin (PG/VG) base to deliver nicotine and flavors (collectively called an “e-liquid”) to the lung via an electronic delivery device that “vaporizes” the e-liquid into an aerosol (1). People inhale tobacco smoke for the psychotropic effects of nicotine (2). The adverse health effects of chronically inhaling combusted tobacco on the lung have been well described and include autophagy, DNA damage, goblet cell metaplasia, increased inflammation, and increased proteolysis in the lung. All of these changes can lead to increased incidences of chronic obstructive pulmonary disease (COPD) and lung cancer, as well as significant extrapulmonary effects including cardiovascular disease (3–7). Whether or not vaping is safe is highly controversial (8). Proponents of vaping suggest that it is safer than smoking and constitutes harm reduction (9, 10). Opponents of vaping have highlighted that vaped e-liquids contain toxic chemicals including formaldehyde and heavy metals, which can cause respiratory disease (11–13). The British government announced that e-cigarettes are 95% safer than traditional tobacco products and recommended that people switch from tobacco to e-cigarettes (14). In contrast, the U.S. Surgeon General’s report (2016) stated “E-cigarette aerosol is not harmless. It can contain harmful and potentially harmful constituents including nicotine,” suggesting that the U.S. government is taking an alternative stance (15). Moreover, this report noted a concerning increase in vaping among adolescents and never-before smokers in young adults (15).
Several academic laboratories have demonstrated that e-cigarette exposure causes inflammation, oxidative stress, and/or is toxic to multiple cell types including pulmonary, endothelial, and stem cells in vitro (16–18). Similarly, murine e-cigarette exposures also caused inflammation and oxidative stress (17, 18). In contrast, emerging in vitro data from the tobacco industry purport that vaping is safer than conventional tobacco exposure (19). However, the tobacco industry has previously made erroneous claims of modified risk, that is, that low-tar cigarettes are safer (20). Thus, given the growing popularity of vaping, it will be vital to independently and agnostically determine the potential for modified risk versus harm of e-cigarettes. Martin and colleagues performed a limited gene expression study on nasal mucosa and found that 305 genes were uniquely downregulated in vapers, suggesting immunosuppression (21). In an epidemiological study, McConnell and colleagues found that “bronchitic symptoms were associated with use of e-cigarettes among adolescents” (22) and it has been shown that vaper’s sputum has proteomic alterations that are distinct from tobacco exposure (23). However, to date, in vivo data on the effects of e-cigarettes on the lower airways are lacking. Because of the paucity of information in this field, we recruited healthy smokers, e-cigarette users (vapers), and nonsmoking control subjects and performed research bronchoscopies to obtain brush biopsy and lavage samples. In parallel, we also vaped human bronchial epithelial cultures and mice to test whether we could replicate our in vivo observations.
Methods
Detailed methods are shown in the online supplement.
Subject Recruitment and Bronchoscopy
We recruited healthy subjects as nonsmokers, smokers, and vapers for our study. Fiberoptic bronchoscopy was performed according to American Thoracic Society guidelines (24). Full details are provided in the online supplement.
Merocyanine 540 Emission Scan
HEK293T cells were seeded into 96-well plates (Corning) and human bronchial epithelia were cultured on semipermeable inserts in 24-well plates (Corning). Cells were loaded with 100 μM merocyanine 540 (M540; Sigma-Aldrich) at 37°C for 30 minutes, washed, and placed in Ringer’s solution and emission spectra read (excitation, 540 nm; emission, 550–700 nm), using an Infinite plate reader (Tecan). Data were background subtracted, using unlabeled cultures, and normalized to baseline emission fluorescence.
Fluorescence Recovery after Photobleaching
HEK293T cells were seeded on #1.5 glass coverslips and transiently transfected with constructs, using Lipofectamine 2000 (Thermo Fisher) as per the manufacturer’s instructions. Cells were pretreated with 3% PG/VG or vehicle for 1 hour at 37°C before imaging. Fluorescence recovery after photobleaching was performed 24–48 hours after transfection, using a Leica SP5 confocal microscope with a 63 × 1.30 numerical aperture glycerol immersion lens as described (25).
Intracellular E-Liquid Imaging
HEK293T cells were seeded onto #1.5 glass coverslips for 24 hours, and primary human bronchial epithelia were cultured at the air–liquid interface for 4 weeks on 12-mm Transwell-clear membranes (Corning). Cells were stained with 3 μM calcein-AM (Thermo Fisher) for 30 minutes at 37°C and mounted in Attofluor imaging chambers (Thermo Fisher). Images were acquired before and after treatment with 150 μl of 3% (vol/vol) Pixie Dust (The Vapor Girl) after exposure with the 405-nm laser line and collected between 409 and 459 nm, using a Leica SP8 confocal microscope with a 63 × 1.40 numerical aperture glycerol immersion lens. The dose of 3% e-liquid was selected as previously it was shown that this causes moderate toxicity (26).
Statistical Analysis
For statistical analysis, P ≤ 0.05 was taken as significant. Full statistical methods are shown in the online supplement. In vivo data are shown as mean ± SD and in vitro data are shown as mean ± SE, where n = the number of subjects, donors, or replicates as applicable.
Results
Chronic Vaping Causes Erythematous Airway Mucosa
We recruited healthy smokers, e-cigarette vapers, and nonsmokers who met the inclusion criteria. Spirometry was normal in all groups, and no obvious underlying abnormalities were detected (Table 1). To confirm smoking/vaping patterns, each subject completed a 2-week smoking diary and we measured serum cotinine/hydroxycotinine. Serum cotinine and hydroxycotinine levels in smokers (140.0 ± 100.7 and 43.3 ± 30.6, respectively; n = 11) were similar to those in vapers (97.2 ± 72.2 and 26.1 ± 21.7, respectively; n = 10). Nonsmokers exhibited no detectable level of either cotinine or hydroxycotinine (n = 13). Smokers consumed 10.1 ± 4.1 cigarettes per day and 9.5 ± 6.2 pack-years (n = 9). E-cigarette use was reported in both puff numbers and milliliters of e-liquid consumed. Mean e-cigarette consumption per day was 44.1 ± 82.2 puffs or 11.4 ± 17.0 ml/day. Although the age of the smokers was significantly higher (P = 0.019) than that of nonsmokers and vapers, no significant difference was observed in sex, race, or body mass index (Table 1). Analysis of BAL cytology showed no significant difference (P > 0.05) in cell types among subject cohorts (Table 1). Nonsmokers tolerated the procedure well, with minimal (if any) cough after intubation of the vocal cords, and their airway mucosa were healthy in appearance (uniformly pale pink). In contrast, e-cigarette vapers and healthy smokers generally coughed throughout the procedure, and e-cigarette vapers had more erythematous and irritable airway mucosa than did nonsmokers (see Figure E1 in the online supplement).
Table 1.
Demographic Details and Cytological Characteristics of BAL from Subjects Categorized as Nonsmokers, Smokers, and Vapers
| Nonsmokers | Smokers | Vapers | |
|---|---|---|---|
| Male/female, n | 8/10 | 7/6 | 7/3 |
| Ethnicity (African American/Asian/Hispanic/white/other), n | 5/1/3/8/1 | 6/0/0/7/0 | 1/2/0/7/0 |
| Age at bronchoscopy, yr | 27.33 ± 7.71 | 34.00 ± 8.02 | 26.80 ± 8.53 |
| Height, cm | 166.86 ± 8.44 | 169.33 ± 9.96 | 172.23 ± 7.75 |
| Weight, kg | 79.04 ± 22.38 | 81.37 ± 19.84 | 85.94 ± 15.16 |
| Body mass index, kg/m2 | 28.13 ± 6.54 | 28.41 ± 6.65 | 29.10 ± 5.35 |
| FVC% | 100.17 ± 20.17 | 105.46 ± 11.79 | 101.00 ± 6.48 |
| FEV1% | 101.89 ± 13.31 | 99.38 ± 13.90 | 100.60 ± 9.12 |
| Polymorphonuclear cells, % | 2.67 ± 2.26 | 2.69 ± 2.35 | 2.88 ± 2.16 |
| Macrophages, % | 96.34 ± 2.08 | 96.62 ± 2.40 | 96.08 ± 2.41 |
| Eosinophils, % | 0.43 ± 0.84 | 0.51 ± 0.59 | 0.40 ± 0.49 |
| Lymphocytes, % | 0.12 ± 0.31 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Bronchial epithelial cells, % | 0.43 ± 0.63 | 0.18 ± 0.31 | 0.64 ± 0.73 |
| Squamous epithelial cells, % | 0.16 ± 0.22 | 0.40 ± 0.58 | 0.20 ± 0.23 |
Data presented are mean ± SD, unless specified otherwise.
Vaping Alters Protein Expression in Bronchial Epithelia
We used bronchoscopy to obtain brush biopsies from the left and right main bronchi or right bronchus intermedius. Protein was extracted from the brushes and stored at −80°C until all samples could be concurrently analyzed by liquid chromatography–tandem mass spectrometry. For this analysis, samples without visual blood contamination were selected to avoid possible interference from blood proteins, and hemoglobin levels were used as covariate in the statistical model to account for possible blood contamination. The demographics and cytology pertaining to the subjects selected for proteomic analyses are shown in Table E1. After mass spectrometry and normalization of label-free quantification intensity data, three samples were identified as outliers due to higher nondetection rate in the hierarchical clustering, principal component analysis, and histogram of imputed data and were removed. The final analysis of differential protein expression included eight nonsmokers, nine smokers, and nine vapers.
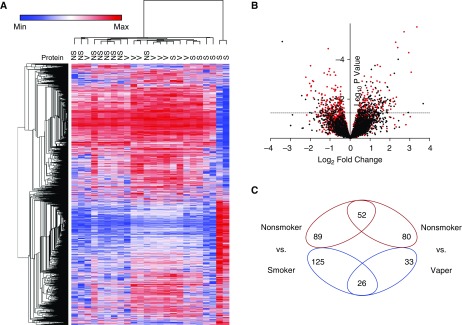
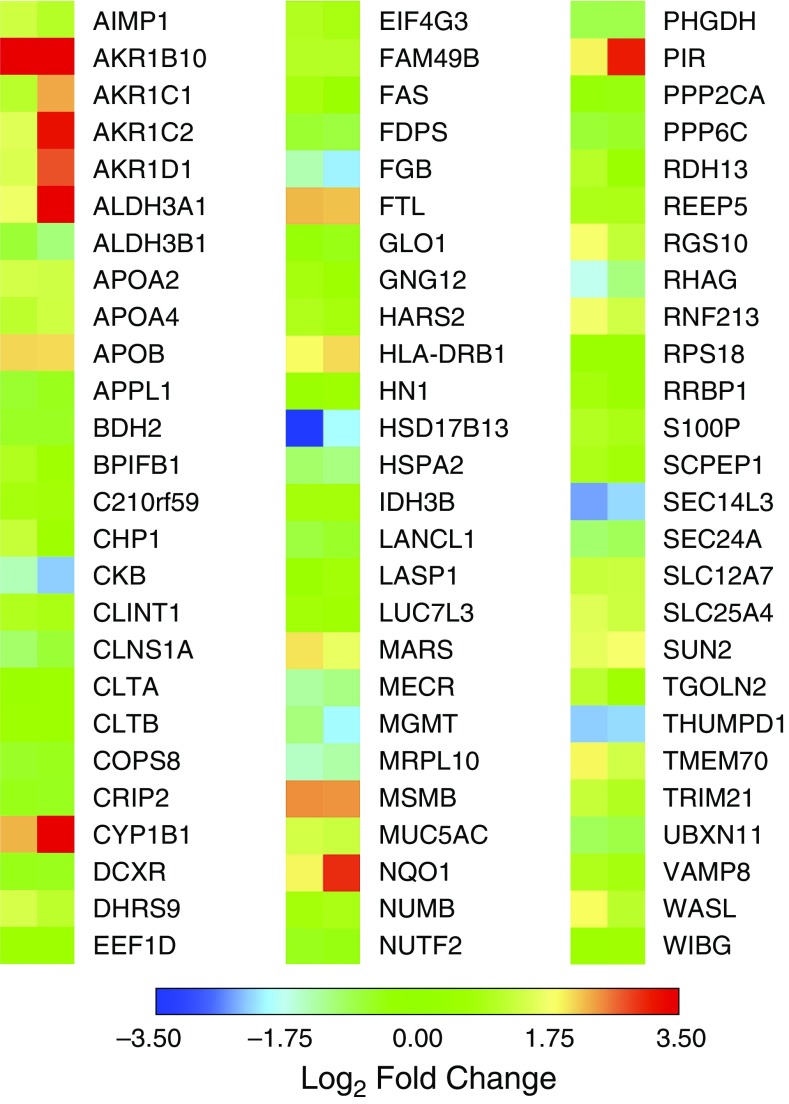
Changes in overall protein abundance in smokers and vapers were different, as evidenced by subject clustering versus protein expression (Figure 1A). A volcano plot also indicated the unique changes in expression of smokers’ and vapers’ proteins (Figure 1B). Our data revealed that 191 proteins were significantly (P < 0.05) up- or downregulated in the vapers, compared with 292 altered proteins in the smokers (Figure 1C and Tables E3 and E4). Only 78 proteins were altered in both groups (Figure 1C). Furthermore, vapers had 132 upregulated and 59 downregulated proteins whereas smokers had 141 and 151 upregulated and downregulated proteins, respectively (Figure 1C). The significantly changed proteins common to smokers and vapers are shown in Figure 2. Select protein changes, along with a housekeeping protein (GAPDH), were confirmed by Western blot, and also shown as label-free quantification (Figure E2). Of note, the secreted/gel-forming mucin MUC5AC, and VAMP8 (vesicle-associated membrane protein 8), a SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) trafficking protein that facilitates mucin secretion, were upregulated in both groups (Figure 2, Figure E2, and Tables E3 and E4). In contrast, MUC4 was upregulated only in the vapers (Table E3) whereas MUC5B was significantly downregulated in the smokers (Table E4). We then used pathway analysis to further investigate these changes. Interestingly, intracellular proteins from many organelles were altered by both vaping and smoking, with proteins from “membrane-bound organelles” forming the largest group that were altered (150 proteins). However, 14 pathways that included proteins involved in organelle membranes, early endosomes/trafficking, macromolecular complexes, and mitochondria were uniquely altered in vapers (Table E5).
Figure 1.
Vaping leads to unique changes in the airway proteome. (A) Heat map of differentially expressed proteins from bronchial brush biopsies from the various groups. Vapers cluster together and are different from nonsmokers and smokers. (B) Log2 fold change in abundance versus significance for all identified proteins for smokers (red dots) and vapers (black dots) relative to nonsmokers. The dashed line indicates a significance (P) value of 0.05. (C) Venn diagram of increased (red) or decreased (blue) proteins in smokers and vapers relative to nonsmokers. NS = nonsmokers; S = smokers; V = vapers.
Figure 2.
Proteins commonly altered in vapers and smokers. Heat map of protein expression (log2 fold change and P < 0.05) for proteins that are commonly altered in vapers and smokers. Data from bronchial brushings of nonsmokers (n = 8), smokers (n = 9), and vapers (n = 9).
PG/VG Exposure Alters Protein Levels in Cultured Primary Human Airway Epithelia and Murine Nasal Epithelia
In the proteomic study, the subjects were allowed to vape their preferred e-liquid. Given the heterogeneity in the more than 7,000 available flavors, we therefore hypothesized that the observed changes were due to either PG/VG and/or nicotine rather than to the flavors. Thus, to better understand what drove the protein changes in vapers’ airways, we acutely exposed primary human bronchial epithelial cultures (HBECs) to aerosolized PG/VG mixed at a 55/45 ratio, which is a common ratio that is locally available. We exposed HBECs to 36 puffs per day because 1) we have previously shown that this dose affects airway epithelia (26) and 2) this was close to the median puffs per day in our vape study group (i.e., 44 puffs). The aerosol was diluted at various percentages of ambient air, and cellular MUC5AC levels were measured 24 hours later by immunostaining. Our data indicated that a 30-minute exposure to PG/VG was sufficient to drive a three- to fourfold increase in MUC5AC levels (Figures 3 and E3). Exposure to PG/VG containing nicotine at 18 mg/ml (e.g., 111 mM nicotine) had no further effect on MUC5AC expression, indicating that these changes were driven by PG/VG and not by nicotine (Figure 3). We then subchronically (4 d) exposed HBECs to PG/VG, which also increased cellular MUC5AC levels (Figure E4). Because CYP1B1 (cytochrome P450 family 1 subfamily B member 1) was upregulated in vapers’ airways, we also tested whether this protein was upregulated in vitro. Again, using the acute vape protocol, we found that a 30-minute exposure significantly upregulated CYP1B1 protein in HBECs (Figure E5).
Figure 3.
Acute PG/VG exposure increases intracellular MUC5AC levels in cultured human bronchial epithelia. (A) Typical hematoxylin and eosin and Alcian blue–periodic acid–Schiff staining of fixed bronchial cultures after acute air versus 36.8% PG/VG/nicotine exposures. (B) Representative confocal microscopy images of fixed cultures stained with Hoechst (nuclei; blue), MUC5AC (green), and cilia (α-tubulin; white). (C) Mean log2 fold changes in MUC5AC fluorescence after exposure to various doses of PG/VG (shaded columns) or PG/VG + nicotine (solid columns). *P < 0.05, different air exposure (i.e., 0% PG/VG). Data shown as means ± SEM. AB/PAS = Alcian blue–periodic acid–Schiff; H&E = hematoxylin and eosin; MUC5AC = mucin 5AC; PG/VG = propylene glycol/vegetable glycerin.
Because PG/VG alone was sufficient to increase intracellular MUC5AC levels in HBECs, we then exposed wild-type mice to aerosolized PG/VG. Mice are obligate nose breathers and exhibit a high level of filtration in the upper respiratory tract/nasal passages (27). Accordingly, we then measured intracellular MUC5AC levels in murine nasal epithelia. We exposed mice to PG/VG or air for 3 hours and harvested nasal tissue samples 24 hours later. We found that PG/VG increased MUC5AC levels compared with air-exposed littermate mice (Figures 4A and 4B). To validate the antibody specificity, we ran samples from a MUC5AC knockout mouse (Figure 4A). As an additional validation, because the Ca2+-regulating protein STIM1 (stromal interacting molecule 1) was specifically upregulated in vapers, we also measured this protein and found that it was also upregulated by PG/VG exposure (Figures 4C and 4D). Of note, Garcia-Arcos and colleagues showed that prolonged exposure to vape causes airspace enlargement with mucin, cytokine, and protease expression in mouse lung (28).
Figure 4.
Acute PG/VG exposure increases upper airway MUC5AC and STIM1 expression in mice. (A) Left: Representative Western blot of MUC5AC from the nasal epithelia of wild-type mice after 3 hours of acute exposure to PG/VG or air (control). Right: Western blot probing for MUC5AC in wild-type and MUC5AC knockout mice as an antibody control. (B) Densitometric analysis of MUC5AC expression in PG/VG- and air-exposed mice (n = 6 per group). (C) Representative Western blot of STIM1 with GAPDH as the loading control in nasal epithelia of mice after 3 hours of exposure to PG/VG or air. (D) Densitometric analysis of STIM1 expression normalized to GAPDH in PG/VG-exposed mice compared with air (both n = 6). *P ≤ 0.05 in PG/VG compared with air. Data shown as means ± SD. The numbers on Western blots indicate protein from individual mice. KO = knockout; MUC5AC = mucin 5AC; PG/VG = propylene glycol/vegetable glycerin; STIM1 = stromal interaction protein 1; WT = wild type.
PG/VG Affects Plasma Membrane Fluidity and Impairs Protein Diffusion
Because our proteomic data indicated that proteins involved in membrane biology were most affected by vaping (Table E5), we hypothesized that PG/VG could incorporate into cellular membranes and alter protein function. We have previously shown that many e-liquids are autofluorescent in the ultraviolet range (29) and that 3% e-liquid addition typically causes moderate toxicity (26). Using autofluorescence as an indicator, we found that 3% Pixie Dust e-liquid could rapidly enter HBECs (Figure 5A). For HEK293T cells, penetration was also observed to occur in a dose-dependent fashion (Figure 5B and Figure E6). Merocyanine 540 (M540) is a fluorescent dye that incorporates into lipid bilayers, including the plasma membrane, and increases in fluorescence in direct proportion to membrane fluidity (30). We loaded M540 into HBECs to assess the effects of PG/VG on membrane fluidity. To calibrate the dye, we decreased membrane fluidity by reducing the temperature with ice-cold Ringer solution, which significantly diminished M540 fluorescence (Figure 5C). We then read M540 fluorescence in HBECs loaded with M540, added PG/VG, and reread fluorescence at 37°C. Using this approach, we found that PG/VG produced a similar diminution of M540 fluorescence as seen with reduced temperature (Figure 5C). M540 fluorescence was also sensitive to PG/VG in HEK293T cells in a dose-dependent manner (Figure E7). Taken together, these data suggested that PG/VG increased plasma membrane rigidity. To further test the effects of PG/VG, we used fluorescence recovery after photobleaching to measure diffusion of the plasma membrane proteins anoctamin 1 (Ano1) and Orai1. In both cases, PG/VG exposure decreased their diffusivity (Figures 5D and 5E), which is consistent with the decreased membrane fluidity seen with M540.
Figure 5.
PG/VG affects membrane fluidity and protein diffusion. (A) Confocal micrographs (x–z plane) of human bronchial epithelial cultures (HBECs) stained with calcein (green) and Pixie Dust e-liquid excited at 405 nm (red). Representative images of three independent experiments were taken before and 10 minutes after addition of e-liquid. The dashed yellow lines indicate the apical surface of the culture. (B) Representative x–y-plane confocal micrograph of HEK293T cells (gray differential interference contrast image) before and after 5 minutes of exposure to Pixie Dust e-liquid (purple). (C) Emission scan of merocyanine 540 (M540) in HBECs. Black circles, vehicle at 37°C; red squares, 3% PG/VG at 37°C; blue triangles, vehicle at 4°C. All n = 9 cultures from three separate donors. (D and E) Fluorescence recovery after photobleaching of (D) Orai1-YFP and (E) Ano1-GFP in HEK293T cells after vehicle or 3% PG/VG exposure. Cells transfected with Orai1-YFP or Ano1-GFP were exposed to PG/VG for 1 hour before FRAP was measured. Data shown as means ± SEM. All n = 12–16 cultures per group from three or four independent experiments. *P ≤ 0.05. Ano1 = anoctamin 1; FRAP = fluorescence recovery after photobleaching; GFP = green fluorescent protein; Orai1 = calcium release-activated calcium modulator 1; PG/VG = propylene glycol/vegetable glycerin; RG = Ringer’s glucose; YFP = yellow fluorescent protein.
Discussion
The lower airways are an important site of pathology for many diseases including asthma, chronic bronchitis, and cystic fibrosis, suggesting that this region of the respiratory tract is relevant to the study of vaping. Researchers have previously performed proteomic and genomic studies on the airways of tobacco smokers (31, 32). In common with these studies, we detected changes in mucins, bacterial permeability–increasing family members, and epidermal growth factor receptor in smokers’ airways, indicating that our approach is valid (Table E4). Importantly, our study is the first to look for proteomic changes in the lower airways of vapers. We deliberately chose younger subjects who were all healthy, as indicated by normal spirometry results, to avoid possible confounders due to the development of COPD or other pathology (Table 1). However, whereas BAL cytology profiles were similar across groups, bronchoscopy revealed that vapers’ airways were more erythematous and irritable to contact, compared with those of nonsmokers (Figure E1), suggesting that vaping is not harmless.
We report here that approximately 300 and approximately 200 proteins were significantly altered in smokers’ and vapers’ bronchial epithelia, respectively, with groups of proteins associated with membranes especially being altered in vapers (Figure 1 and Tables E3–E5). In Figure 2, we focused on the commonly changed proteins, many of which are known to be pathogenic. For example, mucins are generated in goblet cells, and their expression level increases both with tobacco exposure and in COPD, where they are associated with intraluminal obstruction that serves as a nidus for airway disease (33). We observed increased intracellular MUC5AC and MUC4 in vapers, which may be indicative of airway remodeling and goblet cell metaplasia/hyperplasia (Figure 2 and Table E3). Furthermore, whereas MUC5AC was upregulated in both vapers and smokers, MUC4 was upregulated only in vapers and MUC5B was downregulated in smokers and unchanged in vapers. Our in vitro and in vivo data suggested that changes in MUC5AC levels were acutely driven by PG/VG exposure (Figures 3 and 4). Given the established relevance of mucins to airway disease including COPD, the altered mucin expression profile may be useful as a biomarker of exposure, and to differentiate between vapers and smokers. However, secreted MUC5AC levels are also increased in vapers (23) and we hypothesize that MUC5AC levels may also serve as a biomarker of harm, because increased mucin expression may be indicative of impending airway obstruction (34, 35). Given the ubiquity of PG/VG as the base vehicle in most (if not all) e-liquids, we propose that its presumed safety now urgently needs to be reevaluated because it may induce increased mucin production (14).
Although PG/VG does not fluoresce, many e-liquids are autofluorescent (29). Accordingly, we used autofluorescence to probe e-liquid distribution in vitro. Our data indicated that components of the Pixie Dust e-liquid rapidly penetrated cells (Figures 5A and 5B, E6, and E7). Propylene glycol (PG) is a common chemical used to produce polyester and as a deicer/antifreeze, as well as being a base constituent in e-liquids. Intravenous PG can cause acute renal and CNS toxicity (36), and PG inhalation causes renal and liver toxicity (37). PG inhibits renal glucose transport and corneal Na+/K+ ATPase activity (38, 39). Vegetable glycerin (glycerol; VG) forms the backbone of many glycerophospholipids in eukaryotic cell membranes (40) and can incorporate directly into membranes to increase their stiffness (41). Both PG and VG are permeable through aquaporins including AQP3, which is expressed in the lung (42, 43), suggesting that they may reach and disrupt intracellular membranes (44). Although it is possible that nicotine and/or flavors exerted effects, we speculate that at least some of the changes seen in the airways are due to interactions between PG/VG and the lipid bilayers that bound all organelles. Importantly, protein mobility is an important indicator of function. Thus, an increase in membrane rigidity and decrease in protein movement may impair the functions of multiple proteins, and may also impair endocytosis and exocytosis, which are strongly dependent on the production of membrane vesicles that form from existing membranes (45).
In our in vitro studies, we used both neat and vaped e-liquids. Our previous studies did not find any difference between neat and vaped e-liquids, suggesting that such an approach is valid (26). However, we cannot exclude the possibility that some pyrolysis occurred during aerosol generation that could affect some readouts. Using our previous studies as a guide, we used approximately 30-minute exposure sessions to 36 puffs for our in vitro studies. How much e-liquid is deposited in the lungs after vaping is not known, and further studies will be needed to validate or refute our approaches. Interestingly, the predicted deposition of e-cigarette aerosol in the lungs is approximately 25%, suggesting that our exposure regimen using the VC 10 smoking robot (Vitrocell Systems) is valid (46).
The liver is the primary site of xenobiotic metabolism. However, the lung also plays a role in this process, and we found that several xenobiotic enzymes were altered in vapers’ airways (Tables E6 and E7), suggesting that the lung reacted to the chemical burden of vaping by increasing its ability for xenobiotic detoxification. For example, CYP1B1 was upregulated, suggesting increased phase I metabolism, which may lead to the generation of more covalent adducts. Similar upregulation was also observed in vape-exposed HBECs (Figure E5), indicating that this is a direct consequence of vaping. Conversely, phase II metabolism proteins (i.e., glutathione S-transferases) were downregulated, indicating that the ability to conjugate and solubilize chemicals may be diminished (Tables E6 and E7). Covalent adducts damage DNA, leading to cancer-causing mutations, and can also post-translationally alter protein function (47–49). Although vapers are less likely to be exposed to tar-phase metabolites than smokers, CYP1B1 is also involved in the metabolism of steroids, bioflavonoids, and xenobiotic oxidation, and the chemical components of e-liquids may also be substrates for P450 proteins (50–52). However, the metabolism of e-liquid constituents in the lung and elsewhere and their propensity for mutagenesis are poorly understood. Furthermore, although we recruited vapers with minimal current combustible tobacco use, a large number of vapers still smoke combustible tobacco products (53), suggesting that the altered xenobiotic metabolism will be especially relevant to dual or mixed users.
We also observed that 113 proteins were uniquely changed in vapers (Figure 1 and Tables E3 and E4), and further studies will be needed to understand their implications. However, STIM1, an endoplasmic reticulum–resident Ca2+-regulating protein, was uniquely upregulated in vapers (Table E3 and Figure E2), and this change was reprised after PG/VG exposure in mice (Figures 4C and 4D). STIM1 is ubiquitously expressed and plays a critical role in Ca2+ homeostasis (54). Cellular Ca2+ controls multiple aspects of cell function including apoptosis, cell division, gene expression, and protein secretion (54). Altered STIM1 levels may thus be indicative of altered Ca2+ metabolism, which could have wide-ranging implications. We also observed that Toll-like receptor 3 was uniquely downregulated in vapers (Table E3). Toll-like receptor 3 recognizes double-stranded RNA and plays a role in defense against viral infections (55), suggesting that innate defense against viruses may be impaired in vapers. These data are consistent with epidemiological observations that vapers are three times more likely to have a respiratory infection than are nonsmokers (22).
Our study has two possible limitations: First, we acknowledge that our sample size is relatively low, resulting in limited statistical power. However, we confirmed important proteomic “hits” by an additional technique (i.e., Western blotting) and our data provide impetus for conducting larger, multicenter studies to examine the effects of vaping on the lung. Second, the vapers were mostly ex-smokers, and so changes should be interpreted in the context of this background. Importantly, we could replicate the changes in CYP1B1, MUC5AC, and STIM1 experimentally (Figures 3, 4, E3, and E6), suggesting that at least some of the proteomic changes were a direct consequence of vaping. For future studies, it would be best to recruit vapers who are never-smokers, but although this is ideal, there are practical limitations from a recruiting standpoint because many vapers also have a significant history of tobacco use. However, Beane and colleagues demonstrated that smoking cessation reverses approximately 80% of tested genes altered by smoking (56). Of note, CYP1B1 and some aldo-keto reductases were rapidly reversible after smoking cessation, suggesting that the changes in detoxifying proteins are due to vaping (56).
E-cigarettes have been recommended by some physicians as a smoking alternative/cessation device (57). Here, we demonstrate that vaping exerts marked and extensive biological effects on human airways, suggesting 1) that inhalation of e-cigarette vapor is not without consequences and is by no means innocuous and 2) that they should not be prescribed as a safe or harmless tobacco alternative. Finally, although the implications of our proteomic data are not yet fully understood, they have generated several testable hypotheses to be further explored. Given the extent of the changes observed with vaping, we propose that vaping be carefully monitored and not promoted as a “safe” smoking alternative and that the implications of these changes should be extensively investigated.
Acknowledgments
Acknowledgment
The authors thank Martha Almond, Carol Robinette, and Heather Wells for technical assistance and gratefully acknowledge Dr. Neal Benowitz (UCSF) for analyzing serum cotinine/hydroxycotinine (P50-CA180890, S10R.5R026437). The authors also thank Leslie Fulcher, Mariam Lam, and John Minges for assistance with in vitro exposures and thank the UNC CF Center Histology Core.
Footnotes
Supported by NIH grants HL120100, HL135642, and CA016086. Research reported in this publication was in part supported by the NIH and the Food and Drug Administration (FDA) Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Author Contributions: A.G., T.M., T.R.R., E.S.D., K.R., M.J.W., L.E.H., M.A.H., and A.L.-B. performed experiments. R.C.C. interviewed subjects and performed bronchoscopies. A.G., E.S.D., K.R., M.J.W., H.D., L.E.H., M.F.S., and S.K.V.B. analyzed data. L.M.G., S.H.R., N.E.A., and R.T. designed experiments. A.G. and R.T. wrote the manuscript, and all other authors edited/approved the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201710-2033OC on February 26, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Grana R, Benowitz N, Glantz S. Background paper on e-cigarettes (electronic nicotine delivery systems) San Francisco: UCSF Center for Tobacco Control Research and Education; 2013. [accessed 2018 Jan 1]. Available from: https://escholarship.org/uc/item/13p2b72n. [Google Scholar]
- 2.Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- 3.Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, Tsaprouni L, et al. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66:521–527. doi: 10.1136/thx.2010.156448. [DOI] [PubMed] [Google Scholar]
- 4.Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease–antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413–421. doi: 10.2147/COPD.S10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 6.Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9:375–383. doi: 10.1016/j.coph.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367:537–550. doi: 10.1007/s00441-016-2562-z. [DOI] [PubMed] [Google Scholar]
- 8.Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann N Y Acad Sci. 2015;1340:65–74. doi: 10.1111/nyas.12609. [DOI] [PubMed] [Google Scholar]
- 9.Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18. doi: 10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nutt DJ, Phillips LD, Balfour D, Curran HV, Dockrell M, Foulds J, et al. E-cigarettes are less harmful than smoking. Lancet. 2016;387:1160–1162. doi: 10.1016/S0140-6736(15)00253-6. [DOI] [PubMed] [Google Scholar]
- 11.Farsalinos KE, Voudris V, Poulas K. Are metals emitted from electronic cigarettes a reason for health concern? A risk-assessment analysis of currently available literature. Int J Environ Res Public Health. 2015;12:5215–5232. doi: 10.3390/ijerph120505215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schüssler J, Hahn H, et al. Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12:23. doi: 10.1186/s12971-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeill A, Brose LS, Calder R, Hitchman SC, Hajek P, McRobbie H.E-cigarettes: an evidence update London: Public Health England; 2015. PHE Publications Gateway Number 2015260 [Google Scholar]
- 15.U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. [accessed 2018 Jan 1]. Available from: https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. [Google Scholar]
- 16.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweitzer KS, Chen SX, Law S, Van Demark M, Poirier C, Justice MJ, et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol. 2015;309:L175–L187. doi: 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haswell LE, Baxter A, Banerjee A, Verrastro I, Mushonganono J, Adamson J, et al. Reduced biological effect of e-cigarette aerosol compared to cigarette smoke evaluated in vitro using normalized nicotine dose and RNA-seq–based toxicogenomics. Sci Rep. 2017;7:888. doi: 10.1038/s41598-017-00852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foy JW, Bombick BR, Bombick DW, Doolittle DJ, Mosberg AT, Swauger JE. A comparison of in vitro toxicities of cigarette smoke condensate from Eclipse cigarettes and four commercially available ultra low-“tar” cigarettes. Food Chem Toxicol. 2004;42:237–243. doi: 10.1016/j.fct.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Martin EM, Clapp PW, Rebuli ME, Pawlak EA, Glista-Baker E, Benowitz NL, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2016;311:L135–L144. doi: 10.1152/ajplung.00170.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, et al. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med. 2017;195:1043–1049. doi: 10.1164/rccm.201604-0804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reidel B, Radicioni G, Clapp P, Ford AA, Abdelwahab S, Rebuli ME, et al. E-cigarette use causes a unique innate immune response in the lung involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197:492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Thoracic Society; Medical Section of the American Lung Association. Guidelines for fiberoptic bronchoscopy in adults. Am Rev Respir Dis. 1987;136:1066. doi: 10.1164/ajrccm/136.4.1066. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan JT, Gilmore RC, Watson MJ, Archer CB, Tarran R. 17β-Estradiol inhibits phosphorylation of stromal interaction molecule 1 (STIM1) protein: implication for store-operated calcium entry and chronic lung diseases. J Biol Chem. 2013;288:33509–33518. doi: 10.1074/jbc.M113.486662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowell TR, Reeber SL, Lee SL, Harris RA, Nethery RC, Herring AH, et al. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2017;313:L52–L66. doi: 10.1152/ajplung.00392.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman I, De Cunto G, Sundar IK, Lungarella G. Vulnerability and genetic susceptibility to cigarette smoke–induced emphysema in mice. Am J Respir Cell Mol Biol. 2017;57:270–271. doi: 10.1165/rcmb.2017-0175ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax. 2016;71:1119–1129. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis ES, Sassano MF, Goodell H, Tarran R. E-liquid autofluorescence can be used as a marker of vaping deposition and third-hand vape exposure. Sci Rep. 2017;7:7459. doi: 10.1038/s41598-017-07862-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langner M, Hui SW. Merocyanine 540 as a fluorescence indicator for molecular packing stress at the onset of lamellar–hexagonal transition of phosphatidylethanolamine bilayers. Biochim Biophys Acta. 1999;1415:323–330. doi: 10.1016/s0005-2736(98)00185-0. [DOI] [PubMed] [Google Scholar]
- 31.Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet. 2011;88:450–457. doi: 10.1016/j.ajhg.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiling K, Kadar AY, Bergerat A, Flanigon J, Sridhar S, Shah V, et al. Comparison of proteomic and transcriptomic profiles in the bronchial airway epithelium of current and never smokers. PLoS One. 2009;4:e5043. doi: 10.1371/journal.pone.0005043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 34.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 35.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, et al. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 36.Yaucher NE, Fish JT, Smith HW, Wells JA. Propylene glycol–associated renal toxicity from lorazepam infusion. Pharmacotherapy. 2003;23:1094–1099. doi: 10.1592/phco.23.10.1094.32762. [DOI] [PubMed] [Google Scholar]
- 37.Doi AM, Roycroft JH, Herbert RA, Haseman JK, Hailey JR, Chou BJ, et al. Inhalation toxicology and carcinogenesis studies of propylene glycol mono-t-butyl ether in rats and mice. Toxicology. 2004;199:1–22. doi: 10.1016/j.tox.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Blake DA, Whikehart DR, Yu H, Vogel T, Roberts DD. Common cryopreservation media deplete corneal endothelial cell plasma membrane Na+,K+ ATPase activity. Curr Eye Res. 1996;15:263–271. doi: 10.3109/02713689609007620. [DOI] [PubMed] [Google Scholar]
- 39.Morshed KM, Jain SK, McMartin KE. Acute toxicity of propylene glycol: an assessment using cultured proximal tubule cells of human origin. Fundam Appl Toxicol. 1994;23:38–43. doi: 10.1006/faat.1994.1076. [DOI] [PubMed] [Google Scholar]
- 40.Madeira A, Moura TF, Soveral G. Aquaglyceroporins: implications in adipose biology and obesity. Cell Mol Life Sci. 2015;72:759–771. doi: 10.1007/s00018-014-1773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pocivavsek L, Gavrilov K, Cao KD, Chi EY, Li D, Lin B, et al. Glycerol-induced membrane stiffening: the role of viscous fluid adlayers. Biophys J. 2011;101:118–127. doi: 10.1016/j.bpj.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verkman AS. Aquaporins in clinical medicine. Annu Rev Med. 2012;63:303–316. doi: 10.1146/annurev-med-043010-193843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- 44.Malajczuk CJ, Hughes ZE, Mancera RL. Molecular dynamics simulations of the interactions of DMSO, mono- and polyhydroxylated cryosolvents with a hydrated phospholipid bilayer. Biochim Biophys Acta. 2013;1828:2041–2055. doi: 10.1016/j.bbamem.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 45.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sosnowski TR, Kramek-Romanowska K. Predicted deposition of e-cigarette aerosol in the human lungs. J Aerosol Med Pulm Drug Deliv. 2016;29:299–309. doi: 10.1089/jamp.2015.1268. [DOI] [PubMed] [Google Scholar]
- 47.Lodovici M, Luceri C, Guglielmi F, Bacci C, Akpan V, Fonnesu ML, et al. Benzo(a)pyrene diolepoxide (BPDE)–DNA adduct levels in leukocytes of smokers in relation to polymorphism of CYP1A1, GSTM1, GSTP1, GSTT1, and mEH. Cancer Epidemiol Biomarkers Prev. 2004;13:1342–1348. [PubMed] [Google Scholar]
- 48.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 49.Pastorelli R, Guanci M, Cerri A, Negri E, La Vecchia C, Fumagalli F, et al. Impact of inherited polymorphisms in glutathione S-transferase M1, microsomal epoxide hydrolase, cytochrome P450 enzymes on DNA, and blood protein adducts of benzo(a)pyrene-diolepoxide. Cancer Epidemiol Biomarkers Prev. 1998;7:703–709. [PubMed] [Google Scholar]
- 50.Doostdar H, Burke MD, Mayer RT. Bioflavonoids: selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology. 2000;144:31–38. doi: 10.1016/s0300-483x(99)00215-2. [DOI] [PubMed] [Google Scholar]
- 51.Lewis DF, Gillam EM, Everett SA, Shimada T. Molecular modelling of human CYP1B1 substrate interactions and investigation of allelic variant effects on metabolism. Chem Biol Interact. 2003;145:281–295. doi: 10.1016/s0009-2797(03)00021-8. [DOI] [PubMed] [Google Scholar]
- 52.Shimada T, Gillam EM, Sutter TR, Strickland PT, Guengerich FP, Yamazaki H. Oxidation of xenobiotics by recombinant human cytochrome P450 1B1. Drug Metab Dispos. 1997;25:617–622. [PubMed] [Google Scholar]
- 53.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102:1758–1766. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harraz OF, Altier C. STIM1-mediated bidirectional regulation of Ca2+ entry through voltage-gated calcium channels (VGCC) and calcium-release activated channels (CRAC) Front Cell Neurosci. 2014;8:43. doi: 10.3389/fncel.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, et al. Structural basis of Toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beane J, Sebastiani P, Liu G, Brody JS, Lenburg ME, Spira A. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biol. 2007;8:R201. doi: 10.1186/gb-2007-8-9-r201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh JS, Bullen C, Glantz SA. Clinical decisions: e-cigarettes and smoking cessation. N Engl J Med. 2016;374:2172–2174. doi: 10.1056/NEJMclde1602420. [DOI] [PubMed] [Google Scholar]